Abstract
Allergic conjunctivitis (AC) and airway hyperreactivity exacerbate corneal allograft rejection. Because AC and airway hyperreactivity are allergic diseases of mucosal tissues, we determined whether an allergic disease of a nonmucosal tissue would affect corneal allograft rejection and whether Th2 cells alone accounted for accelerated graft rejection in allergic mice. Hosts sensitized cutaneously with short ragweed pollen developed cutaneous immediate hypersensitivity but rejected corneal allografts at the same tempo and incidence as naive mice. Th2 immune deviation induced with keyhole limpet hemocyanin and IFA did not affect corneal allograft rejection. Thus, Th2 immune deviation alone does not account for the exacerbation of corneal allograft rejection that occurs in mice with AC. CD4+ T cells from AC mice elaborated Th1 (IFN-γ) and Th2 (IL-13) cytokines when challenged with donor alloantigens. Adoptive transfer of Th1 or Th2 cells to nude mice, from AC mice that had rejected corneal allografts, produced graft rejection in 70% and 20% of the hosts, respectively. In contrast, adoptive transfer of a combination of Th1 and Th2 cells produced 100% rejection. Administration of exogenous IFN-γ could substitute for Th1 cells and produced 100% corneal allograft rejection in recipients of Th2 cells alone. By contrast, IFN-γ did not significantly enhance corneal allograft rejection mediated by Th1 cells. Thus, exacerbation of corneal allograft rejection in mice with AC is associated with a mixed Th1 and Th2 alloimmune response, and the contribution of Th1 cells is through their production of IFN-γ.
Corneal transplantation is the oldest, most common, and arguably the most successful form of solid tissue transplantation (1–3). Although it is widely acknowledged that corneal allografts enjoy immune privilege, closer scrutiny reveals that this privilege is restricted to certain categories of hosts. For patients who require corneal transplants for correcting keratoconus, a condition in which the corneal graft bed is not inflamed and retains it avascularity, an acceptance rate of 90% at 10 y posttransplantation is not uncommon (3). However, in patients with underlying corneal inflammation, the long-term survival rate is considerably less; it decreases to 74% at 5 y and 62% at 10 y (4). Corneal allograft survival is even less in patients who are considered to be high risk based on the presence of pre-existing corneal vascularization or a history of corneal allograft rejection. In such high-risk hosts, the 10-y survival rate decreases to 35% (5).
Rodent models of penetrating keratoplasty have confirmed the exacerbation of corneal graft rejection in hosts that are deemed high-risk based on the presence of a prevascularized corneal graft bed or a history of graft rejection (6–8). Prospective studies in the mouse model of penetrating keratoplasty demonstrated that allergic conjunctivitis (AC) also creates a high-risk setting for corneal allograft rejection (9, 10). The exacerbation of corneal allograft rejection in mice with ongoing AC is a systemic effect, rather than the result of ocular inflammation related to the allergic reaction in the conjunctiva (i.e., mice in which AC is restricted to one eye still demonstrate a >90% incidence of rejection, even if corneas are transplanted to the contralateral “quiet” eye that displays no evidence of AC) (9). Recently, we reported that airway hyperreactivity (AHR), which is a model of allergic asthma, exacerbates corneal allograft rejection (11). The increased incidence and faster tempo of corneal allograft rejection in mice with an ongoing allergic disease in an organ distant from the eye (i.e., the lung) is additional evidence that this is the consequence of a perturbation in the systemic alloimmune response. Mice with AC produce Th1 and Th2 cytokines when confronted with donor alloantigens in vitro, suggesting that the presence of an ongoing Th2-mediated allergic disease does not result in the downregulation of Th1 immune responses to the donor alloantigens that are expressed on the corneal allograft (9). In contrast, it was reported that tilting the host’s immune response toward a Th2 pathway by immunization with keyhole limpet hemocyanin (KLH) plus IFA promoted the generation of donor-specific Th2 cells that cross-regulated allospecific Th1 cells and resulted in a significant reduction in corneal allograft rejection, even in hosts that were at high risk for rejection because of pre-existing vascularized corneal graft beds (12). Thus, hosts with Th2-based allergic diseases have an increased risk for corneal allograft rejection, whereas hosts with a Th2-biased immune response display enhanced corneal allograft acceptance. In attempting to reconcile these seemingly contradictory findings, two explanations come to mind. First, AC and AHR are Th2-based diseases that are elicited by the repeated application of allergens to mucosal surfaces, whereas the Th2 immune responses in mice immunized with KLH + IFA are evoked by immunization through nonmucosal tissues. Second, mice with AC display a mixed Th1 and Th2 immune response (13). Moreover, the Th1 cytokine IFN-γ is required for maximum expression of AC (13). In the current study, we asked whether an allergic disease elicited in a nonmucosal tissue (i.e., the skin) would exacerbate or mitigate corneal allograft rejection. We also examined the hypothesis that the mixed Th1 and Th2 alloimmune response that occurs in mice with AC is responsible for the exacerbation of corneal allograft rejection.
Materials and Methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Taconic Farms (Germantown, NY). Animals used in grafting experiments were female, 8–12 wk of age. BALB/c nude mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals used in these experiments were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Orthotopic corneal transplantation
Full-thickness penetrating orthotopic corneal grafts were performed as previously described (14), with a few modifications. Mice were anesthetized systemically with an i.p. injection of 115 mg/kg ketamine HCl (Fort Dodge Laboratories, Fort Dodge, IA) and 5.6 mg/kg xylazine (Bayer, Shawnee Mission, KS). Proparacaine HCl ophthalmic solution (USP 0.5%; Alcon Laboratories, Ft.Worth, TX) was used as a topical anesthetic. Donor grafts and recipient graft beds were scored with 2.0-mm trephines, and the corneas were excised with Vannas scissors. Donor grafts were sewn into place using running 11-0 nylon sutures (Ethicon, Sommerville, NJ), and sutures were removed on day 7 posttransplantation. Topical antibiotic (Akorn, Decatur, IL) was applied immediately after surgery, as well as immediately after the removal of sutures. No immunosuppressive drugs were used, either topically or systemically.
Clinical evaluation of grafted corneas
Corneal grafts were examined two or three times a week with a slit-lamp biomicroscope (Carl Zeiss, Oberkochen, Germany). Graft opacity was scored using a scale of 0–4. The degree of graft opacity was scored as follows: 0, clear; 1+, minimal superficial opacity; 2+, mild deep stromal opacity with pupil margin and iris visible; 3+, moderate stromal opacity with pupil margin visible, but iris structure obscured; and 4+, complete opacity, with pupil and iris totally obscured. Corneal grafts were considered rejected upon two successive scores of 3+.
