Abstract
Introduction:
The increase in blood nicotine after smoking a single cigarette is nicotine boost. We hypothesized that smokers with schizophrenia (SCZ) have a greater nicotine boost than controls without this disorder.
Methods:
Twenty-one subjects (11 SCZ and 10 controls, CON) had repeated venous blood sampling before, during, and after smoking a single cigarette after 12-hr abstinence to measure nicotine concentrations. Blood samples were drawn at baseline (before smoking) and 1, 2, 4, 6, 8, 10, 20, 30, 60, 90, and 120 min after the first puff. Groups were similar in baseline characteristics, including gender and level of dependence, and all smoked 20–30 cigarettes/day. Area under the serum nicotine concentration-time curve (AUC20) was calculated for time up to 20 min after the start of smoking.
Results:
The mean difference in AUC20 was significantly greater for SCZ versus CON (135.4 ng-min/ml; 95% CI = 0.45–283.80). The shape of the nicotine concentration-time curve for SCZ was significantly different compared with controls (p < .01). Nicotine boost in the first 4 min of smoking was higher in SCZ versus CON (25.2 vs. 11.1 ng/ml, p < .01) with no difference in the total time spent smoking.
Discussion:
This technique improves on methods, which draw only two blood specimens to assess nicotine intake. Understanding how nicotine boost differs in SCZ from CON may explain high levels of addiction and low success in cessation in smokers with SCZ.
Introduction
International studies have confirmed the higher prevalence of smoking in schizophrenia compared with the general population (de Leon & Diaz, 2005). Studies of smokers with schizophrenia show higher levels of nicotine and its metabolites (Strand & Nyback, 2005; Williams et al., 2005) and altered cigarette puffing (Tidey, Rohsenow, Kaplan, & Swift, 2005) despite normal rates of nicotine metabolism (Williams et al.).
The increase in levels of blood nicotine from smoking a single cigarette is referred to as “nicotine boost,” and there are theories about different smoking patterns and what they represent. So called, “peak seekers” who achieve a high nicotine boost may be seeking more positive reinforcement or arousal, whereas trough maintainers are suspected to smoke for reasons of negative reinforcement to avoid withdrawal (Russell & Feyerabend, 1978). Greater nicotine boost may be linked to increased addictive potential and increased risk for relapse after a quit attempt (Patterson et al., 2003). We hypothesized that smokers with schizophrenia would be more likely to be peak seekers with higher levels of nicotine boost from a single cigarette. The objective of this study was to measure venous nicotine blood levels after smoking a single cigarette in a laboratory-based design in smokers with schizophrenia and those without a current mental illness.
Methods
Subjects
Subjects were 21 smokers (11 schizophrenia, SCZ and 10 controls, CON) who also participated in a larger ongoing study of nicotine levels and smoking topography. Smokers were recruited sequentially if they smoked 20–30 cigarettes/day of a regular (no light or ultra-light) brand of cigarettes. All participants gave signed informed consent. The Institutional Review Board of Robert Wood Johnson Medical School approved the protocol.
All subjects with schizophrenia were enrolled in mental health treatment, stable on antipsychotic medications, and had their diagnosis confirmed with the Structured Clinical Interview for DSM-IV (Spitzer & Williams, 1985). Individuals with schizoaffective disorder or serious cognitive impairment were excluded. Controls smokers had to be without any mental illness within the last year and could not be taking an antidepressant, mood stabilizer, or anxiolytic for any reason.
Subjects using tobacco products other than cigarettes, pregnant smokers, or anyone with problematic substance use were excluded. Use of any tobacco treatment medications was also an exclusion. Participants were paid $15 for baseline assessments and $85 for the completion of all blood draws on Day 2. Three consented smokers were later excluded (one CON and one SCZ did not wish to participate and one SCZ for failing to meet criteria for overnight abstinence) leaving 21 subjects for analysis.
