Abstract
This study used quantitative volumetric magnetic resonance imaging techniques to explore the neuroanatomic correlates of chronic, combat-related posttraumatic stress disorder (PTSD) in seven Vietnam veterans with PTSD compared with seven nonPTSD combat veterans and eight normal nonveterans. Both left and right hippocampi were significantly smaller in the PTSD subjects compared to the Combat Control and Normal subjects, even after adjusting for age, whole brain volume, and lifetime alcohol consumption. There were no statistically significant group differences in intracranial cavity, whole brain, ventricles, ventricle:brain ratio, or amygdala. Subarachnoidal cerebrospinal fluid was increased in both veteran groups. Our finding of decreased hippocampal volume in PTSD subjects is consistent with results of other investigations which utilized only trauma-unexposed control groups. Hippocampal volume was directly correlated with combat exposure, which suggests that traumatic stress may damage the hippocampus. Alternatively, smaller hippocampi volume may be a pre-existing risk factor for combat exposure and/or the development of PTSD upon combat exposure.
Keywords: Stress disorders, posttraumatic, magnetic resonance imaging, hippocampus
Introduction
Animal research indicates that the hippocampus may be damaged by stress. Uno et al (1989) performed postmortem examinations on monkeys who died spontaneously after a period of sustained social stress. All showed evidence of marked 1and preferential hippocampal degeneration. Watanabe et al (1992c) reported that repeated daily restraint stress in rodents caused atrophy of the apical dendrites of CA3 hippocampal pyramidal neurons. Mizoguchi et al (1992) found significant loss of hippocampal CA3 and CA4 neurons in castrated rats stressed by restraint and water immersion.
Exposure to glucocorticoids has also been shown to damage the hippocampus, probably in interaction with excitatory amino acids. Sapolsky et al (1985) injected rats on a daily basis with sufficient corticosterone to produce prolonged elevations of circulating titers within the high physiologic range. Animals treated in this manner for three months showed loss of corticosterone-concentrating cells, especially in the CA3 cell field of the hippocampus. Clark et al (1995) found 15% cell loss in the CA3b hippocampal subfield in rats treated for twelve weeks with corticosterone. In another study, Sapolsky et al (1990) implanted cortisol pellets into the hippocampi of vervet monkeys. Postmortem examinations a year later showed damage restricted to the CA3/CA2 hippocampal cell fields, agreeing with the cellular features of stress-induced hippocampal damage. Uno et al (1990) found that near-term fetal rhesus monkeys exposed to even a single maternal dose of the synthetic glucocorticoid dexamethasone showed dose-dependent histologic CA3 cell field hippocampal damage after caesarean section 2–3 days later. In Cushing’s syndrome in humans, hippocampal volume has been found to vary inversely with plasma cortisol (Starkman et al 1992).
The above findings motivated Bremner et al (1995a) to use magnetic resonance imaging (MRI) to measure hippocampal volume in 26 Vietnam combat veterans with posttraumatic stress disorder (PTSD) and 22 noncombat, normal control subjects of similar age, sex, race, years of education, socioeconomic status, body size, and years of alcohol abuse. Volume of a segment of the hippocampal body was derived from five 3 mm contiguous coronal slices with no gaps. The results indicated an 8% decrease in right hippocampal volume in PTSD relative to control subjects, with no significant decreases for left hippocampus, right or left caudate nucleus, or right or left temporal lobe. The Bremner et al volumetric data, however, were not adjusted for whole brain volume.
Focusing on a different kind of traumatic event, Stein et al (1995) measured MRI hippocampal volume in 20 female victims of childhood sexual abuse, 14 with current PTSD and four with past but not current PTSD, vs 18 nonabused control subjects. Hippocampal volume was derived from seven 4mm coronal slices with 0.4 mm gaps between slices. No other brain structures were quantified. The abuse victims showed 7% smaller hippocampi than control subjects. This group difference remained significant after adjusting for brain volume as determined from the slices that were used to measure the hippocampus.
Using a similar MRI technique as in their first study (Bremner et al 1995a), Bremner et al (1995b) found left hippocampal volume to be 12.5% lower in 17 childhood physical and/or sexual abuse victims than in 17 normal controls. No further details are available regarding this unpublished study.
Hippocampal atrophy has also been found in neuropsychiatric conditions other than PTSD. It has, for example, been reported in 33% of MRIs in normal elderly persons (Golomb et al 1993). Hippocampal atrophy has also been proposed as an early marker for Alzheimer’s disease (Desmond et al 1992; O’Brien et al 1994). In schizophrenia, Chua and McKenna (1995) reviewed 14 MRI investigations that measured the amygdala–hippocampal complex; five found diminution in both hippocampus and amygdala, three in hippocampus only, and six in neither. Hippocampal atrophy has not been found in depressives (Axelson et al 1993), even those with cognitive impairment (O’Brien et al 1994), nor in alcoholic Korsakoff’s syndrome (Squire et al 1990).
