Abstract
Human embryonic stem cells (hESCs) may offer an unlimited supply of cells that can be directed to differentiate into all cell types within the body and used in regenerative medicine for tissue and cell replacement therapies. Previous work has shown that exposing hESCs to exogenous factors such as dexamethasone, ascorbic acid and β-glycerophosphate can induce osteogenesis. The specific factors that induce osteogenic differentiation of hESCs have not been identified yet, however, it is possible that differentiated human bone marrow stromal cells (hMBSCs) may secrete factors within the local microenvironment that promote osteogenesis. Here we report that the lineage progression of hESCs to osteoblasts is achieved in the presence of soluble signaling factors derived from differentiated hBMSCs. For 28 days, hESCs were grown in a transwell co-culture system with hBMSCs that had been previously differentiated in growth medium containing defined osteogenic supplements for 7-24 days. As a control. hESCs were co-cultured with undifferentiated hBMSCs and alone. Von Kossa and Alizarin Red staining as well as immunohistochemistry confirmed that the hESCs co-cultured with differentiated hBMSCs formed mineralized bone nodules and secreted extracellular matrix protein osteocalcin (OCN). Quantitative Alizarin Red assays showed increased mineralization as compared to the control with undifferentiated hBMSCs. RT-PCR revealed the loss of pluripotent hESC markers with the concomitant gain of osteoblastic markers such as collagen type I, runx2, and osterix. We demonstrate that osteogenic growth factors derived from differentiated hBMSCs within the local microenvironment may help to promote hESC osteogenic differentiation.
Keywords: Human embryonic stem cells, bone marrow stromal cells, osteoblasts, stem cell-microenvironment
Introduction
Human embryonic stem cells (hESCs) present a potentially unlimited supply of cells that may be directed to differentiate into all cell types within the body and used in regenerative medicine for tissue and cell replacement therapies. An area of particular interest is stem cell transplantation for bone tissue regeneration where hESCs may be used to repair skeletal defects. One of the major gaps in the knowledge regarding hESCs is the lack of understanding of the growth factors and three-dimensional signals that control differentiation. Current techniques used for bone tissue repair employ the use of auto- and allografting methods, however, these methods have inherent limitations that restrict their universal application. The limitations of these reparative strategies suggest that an alternative approach is required, and hESCs may provide a repository of cells for such an approach. Previous work has shown that exposing hESCs to exogenous factors such as dexamethasone, ascorbic acid and β-glycerophosphate can induce osteogenesis in vitro [1-3]. However, the specific factors that regulate and influence the commitment of hESCs along the osteoblast lineage have not yet been identified. It is possible that soluble factors secreted by human bone marrow stromal cells (hBMSCs) may provide the necessary signaling molecules to direct osteogenic differentiation of hESCs.
When bone marrow is cultured in vitro, adherent non-hematopoietic cells proliferate and exhibit characteristics of bone marrow stroma in vivo. Within this diverse population of hBMSCs there exist early progenitor mesenchymal stem cells that are capable of self-renewal and have multi-lineage differentiation potential into cell types such as osteoblasts, chondrocytes, and adipocytes. In the presence of dexamethasone, ascorbic acid and β-glycerophosphate, it has been demonstrated that hBMSCs can be differentiated readily into mineralizing osteoblasts both in vitro and in vivo [4-7]. The in vitro equivalent of bone formation is characterized by the formation of mineralized nodules, increased alkaline phosphatase activity, and up regulation of osteoblastic genes such as runx2, osteocalcin, bone sialoprotein, and collagen type I [5, 8-10]. The use of this well-defined in vitro model allows the control of the differentiation state of hBMSCs at varying time points within their lineage progression towards functional osteoblasts. Subsequently, soluble factors derived from hBMSCs may be controlled, thus enabling the establishment of a co-culture system that stimulates hESC differentiation.
