Abstract
Diverticulosis is extremely common in Western societies and is associated with complications in up to 15 percent of cases. Altered motility is an important feature of the pathogenesis of diverticular disease, and serotonin (5-HT) release is a primary trigger of gut motility. This study aims to determine whether colonic 5-HT signaling is altered in patients with diverticulosis or diverticulitis, and whether differences in serotonin signaling may distinguish patients with asymptomatic diverticulosis from those who develop disease specific complications.
Sigmoid colon biopsies were obtained from healthy control subjects, individuals with asymptomatic diverticulosis, and those with a history of CT-proven diverticulitis within the preceding 6 months. The key elements of 5-HT signaling including content, release and 5-HT transporter (SERT) expression were analyzed.
A significant decrease in SERT transcript levels was present in the mucosa of patients with a history of diverticulitis when compared with controls, but not in those with asymptomatic diverticulosis. Mucosal 5-HT content, enterochromaffin (EC) cell numbers, and TpH-1 mRNA levels were comparable amongst the groups, as were basal and stimulated 5-HT release. Alterations in 5-HT signaling do not appear to be responsible for the development of diverticula. However, patients with a recent history of acute diverticulitis have a significant attenuation in SERT expression and function, likely secondary to previous inflammation. Our findings may explain the persistent symptoms of pain and altered motility so often observed in patients with diverticulitis long after recovery from the acute inflammatory response.
Keywords: Diverticulosis, diverticulitis, serotonin transporter (SERT), enterochromaffin cells
INTRODUCTION
Diverticular disease is the most common abnormality of colonic structure, with a prevalence in a postmortem surveys of 35%–50%.1–3 Complications can affect 10%–25% of those with diverticulosis and include bleeding, acute or chronic abdominal pain, intra-abdominal abscesses, bowel perforation, fistulae, sepsis, and death.4, 5 Pathogenic factors that have been associated with diverticula include a low-residue diet, elastin and collagen redistribution, compartment segmentation, and disordered motility.3, 6 However, remarkably little is known about the exact pathophysiology of disease, and why specific complications occur in some patients while most remain asymptomatic.
Serotonin (5-HT) is an important chemical mediator involved in bowel function, secretion, and motility independent of the CNS.7, 8 Over 95% of the body’s 5-HT stores are located in the intestines, where it is synthesized by a subset of enteroendocrine cells called enterochromaffin (EC) cells. Serotonin is secreted from EC cells into the lamina propria in response to mechanical, chemical, and noxious stimuli7, and 5-HT release is a primary trigger for peristalsis, secretion, and visceral sensation.9 Mucosal 5-HT signaling involves the following sequence of events: (1) luminal stimulation (for example; acidity, mechanical or sheer stress) causes EC cells to release 5-HT into the lamina propria; (2) activation of 5-HT receptors on nearby nerve fibers; (3) removal of 5-HT from the interstitial space via the serotonin-selective reuptake transporter (SERT), which is expressed by all epithelial cells; and (4) intracellular degradation by type A monoamine oxidase.7, 10–12
It is becoming increasingly clear that key elements of 5-HT signaling are altered in disease states characterized by disordered motility and secretion such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS).10, 13, 14 Diminished SERT activity appears to be a crucial factor in altered 5-HT signaling.10 We hypothesized that similar derangements in 5-HT signaling would be detected in patients with diverticulosis. Further, we hypothesized that patients with a history of diverticulitis would greater alterations in 5-HT signaling, possibly explaining a predisposition to disease specific complications.
MATERIALS AND METHODS
Tissue Acquisition
Human sigmoid colonic mucosal samples were obtained from asymptomatic volunteers who presented for screening colonoscopy after informed consent. Patients were designated as either normal control or asymptomatic diverticulosis based on the presence of diverticula at the time of the procedure. Subjects who had an episode of CT-proven diverticulitis within the prior 6 months, as identified by one of three surgical specialists, were referred for the study if they were undergoing colonoscopic or sigmoidoscopic evaluation. Patients were excluded if the researcher felt that they were unable to provide informed consent (i.e. language or mental status barriers), if they had a history of IBS or IBD, pelvic radiation, history of diverticulitis greater than 1 year prior, bleeding disorders, or if they had not stopped anticoagulation for seven days prior to the procedure. Five sigmoid colon mucosal biopsies were obtained in succession from each individual identified as control or diverticulosis using the large capacity biopsy forceps. In patients designated as the diverticulitis group, 3 biopsies were taken from the area of previous inflammation based on endoscopic, surgical, and radiologic findings, and 3 were taken from the normal appearing sigmoid colon distal to the area of previous inflammation. We elected to take biopsies in the distal sigmoid as 5-HT signaling varies throughout the colon.15, 16 Each sample was immediately prepared and processed as described below. The study was approved by the Institutional Review Board of the University Of Vermont College Of Medicine.