Study procedures
Subjects completed an assessment battery including a smoking history, demographic and medication questionnaire, the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), and assessments of symptom severity (Positive and Negative Syndrome Scale; Kay, Opler, & Lindenmayer, 1989; SCZ only). Subjects had a baseline expired carbon monoxide (CO) reading using an EC-50 Smokerlyzer (Bedfont Scientific, Williamsburg, VA). Subjects were instructed to abstain completely from cigarettes after 8 p.m. that night, and the remainder of data collection took place at the Environmental and Occupational Health Sciences Institute Controlled Environment Facility, an indoor environment for smoking. Upon arrival on Day 2, subjects were required to provide an expired CO reading of less than 15 parts per million to verify overnight abstinence, which has been used by other investigators (Loughead et al., 2009; McBride, Barrett, Kelly, Aw, & Dagher, 2006; Shiffman et al., 2003), and was selected since the study included heavier smokers. Any participant who did not meet abstinence criteria was sent home and given one opportunity to reschedule Day 2 testing.
An indwelling venous catheter was placed and a baseline blood sample obtained prior to smoking. Subjects completed questionnaires assessing their urges to smoke (Questionnaire on Smoking Urges [QSU] Brief Form; Cox, Tiffany, & Christen, 2001), mood states (Positive and Negative Affect Schedule [PANAS]; Watson, Clark, & Tellegen, 1988), and nicotine withdrawal symptoms (Wisconsin Smoking Withdrawal Scale [WSWS]; Welsch et al. 1999). Subjects were then instructed to smoke one of their own cigarettes ad libitum. Time spent smoking was measured from first to last puff. Subjects underwent repeated venous blood measures from the indwelling catheter at 1, 2, 4, 6, 8, 10, 20, 30, 60, 90, and 120 min after the first puff and had an expired CO reading at 60 min after smoking. Serum was frozen at −20 °C and then sent to the Clinical Pharmacology Laboratory at the University of California, San Francisco, for analysis of nicotine, cotinine (COT), and 3-hydroxycotinine (3HC), which were quantified by liquid chromatography–mass spectrometry (Dempsey et al., 2004).
Statistical analysis
Two-sample t tests and chi-square tests were used to compare the differences in sociodemographic variables and symptom scores between groups. A ratio of 3HC to COT (3HC/COT) was calculated. The time to peak, serum nicotine, and CO values were compared between groups using the nonparametric Wilcoxon test. The area under the serum nicotine concentration-time curve (AUC) was calculated using the trapezoidal rule for time up to 20 min (AUC20) and 120 min (AUC120). Twenty minutes was selected since nicotine blood levels decline rapidly in 20 min after smoking due to tissue distribution (Benowitz et al., 1990). Last value carried forward was applied in the calculation of AUC for any missing data. Between-group differences were calculated and the bootstrap method (Efron & Tibshirani, 1993) with 1,000 resamplings of data used to derive 95% CIs.
Continuous nicotine concentration-time curves for SCZ and CON smokers were modeled by the polynomial random effects regression analysis. Polynomials of time were used to model the shape of the curves, and a random intercept was applied to account for the intrasubject correlation in repeated measures. Shapes of the curves were compared between SCZ and CON by testing the difference in the polynomial coefficients using the Wald’s chi-square tests. Estimated nicotine peaks and time to peak were then derived from the polynomial curves based on the quasi–Newton method (Fletcher, 1987) and the bootstrap method for the 95% CIs. All statistical analysis was performed using SPSS v.16.0 and R package.
Results
Baseline comparisons between smokers with schizophrenia versus controls
No differences were detected between groups on smoking or demographic characteristics except baseline CO, which was not controlled for time of day or time since last cigarette (Table 1). CO values at 8:30 a.m. on Day 2 were consistent with 12-hr abstinence, and all subjects had at least a 50% reduction in CO level from baseline. Nicotine levels at 8:30 a.m. on Day 2 were not different between groups and consistent with abstinence. 3HC/COT ratios were not different between groups. All SCZ subjects were taking antipsychotic medications; 91% were taking an atypical antipsychotic.
