Abstract
Purpose
To examine whether the noninvasive technique of blood oxygenation level dependent magnetic resonance imaging (BOLD MRI) can detect changes in renal medullary oxygenation following administration of a nitric oxide (NO) synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). Hypertension is associated with endothelial dysfunction and is characterized by a lack of response to endothelial-dependent vasoactive substances, including nitric oxide synthase inhibitors. We hypothesized that the magnitude of the change would be reduced in the kidneys of hypertensive subjects relative to normal controls.
Materials and Methods
To test this hypothesis, data were obtained in spontaneously hypertensive rats (SHR, n = 6). Wistar-Kyoto rats (WKY, n = 7) were used as normotensive controls.
Results
As expected, WKY rats showed a significant response to L-NAME (R2* increasing from 23.6±1.5 Hz to 32.5±2.2 Hz, P < 0.05), while SHR exhibited a minimal change in medullary oxygenation (R2* measuring 31.9±2.8 Hz pre- and 35.5±2.2 Hz post-L-NAME). The baseline R2* in SHR is found to be comparable to post-L-NAME values in WKY rats, suggesting a basal deficiency of nitric oxide in SHR.
Conclusion
Based on the differential effect of NO synthase inhibition on medullary oxygenation, BOLD MRI can distinguish hypertensive from normal kidney. Our results are consistent with previously reported observations using invasive methods.
Keywords: kidney, medulla, nitric oxide, L-NAME, hypertension, BOLD MRI
THE KIDNEY IS BELIEVED TO PLAY A ROLE in the pathogenesis of essential hypertension (1,2). In particular, reduced renal medullary blood flow is thought to be one of the important factors in the development of the disease (3–8). Animal studies have shown that medullary blood flow is decreased in hypertension and, more importantly, that reduced medullary blood flow is sufficient to produce hypertension (9).
While significant advances have been made in understanding the causes of essential hypertension through the use of such animal studies, it has proven difficult to extend the research to humans because of the lack of suitable monitoring techniques. In animals, medullary blood flow is typically measured using invasive laser Doppler probes (1) and to date there is no noninvasive alternative for use in humans.
The motivation behind the present work was to determine whether blood oxygen level dependent magnetic resonance imaging (BOLD MRI) could provide such an alternative. While BOLD MRI does not measure blood flow directly, a large body of evidence now exists, derived primarily from functional MRI studies in the brain (10–12), suggesting a strong correlation between BOLD measurements and blood flow.
The BOLD MRI technique exploits the fact that the magnetic properties of hemoglobin vary depending on whether it is in the oxygenated or deoxygenated form. This affects the T2* relaxation time of the neighboring water molecules and in turn influences the MRI signal on T2*-weighted images. Because the ratio of oxyhemoglobin to deoxyhemoglobin is related to the pO2 of blood, and since the pO2 of capillary blood is thought to be in equilibrium with the surrounding tissue, changes estimated by BOLD MRI can be interpreted as changes in tissue pO2 (13). Tissue pO2 is in turn correlated with blood flow since an increase in blood flow, unless it is compensated by a corresponding increase in metabolic activity, will oversupply the tissue with oxygen resulting in an increase in tissue pO2.
BOLD MRI has previously been used to monitor changes in intrarenal blood flow and medullary oxygenation in both human (13) and animal (14) studies. In humans, attenuated responses to waterload have been observed using BOLD MRI in elderly subjects (15), diabetics (16), and in young healthy subjects pretreated with a prostaglandin inhibitor (15). The technique has also been used in rats to monitor changes in renal oxygenation following the administration of various pharmacologic agents that either increase (17) or decrease (14) intrarenal blood flow.
The goal of our current work is to determine whether BOLD MRI can be used to detect differences in medullary blood flow between hypertensive subjects and normotensive controls. In this preliminary study, we made use of a nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). Nitric oxide (NO) is a soluble gas that is continuously synthesized by the endothelium (18) and has a wide range of biological properties including relaxation of vascular tone. In healthy subjects, administration of L-NAME blocks the synthesis of nitric oxide and results in a constriction of the vessels. There is also a corresponding reduction in blood flow and an increase in blood pressure (6,8,19–22). In hypertensive subjects, however, it is known that both NO production and basal NO bioavailablity is reduced (23), due to either impaired synthesis or excessive oxidative degradation (24). Administration of nitric oxide synthase inhibitors such as L-NAME, therefore, should have little effect in such individuals.