Gurvits et al (1993) reported neurologic compromise in Vietnam veterans with PTSD, manifested by increased neurologic soft signs and a history of neurodevelopmental abnormalities. The study reported here was undertaken to explore the anatomic substrate of functional neurologic compromise in PTSD by means of MRI. A specific goal was to replicate findings of diminished hippocampal volume in PTSD and to extend this effort to include nonPTSD, trauma-exposed control subjects. One and one- half mm, contiguous MR slices were used to evaluate the entire amygdala–hippocampal complex, affording higher resolution than in previous studies.
Methods and Materials
Subjects
Participants included seven male Vietnam combat veteran outpatients who met DSM-III-R criteria for PTSD utilizing the Clinician-Administered PTSD Scale (CAPS; Blake et al 1995) and seven male Vietnam combat veterans with no lifetime PTSD (combat controls). No veteran had been a prisoner of war or exposed to prolonged malnutrition. Excluded were individuals with current organic mental, bipolar, or psychotic disorder, or with alcohol or other substance dependence or abuse within the past year, determined according to the Structured Clinical Interview for DSM-III-R (SCID; Spitzer et al 1989), neurologic disorder, or history of major head trauma, defined as involving loss of consciousness for more than 10 minutes. Subjects were free of psychotropic medications for at least two weeks prior to the onset of testing and for its duration.
Comorbid current or past lifetime Axis I disorders, determined by the SCID, in the seven PTSD subjects included one bipolar II, four major depression, two dysthymia, two panic, one simple phobia, one social phobia, one obsessive compulsive, and four generalized anxiety. Comorbid current or past Axis I disorders in the Combat Control subjects included three major depression. (Some subjects had more than one comorbid disorder). Of the seven PTSD subjects, two had past dependence or abuse of alcohol and another substance; three had past dependence or abuse of alcohol only. Of the seven combat control subjects, one had past dependence or abuse of alcohol and another substance; three had past dependence or abuse of alcohol only.
The PTSD subjects were all studied in the MRI laboratory prior to the combat control subjects, who were only added after additional funds became available. One of the authors (MES) also provided MRI data from eight normal, nonveteran control subjects drawn from subsequent investigation. These subjects were not available for neurologic examination or neuropsychologic testing, but they had been screened for the absence of disorders that could adversely affect brain function. None had a history of neurologic or psychiatric disorder or alcohol dependence/abuse.
Written informed consent for participation was obtained from all subjects after a complete description of the procedures.
Psychometric Tests
In addition to using the CAPS as a categorical measure to determine the presence or absence of the DSM-III-R PTSD diagnosis in the PTSD and combat control subjects, total CAPS score was also used as a continuous measure of PTSD severity, as was the score on the Mississippi Scale for combat-related PTSD (Keane et al 1988). PTSD and combat control subjects also completed the Combat Exposure Scale (Keane et al 1989).
Neuropsychologic Tests
In light of the well recognized role of the hippocampus in memory, PTSD and combat control subjects completed the Wechsler Memory Scale-Revised (WMS-R; Wechsler 1987) and Benton Visual Retention Test (Benton 1992), in addition to five subscales of the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler 1981).
Neurologic/Neuropsychiatric Evaluation
PTSD and Combat Control subjects underwent a videotaped examination for neurologic soft signs performed by TVG according to an expanded version of a previously published procedure (Gurvits et al 1993). Three independent raters, blind to group membership, used a scoring manual (available on request) to rate 41 neurologic soft signs each on a 0–3 scale. Scores were summed for a total measure of soft neurologic impairment (r = 0.86, intraclass correlation coefficient). TVG also obtained a standardized neuropsychiatric history (available on request), with special attention to developmental problems and central nervous system insults, including minor head injury, defined as involving loss of consciousness for less than 10 minutes. Subjects were asked to estimate the number of months of their lives they had engaged in excessive drinking, defined as daily intake of 70 g or more of ethanol (Baydena et al 1989).