Reagents and Abs
Anti-Tim3 mAb and anti-T1/ST2 mAb were purchased from eBioscience (San Diego, CA). Anti–IFN-γ Ab was isolated from cultures of R4-6A2 (American Type Culture Collection, Manassas, VA). Recombinant murine IFN-γ was purchased from R&D Systems (Minneapolis, MN).
Induction of AC
The protocol used to sensitize and challenge mice was modified from Magone et al. (15). BALB/c mice were immunized with 50 µg short ragweed (SRW) pollen (Ambrosia artemisiifolia; International Biologicals, Piedmont, OK) in 5 mg alum (Imject; Thermo Scientific, Rockford, IL) by footpad injection on day 0. AC was induced by a multi-hit topical challenge method in which immunized mice were given topical applications of SRW pollen (1.5 mg in 10 µl PBS) to the right eye once per day from days 10–16. Animals were examined clinically for signs of immediate hypersensitivity responses 20 min after each topical challenge. A clinical scoring scheme, similar to that described by Magone et al. (15), was used to evaluate chemosis, conjunctival redness, lid edema, and tearing. Each parameter was graded on a scale from 0 to 3+. Mice were challenged on day 17 with C57BL/6 corneal allografts.
Induction and assessment of cutaneous immediate hypersensitivity
Cutaneous immediate hypersensitivity was induced in mice by i.p. immunization with 40 µg SRW extract (Greer, Lenoir, NC) mixed with alum given on days 0 and 7. On days 14, 15, 21, and 22, mice were challenged s.c. with SRW extract (125 µg in 25 µl). On day 23, each mouse was injected i.v. with 25 µl 0.5% Evans blue and challenged in the right ear with a s.c. injection of 125 µg SRW extract in 10 µl PBS. The left ear served as a negative control and was injected with 10 µl PBS. Both ears were measured with an engineer’s micrometer immediately prior to s.c. ear challenge with SRW extract or PBS and 30 min after the s.c. injections. Results were expressed as specific ear swelling = (30-min measurement − baseline measurement for experimental ear) − (30-min measurement − baseline measurement for the negative control ear).
Mice were killed after ear measurements were completed, and both ears were removed for the Van Gogh spectrophotometric assays (16). Each ear was placed into a test tube containing 500 µl formamide (Sigma-Aldrich, St. Louis, MO) and incubated for 24 h in a 56°C water bath to leach out the Evans blue. Tubes were vortexed, and 100 µl of the fluid was removed and placed into four wells of a 96-well microtiter plate. OD values were determined on a spectrophotometric plate reader set at 590 nm. Results were expressed as OD readings for control and SRW extract ears.
Orthotopic skin grafting
Full-thickness skin grafts were transplanted orthotopically to the lateral thorax of female mice, as described previously (17). Male C57BL/6 skin grafts were transplanted orthotopically to female C57BL/6 recipients. Female C57BL/6 skin grafts were transplanted orthotopically to female BALB/c recipients. Skin grafts were protected with plaster of Paris bandages. Casts were removed 7 d later, and the grafts were inspected for evidence of rejection. Graft rejection was deemed complete when all remnants of surface epidermis were gone (17). Median survival times (MSTs) were calculated, and the statistical significance between groups was determined by the Mann–Whitney U test.
CD4+ T cell isolation
CD4+ T cells were harvested by positive selection using MACS CD4 (L3T4) MicroBeads (Miltenyi Biotec, Auburn, CA), magnetic microbeads conjugated to monoclonal rat anti-mouse CD4 (L3T4) Abs, as described elsewhere (18). Briefly, single-cell suspensions of lymphocytes in HBSS were prepared from experimental or control host spleens, as described earlier. Lymphocytes were washed once in PBS and then resuspended in 90 µl ice-cold 0.5% BSA in PBS (buffer solution) per 107 cells. Ten microliters of the MicroBeads was added per 107 cells, mixed well, and incubated for 15 min at 6–12°C. The cell/magnetic bead suspension was washed once with 10–20 times the labeling volume of buffer solution by centrifuging at 300 × g for 10 min, before resuspending to a concentration of 2 × 108 cells/ml in buffer solution. Five hundred microliters of buffer solution was allowed to run through a MACS mini-separation column (Miltenyi Biotec), which was placed in the magnetic field of a MACS separator, before a maximum of 2 × 108 cells was carefully loaded onto the column. The column was washed three times with 500 µl buffer solution so that any unlabeled CD4− cells would run through the column, whereas CD4+ cells were retained. The column was then removed from the magnetic field and held over a collection tube, while 1 ml cold buffer solution was added to the top of the column. CD4+ cells were collected in the collection tube by inserting a plunger at the top of the separation column and depressing it quickly and firmly.
Th1 and Th2 cell isolation
Tim-3 is a cell surface molecule that is a member of the T cell Ig domain, mucin domain superfamily and is expressed on activated Th1 cells but not Th2 cells (19, 20). The orphan receptor T1/ST2 is a member of the IL-1R family and is preferentially expressed on the surfaces of activated murine Th2 cells, but not Th1 cells (21). CD4+ T cells were isolated from spleen-cell suspensions from BALB/c mice 7–10 d after they rejected their C57BL/6 corneal allografts. CD4+ cell suspensions were incubated with anti–Tim-3 or anti-T1/ST2 rat IgG Abs, and the respective cell populations were enriched using anti-rat IgG-coated magnetic beads, as described above. The enrichments of Th1 and Th2 cells were confirmed by detection of T-bet and GATA-3 transcription factors, respectively, by RT-PCR.
MLRs and cytokine ELISA
An indirect MLR was used to determine the cytokine profile of CD4+ T cells from the various experimental groups. The indirect MLR was selected instead of the conventional MLR, because the indirect pathway of alloantigen presentation is the predominant pathway in the immune rejection of corneal allografts in low-risk eyes (8, 22). For the indirect MLR, spleen cells from naive C57BL/6 mice were treated with NH4Cl erythrocyte lysis solution, washed, and resuspended in 10 ml complete RPMI 1640 without FBS. C57BL/6 cell lysates were prepared by freezing C57BL/6 spleen cells at −80°C for 10 min, thawing at 37°C for 5 min, and vortexing vigorously for two cycles. BALB/c splenocytes were isolated, treated with NH4Cl erythrocyte lysis solution, washed, and resuspended in 10 ml complete RPMI 1640 containing 10% FBS. The cell suspension was plated onto two 100-mm Primaria plates (5 ml each plate) and incubated at 37°C for 1 h. Nonadherent cells were removed by gentle washing. Adherent APCs were cultured in one 100-mm Primaria plate containing 5 ml complete RPMI 1640 containing 10% FBS and pulsed by the addition of C57BL/6 cell lysates (5 ml) overnight at 37°C (23). As a control, non-pulsed APCs were cultured in a 100-mm Primaria plate containing 5 ml complete RPMI 1640 supplemented with 10% FBS overnight at 37°C. Supernatants were collected 72 h later and examined for the presence of Th1 and Th2 cytokines by capture ELISA (R&D Systems), as previously described (24).