The hypothesis of this study was that a response to L-NAME administration should be detectable in normotensive subjects using BOLD-MRI, but that the response should be diminished in hypertensive individuals. Because L-NAME has not yet been approved for human use, the study was performed in spontaneously hypertensive rats (SHR), a commonly used animal model of essential hypertension. SHR were originally derived from Wistar-Kyoto (WKY) rats. They exhibit an upregulation of nitric oxide synthase (NOS) (25), an enzyme present in endothelial cells that metabolizes L-arginine to produce NO. However, due to excessive oxidative degradation, SHR actually have reduced bioavailability and activity of NO (26,27). Inhibition of NO synthase, therefore, should have minimal effect on their renal blood flow, a result that has been confirmed using invasive measurements (5,6,8,19–22,28). It was our hypothesis that a reduced response to NOS inhibition should also be detectable with BOLD MRI. In our experiments, we performed BOLD imaging in SHR before and after administration of L-NAME. We then compared the results with data obtained in WKY rats used as normotensive controls.
MATERIALS AND METHODS
All imaging was performed on a 1.5T scanner (CV/i, General Electric Medical Systems, Milwaukee, WI) using a multiple gradient recalled echo (mGRE) sequence (TR/TE/flip angle/bandwidth = 70 msec/8–50.4 msec/20°/±62.5 kHz) to acquire 16 T2* weighted images. The field of view was 13 cm, matrix size was 256×256, slice thickness was 3 mm, and slice separation was 1 mm. Due to the small voxel size, the signal was averaged over four repetitions (i.e., NEX=4). A standard quadrature extremity coil was used for signal reception and the animal was positioned in a right lateral position in order to minimize susceptibility artifacts from bowel gas. The signal intensity vs. time data were fitted to a single decaying exponential function to determine the value of R2* (=1/T2*) that was used as a semiquantitative measure of relative tissue oxygenation (13). An increase in R2* indicates a decrease in tissue pO2.
All experiments were performed in anesthetized rats (Inactin 100 mg/kg) and were approved by the Institutional Animal Care and Use Committee of our institution. For validation purposes, imaging was first performed on Sprague-Dawley (SD) rats (n=5; wt: 494±25 gm) in order to confirm results previously obtained on a different scanner (14). The studies were then repeated on SHR (n=6; wt: 270±26 gm) and WKY rats (n=7, wt: 276±33 gm). All animals were purchased from Harlan Laboratories (Madison, WI) and the SHR were at least 9 weeks old, the age by which they are known to become hypertensive (29). The femoral vein was catheterized for administration of the NO synthase inhibitor L-NAME. After obtaining a set of baseline T2*-weighted images, L-NAME (10 mg/kg) was administered i.v. Further sets of T2*-weighted images were obtained every 2 to 3 minutes for about 30 minutes post-L-NAME.
R2* maps were generated from the T2*-weighted images using the inbuilt “FuncTool” feature on the scanner. Regions of interest (ROIs) of at least 4 pixels were chosen on the maps to obtain values for the mean and standard deviation of R2* in the renal medulla and cortex. The statistical significance of the differences between pre- and post-L-NAME R2* values was assessed using the two-tailed paired Student’s t-test.