MRI Image Acquisition
MRIs were performed at the Brigham and Women’s Hospital in Boston, MA. The scanning and image processing methodologies employed have been described in detail elsewhere (Shenton et al 1992). In brief, patients lay in the scanner in the neutral supine position. MR scans were acquired without tilting parallel to the three main axes on a 1.5 Tesla General Electric SIGNA system (GE Medical Systems, Milwaukee, WI). For the entire extent of the brain, 108 contiguous double echo spin-echo axial slices (54 for each level) of 3 mm slice thickness were obtained. The imaging parameters were: echo time (TE) = 30 and 80 msec, repetition time (TR) = 3000 msec, field of view = 24 cm, acquisition matrix = 256 × 256, voxel dimensions = 0.9375 × 0.9375 × 3. For the amygdala-hippocampal complex, a 3-D Fourier transform spoiled gradient-recalled acquisition in steady state was used to obtain scans throughout the entire brain and this 3-D acquisition was then reformatted into 124 contiguous 1.5 mm coronal slices.. The imaging parameters were: TE = 5 msec, TR = 35 msec, one repetition, nutation angle = 45 degrees, field of view = 24 cm, acquisition matrix = 256 × 256 × 124, voxel dimensions = 0.9375 × 0.9375 × 1.5.
Volumetric analyses
The postprocessing steps for the MR scans were performed according to a previously established protocol (Cline et al 1987, 1988, 1990; Kikinis et al 1992; Shenton et al 1992); only a summary is provided here. The steps included: 1) the use of a preprocessing filter to enhance the boundaries between tissues (Gerig et al 1992); 2) a nonparametric clustering algorithm for classifying voxels as to tissue type (Cline et al 1988); 3) a connectivity algorithm for linking regions of similar type (Cline et al 1987, 1988); 4) a voxel summation for computing volumes; and 5) a 3-D reconstruction to visualize the segmented tissue classes (Cline et al 1990).
For the hippocampal-amygdala complex, postprocessing steps were completed as above, followed by manual volumetric analyses performed by one of three raters, blind to any diagnostic information, in order to further delineate this region of interest. The same rater (HH) analyzed all PTSD and Combat Control subjects’ scans; another rater (HO) analyzed the Normal subjects’ scans.
The most anterior slice of the amygdala was defined by the white-matter tract (temporal stem) that links the temporal lobe with the rest of the brain and which is easily visualized in the coronal plane. The most posterior slice of the amygdala was defined as the last slice before the appearance of the mammillary bodies. The anterior boundary of the hippocampus was the slice with the first appearance of the mammillary bodies. The posterior boundary of the hippocampus was the slice with the last appearance of the fibers coursing the crux of the fornix. On average, 28 contiguous 1.5 mm slices were used to derive the volume of the amygdala-hippocampal complex. As has successfully been done in other studies (Bogerts et al 1993; Shenton et al 1992; Stein et al 1995), the mammillary bodies were used to divide the amygdala-hippocampal complex. This which resulted in the inclusion of a portion of the anterior hippocampus with the amygdala; however, this portion was too small to confound the results reported below.
Results
Demographic and Psychometric Data
Group means (and SDs) were as follows: age, PTSD 44.4 (1.7), combat control 47.6 (2.9), normal 38.1 (10.0), F(2,19) = 4.4, p = 0.03; education (grades completed), PTSD 14.7 (1.1), combat control 17.0 (1.7), normal 14.5 (2.6), F(2,19) = 3.6, p = 0.05. Psychometric data (available only for the PTSD and combat control subjects) appear in Table 1.
Table 1.
Psychometric, Neurologic, and Neuropsychologic Data and Correlations with Hippocampal Volume
| PTSD (n = 7) |
Combat Control (n = 7) |
t(12) | p | Correlations with total hippocampal volume |
||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | r(12) | p | |||
| Psychometric | ||||||
| Combat exposure scale | 33.1 (5.0) | 24.4 (9.9) | 2.1 | .06 | −.72 | .003 |
| Clinician-administered PTSD scale | 70.3 (17.1) | 15.3 (17.7) | 5.9 | <.001 | −.78 | .001 |
| Mississippi scale for combat-related PTSD | 120.7 (11.9) | 72.0 (24.9) | 4.7 | <.001 | −.57 | .03 |
| Neurologic | ||||||
| Total soft sign score | 31.1 (9.5) | 14.7 (4.2) | 4.2 | .001 | −.47 | .09 |
| Neuropsychologic | ||||||
| WAIS-R | ||||||
| Information | 11.07 (1.9) | 12,7 (1.9) | 1.6 | .14 | .38 | .20 |
| Digit span | 9.0 (2.7) | 10.6 (1.5) | 1.3 | .20 | .52 | .05 |
| Arithmetic | 11.6 (2.4) | 13,1 (2.3) | 1.2 | .23 | .58 | .02 |