Cytokine production was also assessed in mice sensitized s.c. with SRW extract. CD4+ spleen cells (5 × 105/ml) were cocultured for 72 h with splenic APCs pulsed with 125 µg SRW extract/ml. Supernatants were collected and examined for the presence of IL-4, -5, and -13; IFN-γ; and TNF-α by capture ELISA, as described above.
In vivo Th2 polarization with KLH and IFA
Mice were immunized with KLH plus IFA as a means to develop CD4+ T cells that produced IL-4, -5, and -6 and IFN-γ when stimulated in vitro with KLH (25). This protocol was modified to promote the generation of allospecific Th2 cells in BALB/c mice challenged with orthotopic C57BL/6 corneal allografts (12). Accordingly, BALB/c mice were immunized i.p. with 50 µg KLH (Calbiochem, San Diego, CA) emulsified in IFA (Difco, Detroit, MI). Control mice were injected with PBS plus IFA.
Two weeks later, high-risk graft beds were produced in one group of KLH-treated BALB/c mice by inserting three interrupted 11-0 silk sutures into the central cornea (12). The other group of KLH-sensitized mice was not subjected to suturing. Four weeks later, both groups of mice were immunized s.c. in the nape of the neck with 50 µg KLH emulsified in CFA and challenged with C57BL/6 orthotopic corneal allografts.
Statistical analysis
The statistical significance of ELISAs, ear swelling assays, and van Gogh spectrophotometric assays was determined using the Student t test. MSTs for corneal allografts were determined and compared using the Mann–Whitney U test.
Results
Heightened risk for corneal allograft rejection dissipates if exposure to the allergen is terminated
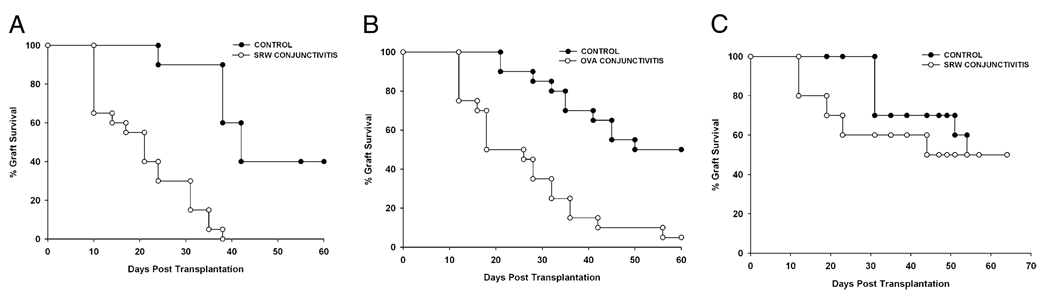
We and other investigators showed that AC induced with SRW pollen induces a sharp increase in the incidence and tempo of corneal allograft rejection (9, 10). Experiments were performed to confirm that this effect was not restricted to SRW pollen-induced disease and that a similar effect occurs if AC were induced with a different allergen. Accordingly, AC was induced in BALB/c mice using SRW pollen or OVA prior to the application of C57BL/6 corneal allografts. The results demonstrated that AC induced with SRW pollen (Fig. 1A) or OVA (Fig. 1B) resulted in an accelerated tempo and increased incidence of corneal allograft rejection.
FIGURE 1.
Corneal allograft rejection in mice with AC. AC was induced in BALB/c mice using SRW pollen (A) or OVA (B). C57BL/6 corneal allografts were applied 17 d after initial SRW sensitization. In a separate experiment, BALB/c mice were sensitized with SRW pollen for 17 d and rested for an additional 30 d without additional exposure to SRW pollen. C57BL/6 corneal allografts were applied 30 d after allergen exposure was terminated (C). n = 20–30 mice per group; p = 0.003 in A and B; p > 0.05 in C.
From a pragmatic standpoint, it was important to ascertain whether a history of AC permanently altered the immune response to corneal allografts or whether the risk would return to normal levels if the hosts were isolated from the allergen for a sufficient period of time. Accordingly, AC was induced with SRW pollen, as described above (i.e., BALB/c mice were immunized in the footpad with SRW pollen suspended in alum on day 0). Mice were given topical applications of SRW pollen in the right eye once per day from days 10–16. However, instead of applying a C57BL/6 corneal allograft on day 17, the mice were returned to the vivarium and were not subjected to additional exposure to SRW pollen. Thirty days later, mice were challenged with C57BL/6 corneal allografts. The results showed that within 30 d of terminating the exposure to SRW, the risk for corneal allograft rejection had dissipated, and the hosts’ ocular surface homeostasis had returned to normal levels. The tempo and incidence of corneal allograft rejection were no different from untreated hosts (Fig. 1C). Thus, the increased risk for corneal allograft rejection in hosts with AC is transient and can be manipulated by simply isolating the allergic host from the offending allergen.