Immunohistochemistry
At the time of biopsy, the samples were carefully oriented on a piece of filter paper placed between 2 mini-sponges and fixed in 4% paraformaldehyde and 0.2% picric acid for 24 hours. The biopsies were then cut into 5µm sections and mounted in paraffin. Standard deparaffinization techniques were performed and the primary antiserum of mouse anti-human monoclonal antibodies to 5-HT (1:500 Dako Diagnostics, Belgium) were applied followed by the secondary goat anti-mouse antibodies labeled with 7-amino-4-methyl-coumarin-3 acetic acid (AMCA; 1:250; Jackson Immunoresearch, West Grove, PA). Sections were then incubated with a 1:30,000 dilution of the nucleic acid stain, yo-yo (Molecular Probes, Eugene, OR).
Quantitative Measurement
Tissue sections from each individual were evaluated under the fluorescence microscope and digitized at a single exposure with a 20X objective. Care was taken to count longitudinal sections of bowel wall to include multiple intact crypts. Epithelial cell hyperplasia or hypoplasia was determined by counting the number of stained nuclei in epithelial cells for a given length of colon and quantifying the number of epithelial cells per crypt (3–6 crypts). 5-HT–containing epithelial cells (EC cells) were counted as a function of muscularis mucosa length, as a function of 5-HT-positive cells per colonic gland, and as a proportion of the total number of epithelial cells in a region of colon.
Histological Assessment
One portion of the above fixed tissue was stained with H&E. Each individual was blindly assigned an inflammatory score (0=none, 1=mild, 2=moderate, 3=severe) by an experienced gastrointestinal pathologist. The scoring system was as follows: (0), normal numbers of inflammatory cells in the lamina propria with no active inflammation. (1) normal or minimally increased numbers of inflammatory cells in the lamina propria and/or a rare neutrophil/eosinophil within the crypt epithelium; (2) increased numbers of inflammatory cells in the lamina propria and/or mild active inflammation; (3) significantly increased numbers of inflammatory cells in the lamina propria and/or moderate/severe active inflammation. Tissue samples with a score of 0 or 1 were considered normal. Any tissue samples scored 2 or 3 were excluded in all groups.
Measurement of 5-HT Content of Colonic Mucosa
One of the biopsy specimens from each individual was homogenized in 0.1M perchloric acid and buffered in an equivalent amount of 0.5M borate buffer. The 5-HT content was analyzed using an enzyme immunoassay kit according to the manufacturer’s instructions (Beckman Coulter, Fullerton, CA).
Measurement of 5-HT Released from Colonic Mucosa
Two biopsy specimens from each individual were used to assess 5-HT release in the control and diverticulosis groups. 5-HT release was not determined in the diverticulitis group secondary to the number of additional biopsies required. Specimen were processed under 2 experimental conditions: 1) basal release – biopsies were kept for 5 minutes in HEPES solution without glucose at 37° C; 2) stimulated release – biopsies were kept in HEPES solution without glucose at 37° C for 2 minutes, followed by 3 minutes of mild agitation on a vortex machine at room temperature (VWR vortexer 2 set at level 4). After the 5 minutes of either treatment, 400 µL of each solution were removed and designated separately. For both specimens, the 5-HT release was analyzed using an enzyme immunoassay kit according to the manufacturer’s instructions (Beckman Coulter, Fullerton, CA).