| Picture arrangements | 11.0 (3.9) | 11.7 (2.8) | 0.4 | .70 | − .06 | .83 |
| Block design | 9.3 (1.8) | 12,0 (2.8) | 2.2 | .05 | .41 | .14 |
| Wechsler memory scale | ||||||
| Verbal memory index | 98.1 (9.5) | 103,1 (6.6) | 1.1 | .28 | .22 | .44 |
| Visual memory index | 104.6 (18.8) | 111.1 (12.4) | 0.8 | .45 | .13 | .64 |
| General memory index | 100.1 (14.0) | 106.3 (8.9) | 1.0 | .35 | .18 | .59 |
| Delayed recall index | 98.4 (12.5) | 104.6 (5.2) | 1.2 | .27 | .25 | .32 |
| Attn./conc. index | 94.0 (13.8) | 109.9 (10.6) | 2.4 | .03 | .65 | .01 |
| Benton visual retention test | ||||||
| Immediate recall errors | 5.6 (3.9) | 2.9 (1.6) | 1.7 | .11 | −.43 | .12 |
| Delayed recall errors | 7.4 (6.4) | 2.1 (1.9) | 2.1 | .06 | −.58 | .03 |
Neurologic and Neuropsychiatric Data
Total neurologic soft sign score was significantly higher for PTSD subjects than combat controls (Table 1). The numbers of PTSD vs combat control subjects who were positive for selected neuropsychiatric history items appear in Table 2. Mean months per lifetime of excessive drinking (and SDs) were: PTSD 98 (108), combat control 36 (44), t(12) = 1.4, p = 0.19.
Table 2.
Neuropsychiatric History Data
| PTSD (n = 7) | Combat Control (n = 7) | p* | |
|---|---|---|---|
| Right-handed | 4 | 7 | .10 |
| Delayed walking/talking | 2 | 0 | .23 |
| Attention deficit | 3 | 1 | .28 |
| Learning difficulties | 2 | 0 | .23 |
| Enuresis | 5 | 0 | .01 |
| Physical abuse | 4 | 2 | .30 |
| Minor head injury | 4 | 4 | .70 |
| Alcohol abuse | 5 | 4 | .50 |
| Other substance abuse | 2 | 1 | .50 |
Fisher’s exact test, one-tailed.
Neuropsychologic Data
There were trends for PTSD subjects’ performance to be worse than combat control subjects’ on most tests (Table 1); however, this was statistically significant (p < 0.05) only for WMS-R Attention/Concentration Index.
Computerized MRI Volumetric Data
Table 3 presents group means for the absolute volumes of the various structures measured, along with ANOVA results and pairwise group differences significant at p < 0.05. (The reliability of these analyses has been previously reported; Shenton et al 1992.) There were no statistically significant group effects for intracranial cavity, whole brain, ventricles, ventricle:brain ratio, or amygdala. Subarachnoid cerebrospinal fluid volume was increased in both PTSD and combat control compared with normal subjects; this difference remained significant after adjusting for age by means of analysis of covariance (ANCOVA): F′ (2,18) = 4.8, p = 0.02.
Table 3.
Volumes (ml) of the Brain Structures Examined
| PTSD (P) (n = 7) |
Combat control (C) (n = 7) |
Normal (N) (n = 8) |
F(2,19) | p | Tukey’s test | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Intracranial cavity | 1432 (181) | 1514 (119) | 1447 (80) | 0.8 | .47 | |
| Whole brain | 1227 (157) | 1290 (100) | 1314 (100) | 1.0 | .38 | |
| Subarachnoidal CSF | 180 (28) | 196 (53) | 105 (38) | 10.6 | <.001 | P, C > N |
| Lateral ventricles | 19.9 (9.7) | 23.6 (7.3) | 24.0 (10.7) | 0.4 | .68 | |
| Left temporal horn | .26 (.13) | .28 (.23) | .18 (.23) | 0.5 | .64 | |
| Right temporal horn | .36 (.22) | .31 (.23) | .23 (.19) | 0.7 | .49 | |
| Third ventricle | 2.7 (.9) | 2.7 (.7) | 2.6 (1.1) | 0.1 | .92 | |
| Fourth ventricle | 1.6 (.4) | 2.0 (.6) | 1.5 (.5) | 2.1 | .15 | |
| Ventricle:brain ratio | .020 (.009) | .022 (.006) | .022 (.009) | 0.2 | .85 | |
| Left hippocampus | 3.2 (.3) | 4.3 (.3) | 4.4 (.3) | 29.0 | <.001 | C, N > P |
| Right hippocampus | 3.2 (.6) | 4.1 (.4) | 4.6 (.4) | 14.3 | <001 | C, N > P |
| Left amygdala | 2.2 (.3) | 2.0 (.4) | 2.1 (.3) | 0.6 | .53 | |
| Right amygdala | 2.7 (.5) | 2.5 (.4) | 2.3 (. 1) | 3.0 | .07 |
Manual MRI Volumetric Data for Amygdala-Hippocampal Complex
Interrater reliabilities for determinations of both left and right amygdala-hippocampal complex volume for three raters [MS, HH, HO] on a previous training set were r > 0.87 (intraclass correlation coefficient). Two cases from the present set were randomly selected for volumetric analyses by these three raters in order to further estimate interrater reliability. These cases included all slices through the entire structure; each case was divided in half, so that n = 4 for these reliability analyses. Reliability coefficients for both the left and right amygdala-hippocampal complex were r > 0.89. The anterior and posterior boundaries of the complex for the two randomly selected cases were judged to be within 1 slice (1.5 mm) by the three raters. The technique employed for separating the amygdala from the hippocampus showed 100% reliability.