Effect of AC on skin graft rejection
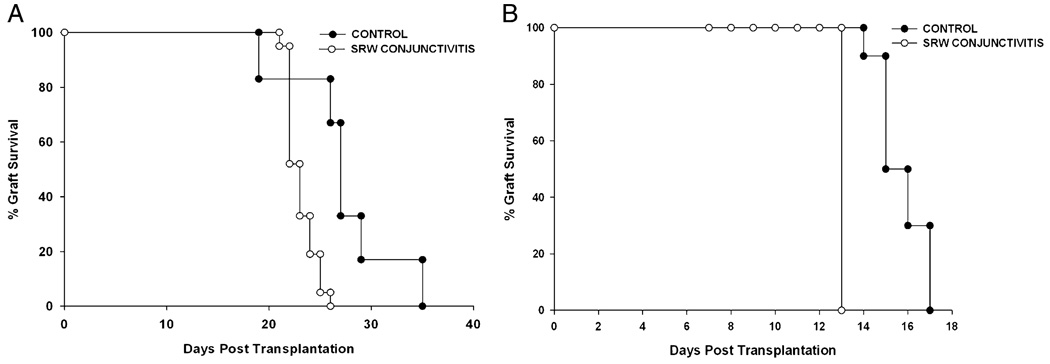
Anecdotal reports suggested that the increased risk for corneal allograft rejection in patients with AC was simply due to the effects of the inflamed eye, which promoted the induction and expression of immune responses to corneal alloantigens at the ocular surface. However, two findings argue against this and suggest that allergic diseases produce a systemic perturbation of the alloimmune response (i.e., hosts in which AC is restricted to one eye still display an increased tempo and incidence of rejection of corneas transplanted onto the contralateral nonallergic eye) (9). Moreover, the risk for corneal allograft rejection soars in hosts with allergic inflammation in an organ distant from the eye (i.e., in hosts with AHR) (11). If the presence of an allergic disease at the time of transplantation has a systemic adjuvant-like effect, one might predict that other categories of allografts would experience a heightened incidence or tempo of rejection. This was tested using two skin graft models. The male-specific, H–Y Ag, is expressed in certain mouse strains (e.g., C57BL/6) and elicits the rejection of syngeneic male skin grafts following orthotopic transplantation to female mice (26).
Experiments were performed to determine whether AC would affect the fate of syngeneic C57BL/6 male skin transplants in female C57BL/6 mice and C57BL/6 skin allografts transplanted to BALB/c mice. As expected, 100% of the male skin grafts were rejected by female C57BL/6 mice (Fig. 2A). Female mice with AC also rejected 100% of their male skin grafts but at a tempo that was significantly faster than normal mice without AC (p = 0.016). The adverse effect of AC was even more pronounced in the rejection of C57BL/6 skin allografts in BALB/c mice. Although skin allograft rejection occurred in 100% of the BALB/c mice in both groups, the rejection was significantly accelerated in BALB/c mice with AC (Fig. 2B).
FIGURE 2.
Effect of AC on skin graft rejection. AC was induced in female C57BL/6 and BALB/c mice with SRW pollen. A, Full-thickness skin grafts prepared from male C57BL/6 mice were transplanted orthotopically to female C57BL/6 1 d after the final topical administration of SRW pollen. n = 15 untreated female C57BL/6 mice; n = 21 female C57BL/6 mice with AC; p = 0.016. B, Full-thickness female C57BL/6 skin grafts were transplanted to female BALB/c mice 1 d after the final topical administration of SRW pollen. n = 10 BALB/c mice in each group; p = 0.0002.
Effect of cutaneous immediate hypersensitivity on corneal allograft rejection
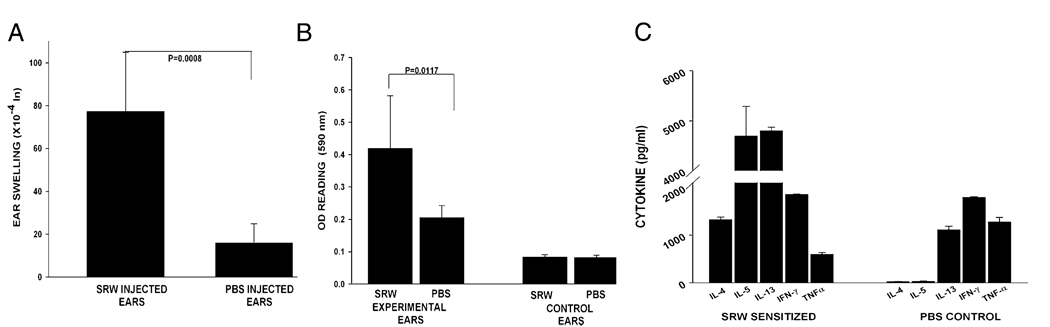
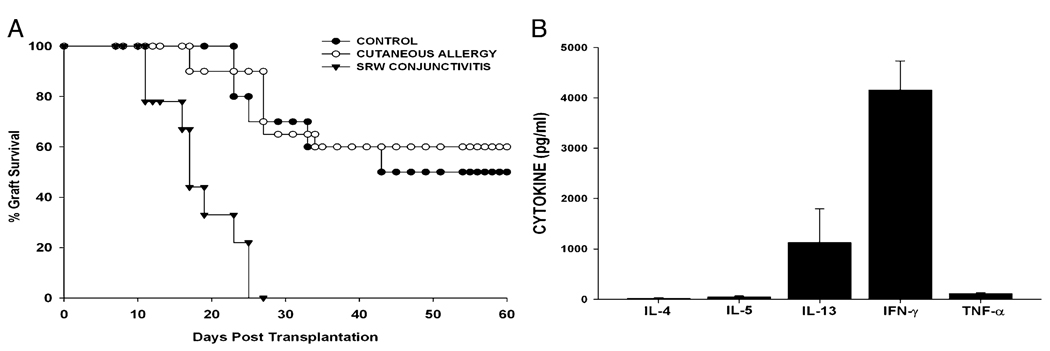
It is noteworthy that AC and AHR are diseases of mucosal tissues. This prompted us to determine whether an allergic disease in a nonmucosal tissue would also exacerbate corneal allograft rejection. Accordingly, cutaneous immediate hypersensitivity was induced using SRW allergens. Mice were immunized i.p. with SRW extract in the presence of alum and subsequently challenged s.c. with SRW extract without alum. One of the hallmarks of an immediate hypersensitivity reaction is vasodilatation and increased vascular permeability in the tissues in which the allergen challenge occurs. The increased vascular permeability leads to edema, which can be quantified by measuring the increased thickness of ears challenged with allergen. The increased vascular permeability in the affected tissue can be quantified by injecting Evans blue dye i.v. and assessing the amount of blue dye that enters the extravascular tissues (16). Accordingly, these parameters were used to confirm the presence of cutaneous immediate hypersensitivity. The right ears of the SRW-sensitized mice were challenged with an s.c. injection of SRW extract, and the ear-swelling responses were measured 30 min later. These ears displayed significant ear swelling, whereas the contralateral ears that were challenged with PBS did not (Fig. 3A). Increased extravasation and vasodilatation were quantified in the van Gogh assay, in which the amount of extravasated Evans blue dye was quantified spectrophotometrically after removing the ears and extracting the dye by immersing the ears in formamide (Fig. 3B). The rapid vasodilatation and extravasation of Evans blue in the SRW extract-injected ears were observed easily with the naked eye (data not shown).To provide further confirmation that this cutaneous immediate-hypersensitivity protocol produced a Th2-mediated response to SRW allergens, splenic CD4+ T cells were removed from mice that had been sensitized s.c. with SRW extract and were cultured in the presence of APCs pulsed with SRW extract. Supernatants were collected 48 h later and assessed for the presence of Th1 and Th2 cytokines. CD4+ T cells from mice sensitized s.c. with SRW extract produced significant quantities of Th2 signature cytokines (IL-4, -5, and -13) and the Th1-related cytokines (IFN-γ and TNF-α) (Fig. 3C). Thus, the weight of evidence indicates that this protocol produces classic cutaneous immune hypersensitivity responses. Accordingly, cutaneous immediate hypersensitivity