Abstract
Industrially produced trans fatty acids (TFAs) consumed in western diets are incorporated into maternal and fetal tissues, and are passed linearly to offspring via breast milk. We hypothesized that TFA exposure in utero and during lactation in infants would promote obesity and poor glycemic control as compared to unmodified fatty acids. We further hypothesized that in utero exposure alone may program for these outcomes in adulthood. To test this hypothesis we fed female C57/BL6 mice identical western diets that differed only in cis- or trans-isomers of C18:1 and then aimed to determine whether maternal transfer of TFAs through pregnancy and lactation alters growth, body composition and glucose metabolism. Mice were unexposed, exposed during pregnancy, during lactation, or throughout pregnancy and lactation to TFA. Body weight and composition (by computed tomography), and glucose metabolism we assessed at weaning and adulthood. TFA exposure through breast milk caused significant early growth retardation (p<0.001) and higher fasting glucose (p=0.01) but insulin sensitivity was not different. Elevated plasma insulin-like growth factor-1 in mice consuming TFA-enriched milk (p=0.02) may contribute to later catch-up growth, leanness and preserved peripheral insulin sensitivity observed in these mice. Mice exposed to TFA in utero underwent rapid early neonatal growth with TFA-free breast milk and had significantly impaired insulin sensitivity (p<0.05) and greater abdominal fat (p=0.01). We conclude that very early catch-up growth resulted in impaired peripheral insulin sensitivity in this model of diet-related fetal and neonatal programming. TFA surprisingly retarded growth and adiposity while still adversely affecting glucose metabolism.
Keywords: Elaidic acid, trans fat, breast feeding, fetal programming, milk fat, mice
1. Introduction
A growing body of evidence suggests that birth weight and early neonatal nutrition is the platform on which an individual’s propensity to develop metabolic syndrome (obesity, insulin resistance and dyslipidemia) is based [1]. This theory is referred to as the ‘common soil’ hypothesis, whereby low birth weight and acceleration of growth in childhood, also known as ‘catch-up growth’, is associated with increased prevalence of cardiovascular disease and insulin resistance later in life [2, 3]. Currently, food products consumed in a typical western diet contain a significant proportion of fats that have been industrially altered. It is estimated that 2–8% of energy needs in the typical western diet come from chemically modified lipid products [4–6]. Trans fatty acids (TFA) are predominantly formed by industrial chemical modification of natural botanical lipids and are currently in widespread use today. Previously we reported that replacing 8% of energy supplied by unmodified lipids with chemically modified lipids results in visceral obesity and altered carbohydrate metabolism in mature male nonhuman primates [7]. The Nurse’s Health Study provides further epidemiological evidence that the incidence of type 2 diabetes (T2DM) could be reduced by greater than 40% if lipids were consumed in their original, unhydrogenated form [5].
Breast milk is the healthier alternative to milk formula [8]; however, milk formula represents a controlled intake of fats which are mostly botanical in nature, whereas breast milk from residents of western countries may contain significant quantities of chemically modified lipids such as TFA. Studies have demonstrated that maternal TFA intake is inversely associated with TFA content of fetal tissues [9, 10]. Natural fats and TFA consumption by the mother are linearly related to their respective content measured within breast milk [10] and are temporally related such that daily variation in TFA intake is reflected in daily breast milk concentrations [11], and TFA transmission from breast milk to the neonate has been documented [9, 12]. Prior reports have indicated that, on average, breast milk contains about 7 g/100 ml milk fat. In a mother consuming a typical western diet, this equates to an infant receiving on average 3.5% (range from 1–10%) of energy supplied as TFA in a milk diet that supplies 50% of energy from fat [13], which is in the range where we have previously reported metabolic effects.
The current prevalence and future predictions for the impact of metabolic syndrome in an increasingly obese population makes investigations into the mechanisms of metabolic disease development critical [14]. How much the in utero environment dictates birth weight and the programming of long term obesity-related disorders is still unclear, especially when compared with that of early neonatal growth rate. We hypothesized that industrially produced TFA exposure in utero and during lactation in infants would promote obesity and poor glycemic control as compared to unmodified fatty acids, as seen in primates and adult people [5, 7, 15]. Rapid development in the first two weeks has been linked to insulin resistance and, conversely, lower nutrient diets predict later cardiovascular disease risk [2]. We further hypothesized that in utero exposure alone may then program for obesity and insulin resistance in adulthood. Maternal fat intake, whether pre- or post-natal and whether unmodified or chemically modified, may affect growth and development and provide an important common link in predisposition of offspring to metabolic disease. The aim of this study was to extend our previous findings that TFA augments visceral obesity and associated insulin resistance in adult nonhuman primates [7] by examining the ‘common soil’ hypothesis of early fetal and neonatal development. We examine our hypothesis by the evaluation of growth, adiposity, and glycemic control of mice known to be prone to obesity and insulin resistance when consuming a high fat diet, following exposure to TFA during pregnancy and lactation.
2. Methods and Materials
2.1. Study Animals
All experimental procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences.
2.2. Experimental Diets
The two experimental diets (cis- and trans-fat; CIS and TRANS hereafter) were formulated in-house (Wake Forest University Primate Center Diet Laboratory, Winston-Salem, NC) and supplied ad libitum to the study mice. Macronutrient content was similar to high fat diets of western consumption [16], with dietary fats constituting 36%, protein 16% and carbohydrate 48%, of total metabolizable energy (ME). Additionally they were high in simple refined sugars (>40% of carbohydrates supplied) and salt (>2 mg/kcal) [16]. Fat was supplied in both diets as a blend of fatty acids (A.C. Humko Foods, Memphis, TN) and made up of differing percentages of sunflower oil, partially hydrogenated and non-hydrogenated soybean oil, cottonseed oil, and palm oil (Table 1). The only major difference between the two diets (36% of calories as fat for each) was in their respective fatty acid compositions for C18:1. Elaidic acid (18:1n-9t) and other 18:1 trans species was supplied solely by partially hydrogenated soybean oil. The goal of the study was to evaluate specific effects of this dietary ingredient when fed in the typical context of the western diet. A small amount of fish oil (OmegaPure, Omega Protein Inc., Houston, TX) supplemented the diets to ensure adequate supplementation of long chain polyunsaturated fatty acids (LC-PUFAs) for maternal and neonatal health. Diets were unbaked and freshly made, thus addition of antioxidants (Tenox 20A [Eastman Kodak Co., Winston-Salem, NC], vitamin E and MTS-50 [ADM, Decatur, IL]) was necessary to permit refrigerated storage until use. All dietary components were sourced from Harlan-Teklad (Madison, WI) or Reidsville Grocery (Reidsville, NC) unless otherwise indicated. The fat blends in the two diet formulations were analyzed four times, coincident with each new batch of diet prepared (see Table 2).