RESULTS
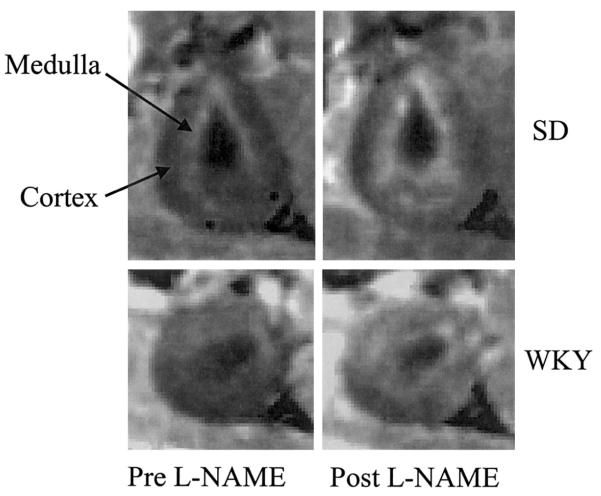
Figure 1 shows representative R2* maps in SD and WKY rats. Owing to the relatively smaller size of the SHR and WKY rats, the images are less impressive compared with SD. In SD and WKY rats, the medulla is brighter than the cortex in the R2* maps because of its lower basal oxygenation. There is a clear increase in R2* in the medulla post-L-NAME in SD rats, indicating a reduction in medullary oxygenation. While a similar trend is observed in WKY rats, no appreciable increase in medullary brightness is observed post-L-NAME in SHR. The absolute magnitude of change observed in SD was higher than in WKY (38.5±5.4 to 63.5±5.7 Hz in the medulla and 29.2±4.3 to 42.8±4.1 Hz in the cortex). The exact cause for this difference is not known.
Figure 1.
Representative pre- and post-L-NAME R2* maps from a SD and WKY rat. Note the relatively brighter medulla in the post-L-NAME map as compared with pre-L-NAME map, signifying a reduction in medullary oxygenation. The window and level settings for both the maps are exactly the same. The trend of increased R2* values in the medulla post-L-NAME is consistent with our previously published data (14).
Figure 2 displays graphs of medullary R2* vs. time for one representative SHR and WKY rat. Note that the SHR exhibits minimal change in R2* over the 30 minute post-L-NAME period during which the data was acquired, while the WKY rat shows a significant increase in R2*. Also note the elevated baseline R2* in the SHR as compared with the WKY rat.
Figure 2.
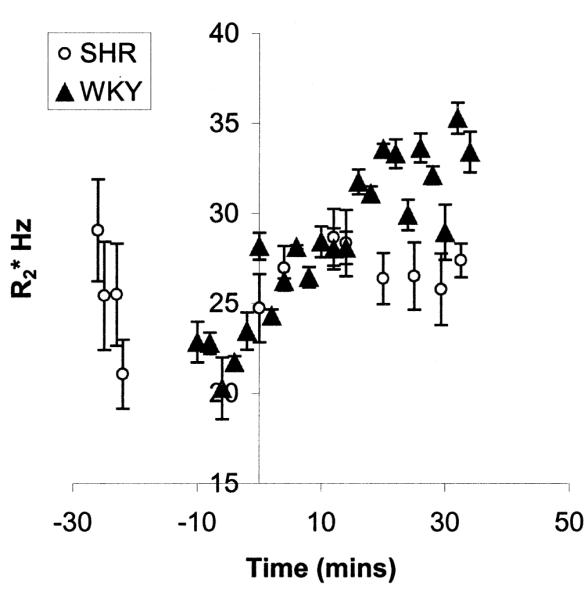
Plot showing medullary R2* vs. time for one representative SHR and WKY rat. Note that the SHR shows a relatively small change in R2*, while the WKY rat shows a significant response to L-NAME (administered at time zero). Also note the difference in baseline values between the two rats. The higher baseline R2* in the SHR is consistent with results reported previously by other groups of inherent reduced bioavailability of NO in the kidneys of hypertensive rats (23). The error bars indicate the standard deviation in each ROI measurement.
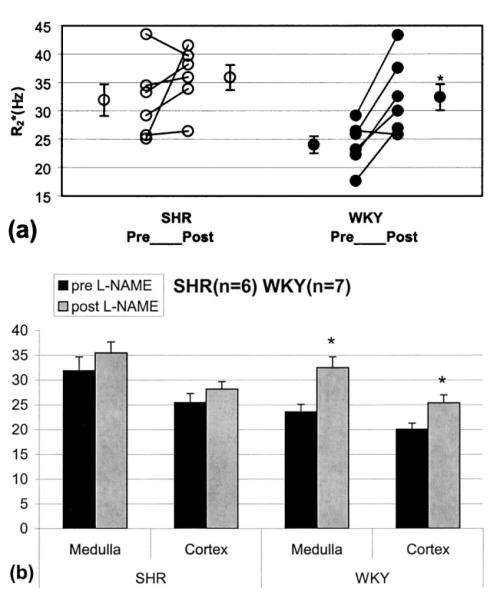
The individual and mean R2* values (averaged over all the rats of each strain) pre- and post-L-NAME are shown in Figure 3. For statistical analysis, the average of all points acquired at least 20 minutes after L-NAME administration was used as post-L-NAME R2*. While the WKY rats showed a statistically significant increase in medullary R2* post-L-NAME, the change in SHR did not reach statistical significance. Note that, even at baseline, SHR rats have higher R2* than WKY rats, suggesting low basal medullary oxygenation. The pre-L-NAME medullary R2* value in SHR (31.9±2.8 s−1) is in fact similar to the post L-NAME R2* value in WKY rats (32.5±2.2 s−1). Although the cortex in WKY also shows an increase in R2*, it may be partly due to partial volume effects from the medulla (14).