was induced using SRW pollen, and the BALB/c mice were challenged with C57BL/6 corneal allografts 23 d later. For comparison, AC was induced with SRW pollen in a separate group of BALB/c mice, which was also challenged with C57BL/6 corneal allografts on day 23. As expected, 100% of the mice with AC rejected their corneal allografts, whereas only 50% of the untreated control mice rejected their C57BL6 corneal allografts (Fig. 4A). Interestingly, mice with cutaneous immediate hypersensitivity rejected their corneal allografts at the same tempo and incidence as did the untreated controls. Mice with cutaneous immune hypersensitivity that were challenged with C57BL/6 corneal allografts were examined to determine whether they had generated a Th2 alloimmune response. Splenic CD4+T cells were isolated from mice 7–10 d after corneal allograft rejection and were cultured in the presence of BALB/c APCs that had been pulsed with sonicated C57BL/6 spleen cells in an indirect MLR. Supernatants were collected 72 h later and examined for the presence of Th1 and Th2 cytokines. The ELISA results indicated the presence of a mixed Th1 and Th2 alloimmune response that was characterized by the absence of detectable IL-4 and -5 but by the significant production of IL-13 and IFN-γ (Fig. 4B).
FIGURE 3.
Induction of cutaneous hypersensitivity in BALB/c mice. Cutaneous immune hypersensitivity was induced with SRW pollen by s.c. injection of SRW pollen plus alum on days 0 and 7. On days 14, 15, 21, and 22, mice were challenged s.c. with SRW extract. On day 23, each mouse was injected i.v. with 25 µl of 0.5% Evans blue and challenged in the right ear with an s.c. injection of SRW extract. The left ear served as a negative control and was injected with PBS. A, Specific ear swelling was measured with an engineer’s micrometer immediately after ear challenge. B, Increased vasodilatation and extravasation were assessed spectrophotometrically in a van Gogh assay by extracting Evan’s blue from the excised ears. C, Cytokine production in mice with cutaneous immediate hypersensitivity was determined in vitro. CD4+ T cells were removed from BALB/c mice that had been sensitized s.c. with SRW extract and were cultured in the presence of APCs pulsed with SRW extract. Supernatants were collected 48 h later and assessed for the presence of Th1 and Th2 cytokines.
FIGURE 4.
Effect of cutaneous immediate hypersensitivity on corneal allograft rejection and cytokine production by CD4+ T cells. A, Cutaneous immediate hypersensitivity or AC was induced in BALB/c mice using SRW allergens. Mice were challenged with C57BL/6 corneal allografts 1 d after final exposure to SRW allergens, and allograft survival was monitored. Cutaneous allergy group (n = 10) was not significantly different from untreated control group (n = 10). p > 0.05. AC group (n = 9) was significantly different from the other two groups. p = 0.001. B, Splenic CD4+ T cells were isolated from BALB/c mice 7–10 d after they had rejected C57BL/6 corneal allografts. CD4+ T cells were cultured in the presence of BALB/c APCs that were pulsed with sonicated C57BL/6 spleen cells. Supernatants were collected 72 h later and examined for the presence of Th1 and Th2 cytokines.
Th2-biased immune deviation does not promote corneal allograft survival
It was reported that mice immunized with KLH in the presence of IFA and subsequently challenged with corneal allografts developed CD4+ T cells that secrete Th2 cytokines (12). Moreover, these Th2-biased mice experience significantly enhanced corneal allograft survival, even when corneas are transplanted into prevascularized, high-risk eyes. These findings differ from the results with SRW AC, AHR, and immediate cutaneous hypersensitivity. Therefore, we examined the effect of KLH plus IFA immune-deviation on corneal allograft survival in our model of penetrating keratoplasty.
BALB/c mice designated as high- or normal-risk hosts were immunized with KLH in the presence of IFA, as previously described, and challenged with orthotopic C57BL/6 corneal allografts 2 wk later (12). High-risk hosts were created by inducing corneal neovascularization by placing three 11-0 interrupted sutures through the cornea 2 wk prior to orthotopic corneal transplantation. Two weeks later, the high- and normal-risk mice were reimmunized in the nape of the neck with KLH; however, this time the KLH was emulsified in CFA. Corneal allografts were applied on the same day as the second KLH immunization.
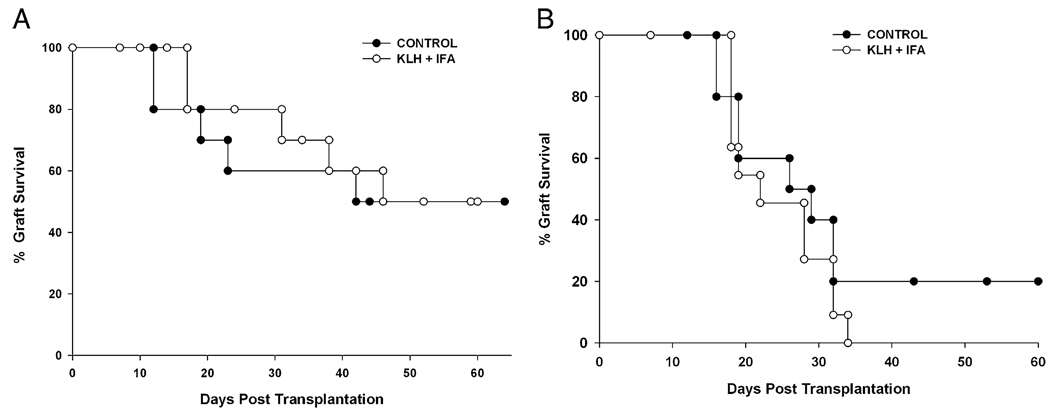
Although a previous report indicated that this form of Th2-immune deviation produced a significant enhancement of corneal allograft survival, we did not observe a prolongation of graft survival or a significant reduction in the incidence of corneal allograft rejection in normal-risk (Fig. 5A) or high-risk hosts (Fig. 5B). In the case of normal-risk hosts, graft rejection occurred in 50% of the KLH-immunized hosts, which was not different from untreated mice or mice with cutaneous immediate hypersensitivity (p > 0.05). Likewise, graft rejection in high-risk KLH-immunized mice was the same as that in untreated high-risk mice (p > 0.05).