As shown in Table 3, both left and right hippocampal volumes were significantly lower in PTSD subjects compared to combat control and normal subjects, who did not differ significantly from each other. The group effect remained significant after adjusting for age and whole brain volume by means of analyses of covariance (ANCOVAs): for left hippocampal volume, F′ (2,17) = 25.2, p < 0.001; for right hippocampal volume, F′ (2,17) = 10.1, p = 0.001. The group effect also remained significant for left hippocampal volume, and nearly significant for right hippocampal volume, after adjusting for lifetime months of excessive drinking (available only for PTSD and combat control subjects) by adding this as a third covariate to the ANCOVAs: for left hippocampal volume, F′ (1,9) = 20.1, p = 0.002; for right hippocampal volume, F′ (1,9) = 4.4, p =07. Finally, the group effect remained significant for left hippocampal volume, but no longer for right hippocampal volume, after adjusting for combat exposure by adding Combat Exposure Scale score (again available only for PTSD and combat control subjects) as a fourth covariate: for left hippocampal volume, F′ (1,8) = 7.9, p = 0.02; for right hippocampal volume, F′ (1,8) = 1.3, p = 0.30.
The total (left plus right) hippocampal volumes of six of the seven PTSD subjects were lower than the volumes of all seven combat control and all eight normal subjects. Figure 1 presents MR images illustrating bilateral reduction in the size of left and right hippocampi in the subject who ranked lowest in total hippocampal volume among the PTSD subjects, compared with the subject who ranked lowest in total hippocampal volume among the Combat Control subjects.
Figure 1.
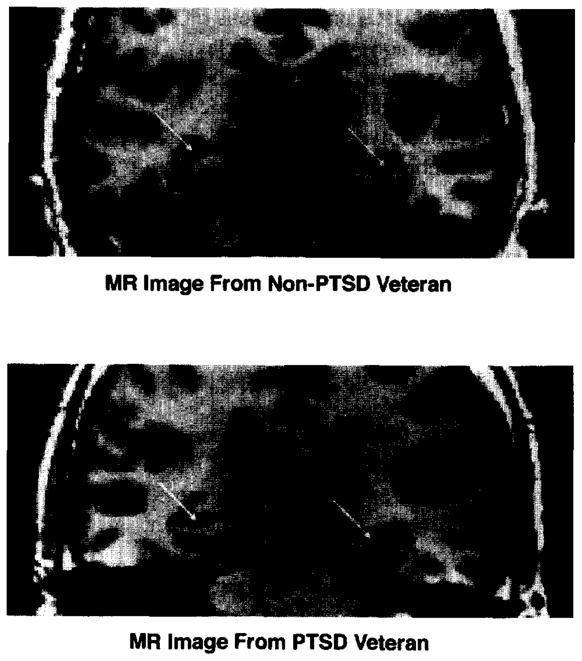
MR images illustrating smaller left and fight hippocampi in a Non-PTSD Veteran (Combat Control, top) and PTSD Veteran (bottom right)
Table 1 shows the zero-order correlations of total hippocampal volume with the psychometric, neurologic, and neuropsychologic data across the PTSD and combat control subjects. It may be seen that total hippocampal volume was highly correlated with combat exposure and PTSD severity as measured by the CAPS. Correlations between total hippocampal volume were in the predicted direction (i.e., lower total hippocampal volume correlated with more impairment) for the neurologic soft sign measure and 13 out of the 14 neuropsychologic measures, but these were statistically significant (p < 0.05) only for WAIS-R arithmetic, WMS-R attention/concentration index, and for Benton Visual Retention Test 15-sec. delayed recall errors.