Table 1.
Ingredient composition (g/100g) of diets fed to mice
| Ingredient | |
|---|---|
| Fat Blend* | 15 |
| Casein, USP | 8.5 |
| Lactalbumin | 6 |
| Dextrin | 9.9 |
| Sucrose | 9.32 |
| Wheat Flour, self-rising | 34 |
| Alphacel | 6.85 |
| Menhaden fish oil | 0.35 |
| Crystalline Cholesterol | 0.05 |
| Complete Vitamin Mix | 2.5 |
| Vitamin Mixture, Teklad | 2.5 |
| Hegsted Salt Mixture | 5 |
| Beta-sitosterol | 0.01 |
| MTS-50 | 0.018 |
| Vitamin E | 0.002 |
| Tenox 20A | 0.004 |
The fat sources (as weight basis) consisted of sunflower oil (45%), non-hydrogenated soybean oil (10%), cottonseed oil (10%) and palm stearine (35%) for the cis diet and partially-hydrogenated soybean oil (55%), cottonseed oil (25%) and palm stearine (20%) for the trans diet.
Table 2.
Fatty acid composition of diets fed to dams and their offspring and the milk from the dams.
| Fatty acid | Cis diet | Trans diet | Cis-fed breast milk | Trans-fed breast milk |
|---|---|---|---|---|
| ≤12:0 | 0.1 ± 0.05 | 0.1 ± 0.2 | 7.6 ± 2.3 | 11.9 ± 1.0 |
| 14:0 | 0.8 ± 0.2 | 0.9 ± 0.2 | 7.3 ± 1.9 | 7.7 ± 0.4 |
| 16:0 | 22.9 ± 2.9 | 23.4 ± 2.2 | 26.3 ± 0.6 | 22.6 ± 0.6 |
| 18:0 | 3.9 ± 0.4 | 5.4 ± 0.2 | 2.5 ± 0.1 | 2.8 ± 0.1 |
| 18:1t | 0.6 ± 0.08 | 26.9 ± 3.4 | 0.7 ± 0.3 | 11.9 ± 0.5 |
| 18:1c | 51.0 ± 2.7 | 18.1 ± 3.9 | 42.3 ± 3.6 | 27.4 ± 1.1 |
| 18:2 | 16.7 ± 1.1 | 17.7 ± 0.7 | 7.6 ± 0.5 | 10.7 ± 0.3 |
| 18:3 | 1.1 ± 0.3 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.02 |
| 20:5 | 0.2 ± 0.05 | 0.2 ± 0.03 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 22:5 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 22:6 | 0.2 ± 0.04 | 0.2 ± 0.03 | 0.2 ± 0.05 | 0.3 ± 0.04 |
| Other | 1.0 ± 0.6 | 6.1 ± 0.3 | 5.5 ± 0.7 | 4.5 ± 0.4 |
Values are means ± SEM of n=6.
The 18:1 trans represents the total 18 carbon trans monoenes.
2.3. Experimental Design
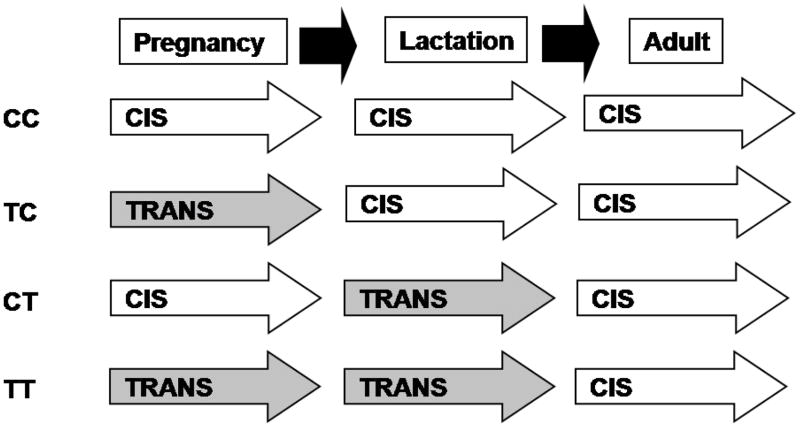
Thirty-two 6-week-old nulliparous adult female C57/BL6 mice, a strain prone to development of obesity and diabetes (Jackson Laboratory, Bar Harbor, ME), were randomly assigned to initially to one of two diets (CIS and TRANS) and then at late gestation, they were randomized again to create four groups (Figure 1). The control group (CC) was fed the CIS diet throughout the study. A second group (TC) was fed the TRANS diet from the onset of the study through gestation only. A third group (CT) began on the CIS diet and then was fed the TRANS diet during lactation only, and a fourth group (TT) was fed the TRANS diet through both gestation and lactation. After weaning, all mice were fed CIS diet until termination of study.
Figure 1.
Study design denoting the dietary type and timing of exposure to CIS (open arrows) and TRANS (grey arrows). Diets were initiated in sibling females at 6 weeks (n=8/group) with breeding attempts from 8 weeks of age. Only litters from primiparous females were evaluated (n= 5–7/group) with endpoints at weaning (day 21) and adulthood (day 60–70) made in offspring from all litters balanced by sex.