FIGURE 5.
Effect of Th2 immune deviation on corneal allograft survival. High-risk hosts were generated by inducing corneal neovascularization by inserting three 11-0 sutures into the central corneas of BALB/c mice 2 wk prior to the induction of Th2 immune deviation. Th2 immune deviation was induced in normal- (A) and high-risk (B) hosts by s.c. immunization with KLH in the presence of IFA, followed by reimmunization with an s.c. injection of KLH in the presence of CFA. This experiment was performed three times with similar results (n = 10 mice per group). p > 0.05 for all groups.
Role of Th1 and Th2 cells in corneal allograft rejection
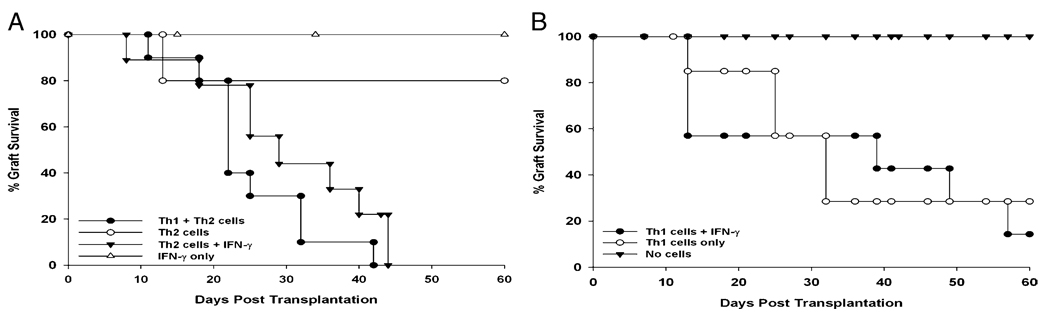
The results reported in this article and elsewhere indicated that the increased incidence and tempo of corneal allograft rejection that occur in mice with AC or AHR are associated with the generation of CD4+ T cells that produce Th1 and Th2 cytokines (9, 11). Therefore, we evaluated the capacity of Th1 and Th2 cells to mediate corneal allograft rejection, independently or together. CD4+ T cells were isolated from the spleens of SRW pollen-sensitized mice that had rejected C57BL/6 corneal allografts. CD4+ T cells were enriched for Th1 cells using immunomagnetic beads and anti–Tim-3 Ab. The enrichment of Th1 cells was confirmed by RT-PCR detection of T-bet transcription factor in the Tim3+ cell suspensions (data not shown). Th2 cells were enriched with the same protocol but using anti-T1/ST2 mAb. Th2 cell-enriched cell suspensions displayed strong GATA-3 transcription factor expression, which is indicative of Th2 cells (data not shown). One donor-equivalent of Th1 cells, Th2 cells, or a combination of Th1 and Th2 cells was infused i.v. into BALB/c nude mice. Mice were challenged with C57BL/6 corneal allografts 1 d later. Corneal allograft rejection occurred in only 20% of the nude mice that received Th2 cells alone (Fig. 6A). However, the combined transfer of Th1 and Th2 cells resulted in 100% corneal allograft rejection, even though the same total number of cells was injected in each of the three experimental groups (i.e., 2 × 106 cells/recipient). The exacerbation of corneal allograft rejection that occurs in mice with Th2-based allergic diseases (i.e., AC and AHR) is associated with the production of the Th1 cytokine IFN-γ (9, 11). Therefore, we suspected that the IFN-γ produced by CD4+ Th1 cells was the major contributing factor in the increased corneal allograft rejection that occurred when Th1 cells were combined with Th2 cells in the adoptive cell-transfer experiments. Accordingly, an additional experiment was performed in which CD4+Tim3+ Th1 cells or CD4+T1/ST2+ Th2 cells were isolated from the spleens of BALB/c mice that had SRW pollen-induced conjunctivitis and had rejected their C57BL/6 corneal allografts. One donor-equivalent of Th1 or Th2 cells was injected i.v. into nude mice, which were immediately challenged with orthotopic C57BL/6 corneal allografts. Mice also received i.p. injection of recombinant murine IFN-γ (1.25 µg) on the day of transplantation and twice per week thereafter. For comparison, nude mice did not receive T cells but were injected with recombinant murine IFN-γ using the same protocols for nude mice that received Th1 or Th2 cells. As expected, BALB/c nude mice that were not reconstituted with T cells failed to reject C57BL/6 corneal allografts (data not shown). Nude mice that were treated with IFN-γ alone did not reject their corneal allografts (Fig. 6A). However, administration of IFN-γ recapitulated the effect of Th1 cells and resulted in 100% graft rejection in nude mice that received Th2 cells plus biweekly injections of recombinant murine IFN-γ (Fig. 6A). Adoptive transfer of Th1 cells alone resulted in 71% rejection. Although nude mice that received Th1 cells plus recombinant murine IFN-γ rejected 86% of their corneal allografts, the MST and incidence of rejection were not significantly different from mice that received Th1 cells alone (Fig. 6B).
FIGURE 6.
Th1 cells or IFN-γ are necessary for the rejection of corneal allografts in mice with AC. Th1 (Tim3+) and Th2 (T1/ST2+) cells were isolated from the spleens of SRW pollen-sensitized mice that had rejected C57BL/6 corneal allografts. A, One donor-equivalent of Th2 cells or a combination of Th1 + Th2 cells was adoptively transferred to BALB/c nude mice. The effect of recombinant murine IFN-γ as a substitute for Th1 cells was evaluated by injecting one donor-equivalent of Th2 cells, followed by i.p. injection of recombinant murine IFN-γ (1.25 µg) on the day of transplantation and twice per week thereafter. B, One donor-equivalent of Th1 cells was adoptively transferred to BALB/c nude mice. One panel of mice received Th1 cells, followed by i.p. injection of recombinant murine IFN-γ (1.25 µg) on the day of transplantation and twice per week thereafter. This experiment was performed twice with similar results (n = 7–10 mice per group). p = 0.003 (Th1 alone versus Th1 + Th2); p = 0.003 (Th2 versus Th1 + Th2); p > 0.05 (Th2 + Th1 versus Th2 + IFN-γ); p > 0.05 (Th1 + IFN-γ versus Th1 alone).