Table 1.
Baseline characteristics and laboratory measures of smokers with schizophrenia compared with control smokers (N = 21)
| Schizophrenia group (n = 11) |
Control group (n = 10) |
||
| M (SD) | M (SD) | p valuea | |
| Cigarettes per day | 21.8 (4.0) | 21.5 (3.4) | .848 |
| Baseline CO (ppm) | 25.7 (10.4) | 18.1 (5.9) | .050 |
| FTND | 6.6 (1.3) | 5.4 (2.3) | .137 |
| Age of first smoking (years) | 13.45 (2.7) | 15.80 (3.3) | .089 |
| Age (years) | 40.2 (12.5) | 45.8 (11.1) | .293 |
| PANSS positive | 16.63 (5.18) | — | — |
| PANSS negative | 16.18 (4.62) | — | — |
| Race/ethnicity | Count (%) | Count(%) | .183 |
| Black | 6 (54.5) | 2 (20.0) | |
| Caucasian | 5 (45.5) | 8 (80.0) | |
| Male gender | 8 (72.7) | 7 (70.0) | 1.000 |
| Education | Count (%) | Count(%) | .158 |
| No high school | 4 (36.4) | 0 (0.0) | |
| High school | 4 (36.4) | 5 (50.0) | |
| Some college | 3 (27.3) | 4 (40.0) | |
| Bachelors degree or higher | 0 (0.0) |
1 (10.0) |
|
|
M (SD) |
M (SD) |
p value |
|
| Time spent smoking cigarette | 5 min, 16 s (2 min, 29 s) | 5 min, 12 s (1 min, 8 s) | .934 |
| Pre-cig expired CO (ppm) | 8.4 (3.0) | 6.0 (1.8) | .042 |
| Pre-cig serum nicotine (ng/ml) | 4.4 (5.4) | 4.9 (6.0) | .819 |
| Time to reach nicotine peak (min) | 4.8 (2.2) | 6.4 (3.0) | .179 |
| Measured nicotine peak (ng/ml) | 33.1 (16.0) | 25.9 (16.7) | .324 |
| Post-cig (60 min) expired CO (ppm) | 10.3 (2.2) | 8.7 (2.0) | .108 |
| Four-minute nicotine boost (ng/ml) | 25.2 (13.5) | 11.1 (7.5) | .009 |
| Maximum nicotine boost (ng/ml) | 28.8 (13.2) | 21.0 (13.9) | .181 |
| 3HC/COT ratios | 0.486 (0.148) |
0.579 (0.212) |
.289 |
| Mean (95% CI) |
Mean (95% CI) |
Difference (95% CI) |
|
| AUC20 (ng-min/ml) | 412.7 (303.9–544.8) | 277.3 (219.1–348.4) | 135.4 (0.45–283.8) |
| AUC120 (ng-min/ml) | 1291.8 (882.0–1923.9) | 883.3 (767.6–1012.8) | 408.4 (−25.5 to 1031.5) |
| Derived nicotine peak (ng/ml) | 30.0 (22.3–39.1) | 18.9 (13.8–25.2) | 11.1(1.37–22.1) |
Note. 3HC = 3-hydroxycotinine; AUC = area under the curve; CO = carbon monoxide; COT = cotinine; FTND = Fagerström Test for Nicotine Dependence; PANSS = Positive and Negative Syndrome Scale; ppm = parts per million.
Independent sample t test or chi-square test.
Withdrawal, craving, and affective states after overnight abstinence
No differences were detected between groups on mean values for WSWS subscales or composite score. There was a trend for higher subscale score on impaired concentration for SCZ versus CON (1.94 vs. 1.30, p = .09). Items from the QSU were collapsed into two factors: “intention to smoke” (Factor 1) and “anticipation of relief from withdrawal” (Factor 2). There was a trend for higher subscale score on Factor 2 for SCZ versus CON (58.18 vs. 33.67, p = .06) but no differences for QSU general factor or Factor 1 scores. No significant differences were detected between groups on mean values for PANAS positive or negative subscales although the negative subscale scores for SCZ were twice as high (7.30 vs. 3.00).