Mice were group-housed (4/cage) and allowed to acclimate to the experimental conditions and diet for 2 weeks prior to breeding. Four males of the same age were rotated through the groups. After three days, the males were rotated to a new cage. Each morning during the breeding period, the females were examined for vaginal plugs as evidence of copulation. Toward the end of the gestation period, the females were separated and checked twice a day for new litters and to facilitate required dietary changes anticipated in groups TC and CT. Only first litters were used in this study because of the need to standardize variables related to effects of litter number on milk yield, and dam effects (body weight, dietary exposure, and age [17], litter size, and birth weights) [18]. No attempt to standardize litter sizes was made. Pups were weighed on the day of birth as soon as detected using a Denver Instruments APX-323 balance, and appropriate diet changes made in Groups TC and CC (Figure 1). Thereafter, the pups were weighed weekly.
During lactation, milk samples were obtained by aspiration of stomach contents from one pup that was randomly selected from each litter. Milk samples were collected between post-parturition days 10 to 14 for analysis of fatty acid composition (Table 2). Pups were weaned on day 21. Nineteen to 21 days after birth, pups from each litter were randomly assigned to either a glucose tolerance test (GTT) to measure glucose excursions following exogenous glucose administration, or to an insulin-tolerance test (ITT) whereby glucose disposal as a result of insulin administration is followed over time. The tests were chosen to assess insulin sensitivity. Computed tomography (CT) scanning was performed on at least 2 pups, males and females, from each litter to evaluate body fat composition at the same time. After weaning (21 days), all mice were placed on the CIS diet until the conclusion of the experiment. At 60–70 days, the pups were again randomly separated into groups balanced by sex for another set of CT scans, GTTs, and ITTs.
Blood samples at weaning and adulthood were taken via submandibular venous collection. All blood samples (fasting, glucose and insulin tolerance testing) were taken after an overnight fast, with 2 hours allowed for acclimation to the testing or sampling environment to minimize stress effects. Samples were collected into EDTA-treated vials and processed promptly to collect plasma. Blood was analyzed for glucose using a Precision QID glucometer (Abbott Laboratories, Abbott Park, IL). Plasma insulin and insulin-like growth factor 1 (IGF-1) were measured using ELISA kits (Mercodia, Uppsala, Sweden) and free fatty acids (FFA) by enzymatic determinations (Wako Chemicals USA, Richmond, VA). Whole blood was aliquoted for measurement of glycation of hemoglobin A1c (HbA1c%) levels via HPLC (Primus PDQ, Primus Diagnostics, Kansas City, MO).
2.4. Fatty acid analysis of breast milk and experimental diets
Samples were analyzed via gas chromatography for fatty acid methyl ester (FAME) residues [19] by gas liquid chromatography (GLC) on a CP-Select CB for FAME capillary column (100m × 0.25 mm ID, Part number CP7420, ChromPack) with a deactivated guard column (0.53 mm ID) installed in a temperature programmed HP 5890 Series II gas chromatograph equipped with an on-column capillary inlet, flame ionization detector (FID), and HP7673 autosampler/injector. The chromatographic conditions were: H2 carrier gas, 20 psi head pressure, 1.25 mL/min at 90°C; He make-up gas, 23 mL/min; inlet temperature at 3°C above the oven temperature; and FID at 230°C. The oven temperature was programmed to begin at 90°C and hold for 0.5 min, increase at 10°C per min to 150°C, increase at 2.5°C per min to 200°C, increase at 1.5°C per min to the final temperature, 220°C, and hold at 220°C for 20 min. Total run time is 60 min plus a 5-min equilibration period between runs. This temperature program results in the C18:1 trans fatty acid methyl esters eluting prior to the C18:1 cis fatty acid methyl esters. Chromatographic data collection and analysis is via a serial connection to a 300 MHz Intel Pentium II personal computer running Chrom Perfect™ Spirit Chromatography Data System (Justice Laboratory Software) in Microsoft Windows NT. Each chromatogram is examined for correct identification of constituent fatty acids and quality control. An elaidic acid standard was utilized to characterize the position and assist in quantification of C18:1 trans fatty acid species.
2.5. Glucose and insulin tolerance testing
Methods for GTT and ITT were modified from standard protocols [20] and involved serial measures of glucose following intraperitoneal injection of glucose for the GTT (1 mg/g, administered using 10% dextrose solution via a 29g needle) or insulin for the ITT (0.75 mU/g regular insulin via a 29g needle). In both cases, glucose was then measured by glucometer from a drop of blood obtained by tail-bleed at pre-injection and 10, 20, 30, 60, and 120 minutes post-injection. Areas under the curve above baseline glucose were calculated using the trapezoidal method.
2.6. Body Composition
CT scans were performed both at weaning and adulthood to accurately quantify fat mass, as previously described [7]. Body fat was quantified by identifying the region of interest from the pubic symphysis to a superior border defined as the most cranial slice that excluded the lungs. This region includes the gonadal tissues and associated fat pads. Thresholds of −140 to 40 Hounsfield units were applied to isolate the fat-containing voxels and the total voxel volumes quantified. Scans were performed on a Toshiba 32-slice Aquilion CT scanner (Toshiba America Medical Systems, Tustin, CA) with 0.5 mm slice collimation and settings at 100 kVp and 300 mAs. Fat mass was calculated from volume results (corrected for fat density of 0.918g/cm3) and was expressed as a percentage of the animal’s total body weight measured on that day.
2.7. Statistical Analyses
All results are reported and depicted as means ± standard error of the mean (SEM) with significance defined as P ≤ 0.05. Ten observations or more for each parameter in each group were made unless otherwise indicated. The sample size of 10 observations per group was based on power analysis conducted using abdominal adiposity measures from primate studies [7] which demonstrated that this number of observations would achieve 80% power at the α = 0.05 level for detection of group differences in diet-related adiposity. Statistical analyses were performed using Statistica 6 (StatSoft Inc., Tulsa, OK). Log transformation of FFA concentrations was performed as this variable was not normally distributed and had heterogeneous variances, and 95% confidence intervals are supplied for this parameter rather than SEM. One-way analysis of variance ANOVA was performed to test for group differences. At weaning and adulthood, the effects of diet during the gestation and lactational growth stages, and interaction of these effects were assessed by 2-way ANOVA. If a significant difference was found, post-hoc analysis for group differences was performed using Tukey’s test for honestly significant differences. Sex distribution of offspring was assessed by the binomial test.