Measurement of mRNA in Colonic Mucosa
For RNA extraction, a biopsy sample was placed in 350 µL RNAlater (Qiagen Inc, Valencia, CA). RNA was extracted from the samples using the RNeasy Mini Kits according to manufacturer’s instructions (Qiagen Inc, Valencia, CA). Samples of complementary strands of DNA to the mRNA samples were generated using the Superscript II Reverse Transcription Kit according to the manufacturer’s instructions (Invitrogen Corp, Carlsbad, CA). A 7500 Fast Real-Time PCR Instrument was used for real-time polymerase chain reaction (RT-PCR; Applied Biosystems, Foster City, CA). SYBR Green Jump Start Taq Ready Mix was used for quantitative PCR (Sigma Aldrich Corp, St. Louis, MI). The expression of ClCN3, SERT, and TpH-1 in each sample was normalized to beta-actin. For SERT, the sense primer was 5’ cgtagttgtggcgggctcat 3’ and the antisense primer was 5’ gccgtggtcatcacctgctt 3’. For ClCN3 the sense primer was 5’ tgctttagtggctgcatttg 3’ and the antisense primer was 5’ ccagaacgggatactttcca 3’. For TpH-1 the sense primer was 5’ tgcggacttggctatgaac 3’ and the anti-sense primer was 5’ cacaggacggatggaaaac 3’. For beta-actin the sense primer was 5’ catgtttgagaccttcaacac 3’, and the antisense primer was 5’ ccaggaaggaaggctggaa 3’. No differences in beta-actin message levels were detected amongst the sample groups. The data are reported as fold change in calculated relative expression normalized to beta-actin from the average of the controls.
Statistical Analyses
Statistical analyses were performed using Prism software (v. 4.00; Graphpad software, San Diego, Ca.). Comparisons between the 4 groups (control, diverticulosis, diverticulitis-affected, diverticulitis-adjacent normal) involved a 1-way ANOVA followed by the Newman-Keuls multiple comparisons test. Comparisons between control and diverticulosis were performed using an unpaired t test. Differences were considered statistically significant if the p value was ≤ 0.05.
RESULTS