Figure 3.
a: Illustration of individual changes in medullary R2* post-L-NAME in SHR and WKY rats. Average of all points acquired at least 20 minutes after L-NAME administration was used as post-L-NAME R2*. Mean R2* values pre- and post-L-NAME were averaged over all rats of each strain. b: Mean R2* values pre- and post-L-NAME in the renal medulla and cortex averaged over all rats of each strain. Note the difference in baseline values between the two rats. The higher baseline R2* in the SHR is consistent with results reported previously by other groups of inherent reduced bioavailability of NO in the kidneys of hypertensive rats (23). *P < 0.05 by paired two-tailed Student’s t-test.
Table 1 provides a summary of R2* values in the medulla and cortex of each strain pre- and post-L-NAME. The post-L-NAME values are the average of all points acquired at least 20 minutes after L-NAME administration.
Table 1.
R2* in Medulla and Cortex of SHR and WKY Rat Kidneys*
| SHR (N = 6) R2* Hz (mean ± SE) |
WKY (N = 7) R2* Hz (mean ± SE) |
|||
|---|---|---|---|---|
| Pre-L-NAME | >20 minutes post-L-NAME | Pre-L-NAME | >20 minutes post-L-NAME | |
| Medulla | 31.9 ± 2.8 | 35.5 ± 2.2 | 23.6 ± 1.5 | 32.5 ± 2.2a |
| Cortex | 25.5 ± 1.8 | 28.2 ± 1.5 | 20.1 ± 1.2 | 25.4 ± 1.6 |
Average of all points acquired at least 20 minutes after L-NAME administration was used as post-L-NAME R2*.
P < 0.05 compared to pre-L-NAME by two tailed paired Student’s t-test.
DISCUSSION
The data presented here demonstrate the utility of BOLD MRI in distinguishing hypertensive from normal kidneys based on the differential effect of NO synthase inhibition on medullary oxygenation. Statistically significant changes in R2* in response to L-NAME were observed in WKY rats, but not in SHR. The fact that the baseline R2* value in SHR is similar to the post-L-NAME value in WKY rats suggests that SHR have low basal bioavailability of NO. This conclusion is consistent with previous findings in spontaneously hypertensive rats, obtained using isolated cannulated arterioles (30). It also agrees with the results of studies in humans of ischemia-induced reactive hyperemia in the peripheral vasculature (31). In these studies, subjects with essential hypertension showed reduced hyperemic response compared with normal controls, demonstrating diminished NO bioavailability (23,32–34).
Our results show changes in R2* in the renal cortex in response to L-NAME administration. While it is possible that this reflects a true change in cortical oxygenation that is consistent with observations by Welch et al (35), we suspect it may be partly related to partial volume effects from the medulla. In principle, one would expect little or no response in the cortex because the cortex is well oxygenated (relative to the medulla) and thus falls near the plateau of the hemoglobin oxygen-saturation curve. A change in blood pO2, therefore, produces relatively little variation in the ratio of oxyhemoglobin to deoxyhemoglobin in the cortex as compared with that in the medulla and should have minimal effect on the BOLD signal. The fact that we observed a BOLD response may be due to the fact that the kidney in rats is so small that voxels apparently lying in the cortex may also contain medullary tissue.