3. Results
3.1. Dietary and breast milk TFA
Approximately 9% of total metabolizable energy (ME) was supplied as trans isomers in mother mice fed the TRANS diet (as calculated from the data presented in Table 2). Milk composition in mice is approximately 20% triglycerides; thus, most calories taken in by neonates were derived from fat [18, 21]. Fatty acid composition of milk (Table 2) indicated that 7–8% of ME for the offspring was supplied by TFA in CT and TT during lactation (n=6–7/group), which reflects accurately the dams’ increased TFA intake [11–13].
3.2. Birth weight and growth
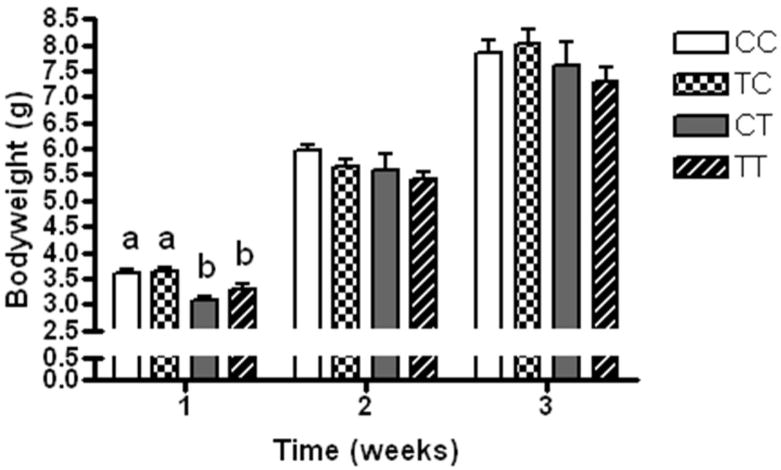
The body weights of the breeding females were comparable at breeding initiation (p=0.41). The sex distribution of offspring was equivalent between CIS and TRANS diet groups (p>0.05). We observed similar birth weights (n=65–75/group 1.28 vs. 1.30 g) with slightly smaller offspring of dams consuming the TRANS diet during pregnancy, which was not statistically significant (p=0.15). We also observed similar but smaller mean litter sizes in dams consuming the TRANS diet during pregnancy, with 6.6±0.5 (range 4–9) pups per litter versus 8.0±0.8 (range 5–13) pups per litter born from CIS-fed pregnancies which were not statistically significant different (n=11–12 litters/gp; p=0.14). CT and TT pups were significantly smaller at 1wk than their CIS-fed counterparts (p<0.0001; Figure 2). In the groups exposed to the TRANS diet during lactation (CT and TT), growth appeared delayed in the first week but accelerated during the second week, such that they had comparable body weights at 2 weeks. No intergroup differences in body weight were observed at weaning.
Figure 2.
Growth of mice exposed to CIS diets during in utero and through breastfeeding during neonatal growth periods (CC; white bar), TRANS diet in utero and CIS diet during breastfeeding (TC; checked bar), CIS diet in utero and TRANS diet during breastfeeding (CT; grey bar) and TRANS diet during in utero and breastfeeding growth periods (TT; diagonal striped bar). Means ± SEM depicted with different letters denoting statistical differences at α < 0.05 following ANOVA with sample sizes of 15–29 for each group.
At adulthood, female offspring exposed to TFA during pregnancy were still smaller (Table 3; p=0.04), but no effect of diet during pregnancy was detected in the males. Instead, we observed a tendency for males to be smaller following TFA exposure during lactation (Table 3; p=0.06).
Table 3.
Body weight recorded at adulthood (60–70 days of age) from mice exposed to CIS (C) and TRANS (T) dietary fatty acids during in utero growth and neonatal development. All offspring were weaned (21 days) to the CIS diet.
| Sex | Measurement | Dietary Groups | P values | |||||
|---|---|---|---|---|---|---|---|---|
| CC | TC | CT | TT | Diet (P) | Diet (L) | Interaction | ||
| Female | Adult body weight (g) | 19.6 ± 0.55 | 18.81 ± 0.30 | 19.54 ± 0.33 | 18.06 ± 0.64 | 0.04 | 0.45 | 0.52 |
| Male | Adult body weight (g) | 25.44 ± 0.55 | 27.13 ± 0.70 | 24.92 ± 0.90 | 24.68 ± 0.61 | 0.35 | 0.06 | 0.22 |
Values are means ± SEM (n=5–16/group).
Two-way ANOVA analysis of diet exposure at each developmental stage with statistics for each effect and their interaction are shown. Females exposed to trans fatty acid during pregnancy were smaller at adulthood.
3.3. Body composition
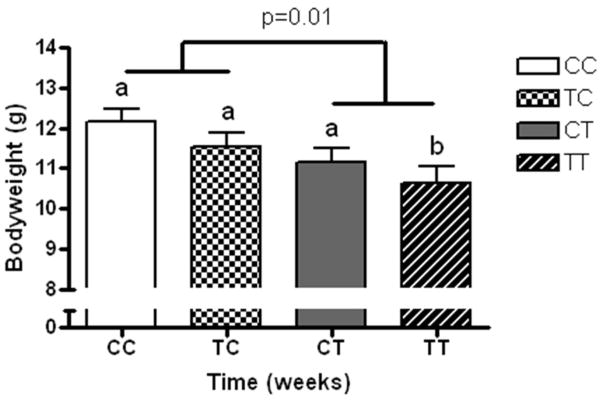
CT scanning at weaning demonstrated that offspring with continuous exposure to the TRANS diet through pregnancy and lactation were significantly leaner (lower abdominal fat as a percentage of body weight (Figure 3; p=0.03)) than all other groups of mice. There was no effect of pregnancy diet on body composition of offspring; however, independent of the type of diet given during pregnancy, there was less abdominal fat in pups exposed to the TRANS diet through lactation (CC and TC vs. CT and TT; p=0.01) than those exposed to the CIS diet during the same period. Body composition changes persisted into adulthood with abdominal fat as a percentage of body weight following a similar pattern: mice exposed to the TRANS diet during lactation (CT and TT) continued to have a significantly reduced percentage of abdominal fat in adulthood (Table 4; p=0.01).
Figure 3.