A total of 51 subjects, identified from December 2005 through May 2006, met inclusion criteria and were recruited for the study. 13 of the subjects had experienced a CT scan proven case of acute diverticulitis 1 to 6 months prior to the time of the colon/sigmoidoscopy. In all 13 cases, a defined area of edema, induration and/or rigidity was identified in the segment of colon that had been radiographically involved with diverticulitis. In a previous study involving 38 healthy controls (18F/16M), no correlation were detected between age, gender or SSRI use and the various elements of serotonin signaling that were investigated in the current study.10 Mucosal inflammatory scores were determined and were not significantly different between groups (Table 1).
Table 1.
Characteristics of 51 patients undergoing biopsies during colon/sigmoidoscopy.
| Group | Control | Diverticulosis | Diverticulitis /Normal Adjacent |
|---|---|---|---|
| n | 22 | 16 | 13 |
| Sex: M | M = 10 | M = 9 | M = 4 |
| F | F = 12 | F = 7 | F = 9 |
| Age Range (Median) |
36–69 (52) |
37–80 (59) |
37–73 (55) |
| # on SSRIs | 2 | 2 | 4 |
| # on benzodiazepines |
2 | 1 | 0 |
| # with Grade 2 or 3 Inflammation |
1 | 1 | 1/2 |
5-HT Content, EC Cell Numbers and TpH-1 mRNA
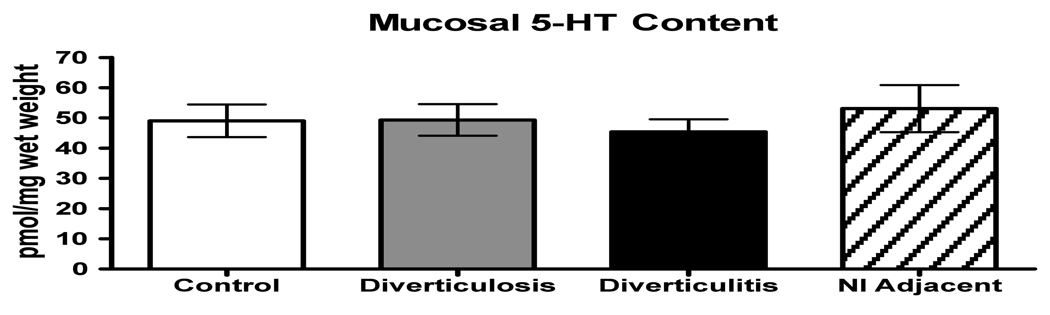
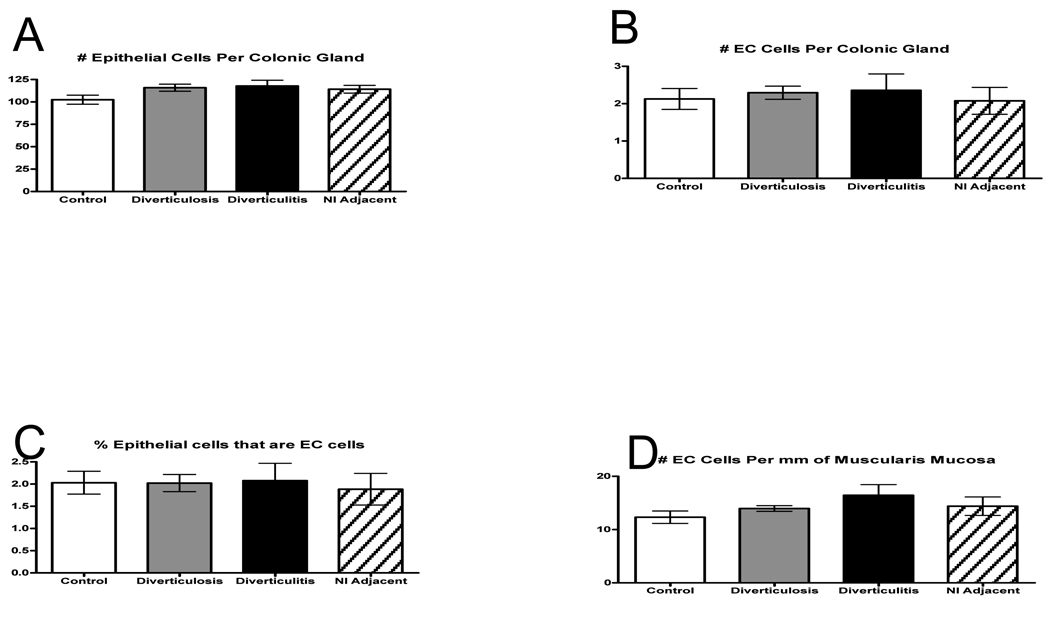
No differences were detected in the mean mucosal 5-HT content between controls, subjects with asymptomatic diverticulosis, or individuals with diverticulitis in either the actively inflamed or adjacent region (Fig. 1). No differences in EC cell numbers were detected amongst the 4 groups with respect to the numbers of epithelial cells per gland, numbers of EC cells per gland, percent EC cells, or the number of EC cells per millimeter muscularis mucosa. (Fig. 2 A–D).
Figure 1.
Mucosal 5-HT content measured enzyme immunoassay demonstrating that no difference in 5-HT content was detected amongst groups. Groups are: control, diverticulosis, diverticulitis = areas of indurated sigmoid, and normal (nl) adjacent = normal sigmoid distal to active inflammation.
Figure 2.
A–D: There were no differences found among the groups either in numbers of EC cells or epithelial cells per gland to suggest an increase in 5-HT content.