Therefore, the goal of this study was to determine if the lineage progression of hESCs toward osteoblasts could be directed by soluble signaling factors derived from differentiated hBMSCs. Because the differentiation of hBMSCs in vitro is so well characterized, the ability to manipulate and control hBMSCs along with the osteogenic factors derived from them will be integral to controlling the osteoblastic differentiation of hESCs. In this study, we demonstrate that osteogenic growth factors secreted by hBMSCs into the local microenvironment can promote osteoblastic differentiation of hESCs, and the secretion of these factors was dependent on the state of cell differentiation.
Materials and Methods
hESC Culture
The BG01 cell line was obtained from Bresagen, Inc. (Atlanta, GA) and cultured on irradiated mouse embryonic fibroblast (MEF) feeder layers at a density of approximately 19,000 cells/cm2 onto 60 mm dishes (0.1% gelatin-coated). The hESC culture medium consisted of 80% (v/v) DMEM/F12, 20% (v/v) knockout serum replacement (KOSR), 200mM L-glutamine, 10mM nonessential amino acids (all obtained from Invitrogen), 14.3M β-mercaptoethanol (Sigma, St. Louis, MO), and 8 ng/ml bFGF (Invitrogen, Carlsbad, CA). Cell cultures were incubated at 37°C in 5% CO2 in air and 95% humidity with medium changes everyday and manually passaged once per week. To induce osteogenic differentiation, the hESCs were made into embryoid bodies (EBs) and then seeded onto 0.1% gelatin-coated 6-well plates and cultured in hBMSC osteogenic medium (OS) consisting of 90% α-MEM (v/v), 10% heat-inactivated fetal bovine serum (FBS), 200 mM L-glutamine, and 10 mM nonessential amino acids (all obtained from Invitrogen) with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 μM ascorbic acid for 4 weeks with medium changes every 48 hours [3].
hESC/hBMSC Transwell Co-Culture
The hBMSCs were isolated from patients at the University of Michigan using IRB approved protocols. The hBMSCs were plated at 2,000/cm2 onto transwell inserts (0.4 μm pore, Corning, Corning, NY) and allowed to differentiate for 7-24 days in OS with complete medium changes every 48 hours. Two days prior to adding the hBMSC transwell inserts, the hESCs were either made into EBs or manually passaged directly from MEFs (omitting EB formation) and then seeded onto gelatin-coated 6-well plates. After 7, 14 or 24 days of differentiation, hBMSCs on transwell inserts were added to the 6-well plates and cultured in hBMSC growth medium (GM) without osteogenic supplements for an additional 28 days with medium changes every 48 hours. This method allows for the passage of soluble molecules while preventing direct cell-cell contact.
RT-PCR
Total RNA was obtained using Trizol (Invitrogen) and purified using the Qiagen RNEasy kit with DNase I treatment (Qiagen, Valencia, CA). Reverse transcription of 1 μg of RNA was performed using the Superscript III kit (Invitrogen). Taq DNA Polymerase (Invitrogen) was used to amplify the cDNA. The PCR conditions were as follows: 2 minutes at 94°C; followed by cycles of 45″ denaturation at 94°C, 45″ annealing at 56°C, and 60″ extension at 72°C. Primer sequences were as follows (forward, reverse): oct4 (GAA GGT ATT CAG CCA AAC, CTT AAT CCA AAA ACC CTG G) [11]; nanog (GAC TGA GCT GGT TGC CTC AT, TTT CTT CAG GCC CAC AAA TC) [12]; runx2 (CAT GGT GGA GAT CAT CGC, ACT CTT GCC TCG TCC ACT C) [11]; osterix (GCA GCT AGA AGG GCG TGG TG, GCA GGC AGG TGA ACT TCT TC) [13]; collagen1-Col-1 (GGA CAC AAT GGA TTG CAA GG, TAA CCA CTG CTC CAC TCT GG) [14]; osteocalcin-OCN (ATG AGA GCC CTC ACA CTC CTC, GCC GTA GAA GCG CCG ATA GGC) [14]; GAPDH (TGA AGG TCG GAG TCA ACG GAT TTG GT, CAT GRG GGC CAT GAG GTC CAC CAC) [12]. PCR products were analyzed on a 1.5% agarose gel with ethidium bromide staining. Imaging was obtained using a Fluor-S system (Biorad).