Body composition of offspring at weaning as measured by computed tomography and calculation of abdominal fat volume and converted to a weight/weight basis for each individual. Data shown represents means ± SEM of the groups, defined as CIS diets during in utero and through breastfeeding during neonatal growth periods (CC; white bar), TRANS diet in utero and CIS diet during breastfeeding (TC; checked bar), CIS diet in utero and TRANS diet during breastfeeding (CT; grey bar) and TRANS diet during in utero and breastfeeding growth periods (TT; diagonal striped bar). Two-way ANOVA analysis of diet exposure at each developmental stage was conducted and different letter superscripts denote statistical differences at α < 0.05 (n=10–18/group). A main effect of diet during lactation (CIS vs. TRANS) is present with TRANS exposure reducing adiposity.
Table 4.
Body composition measured by computed tomography and calculation of abdominal fat volume and converted to a weight/weight basis for each individual at adulthood
| Measurement | Dietary Groups |
P values |
|||||
|---|---|---|---|---|---|---|---|
| CC | TC | CT | TT | Diet (P) | Diet (L) | Interaction | |
| Abdominal fat (% body weight) | 12.15 ± 0.31 | 11.54 ± 0.36 | 11.15 ± 0.36 | 10.62 ± 0.42 | 0.52 | 0.01 | 0.39 |
Values are means ± SEM (n=10–18).
Two-way ANOVA analysis of diet exposure at each developmental stage with statistics for each effect and their interaction are shown. A significant effect of diet during breastfeeding is apparent with trans fatty acid exposure from neonatal growth persisting as leanness in early adulthood. No sex differences in body composition were observed.
3.4. Free fatty acids and insulin-like growth factor-1
Circulating FFA concentrations at weaning were increased in pups exposed to the TRANS diet during pregnancy (TC and TT, 1.24 [1.12–1.37] mEq/L vs.; CC and CT, 1.05 [0.85–1/24] mEq/L; p<0.05), suggesting increased lipolysis. However, there were no intergroup differences in FFA that persisted into adulthood. At weaning, IGF-1 levels were significantly increased in mice exposed to the TRANS diet during lactation (CT and TT, 889±112 ng/mL vs. CC and TC, 523±149 ng/mL; p=0.02) which is consistent with lower body fat despite comparable body weights (Figure 3). Differences in circulating IGF-1 at adulthood were not found.
3.5. Glucose metabolism
We observed significant differences in fasting glucose at the time of weaning, with exposure to TRANS diet through lactation (CT and TT) elevating glucose levels by 17% (p=0.01 vs. CIS; Table 5). For comparison of GTT and ITT, we corrected for variable baseline glucose concentrations between groups by normalizing to a baseline (pre-challenge) concentration of 100 mg/dL. The elevated fasting glucose levels in groups exposed to TRANS diet during lactation were not reflected in an inability to dispose of glucose, since areas under the curve (AUCs) in the GTT were actually decreased relative to the CIS groups (Table 5). This result may reflect the elevated starting point and comparable insulin sensitivity between groups, as AUCs calculated from raw glucose values unadjusted for group differences in fasting glucose were not different (data not shown). Insulin sensitivity (estimated by AUC for glucose following insulin tolerance testing) was comparable among all groups. Any changes in fasting glucose due to TRANS diet exposure did not persist into adulthood, when all groups consumed the CIS diet. No changes were detected in fasting insulin concentrations as a result of diet (Table 5).
Table 5.
Glycemic parameters measured at weaning (day 21) and early adulthood (day 60–70) in mice exposed to CIS diets during in utero and through breastfeeding during neonatal growth periods (CC), TRANS diet in utero and CIS diet during breastfeeding (TC), CIS diet in utero and TRANS diet during breastfeeding (CT) and TRANS diet during in utero and breastfeeding growth periods (TT).
| Age | Measurement | Dietary Groups | P values | |||||
|---|---|---|---|---|---|---|---|---|
| CC | TC | CT | TT | Diet (P) | Diet (L) | Interaction | ||
| Weaning | Glucose (mmol/L) | 7.47 ± 0.40 ab | 6.46 ± 0.43 a | 8.33 ± 0.52 b | 7 7.93 ± 0.45 ab | 0.12 | 0.01 | 0.50 |
| Insulin (pmol/L) | 12.3 ± 2.55 | 17.2 ± 4.39 | 15.1 ± 2.42 | 9.63 ± 0.62 | 0.86 | 0.42 | 0.10 | |
| AUCGTT | 18410 ± 825 ab | 22155 ± 1800 a | 16116 ± 1323 b | 15294 ± 769 b | 0.29 | <0.002 | 0.10 | |
| AUCITT | 6117 ± 438 ab | 8409 ± 774 a | 6354 ± 1042 ab | 5412 ± 664 b | 0.37 | 0.07 | 0.04 | |
| HbA1c (%) | 4.33 ± 0.29 | 5.09 ± 0.37 | 4.55 ± 0.24 | 4.92 ± 0.46 | 0.14 | 0.94 | 0.61 | |
| Adulthood | Glucose (mmol/L) | 12.0 ± 0.43 | 11.0 ± 0.47 | 10.43 ± 0.48 | 12.04 ± 0.67 | 0.60 | 0.65 | 0.02 |
| Insulin (pmol/L) | 17.8 ± 2.94 | 16.9 ± 3.52 | 9.63 ± 1.09 | 13.8 ± 2.86 | 0.79 | 0.20 | 0.32 | |
| AUCGTT | 16341 ± 1934 | 14943 ± 890 | 15719 ± 1075 | 12523 ± 1315 | 0.10 | 0.27 | 0.51 | |
| AUCITT | 9518 ± 889 | 9657 ± 726 | 8138 ± 2086 | 8492 ± 756 | 0.84 | 0.31 | 0.93 | |
| HbA1c (%) | 5.04 ± 0.36 | 4.98 ± 0.26 | 4.82 ± 0.19 | 5.06 ± 0.32 | 0.77 | 0.82 | 0.63 | |
Values are mean ± SEM (n ≥ 10/group).