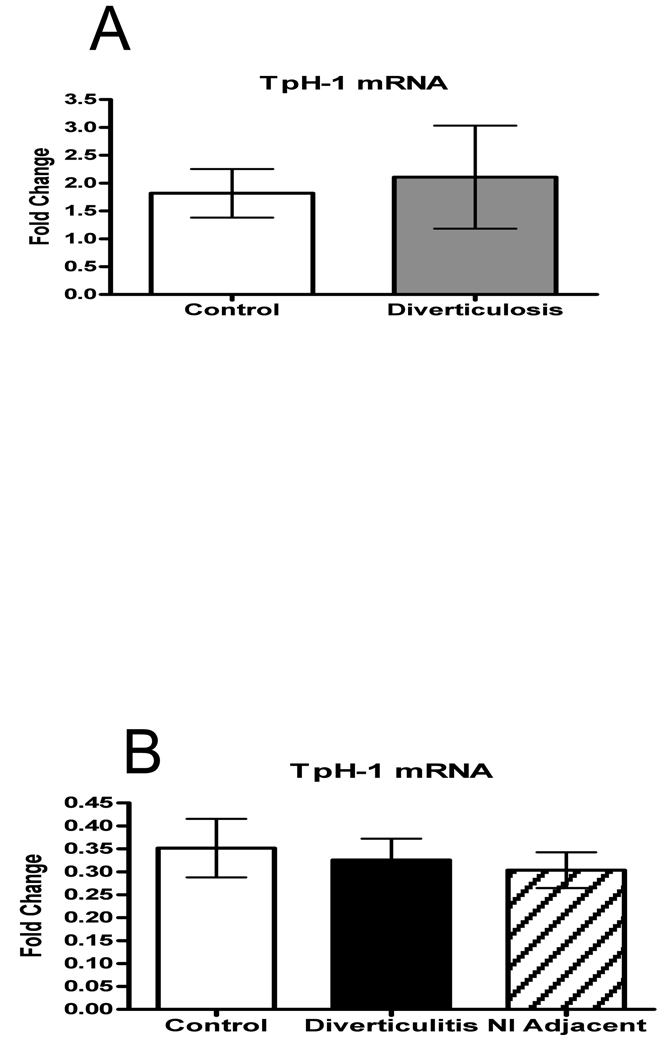
Type 1 tryptophan hydroxylase (TpH-1) is the rate-limiting enzyme for 5-HT biosynthesis in the intestinal mucosa.17 To evaluate the 5-HT synthesis capacity in mucosa of healthy controls vs diverticulosis and diverticulitis samples, we measured TpH-1 transcript levels in mucosal biopsy specimens. Consistent with the lack of differences in 5-HT content and EC cell numbers, no differences in TpH-1 mRNA levels were detected amongst the populations that were studied (Fig. 3A–B).
Figure 3.
A–B: The rate limiting enzyme in 5-HT production, TpH-1 mRNA relative expression was measured using quantitative RT-PCR. There was no difference in TpH-1 mRNA levels between any of the groups.
5-HT Release
An ex vivo model of 5-HT release has been developed in an attempt to evaluate basal and stimulated release of 5-HT from EC cells in human tissue specimens. Mechanical stimulation resulted in a significant increase in 5-HT relative to basal release in both the control and the diverticulosis groups (p<0.0005) However, no differences in basal or stimulated 5-HT release were detected between the control and the diverticulosis specimens.
SERT mRNA
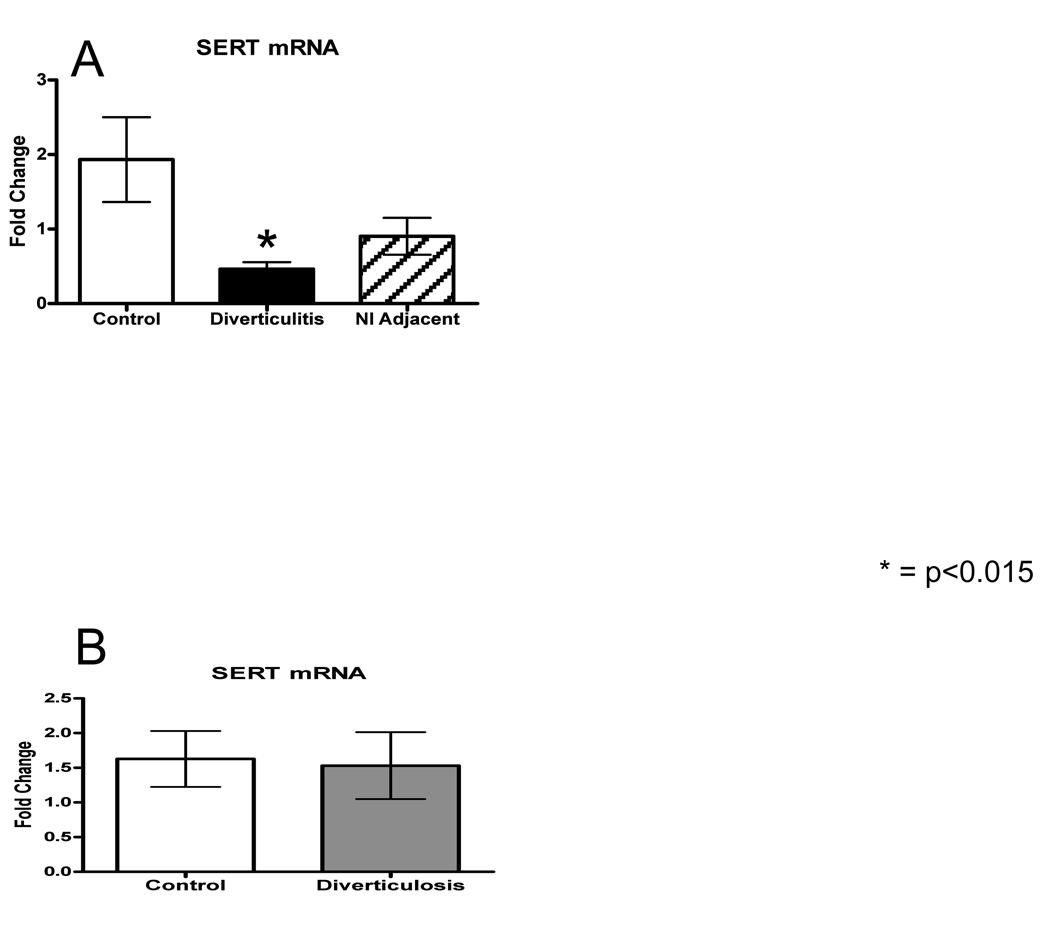
The 5-HT transporter, SERT, is expressed by all epithelial cells and terminates 5-HT signaling in the interstitial space.18 In the current study, a highly significant decrease in SERT transcript levels was detected within the affected area of diverticulitis specimens (<0.01), and a trend towards decreased expression was detected in the normal adjacent mucosa, when compared to control (Fig. 4A). Importantly, no difference in SERT transcript levels were detected between patients with asymptomatic diverticulosis and control samples (Fig. 4B).
Figure 4.
A–B: SERT mRNA relative expression was measured using quantitative RT-PCR and normalized to beta-actin. There was no difference between control and diverticulosis, but there was a statistically significant decrease in the mRNA expression in subjects with diverticulitis while the adjacent bowel was decreased but not significantly. These data are demonstrated on separate graphs as the groups were run separately.