Mineralization Assays by Alizarin Red S and von Kossa Staining
Cell cultures were fixed in 4% paraformaldehyde for 30 minutes, washed twice with PBS and then stained. Alizarin Red S (Sigma) staining was used to determine the presence of mineralized nodules. Fixed cells were incubated for 1 hour in 1% Alizarin Red S solution and then washed twice with water. Von Kossa staining was used to determine the presence of phosphate. Fixed cells were incubated for 1 hour in 5% AgNO3 solution and exposed to bright light for at least 30 minutes. For mineralization quantification, Alizarin Red S precipitate was extracted using a 10% acetic acid/20% methanol solution for 45 minutes. Spectrophotometric measurements of the extracted stain were made at 450 nm.
Immunofluorescence
Cell cultures were fixed for 30 minutes at room temperature with 4% paraformaldehyde. After washing with PBS, cells were permeabilized for 10 minutes with 10% (v/v) Triton X-100 in PBS, then washed twice with a serum wash containing 1% (v/v) sodium azide, followed by a blocking step containing 0.5% (v/v) Triton X-100 and 1% (v/v) sodium azide for 30 minutes at room temperature. Dilution buffer containing 2 μg/ml polyclonal rabbit anti-human osteocalcin (Santa Cruz Biotechnology, Santa Cruz, CA) was added and incubated at 4°C overnight. Following incubation, the cells were rinsed twice with serum wash and incubated in the dark with 8 μg/ml FITC-labeled secondary antibodies for 30 minutes and counterstained with DAP1. The cells were then washed with PBS and fluorescence was observed using a Nikon Eclipse TE3000.
Statistical Analysis
Results were evaluated using the student t test. Statistical significance was set at the 95% confidence level with a p-value < 0.05.
Results
hESCs Undergo Osteogenic Differentiation and Give Rise to Condensed Mineralized Nodules in the Presence of hBMSCs
Human bone marrow stromal cells are able to differentiate into osteoblasts both in vitro and in vivo. Therefore, we postulated that exposing hESCs to hBMSCs undergoing osteoblastic differentiation would stimulate differentiation of hESCs along the osteogenic lineage [4-7]. For the transwell co-culture experiments, we used EB-derived cells. Human ESCs were plated onto gelatin-coated 6-well plates two days prior to co-culture with hBMSCs. Prior to plating the hESCs, hBMSCs were seeded onto gelatin-coated 0.4 μm pore transwell inserts and differentiated in growth medium plus defined osteogenic supplements (OS) for 7 days, 14 days, or 24 days. As controls, hESCs were co-cultured with undifferentiated hBMSCs or cultured alone in growth medium without osteogenic supplements (GM). At each specific time point (7, 14 or 24 days), the differentiated hBMSCs were added to the hESCs that had been plated onto gelatin-coated 6-well plates. The two cell types were then co-cultured in GM for an additional 28 days. Since the two cell types were not in direct physical contact, the hESCs were exposed to soluble signaling factors derived only from differentiated hBMSCs. In addition, hESCs were grown in osteogenic medium as previously described [1,3].
Mineralized nodule formation is the hallmark of in vitro osteogenic differentiation. Therefore, after 28 days the cells and extracellular matrix (ECM) produced by co-cultures were stained by von Kossa and Alizarin Red to detect the presence of phosphate and calcium, respectively [15, 16]. The hESCs in co-culture with 14 day differentiated hBMSCs formed bone nodules that stained positively for von Kossa and Alizarin Red (Figure 1C, D). In contrast, cells grown with undifferentiated hBMSCs only showed minimal levels of mineral deposition and weak staining (Figure 1A, B). As expected, the hESCs grown in the presence of osteogenic supplements for 28 days stained positive for a mineralized matrix (data not shown).
Figure 1.