Two-way ANOVA analysis of diet exposure at each developmental stage with statistics for each effect and their interaction are shown. Different letters indicate group differences at the p<0.05 level. The significance of the main effects of diet during pregnancy and lactation and their interaction variables are indicated. Trans fatty acid exposure during lactation resulted in significantly higher fasting glucose but lower AUC with glucose tolerance testing. No diet effects persisted into adulthood.
Glycemic parameters measured were fasting glucose and insulin concentrations, area under the curve (AUC) for glucose after glucose and insulin tolerance testing, and percent of glycation of hemoglobin A1c chain (HbA1c). All groups were weaned onto the CIS diet until assessment as adults.
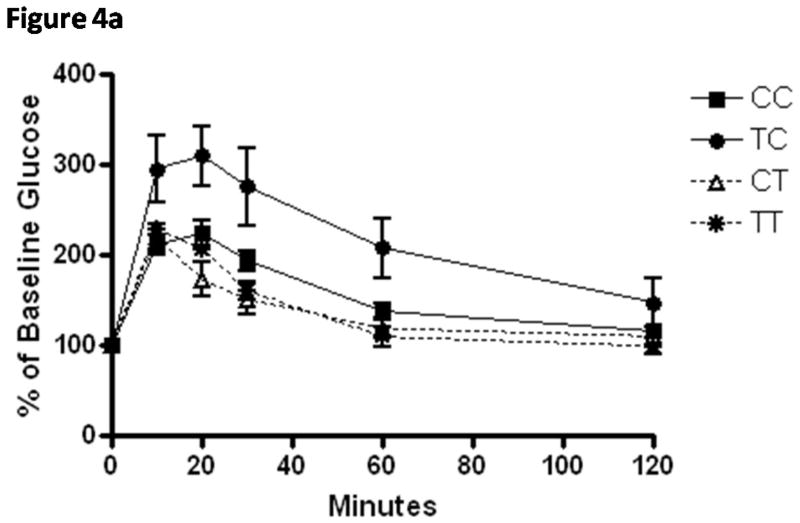
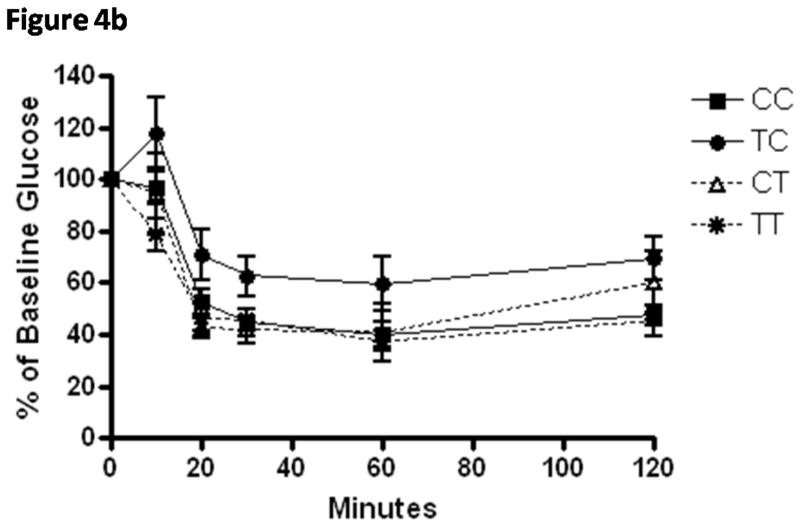
Elevations in fasting glucose at weaning were not reflected in increased HbA1c% (Table 5), suggesting that overall glucose tolerance and post-prandial glucose concentrations were not affected by diet. No group differences were observed at weaning or adulthood in casual non-fasting glucose measurements that were measured (data not shown). Mice exposed to the TRANS diet during pregnancy only (TC) demonstrated glucose intolerance and insulin resistance (Figure 4) and had the highest HbA1c% value. This group also demonstrated the most rapid growth in the first week of neonatal growth (0.33 g/d). This weight gain was 15% faster than pups under the same gestational conditions but weaned onto dams eating TRANS diets.
Figure 4.
A) Means ± SEM normalized glucose values from glucose tolerance testing of weanling mice (n=8–14/group) exposed to CIS diets during in utero and through breastfeeding during neonatal growth periods (CC; white bar), TRANS diet in utero and CIS diet during breastfeeding (TC; checked bar), CIS diet in utero and TRANS diet during breastfeeding (CT; grey bar) and TRANS diet during in utero and breastfeeding growth periods (TT; diagonal striped bar). The TC group demonstrates glucose intolerance and area under the curve calculated from this data series was significantly higher than all other groups (see Table 5).
B) Means ± SEM normalized glucose values from insulin tolerance testing of weanling mice (n=6–11/group) from these same groups demonstrating further evidence that TC mice were significantly more insulin resistant (see Table 5) as determined by calculated areas under the curve.
4. Discussion
This is the first study to specifically address the question of whether industrially produced dietary trans fatty acids, when supplied in utero and through breast milk, have immediate or persistent effects on neonatal growth, body composition and glucose metabolism. Following weaning, animals were maintained on TFA-free diets until adulthood in order to monitor for any programming effects of early perturbations in growth, body composition and glucose metabolism resultant from TFA exposure. Aside from what would be considered a positive effect on body composition, fetal programming effects of TFA were not apparent. In particular, we observed no obesigenic effects, as might be anticipated from epidemiologic and non-human primate studies of TFA [7]. Disturbances in glucose metabolism related to TFA exposure during pregnancy and lactation were seen in our study, but were particularly apparent when dams consumed the TRANS diet during pregnancy and ceased during lactation (analogous to the use of infant formula). We had hypothesized that TFA exposure would result in obesity and insulin resistance, and that these effects may be in part resultant from in utero exposure and fetal programming. We rejected our hypothesis upon finding that although disturbed glucose metabolism was present, obesity was a concomitant feature and glycemic effects did not persist into adulthood.