DISCUSSION
Despite the high prevalence of diverticular disease, little is known about its pathophysiology, and there is currently no way to predict who will develop complications. Nonetheless, altered motility appears to be a key feature associated with diverticulosis. 5-HT signaling alterations are thought to be a cause of the disordered motility, secretion and sensation found in IBD and IBS.10, 19 Our data suggest that similar abnormalities exist in patients who have had an episode of acute diverticulitis but not in those with asymptomatic diverticula.
Disordered serotonergic signaling may result from changes in availability, release, or reuptake of 5-HT. Serotonin availability is measured directly by 5-HT content and indirectly by counting the numbers of EC cells, as well as measuring TpH-1 transcript levels. 5-HT release is simulated ex vivo in our laboratory, as it is currently not feasible to measure 5-HT concentrations in the lamina propria in vivo, and 5-HT released in solution containing mucosa at body temperature is measured. 5-HT reuptake is approximated indirectly by measurement of the expression of SERT mRNA. 5-HT acts on numerous receptors which mediate its signals to the intrinsic neural circuitry of the gut as well as to the central nervous system through spinal nerves and the vagus nerve. This study does not attempt to quantify the effects of increased or decreased 5-HT levels on these receptors.
Our data demonstrate that SERT transcript levels were significantly lower in areas of active inflammation in diverticulitis, and that there was a trend towards decreased expression of SERT mRNA in the adjacent normal bowel of patients with diverticulitis when compared to control. There were no differences detected between those with diverticulitis and control with regards to measures of 5-HT content or release. This study showed no differences detected in any of the studied aspects of 5-HT signaling between diverticulosis and control subjects.