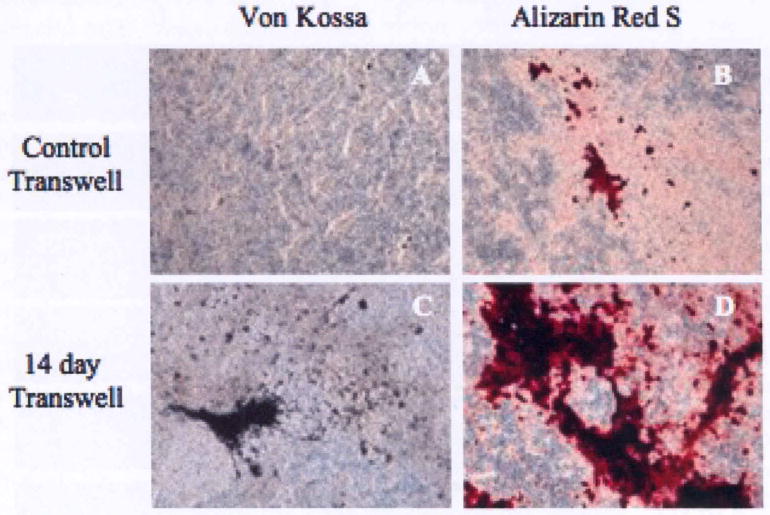
Human embryonic stem cells mineralize when co-cultured with differentiated hBMSCs. hBMSCs were grown on transwell inserts in osteogenic induction medium for 14 days. Differentiated hBMSCs were co-cultured with hESCs for an additional 28 days. Von Kossa (A, C) and Alizarin Red S (B, D) staining of hESCs co-cultured with differentiated or undifferentiated hBMSCs. Magnification = 10×.
Calcium deposition can also be quantified by extracting the Alizarin Red stain and subsequent spectrophotometric readings of Alizarin Red uptake. Therefore, we also performed quantitative Alizarin Red assays to assess the extent of bone nodule mineralization. There was a marked increase in mineralization within the transwell co-cultures with differentiated hBMSCs as compared to the controls. The hESCs exposed to hBMSCs differentiated for 7 days showed a 1.7-fold increase in Alizarin Red concentration (μg/ml) above control, a 2.5-fold increase for 14 day differentiated hBMSCs, and a 1.8-fold increase for cells exposed to hBMSCs differentiated for 24 days (Figure 2). Taken together, the upward trend of Alizarin Red content and greater von Kossa staining indicates that higher levels of mineral deposition could be found in hESCs exposed to differentiating hBMSCs.
Figure 2.
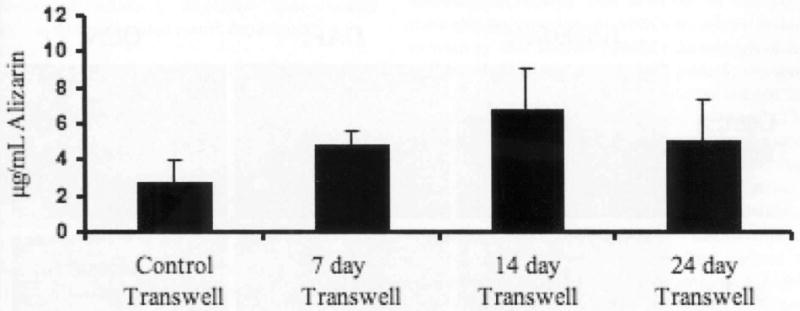
Quantitative analysis of Alizarin Red assays. hBMSCs were grown on transwell inserts in osteogenic induction medium for 7, 14 or 24 days. Differentiated hBMSCs were co-cultured with hESCs for an additional 28 days. Comparison of Alizarin Red staining between co-cultures with undifferentiated hBMSCs and experimental groups consisting of 7, 14 and 24 day differentiated hBMSCs.
hESCs Respond to the hBMSC Transwell Co-Culture System and Express Osteogenic Specific Markers
Osteoblastic lineage commitment can be observed by the expression of bone specific transcription factors runx2 and osterix, and with collagen type I production. Runx2 is a homolog of the Drosophila Runt protein that acts as a transcriptional regulator of osteoblast differentiation, and osterix is a zinc finger containing transcription factor required for osteoblastic differentiation that acts downstream of runx2 [17, 18]. Collagen type I is the most abundant protein found in bone ECM [19].