This study may provide further support to the theory that TFAs affect birth weight and early neonatal growth, and is in agreement with observations in infants whose plasma TFA levels had a significant inverse association with body weight within the first week of life [22]. Consumption of cafeteria style foods versus standard rodent diets was also associated with lower birth weight in preclinical studies [23]. The common use of standard rodent diets as a control diet in fetal programming studies is flawed because it is not nutritionally relevant to people and the comparison of macronutrient- and ingredient-matched diets in this study is one of its strengths, as palatability, programming of satiety effects and maternal insulin sensitivity should be equivalent. This allows specificity of observed effects to be attributed to the trans-isomerization of fatty acid components of the western diet [24].
The current study suggests that maternal diets including lipids in their unmodified form are associated with slightly higher birth weight and slower weight gain in the early post-natal period. These changes translate to measurable improvements in body composition and glucose metabolism as adults [25, 26]. Reduction of litter sizes in the CIS group to match the fewer TRANS offspring would presumably increase nutrient resources and may have accentuated TFA-related impedance of fetal and neonatal growth [18, 24]. The specific mechanisms causing impairment of growth with high fat and TFA-rich diets are unknown.
Elevations in fasting glucose that resulted from TFA exposure during lactation reflects enhanced basal hepatic glucose output, and indicates that TFA intake may induce hepatic insulin resistance. However, casual glucose and insulin concentrations (no control for feeding) and fasting insulin showed no group differences as has been seen previously rodents reared on high fat diets under control or growth restricted conditions [27]. There were also no group differences in overall glycemic control (HbA1c%) and tolerance testing, indicating adequate peripheral insulin sensitivity and compensation for hepatic insulin resistance that is likely due to increased insulin secretion. Glucose challenges were administered on a weight basis and the TFA-fed mice had proportionately more lean tissue as assessed by body compositional analysis of CT scans, which may explain in part their normal glucose disposal despite having nearly 20% higher fasting glucose values. Only the neonates that had TFA-associated in utero growth retardation coupled with enhanced neonatal growth under TFA-free conditions (TC) exhibited peripheral insulin resistance at weaning. This group also demonstrated very early catch-up growth in week 1, suggesting a critical period for mice that is consistent with conclusions drawn from other rodent studies [27].
Our present observation that IGF-1 levels were increased in weanling mice exposed to TFA through breast milk corroborates those seen with junk food diets result increasing IGF-1 expression in fat [28]. Lactational exposure to TFA demonstrated early growth retardation, but then had subsequent catch-up growth (growth rate increase) and proportionately lower fat mass (lipolysis increases to fuel growth), which may reflect increasing IGF-1 or decreasing IGF-1 binding protein levels. High levels of exogenously administered IGF-1 have resulted in greater lipolysis without changes in FFA, similar to results seen in the TFA breast milk-exposed weanling mice of this study [29]. Endogenous IGF-1-mediated insulin receptor substrate activation and enhanced beta cell function may be responsible in part for maintaining low levels of lipolysis and FFA and peripheral insulin sensitivity [3, 30] also seen in this study.
The protective effect against obesity development conferred by breastfeeding is controversial, with exposure and duration of breastfeeding considered less influential than maternal adiposity on subsequent weight [31]. However, the nutritional state of the mother is interconnected with both her adipose mass and composition. Dietary TFA is linearly and highly associated with an individual’s adipose TFA content [32], which will be reflected in the quality of breast milk [7, 15, 33]. The TRANS diet composition used in this study mimics the trend in human food production, as partially hydrogenated soybean oil provides high levels of elaidic acid, which is estimated to constitute most (80–90%) of the TFA in the American diet (9). Actual values of milk TFA in this study (mean 11.9%, range 9.7–12.9%) were at the high end of previously published estimates of breast milk TFA content [10, 11, 34], consistent with the high TFA content of the TRANS diet. LC-PUFA content was very similar to the range of values reported from samples of lactating women in the United States [34, 35], so we conclude that our study was relevant to the current nutritional environment for humans. No differences in maternal supply and milk transfer of LC-PUFAs were detected between diets. In general, reduced milk fat content is seen with high-fat and TFA diets as a result of inhibition of mammary fatty acid synthesis; however, alterations of neonatal growth are not seen in response to variable fat content of mouse milk [21, 36].
Limitations to the current study include the unequal litter sizes and our inability to eliminate potential litter effects by cross-adoption of offspring between dams [37]. To eliminate effects of differing litter size and dam effects, we sampled representative offspring balanced by sex from all litters produced from the eight sibling dams. Further, the inbred nature of the mouse strain minimizes inter-individual differences. The possibility of an elaidic acid effect on food intake cannot be excluded without quantifying diet consumption, nor can an effect on gestational length. Despite carefully matching diet on macronutrient content, our fat blends used differed by percentage breakdown of fat type and thus bioactive phytosterols, oxysterols and other constituents may have differed and had biological actions.
Our study has compared the effects of nutritionally and physiologically relevant supplementation of TFAs during in utero and early neonatal growth periods of mice. We conclude that high TFA consumption during pregnancy and lactation interfered with normal development in these mice. Weanling mice exposed to TFAs had retarded growth compared to their experimental counterparts. Overall impairment in glucose tolerance was not present at this early stage of maturation, in contrast to preclinical and epidemiologic studies involving adults [5, 7]. However, increases in fasting glucose and IGF1 seen with TFA exposure could progress to further metabolic dysregulation with continuous consumption. Specifically growth restriction seen with TFA exposure in utero may set the stage for early catch-up growth during a critical period for determining later glucose tolerance, peripheral insulin resistance and body size.
Acknowledgments
This study was funded by the NIH Office of Dietary Supplements/Center for Botanical Lipids at Wake Forest University Health Sciences and NIH K01AG033641.