Figure 2 presents a scatter plot of total hippocampal volume as a function of Combat Exposure Scale score in the 14 veteran subjects. Statistical analysis supported normal distribution of both Combat Exposure Scale scores (W= 0.96, p = 0.67) and total hippocampal volumes (W = 0.94, p = 0.40) despite the dichotomous classification of subjects into PTSD (closed circles) and nonPTSD (open circles) groups. The partial correlation between Combat Exposure Scale score and total hippocampal volume remained significant after adjusting for age, whole brain volume, and lifetime months of abusive drinking: r = −0.58, F(4,9) = 8.2, p = 0.02. However, the relationship between Combat Exposure Scale score and total hippocampal volume was confounded by PTSD severity, because CAPS total score was highly correlated with both Combat Exposure Scale score, r(12) = 0.82, p < 0.001, and total hippocampal volume, r(12) = −0.78, p = 0.001. Neither the partial correlation of total hippocampal volume with Combat Exposure Scale score, r(11) = −0.25, p = 0.42, nor with total CAPS score, r(11) = −0.46, p = 0.11, was significant when each was adjusted for the other.
Figure 2.
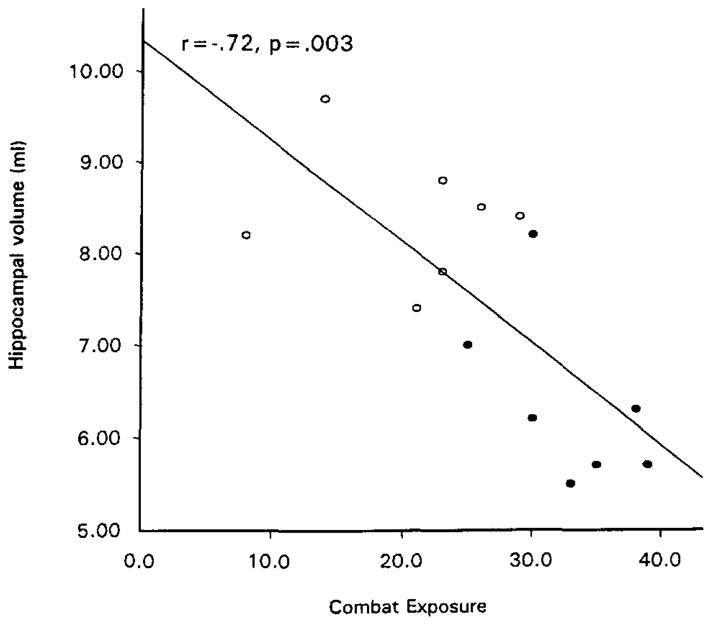
Total hippocampal volume as a function of combat exposure scale score. Closed circles: PTSD subjects; open circles: non-PTSD subjects.
Discussion
The present study found diminished left and right hippocampal volume in PTSD combat veteran subjects in comparison to both combat and noncombat control subjects. These results remained significant after statistically adjusting for age, total brain volume, lifetime months of excessive drinking, and, for left hippocampus, severity of combat exposure. This finding supplements previous evidence of neurologic compromise in PTSD. It is also consistent with other findings of diminished hippocampal volume in subjects with PTSD resulting from combat in Vietnam (Bremner et al 1995a) and child abuse (Bremner et al 1995b; Stein et al 1995) in comparison only to nontrauma exposed control subjects. The 26% total hippocampal diminution in the present study was manifest bilaterally and larger than the percent diminutions described in other studies. This may in part be accounted for by the greater resolution afforded by the larger number of thinner MRI slices employed, and the greater extent of hippocampal structure measured, in the present study.
Results of the present study present modest evidence of an association between hippocampal volume reduction and impaired memory functioning, e.g., in the correlation between hippocampal volume and delayed recall errors.
The present investigation suffers from limitations typical of pilot studies of this nature. Subjects from the different groups were not intermingled in the MRI analyses, so the possibility of subtle methodologic differences between groups cannot be excluded. Sample sizes were small, and the groups were imperfectly matched on age and past alcohol abuse, although when statistically controlled, group differences on these factors did not account for the significantly smaller hippocampi in the PTSD subjects. Furthermore, the credibility of this finding is heightened by its convergence with the results of three other investigations performed in two other laboratories. Nevertheless, replication of the present results is called for in a larger, more carefully controlled study. The greater volume of cerebrospinal fluid in both the PTSD and combat control subjects relative to the normal nonveteran subjects may at least partially have resulted from greater age and past alcohol use.