Therefore, in order to further confirm osteogenic differentiation, we analyzed the expression of these bone markers after 28 days in co-culture (Figure 3). RT-PCR analysis demonstrated that undifferentiated hESCs exhibit strong expression of the hESC pluripotency markers Oct-4 and Nanog, whereas they were absent in the transwell co-cultures and OS conditions (Figure 3, Lane 1).
Figure 3.
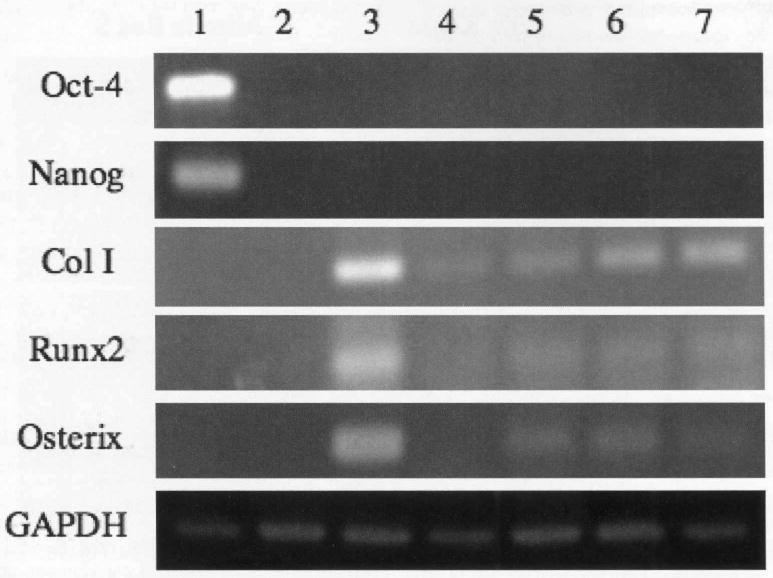
Human embryonic stem cells express osteoblastic markers when co-cultured with differentiated hBMSCs. hBMSCs were grown on transwell inserts in osteogenic induction medium for 7, 14 or 24 days. Differentiated hBMSCs were co-cultured with hESCs for an additional 28 days. Lane 1: undifferentiated hESCs; lane 2: hESC in growth medium; lane 3: hESC in osteogenic medium; lane 4: transwell co-culture with undifferentiated hBMSCs (control); lane 5: transwell co-culture with 7 day differentiated hBMSCs; lane 6: transwell co-culture with 14 day differentiated hBMSCs; lane 7: transwell co-culture with 24 day differentiated hBMSCs.
In the presence of OS, there was a significant induction of osteogenic marker expression in hESCs [3, 15]. The transwell co-cultures also led to the induction of osteogenic gene expression, although it was not as robust. There were modest levels of bone specific gene expression by cells in co-culture with undifferentiated hBMSCs (Figure 3, Lane 4). However, gene expression of osteo-specific markers was upregulated in hESCs in the presence of differentiated hBMSCs (Figure 3, Lanes 5-7).
hESCs Co-Cultured with Differentiated hBMSCs Secrete the ECM Protein Osteocalcin
Fully differentiated osteoblasts have the ability to form three-dimensional nodules with many of the immunohistochemical markers of bone and also form mineralized matrix. These nodules are thought to represent the end stage of differentation of osteoprogenitor cells in vitro [20]. It has also been shown that three-dimensional bone nodules derived from hESCs stain positive for osteocalcin (OCN) [15]. Results from our study show that immunostaining with antibody to OCN had strong immunoreactivity localized to clusters of hESCs co-cultured with 7, 14, and 24 day differentiated hBMSCs, whereas there was no evidence of OCN staining for hESCs exposed to undifferentiated hBMSCs (Figure 4). We believe the clusters that formed in the differentiated hBMSC transwell co-cultures are condensed bone nodules comprised of hESC-derived osteoblasts that secrete osteocalcin. The hESCs readily adhered to the culture plates and proliferated extensively in the presence of hBMSCs as evidenced by DAPI staining (Figure 4). As compared to hESCs grown alone or with undifferentiated hBMSCs, the cells in transwell co-culture with differentiated hBMSCs displayed bone nodule formation and mineralized matrix deposition.