Owing to the influence of geometrical factors, a major limitation of the BOLD MRI technique for the evaluation of oxygenation is the absence of a direct relationship between R2* and blood pO2. This precludes the quantitative interpretation of R2* data in terms of blood, and hence tissue, pO2. However, in the absence of any alternative noninvasive technology to provide such information, BOLD MRI should still have a major impact on the study of ischemic renal disease in humans.
While the present study was performed in an animal model due to the use of L-NAME, the imaging protocol is well suited for clinical applications. Experiments analogous to the one reported here should be easily translated to human studies with a careful choice of vasoactive substances. We are currently considering the use of an alternative NO synthase inhibitor, L-NMMA, that is preferred for human use and is approved for investigational purposes (36–39). The BOLD technique itself is routinely used in humans and, in fact, is easier to apply in human studies than animal experiments due to the larger size and the possibility of breath-holding.
Reports in the literature indicate that the factors that reduce medullary blood flow are those commonly associated with elevations of arterial pressure, such as NO synthase inhibition. Conversely, factors that increase medullary blood flow are those believed to lower blood pressure, such as acetylcholine and prostaglandins (3). Given that BOLD measurements of renal oxygenation are correlated with blood flow, this suggests that BOLD MRI may be a useful tool to evaluate the potential effectiveness of antihypertensive pharmacological therapies. Among these are treatments involving oxygen free-radical scavengers (40). Although the exact mechanism of action of free-radical scavengers is not yet known, it has been shown that they improve endothelium-dependent vasodilation in human subjects (32). BOLD MRI should provide a useful tool to monitor efficacy of such therapies.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Institutes of Health, NIDDK 53221 (PVP). We thank Jason Polzin, Ph.D., GE Medical Systems, Milwaukee, WI for his assistance in implementing the mGRE sequence and FUNCTOOL routine to calculate R2* maps. We also thank Dr. Ai-Ping Zou for his many useful discussions.
Contract grant sponsor: National Institutes of Health; Contract grant number: NIDDK 53221.
Footnotes
Presented at the 10th Annual Meeting of the ISMRM, Honolulu, Hawaii, 2002.
REFERENCES
- 1.Cowley AW, Roman RJ, Fenoy FJ, Mattson DL. Effect of renal medullary circulation on arterial pressure. J Hypertens Suppl. 1992;10:S187–S193. [PubMed] [Google Scholar]
- 2.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW, Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 4.Roman RJ, Kaldunski ML. Renal cortical and papillary blood flow in spontaneously hypertensive rats. Hypertension. 1988;11:657–663. doi: 10.1161/01.hyp.11.6.657. [DOI] [PubMed] [Google Scholar]
- 5.Naess PA, Kirkeboen KA, Christensen G, Kiil F. Inhibition of renal nitric oxide synthesis with NG-monomethyl-L-arginine and NG-nitro-L-arginine. Am J Physiol. 1992;262:F939–F942. doi: 10.1152/ajprenal.1992.262.6.F939. [DOI] [PubMed] [Google Scholar]
- 6.Dananberg J, Sider RS, Grekin RJ. Sustained hypertension induced by orally administered nitro-L-arginine. Hypertension. 1993;21:359–363. doi: 10.1161/01.hyp.21.3.359. [DOI] [PubMed] [Google Scholar]
- 7.Gardiner SM, Kemp PA, Bennett T, Palmer RM, Moncada S. Nitric oxide synthase inhibitors cause sustained, but reversible, hypertension and hindquarters vasoconstriction in Brattleboro rats. Eur J Pharmacol. 1992;213:449–451. doi: 10.1016/0014-2999(92)90636-i. [DOI] [PubMed] [Google Scholar]
- 8.Manning RD, Jr, Hu L, Mizelle HL, Montani JP, Norton MW. Cardiovascular responses to long-term blockade of nitric oxide synthesis. Hypertension. 1993;22:40–48. doi: 10.1161/01.hyp.22.1.40. [DOI] [PubMed] [Google Scholar]
- 9.Mattson DL, Roman RJ, Cowley AW., Jr Role of nitric oxide in renal papillary blood flow and sodium excretion. Hypertension. 1992;19:766–769. doi: 10.1161/01.hyp.19.6.766. [DOI] [PubMed] [Google Scholar]
- 10.Mechelli A, Price CJ, Friston KJ. Nonlinear coupling between evoked rCBF and BOLD signals: a simulation study of hemodynamic responses. Neuroimage. 2001;14:862–872. doi: 10.1006/nimg.2001.0876. [DOI] [PubMed] [Google Scholar]