Discussion
It was previously believed that corneal allograft rejection was mediated by CD4+ Th1 immune responses. This assumption was based on observations indicating that the rejection of corneal allografts was closely correlated with the acquisition of delayed-type hypersensitivity responses to the donor’s histocompatibility Ags and that procedures that specifically downregulated allospecific delayed-type hypersensitivity responses enhanced corneal allograft survival (27–30). In the classical Th cell paradigm, Th2 cells secrete IL-4, -5, and -13, as well as other cytokines that cross-regulate Th1 cells (31). Thus, it was suggested by many investigators that simply tilting the host’s immune response toward a Th2 pathway would downregulate Th1 alloimmune responses and culminate in enhanced allograft survival. However, a number of findings have caused this hypothesis to come unraveled. Deviating the immune response toward a Th2 pathway results in the swift rejection of cardiac and skin allografts in mice (32–34). More recent investigations reported that mice with classic allergic diseases, such as conjunctivitis and AHR, have dramatic increases in the speed and incidence of corneal allograft rejection (9–11). Based on these findings, it is tempting to conclude that the Th2-prone host is categorically at greater risk for corneal allograft rejection than are hosts with an intact Th1-immune repertoire. However, the results reported in this study and elsewhere indicated that not all categories of Th2 immune deviation translate into an increased risk for corneal allograft rejection (12). The present findings demonstrated that mice with cutaneous immediate hypersensitivity produce Th2 cytokines when confronted with the offending allergen or the donor’s histocompatibility Ags in vitro and display the clinical features of immune hypersensitivity when challenged with the relevant allergen in vivo, yet neither the incidence nor tempo of corneal allograft rejection is affected in these hosts. Likewise, corneal allograft rejection was unaltered in hosts subjected to a conventional Th2 immune-deviation protocol using KLH and IFA. We are at a loss to explain our inability to recapitulate previous findings that Th2 immune deviation using KLH and IFA enhanced corneal allograft survival, even though we performed these experiments on three separate occasions using the same protocols, reagents from the same vendors, and mice from the same sources as in the previous report (12).
The underlying mechanisms responsible for the increased risk for corneal allograft rejection that occurs in mice with SRW pollen-induced immediate hypersensitivity of mucosal surfaces, but not in mice with cutaneous immediate hypersensitivity, remain to be clarified. It was reported that CD4+CD25+ T regulatory cells are present in hosts with long-term successful corneal allografts and disabling T regulatory cells invariably results in corneal allograft rejection (27, 35). In this regard, it is noteworthy that a recent study on OVA-induced allergic AHR in mice revealed that IL-4 produced during the course of allergic AHR rendered Th cells resistant to the suppressive effects of CD4+CD25+ T regulatory cells (36). We previously demonstrated that CD4+ T cells from mice with AC produce IL-4 when stimulated with alloantigen-pulsed APCs (9). In contrast, the present findings indicated that CD4+ T cells from mice with cutaneous immediate hypersensitivity failed to produce detectable quantities of IL-4 when stimulated with alloantigen-pulsed APCs. Thus, one possible explanation for the discordance in corneal allograft survival in hosts with allergic responses in mucosal tissues versus cutaneous immediate hypersensitivity might be related to the effect of IL-4 on T regulatory cells associated with corneal allograft survival. This mechanism might also explain the restoration of the normal allograft-rejection phenotype that was observed when mice with AC were isolated from SRW pollen exposure for 30 d. We are actively exploring this hypothesis and others relating to the possibility that allergic diseases of mucosal tissues handicap the function of T regulatory cells in corneal allograft recipients.
The present findings add to a growing body of evidence that some diseases that were previously viewed as solely Th2-mediated are, in fact, a blend of Th1 and Th2 cytokine-dependent inflammatory disorders (13, 37–40). The results reported in this article indicated that merely invoking a Th2-dominated disease in itself does not jeopardize corneal allograft survival. Previous reports showed that production of the Th1 cytokine IFN-γ was necessary for the full expression of allergic diseases, such as AHR and AC (13, 37, 39, 40). In the current study, we found evidence that Th1 cells were crucial for the exacerbation of corneal allograft rejection that occurs in hosts with AC, because adoptive transfer of Th1 or Th2 cells alone failed to produce the same incidence of corneal allograft rejection that occurred in hosts with AC. However, adoptively transferring a combination of Th1 and Th2 cells resulted in 100% corneal allograft rejection. The role of the Th1 cell in corneal allograft rejection in mice with AC seems to be dependent upon the production of IFN-γ, because infusion of recombinant murine IFN-γ substituted for Th1 cells promoted the rejection of corneal allografts in nude mice that received Th2 cells from allograft rejecter donors, but it did not affect the corneal allograft rejection in hosts receiving Th1 cells. IFN-γ is a pleiotropic cytokine that influences multiple processes that might affect corneal allograft rejection. For example, IFN-γ upregulates VCAM-1, which is an important ligand for facilitating the extravasation of inflammatory cells (41). IFN-γ might also contribute to the effector stage of corneal allograft rejection, because it can induce apoptosis of corneal endothelial cells (J.Y. Niederkorn, unpublished observations). IFN-γ also activates macrophages, which are required for the rejection of corneal allografts in rats and mice (18, 42). The present findings, along with numerous previous reports, remind us that multiple mechanisms can culminate in allograft rejection. Merely deviating the alloimmune response in a Th1 or a Th2 pathway alone does not guarantee allograft survival, and in some cases it can exacerbate rejection. Moreover, simultaneously invoking Th1 and Th2 alloimmune responses due to allergic diseases of mucosal tissues can exacerbate rejection. The present results also suggested that using pharmaceutical agents that target the end-stage effector molecules of allergy, such as histamine, that do not affect the production of Th2 cytokines, such as IL-4, will not remove the risk for corneal allograft rejection that is associated with atopic diseases. However, isolating atopic patients from their allergens or delaying keratoplasty until seasonal allergies have dissipated may hold greater promise for mitigating the risk for rejection that is associated with diseases involving immediate hypersensitivity reactions.
Acknowledgments
This work was supported by National Institutes of Health Grants EY007641 and EY016664 and an unrestricted grant from Research to Prevent Blindness.