Nicotine peak concentrations, area under nicotine concentration-time curve, and time to peak concentration
The length of time spent smoking the laboratory cigarette was not significantly different between groups. The serum nicotine peak was higher in SCZ versus CON but not statistically significant (33.1 vs. 25.9 ng/ml). Time to reach the peak was also shorter in SCZ (4.8 vs. 6.4 min). Expired CO values were measured at 60 min after smoking, so a true CO boost could not be determined.
We first calculated the nicotine boost as the mean of the differences between the observed nicotine value taken at 4 min and the baseline (pre-cig) nicotine level. Four minutes was chosen since this was the mean time to reach the peak in SCZ. Four-minute nicotine boost was higher in SCZ versus CON (25.2 vs. 11.1 ng/ml, t = 2.92, df = 19, p < .01). We also calculated the maximum nicotine boost as the mean of the differences between the highest observed nicotine for each subject and the baseline (pre-cig) nicotine level. Maximum nicotine boost was higher in SCZ versus CON but not statistically significant (28.8 vs. 21.0 ng/ml).
Smokers with SCZ had higher mean values for AUC20 compared with CON (412.7 vs. 277.3 ng-min/ml). The mean difference in AUC20 was significantly greater for SCZ versus CON (135.4 ng-min/ml, 95% CI = 0.5–283.8). There was a trend for higher mean values for AUC120 in SCZ (1291.8 ng-min/ml, 95% CI = 882.0–1923.9) versus CON (883.3 ng-min/ml, 95% CI = 767.6–1012.8). The mean difference in AUC120 for SCZ versus CON was not significant (408.4 ng-min/ml, 95% CI = −25.5 to 1031.5).
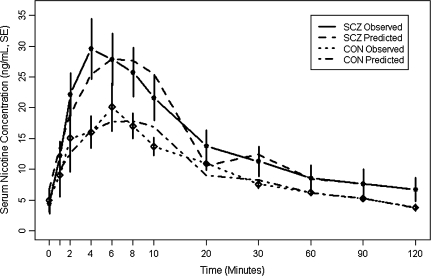
We next modeled the serum nicotine concentration curve as a function of time for SCZ and CON separately using the polynomial random effects models. The shape of these two curves was statistically different by comparing the polynomial coefficients using the Wald test (chi-square = 19.84, df = 6, p < .01); this implied that the slope of the nicotine intake curve for SCZ was significantly different compared with controls (Figure 1). Estimated nicotine peak concentrations and time to peak based on the fitted curves were higher in SCZ (28.1 ng/ml, 95% CI = 21.1–36.5) versus CON (17.9 ng/ml, 95% CI = 13.5–23.3). Nicotine peaks estimated from the polynomial curves were 10.1 ng/ml higher (95% CI = 1.07–19.68) for SCZ than CON. Time to peak was 0.46 min sooner in SCZ versus CON but not statistically significant (95% CI = −2.84 to 2.00). Results were unchanged when we repeated analyses adjusting for PANAS negative and QSU Factor 2 scores.
Figure 1.
Predicted values of SCZ and CON were obtained from the polynomial mixed model analysis with fixed effects (p < .001 for time to the sixth power for both SCZ and CON): y = 11.67 + 0.97 × (time − 29.25) + 0.07 × (time − 29.25)2 − 0.003 × (time − 29.25)3 − 7.01 × 10−05 × (time − 29.25)4 + 2.87 × 10−6 × (time − 29.25)5 − 2.01 × 10−8 × (time − 29.25)6 for SCZ and y = 8.01 + 0.30 × (time − 29.25) + 0.03 × (time − 29.25)2 − 0.001 × (time − 29.25)3 − 3.01 × 10−5 × (time − 29.25)4 + 1.34 × 10−6 × (time − 29.25)5 − 9.17 × 10−8 × (time − 29.25)6 for CON. To minimize extrapolation error due to no measurements between the following intervals, the polynomial curves over these intervals were connected by straight line segments between 10 and 20, 20 and 30, 30 and 60, 60 and 90, and 90 and 120 min.