- 11.Kastrup A, Kruger G, Neumann-Haefelin T, Glover GH, Moseley ME. Changes of cerebral blood flow, oxygenation, and oxidative metabolism during graded motor activation. Neuroimage. 2002;15:74–82. doi: 10.1006/nimg.2001.0916. [DOI] [PubMed] [Google Scholar]
- 12.Rees G, Howseman A, Josephs O, et al. Characterizing the relationship between BOLD contrast and regional cerebral blood flow measurements by varying the stimulus presentation rate. Neuroimage. 1997;6:270–278. doi: 10.1006/nimg.1997.0300. [DOI] [PubMed] [Google Scholar]
- 13.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 14.Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13:744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55:294–298. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care. 2002;25:575–578. doi: 10.2337/diacare.25.3.575. [DOI] [PubMed] [Google Scholar]
- 17.Priatna A, Epstein FH, Spokes K, Prasad PV. Evaluation of changes in intrarenal oxygenation in rats using multiple gradient-recalled echo (mGRE) sequence. J Magn Reson Imaging. 1999;9:842–846. doi: 10.1002/(sici)1522-2586(199906)9:6<842::aid-jmri12>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 19.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol. 1993;264:F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- 20.Salazar FJ, Alberola A, Pinilla JM, Romero JC, Quesada T. Salt-induced increase in arterial pressure during nitric oxide synthesis inhibition. Hypertension. 1993;22:49–55. doi: 10.1161/01.hyp.22.1.49. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi K, Mattson DL, Cowley AW., Jr Role of renal medullary blood flow in the development of L-NAME hypertension in rats. Am J Physiol. 1995;268:R317–R323. doi: 10.1152/ajpregu.1995.268.2.R317. [DOI] [PubMed] [Google Scholar]
- 22.Mattson DL, Lu S, Nakanishi K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–H1926. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 23.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 24.Cannon RO., III Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 25.Vaziri ND, Ni Z, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- 27.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 28.Baylis C, Engels K, Samsell L, Harton P. Renal effects of acute endothelial-derived relaxing factor blockade are not mediated by angiotensin II. Am J Physiol. 1993;264:F74–F78. doi: 10.1152/ajprenal.1993.264.1.F74. [DOI] [PubMed] [Google Scholar]
- 29.Krinke GJ. The Laboratory Rat. Academic Press; New York: 2000. p. 352. [Google Scholar]
- 30.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen KE, Celermajer DS, Spiegelhalter DJ, et al. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 33.McAllister AS, Atkinson AB, Johnston GD, Hadden DR, Bell PM, McCance DR. Basal nitric oxide production is impaired in offspring of patients with essential hypertension. Clin Sci (Lond) 1999;97:141–147. [PubMed] [Google Scholar]
- 34.Calver A, Harris A, Maxwell JD, Vallance P. Effect of local inhibition of nitric oxide synthesis on forearm blood flow and dorsal hand vein size in patients with alcoholic cirrhosis. Clin Sci (Lond) 1994;86:203–208. doi: 10.1042/cs0860203. [DOI] [PubMed] [Google Scholar]
- 35.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59:230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 36.Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- 37.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, III, Panza JA. Selective defect in nitric oxide synthesis may explain the impaired endothelium-dependent vasodilation in patients with essential hypertension. Circulation. 1998;97:851–856. doi: 10.1161/01.cir.97.9.851. [DOI] [PubMed] [Google Scholar]
- 38.Berger ED, Bader BD, Rub N, et al. Changes in renal and systemic hemodynamics after NO-synthase inhibition in males with family history of hypertension. Kidney Blood Press Res. 2002;25:42–49. doi: 10.1159/000049434. [DOI] [PubMed] [Google Scholar]
- 39.Delles C, Jacobi J, Schlaich MP, John S, Schmieder RE. Assessment of endothelial function of the renal vasculature in human subjects. Am J Hypertens. 2002;15:3–9. doi: 10.1016/s0895-7061(01)02242-7. [DOI] [PubMed] [Google Scholar]
- 40.Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]