Abbreviations used in this paper
- AC
allergic conjunctivitis
- AHR
airway hyperreactivity
- KLH
keyhole limpet hemocyanin
- MST
median survival time
- SRW
short ragweed
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–1508. doi: 10.1097/00007890-199906270-00001. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY. The immune privilege of corneal grafts. J. Leukoc. Biol. 2003;74:167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 3.Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84:806–813. doi: 10.1097/01.tp.0000285489.91595.13. [DOI] [PubMed] [Google Scholar]
- 4.Waldock A, Cook SD. Corneal transplantation: how successful are we? Br. J. Ophthalmol. 2000;84:813–815. doi: 10.1136/bjo.84.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. Long-term outcome in corneal allotransplantation. The Australian Corneal Graft Registry. Transplant. Proc. 1997;29:983. doi: 10.1016/s0041-1345(96)00335-1. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Callanan D, Kunz H, Niederkorn J. Evidence that the fate of class II-disparate corneal grafts is determined by the timing of class II expression. Transplantation. 1991;51:532–536. doi: 10.1097/00007890-199102000-00049. [DOI] [PubMed] [Google Scholar]
- 7.Ross J, He YG, Niederkorn JY. Class I disparate corneal grafts enjoy afferent but not efferent blockade of the immune response. Curr. Eye Res. 1991;10:889–892. doi: 10.3109/02713689109013885. [DOI] [PubMed] [Google Scholar]
- 8.Sano Y, Ksander BR, Streilein JW. Murine orthotopic corneal transplantation in high-risk eyes. Rejection is dictated primarily by weak rather than strong alloantigens. Invest. Ophthalmol. Vis. Sci. 1997;38:1130–1138. [PubMed] [Google Scholar]
- 9.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J. Immunol. 2005;174:6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 10.Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest. Ophthalmol. Vis. Sci. 2007;48:4044–4049. doi: 10.1167/iovs.06-0973. [DOI] [PubMed] [Google Scholar]
- 11.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. Am. J. Transplant. 2009;9:1017–1026. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada J, Yoshida M, Taylor AW, Streilein JW. Mice with Th2-biased immune systems accept orthotopic corneal allografts placed in “high risk” eyes. J. Immunol. 1999;162:5247–5255. [PubMed] [Google Scholar]
- 13.Stern ME, Siemasko K, Gao J, Duong A, Beauregard C, Calder V, Niederkorn JY. Role of interferon-gamma in a mouse model of allergic conjunctivitis. Invest. Ophthalmol. Vis. Sci. 2005;46:3239–3246. doi: 10.1167/iovs.05-0138. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Mellon J, Apte R, Niederkorn JY. Effect of LFA-1 and ICAM-1 antibody treatment on murine corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 1994;35:3218–3225. [PubMed] [Google Scholar]
- 15.Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 1998;87:75–84. doi: 10.1006/clin.1997.4507. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J. Biol. Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 17.Niederkorn J, Streilein JW, Shadduck JA. Deviant immune responses to allogeneic tumors injected intracamerally and subcutaneously in mice. Invest. Ophthalmol. Vis. Sci. 1981;20:355–363. [PubMed] [Google Scholar]
- 18.Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79:23–31. doi: 10.1097/01.tp.0000147196.79546.69. [DOI] [PubMed] [Google Scholar]
- 19.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 20.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 21.Meisel C, Bonhagen K, Löhning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, Kamradt T. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J. Immunol. 2001;166:3143–3150. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- 22.Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J. Immunol. 2001;167:1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 23.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. Generation in vitro of B-cell chronic lymphocytic leukaemia-proliferative and specific HLA class-II-restricted cytotoxic T-cell responses using autologous dendritic cells pulsed with tumour cell lysate. Clin. Exp. Immunol. 2001;126:16–28. doi: 10.1046/j.1365-2249.2001.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 25.DeKruyff RH, Fang Y, Umetsu DT. IL-4 synthesis by in vivo primed keyhole limpet hemocyanin-specific CD4+ T cells. I. Influence of antigen concentration and antigen-presenting cell type. J. Immunol. 1992;149:3468–3476. [PubMed] [Google Scholar]
- 26.Simpson E. The role of H–Y as a minor transplantation antigen. Immunol. Today. 1982;3:97–106. doi: 10.1016/S0167-5699(82)80025-X. [DOI] [PubMed] [Google Scholar]
- 27.Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 1996;37:2700–2707. [PubMed] [Google Scholar]
- 28.She SC, Steahly LP, Moticka EJ. Intracameral injection of allogeneic lymphocytes enhances corneal graft survival. Invest. Ophthalmol. Vis. Sci. 1990;31:1950–1956. [PubMed] [Google Scholar]
- 29.Sonoda Y, Streilein JW. Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J. Immunol. 1993;150:1727–1734. [PubMed] [Google Scholar]
- 30.Yao YF, Inoue Y, Miyazaki D, Hara Y, Shimomura Y, Tano Y, Ohashi Y. Correlation of anterior chamber-associated immune deviation with suppression of corneal epithelial rejection in mice. Invest. Ophthalmol. Vis. Sci. 1997;38:292–300. [PubMed] [Google Scholar]
- 31.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 32.Le Moine A, Flamand V, Demoor FX, Noël JC, Surquin M, Kiss R, Nahori MA, Pretolani M, Goldman M, Abramowicz D. Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. J. Clin. Invest. 1999;103:1659–1667. doi: 10.1172/JCI5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J. Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 34.VanBuskirk AM, Wakely ME, Orosz CG. Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. Transplantation. 1996;62:229–238. doi: 10.1097/00007890-199607270-00014. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J. Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J. Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahl ME, Dabbagh K, Liggitt D, Kim S, Lewis DB. Viral-induced T helper type 1 responses enhance allergic disease by effects on lung dendritic cells. Nat. Immunol. 2004;5:337–343. doi: 10.1038/ni1041. [DOI] [PubMed] [Google Scholar]
- 38.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat. Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 39.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Co-operation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern ME, Siemasko KF, Niederkorn JY. The Th1/Th2 paradigm in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2005;5:446–450. doi: 10.1097/01.all.0000182547.60595.64. [DOI] [PubMed] [Google Scholar]
- 41.Parr MB, Parr EL. Interferon-gamma up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99:540–545. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Veen G, Broersma L, Dijkstra CD, Van Rooijen N, Van Rij G, Van der Gaag R. Prevention of corneal allograft rejection in rats treated with subconjunctival injections of liposomes containing dichloromethylene diphosphonate. Invest. Ophthalmol. Vis. Sci. 1994;35:3505–3515. [PubMed] [Google Scholar]