Discussion
This is the first study of nicotine intake in schizophrenia that has repeated blood measures from cigarette smoking after an overnight period of abstinence. This design allows for precise measurement of nicotine peak concentration and the boost that comes from smoking a single cigarette. In addition, we are able to characterize nicotine levels for 2 hr after smoking in order to estimate the total nicotine dose obtained by each smoker. Assuming that clearance of nicotine is similar across subjects, the systemic intake of nicotine is proportional to the serum nicotine AUC. Clearance is in fact quite variable from person to person, but average clearance values for groups are expected to be similar. In particular, we have found no difference in nicotine clearance based on a biomarker of rate of nicotine metabolism in SCZ versus CON in our prior studies (Williams et al., 2005) and in this sample.
Based on the AUC data, SCZ tended toward a higher dose of nicotine per cigarette than CON. The modeled peak serum nicotine concentration and the observed 4-min nicotine boost were also higher in SCZ. The slope of the nicotine intake curve for SCZ, modeled by cubic polynomial functions, was significantly different compared with controls. The modeled curve has advantages compared with the measured values when determining nicotine intake and peak. Even with frequent nicotine sampling as described in our sample, it is possible that we missed the actual peak level of subjects, whereas estimates derived from the curve are continuous and likely a more accurate reflection of nicotine peak concentration and peak time. Sample size may have limited our ability to detect significant differences in other variables, including time to peak and AUC120. A potential limitation of this study is that all subjects with SCZ were taking antipsychotic medications. Overall, our data suggest that SCZ smoke cigarettes differently than CON, resulting in higher peak nicotine levels, faster rate of rise of nicotine levels, and higher systemic doses of nicotine from the first cigarette of the day, which may have implications for higher levels of dependence and success in quitting smoking.
Funding
This work was supported by a grant from the National Institute of Mental Health (MH076672-01A1 to JMW) and from the National Institute on Drug Abuse (DA12393 to NLB). This research was also supported in part by the NIEHS sponsored University of Medicine and Dentistry of New Jersey Center for Environmental Exposures and Disease, Grant no. NIEHS P30ES005022.
Declaration of Interests
JMW receives research support from Pfizer.
References
- Benowitz NL, Porchet H, Jacob P. Pharmacokinetics, metabolism and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine psychopharmacology: Molecular, cellular, and behavioural aspects. Oxford, UK: Oxford University Press; 1990. pp. 112–157. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology and Therapeutics. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Fletcher R. Practical methods of optimization. 2nd ed. New York: John Wiley & Sons; 1987. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale and standardisation. British Journal of Psychiatry Supplement. 1989;7:59–67. [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, et al. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Molecular Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, et al. Individual differences in nicotine intake per cigarette. Cancer Epidemiology, Biomarkers & Prevention. 2003;12:468–471. [PubMed] [Google Scholar]
- Russell MA, Feyerabend C. Cigarette smoking: A dependence on high-nicotine boli. Drug Metabolism Reviews. 1978;8:29–57. doi: 10.3109/03602537808993776. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shadel WG, Niaura R, Khayrallah MA, Jorenby DE, Ryan CF, et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology (Berlin) 2003;166:343–350. doi: 10.1007/s00213-002-1338-1. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured clinical interview for DSM-III-R. New York: Biometrics Research Department, New York State Psychiatric Institute; 1985. [Google Scholar]
- Strand JE, Nyback H. Tobacco use in schizophrenia: A study of cotinine concentrations in the saliva of patients and controls. European Psychiatry. 2005;20:50–54. doi: 10.1016/j.eurpsy.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug and Alcohol Dependence. 2005;80:259–265. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophrenia Research. 2005;79:323–335. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]