Abbreviations
- TFA
trans fatty acid
- T2DM
Type 2 diabetes mellitus
- GTT
glucose tolerance testing
- ITT
insulin tolerance testing
- AUC
area under the curve
- CT
computed tomography
- EDTA
ethylenediaminetetraacetate
- ELISA
enzyme linked immunosorbant assay
- FFA
free fatty acids
- IGF-1
insulin like growth factor 1
- ANOVA
analysis of variance
- LC-PUFA
long chain polyunsaturated fatty acids
- FAME
fatty acid methyl ester
- FID
flame ionization detector
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Singhal A, Lucas A. Early origins of cardiovascular disease: Is there a unifying hypothesis? Lancet. 2004;363:1642–1645. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 3.Dunger DB, Salgin B, Ong KK. Session 7: Early nutrition and later health early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66:451–457. doi: 10.1017/S0029665107005721. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmeron J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73:1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 6.Craig-Schmidt MC. World-wide consumption of trans fatty acids. Atheroscler Suppl. 2006;7:1–4. doi: 10.1016/j.atherosclerosissup.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15:1675–1684. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 8.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, Eidelman AI. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 9.Elias SL, Innis SM. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001;73:807–814. doi: 10.1093/ajcn/73.4.807. [DOI] [PubMed] [Google Scholar]
- 10.Larque E, Zamora S, Gil A. Dietary trans fatty acids in early life: A review. Early Hum Dev. 2001;65 (Suppl):S31–41. doi: 10.1016/s0378-3782(01)00201-8. [DOI] [PubMed] [Google Scholar]
- 11.Craig-Schmidt MC, Weete JD, Faircloth SA, Wickwire MA, Livant EJ. The effect of hydrogenated fat in the diet of nursing mothers on lipid composition and prostaglandin content of human milk. Am J Clin Nutr. 1984;39:778–786. doi: 10.1093/ajcn/39.5.778. [DOI] [PubMed] [Google Scholar]
- 12.Innis SM. Trans fatty intakes during pregnancy, infancy and early childhood. Atheroscler Suppl. 2006;7:17–20. doi: 10.1016/j.atherosclerosissup.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Innis SM, King DJ. Trans fatty acids in human milk are inversely associated with concentrations of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants. Am J Clin Nutr. 1999;70:383–390. doi: 10.1093/ajcn/70.3.383. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the U.S. obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 15.Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz G, Willett W, Rimm E. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16 587 U.S. men. Am J Clin Nutr. 2003;78:719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 16.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 17.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight CH, Maltz E, Docherty AH. Milk yield and composition in mice: Effects of litter size and lactation number. Comp Biochem Physiol A Comp Physiol. 1986;84:127–133. doi: 10.1016/0300-9629(86)90054-x. [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipid gas chromatography analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- 20.Heikkinen S, Argmann CA, Champy MF, Auwerx J. Evaluation of glucose homeostasis. Curr Protoc Mol Biol. 2007;Chapter 29(Unit 29B):23. doi: 10.1002/0471142727.mb29b03s77. [DOI] [PubMed] [Google Scholar]
- 21.Aoki N, Yamaguchi Y, Ohira S, Matsuda T. High fat feeding of lactating mice causing a drastic reduction in fat and energy content in milk without affecting the apparent growth of their pups and the production of major milk fat globule membrane components mfg-e8 and butyrophilin. Biosci Biotechnol Biochem. 1999;63:1749–1755. doi: 10.1271/bbb.63.1749. [DOI] [PubMed] [Google Scholar]
- 22.Koletzko B. Trans fatty acids may impair biosynthesis of long-chain polyunsaturates and growth in man. Acta Paediatr. 1992;81:302–306. doi: 10.1111/j.1651-2227.1992.tb12230.x. [DOI] [PubMed] [Google Scholar]
- 23.Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98:843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- 24.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: Consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prior LJ, Velkoska E, Watts R, Cameron-Smith D, Morris MJ. Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: A protective effect? Int J Obes (Lond) 2008;32:1585–1594. doi: 10.1038/ijo.2008.141. [DOI] [PubMed] [Google Scholar]
- 28.Bayol SA, Simbi BH, Bertrand JA, Stickland NC. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol. 2008;586:3219–3230. doi: 10.1113/jphysiol.2008.153817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vickers MH, Ikenasio BA, Breier BH. IGF-1 treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–3973. doi: 10.1210/endo.142.9.8390. [DOI] [PubMed] [Google Scholar]
- 30.Holly J, Sabin M, Perks C, Shield J. Adipogenesis and IGF-1. Metab Syndr Relat Disord. 2006;4:43–50. doi: 10.1089/met.2006.4.43. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Kaur H, Choi WS, Huang TT, Lee RE, Ahluwalia JS. Additive interactions of maternal prepregnancy BMI and breast-feeding on childhood overweight. Obes Res. 2005;13:362–371. doi: 10.1038/oby.2005.48. [DOI] [PubMed] [Google Scholar]
- 32.Enig MG, Atal S, Keeney M, Sampugna J. Isomeric trans fatty acids in the U.S. Diet. J Am Coll Nutr. 1990;9:471–486. doi: 10.1080/07315724.1990.10720404. [DOI] [PubMed] [Google Scholar]
- 33.Muskiet FA, van Goor SA, Kuipers RS, Velzing-Aarts FV, Smit EN, Bouwstra H, Dijck-Brouwer DA, Boersma ER, Hadders-Algra M. Long-chain polyunsaturated fatty acids in maternal and infant nutrition. Prostaglandins Leukot Essent Fatty Acids. 2006;75:135–144. doi: 10.1016/j.plefa.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Mosley EE, Wright AL, McGuire MK, McGuire MA. Trans fatty acids in milk produced by women in the United States. Am J Clin Nutr. 2005;82:1292–1297. doi: 10.1093/ajcn/82.6.1292. [DOI] [PubMed] [Google Scholar]
- 35.Szabo E, Boehm G, Beermann C, Weyermann M, Brenner H, Rothenbacher D, Decsi T. Trans octadecenoic acid and trans octadecadienoic acid are inversely related to long-chain polyunsaturates in human milk: Results of a large birth cohort study. Am J Clin Nutr. 2007;85:1320–1326. doi: 10.1093/ajcn/85.5.1320. [DOI] [PubMed] [Google Scholar]
- 36.Teter BB, Sampugna J, Keeney M. Lactation curves and effect of pup removal on milk fat of C57BL/6J mice fed different diet fats. Lipids. 1992;27:912–916. doi: 10.1007/BF02535872. [DOI] [PubMed] [Google Scholar]
- 37.Wainwright PE. Issues of design and analysis relating to the use of multiparous species in developmental nutritional studies. J Nutr. 1998;128:661–663. doi: 10.1093/jn/128.3.661. [DOI] [PubMed] [Google Scholar]