Additional studies are also required to test the anatomic specificity of lower hippocampal volume in PTSD. The present study included only one comparison structure, viz., amygdala. Bremner et al (1995a) compared hippocampus with caudate nucleus and temporal lobe. Although only the right hippocampus was found to be significantly smaller in the PTSD subjects, its differential reduction over the comparison structures was less apparent when expressed in percentage terms than in terms of statistical effect size. Stein et al (1995) did not measure any comparison brain structures. It remains to be investigated whether the volume of other structures, e.g., the frontal lobes, may also be lower in PTSD.
In light of the strong negative correlation between severity of past combat exposure and hippocampal volume, a straightforward interpretation of the results of the present study is that the severe stress of military combat both damages the hippocampus and causes PTSD. This conclusion would bring the present findings in line with those from studies indicating that stress and glucocorticoids damage the rodent and primate hippocampus. Bremner et al (1995a) did not find a significant correlation between hippocampal volume and combat exposure within the PTSD veterans they studied. However, the power to detect such a correlation in that study may have been reduced by the smaller decreases in hippocampal volume that were observed, and by a narrower range of combat exposure scores due to the absence of a nonPTSD combat control group.
There are, however, at least three alternative explanations for the association between combat exposure and smaller hippocampi found here. First, it may be that this relationship is not directly causal but rather results from the association of both these variables with a third factor, i.e., PTSD. Indeed, the correlation between combat exposure and hippocampal volume lost its significance after adjusting for PTSD severity. This makes sense, because a psychologic stressor can only impact on the organism insofar as it induces a stress response. It may be that combat stress causes PTSD, and PTSD, and/or its biopsychosocial concomitants, causes shrinkage of the hippocampus.
Second, it is possible that diminished hippocampal volume represents a risk factor for exposure to combat. In this regard, the history of significantly more precombat enuresis, and the nonsignificant trends toward more delayed walking/talking, attention deficit, and learning difficulties, in the PTSD subjects, is worth noting. In a previous neuropsychiatric study of PTSD, Gurvits et al (1993) also found evidence of pretrauma, neurodevelopmental impairment in PTSD Vietnam veterans. Individuals with neurodevelopmental impairment, possibly including smaller hippocampi, may be less likely to be assigned to higher-skill military occupational specialties that isolate them from combat as opposed to infantry or other high-combat units.
Third, low hippocampal volume may represent a precombat vulnerability factor for the development of PTSD upon combat exposure. Theoretically, this possibility would not predict a statistical association between combat exposure and decreased hippocampal volume; however, if precombat vulnerability, manifest (among other things) in smaller hippocampi on the one hand, and high combat exposure on the other hand, were independently to increase the likelihood of PTSD, the selective recruiting of PTSD subjects in the present study might have led to a spurious, i.e., noncausal, association between combat exposure and hippocampal volume. Such an explanation could potentially be disproven in a study that selected subjects independent of PTSD status. Although no such study has yet been undertaken in combat veterans, Stein et al (1995) selected their subjects on the basis of a history of exposure vs. no exposure to child abuse, irrespective of PTSD. Thus their finding of smaller hippocampi in the exposed group is supportive of a causal relationship between traumatic exposure and hippocampal reduction.
The ultimate test of whether combat, or any other traumatic exposure, causes hippocampal damage, must be a prospective study employing a within subjects, repeated measures design that examines the development of de novo hippocampal reduction, and possibly PTSD, following exposure to traumatic stress. Meanwhile, animal studies indicate that stress and steroid induced hippocampal damage may be prevented by the administration of certain pharmacologic agents, e.g., phenytoin (Watanabe et al 1992a) or tianeptine (Watanabe et al 1992b). Should it be established that traumatic stress does indeed damage the human hippocampus, these animal findings raise the intriguing possibility of secondary, i.e., posttraumatic event, pharmacologic prevention of hippocampal damage and possibly PTSD.
Acknowledgments
Supported by a VA Research Career Development Award (TVG); VA Medical Research Service Grants (SPO, RWM, RKP); NIMH Research Scientist Development Award #KO2MH01110-01 (MES); NIMH Grant #R29MH50740-01 (MES); NIMH Grant #R01MH40799 (RWM); the VA Research Center for Basic Science and Clinical Studies of Schizophrenia (RWM); and the Stanley Foundation (MES, RK).
The authors thank Maureen Ainslie, Michelle Ballard, Brian Chiango, Eve Chiango, Heike Croteau, Marianna Jakob, and Adam Shostack for technical and administrative assistance and Michael Macklin for subject recruitment assistance.