Our results are consistent with data from other studies of mucosal inflammation in animals, as well as in human studies of IBD and IBS.10, 13, 14, 20 Furthermore, we have recently reported that SERT expression and function are decreased by TNF-alpha and IFN-gamma in the Caco-2 human epithelial cell line.21 Interestingly, the extramucosal inflammation, which is a hallmark of diverticulitis, appears to cause a similar effect on SERT expression in the mucosa. It is not possible to discern with certainty from this study whether decreased SERT expression predisposes patients with diverticulosis to develop diverticulitis, or if the inflammation associated with diverticulitis causes altered SERT expression. The latter is the more likely hypothesis because it is clear that inflammation down-regulates SERT expression and function.13, 14, 20, 21
Changes in 5-HT reuptake are likely to lead to altered 5-HT availability upon release, and to alterations in 5-HT-activated reflexes. Recently, Bian and colleagues demonstrated that delayed expression of SERT in the mucosa of neonatal guinea pigs is associated with elevated levels of free mucosal 5-HT.22 Furthermore, Chen et al. showed that transgenic mice that lack SERT exhibit diarrhea,23 and the use of serotonin selective reuptake inhibitors is similarly associated with an increased incidence of diarrhea.24 In addition, acute administration of the selective serotonin reuptake inhibitor, citalopram leads to increased motility, including high amplitude propagated contractions that are associated with cramping, as well as compliance, in the human colon.25 Our results may explain why many patients continue to suffer from discomfort and altered gut motility long after an acute flare of diverticulitis despite normalization of the white blood cell count and physical findings. This clinical scenario may mimic postinfectious-IBS, a well documented disorder related to disordered 5-HT signaling after mucosal inflammation from prior campylobacter infection.26
In conclusion, disordered 5-HT signaling does not appear to contribute to the development of colonic diverticula. However, decreased expression of SERT was noted in patients who have had CT proven acute diverticulitis up to six months after resolution of their clinical episode. These findings provide a plausible explanation for the persistent symptoms of altered motility often observed in patients who appear to have recovered from the acute inflammatory phase of diverticulitis.
ACKNOWLEDGMENTS
This work was supported by a New Research Initiative from the University Of Vermont College Of Medicine, NCRR P20 RR16435, and NIH grant DK62267. The authors would like to thank Drs. Peter Cataldo, Gino Trevisani, James Hebert, Peter Moses, Doris Strader, and Steven Willis for assistance with tissue gathering, Cristen Pantano, and Elice Brooks for laboratory assistance, and the COBRE laboratory for assistance and equipment in the RT-PCR.
Footnotes
Presented to the Surgical Forum of the American College of Surgeons, October 9, 2007.
REFERENCES
- 1.Simpson J, Scholefield JH, Spiller RC. Pathogenesis of colonic diverticula. Br J Surg. 2002;89(5):546–554. doi: 10.1046/j.1365-2168.2002.02076.x. [DOI] [PubMed] [Google Scholar]
- 2.Young-Fadok TM, Roberts PL, Spencer MP, Wolff BG. Colonic diverticular disease. Curr Probl Surg. 2000;37(7):457–514. doi: 10.1016/s0011-3840(00)80011-8. [DOI] [PubMed] [Google Scholar]
- 3.Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2(759):450–454. doi: 10.1136/bmj.2.5759.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delvaux M. Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 2003;18 Suppl 3:71–74. doi: 10.1046/j.0953-0673.2003.01720.x. [DOI] [PubMed] [Google Scholar]
- 5.Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4(1):53–69. [PubMed] [Google Scholar]
- 6.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363(9409):631–639. doi: 10.1016/S0140-6736(04)15597-9. [DOI] [PubMed] [Google Scholar]
- 7.Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15–30. [PubMed] [Google Scholar]
- 8.Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52(1):1–30. [PubMed] [Google Scholar]
- 9.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288(1):93–97. [PubMed] [Google Scholar]
- 10.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20 Suppl 7:3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 12.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115(2):370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 13.Linden DR, Foley KF, McQuoid C, et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17(4):565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 15.Penttila A, Lempinen M. Enterochromaffin cells and 5-hydroxytryptamine in the human intestinal tract. Gastroenterology. 1968;54(3):375–381. [PubMed] [Google Scholar]
- 16.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85(5):1120–1130. [PubMed] [Google Scholar]
- 17.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 18.Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23(8):1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 19.Costedio MM, Hyman N, Mawe GM. Serotonin and Its Role in Colonic Function and in Gastrointestinal Disorders. Dis Colon Rectum. 2006 doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 20.O'Hara JR, Ho W, Linden DR, et al. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287(5):G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- 21.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 22.Bian X, Patel B, Dai X, et al. High mucosal serotonin availability in neonatal guinea pig ileum is associated with low serotonin transporter expression. Gastroenterology. 2007;132(7):2438–2447. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21(16):6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorard DA, Libby GW, Farthing MJ. 5-Hydroxytryptamine and human small intestinal motility: effect of inhibiting 5-hydroxytryptamine reuptake. Gut. 1994;35(4):496–500. doi: 10.1136/gut.35.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tack J, Broekaert D, Corsetti M, et al. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther. 2006;23(2):265–274. doi: 10.1111/j.1365-2036.2006.02724.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98(7):1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]