References
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH, Krishna KRR. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- Baydena L, Branchey MH, Noumair D. Age of alcoholism onset I: Relationship to psychopathology. Arch Gen Psychiatry. 1989;46:225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- Benton AB. Benton Visual Retention Test. 5. San Antonio: Harcourt Brace Jovanovich, Inc. (The Psychological Corporation); 1992. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Bremner D, Randall P, Scott TN, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarty G, Charney DS, Innis RB. MRI-based measurements of hippocampal volume in combat-related posttraumatic stress disorder. Am J Psychiatry. 1995a;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PK, Southwick SM, Krystal JH, Innis RB, Charney DS. Stress-induced changes in memory and development. American Psychiatric Association Syllabus and Proceedings Summary. 1995b;148:112. [Google Scholar]
- Chua SE, McKenna PJ. Schizophrenia-a brain disease: A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry. 1995;166:563–582. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 1995;679:64–71. doi: 10.1016/0006-8993(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Cline HE, Dumoulin CL, Hart HR, Jr, Lorensen WE, Ludke S. 3D surface reconstruction of the brain from magnetic resonance images using a connectivity algorithm. J Mag Reson Imaging. 1987;5:345–52. doi: 10.1016/0730-725x(87)90124-x. [DOI] [PubMed] [Google Scholar]
- Cline HE, Lorensen WE, Kikinis R, Jolesz FA. Three dimensional segmentation of MR images of the head using probability and connectivity. J Comput Assist Tomogr. 1990;14:1037–1045. doi: 10.1097/00004728-199011000-00041. [DOI] [PubMed] [Google Scholar]
- Cline HE, Lorensen WE, Ludke S, Crawford CR, Teeter BC. Two algorithms for the three-dimensional reconstruction of tomograms. Med Phys. 1988;15:320–327. doi: 10.1118/1.596225. [DOI] [PubMed] [Google Scholar]
- Desmond PM, Tress B, Ames D, Clement JG, Clement P, Schweitzer I, Robinson GS. Volumetric and visual assessment of the mesial temporal structures in Alzheimer’s Disease. American Society of Neuroradiology Annual Meeting. 1992 doi: 10.1111/j.1445-5994.1994.tb01756.x. (Abstract 102(a):68) [DOI] [PubMed] [Google Scholar]
- Gerig G, Kubler O, Kikinis R, Jolesz FA. Nonlinear anisotropic filtering of MRI data. IEEE Trans Med Imaging. 1992;11:221–232. doi: 10.1109/42.141646. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging: An association with recent memory impairment. Arch Neurol. 1993;50:967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Gurvits TG, Lasko NB, Schachter SC, Kuhne AA, Orr SP, Pitman PK. Neurological status of Vietnam veterans with chronic post-traumatic stress disorder. J Neuropsychiatry Clin Neurosci. 1993;5:183–188. doi: 10.1176/jnp.5.2.183. [DOI] [PubMed] [Google Scholar]
- Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: Three studies in reliability and validity. J Consult Clin Psychol. 1988;56:85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- Keane TM, Fairbank JA, Caddell JM, Zimering RT, Taylor KL, Mora CA. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1:53–55. [Google Scholar]
- Kikinis R, Shenton ME, Jolesz FA, Gerig G, Martin J, Anderson M, Metcalf D, Guttman C, McCarley RW, Lorensen W, Cline H. Routine quantitative analysis of brain and cerebrospinal fluid spaces with MR imaging. J Mag Reson Imaging. 1992;2:619–629. doi: 10.1002/jmri.1880020603. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Kunishita T, Chui DH, Tabira T. Stress induces neuronal death in the hippocarnpus of castrated rats. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Desmond P, Ames D, Schweitzer I, Tuckwell V, Tress B. The differentiation of depression from dementia by temporal lobe magnetic resonance imaging. Psychol Med. 1994;24:633–640. doi: 10.1017/s0033291700027781. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton MA, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: A quantitative magnetic resonance imagery study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. New York: Biometrics Research Department, New York State Psychiatric Institute; 1989. [Google Scholar]
- Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci. 1990;10:3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stein MB, Hannah C, Koverola C, McClarty B. Neuroanatomic and cognitive correlates of early abuse. American Psychiatric Association Syllabus and Proceedings Summary. 1995;148:113. [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques, i. hippocampus. Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else J, Suleman M, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe YE, Gould H, Cameron D, Daniels D, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe YE, Gould H, Daniels D, Cameron D, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharm. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992c;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. New York: Harcourt Brace Jovanovich, Inc. (The Psychological Corporation); 1981. [Google Scholar]
- Wechsler D. WMS-R Manual: Wechsler Memory Scale Revised. New York: Harcourt Brace Jovanovich, Inc. (The Psychological Corporation); 1987. [Google Scholar]