Figure 4.
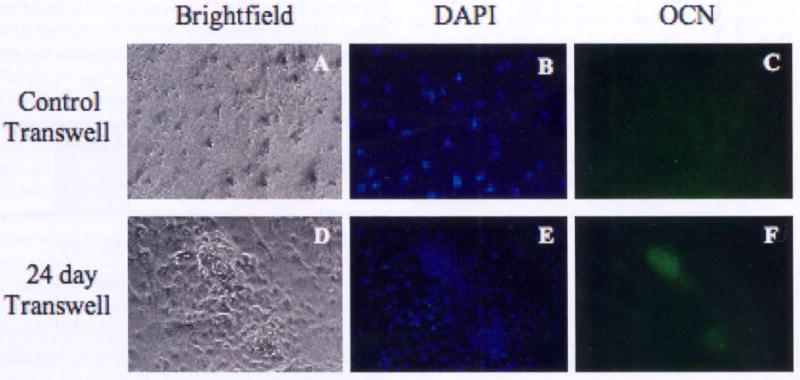
Human embryonic stem cells express osteocalcin when co-cultured with differentiated hBMSCs. hBMSCs were grown on transwell inserts in osteogenic induction medium for 24 days. Differentiated hBMSCs were co-cultured with hESCs for an additional 28 days. Expression of osteocalcin with DAPI counterstaining of hESC co-cultures with undifferentiated hBMSCs (A-C) and 24 day differentiated hBMSCs (D-F). Magnification = 10×.
Discussion
Bone marrow is a complex tissue comprised of hematopoietic precursors, as well as a connective tissue network referred to as bone marrow stroma. It has been demonstrated that culture-adherent cells present in the marrow stroma have the capability to differentiate along multiple mesenchymal lineages [21]. Therefore, within the stroma itself exists a heterogeneous population of cells including osteoprogenitor cells that can proliferate and differentiate into mature osteoblasts. These multipotent cells are referred to as mesenchymal stem cells (MSCs), and have been serially passaged without lineage progression and subsequently shown to form cartilage, bone, fat and mature stromal cell lineages [2, 5, 22-24]. Due to our knowledge about the differentiation potential of human MSCs, we hypothesized that exposing hESCs to hBMSCs at various time points along the osteogenic differentiation pathway would contribute to directed osteoblastic differentiation of hESCs. The temporal expression of cell growth and osteoblast phenotype-related genes during osteoblast growth and differentiation has been established for primary osteoblasts, where three principle periods of osteoblast phenotype development have been identified – proliferation, ECM development and maturation, and mineralization phases [25]. During the proliferation phase (approximately 0-12 days in culture) collagen type I is predominantly expressed. The ECM development and maturation phase (approximately 12-21 days in culture) is characterized by high alkaline phosphatase expression, and within the mineralization phase (approximately 21-35 days in culture) osteocalcin is highly expressed as cell aggregates, or nodules, become mineralized in the presence of dexamethasone, ascorbic acid and β-glycerophosphate [25]. Therefore, we chose to differentiate our hBMSCs for 7, 14, and 24 days to determine if soluble factors secreted at different developmental time points would contribute to hESC differentiation.
Mineralization assays, RT-PCR and immunohistochemistry data described in this study show that by exposing the hESCs to soluble factors secreted by differentiated hBMSCs during each of the osteoblast phenotype development periods, we were able to achieve osteogenic differentation without the addition of any other exogenous factors. The osteogenic response observed can be compared to that of hBMSCs in osteogenic medium [22]. There were substantially higher levels of Alizarin Red staining found in hESC co-cultures with differentiated hBMSCs than co-cultures with undifferentiated hBMSCs. More specifically, the cells exposed to hBMSCs differentiated for 14 days gave rise to highly mineralized bone nodules as evidenced by exhibiting the strongest von Kossa and Alizarin Red staining, as well as the highest Alizarin Red concentration as compared to the other transwell co-cultures. Although the increase in Alizarin Red content was not statistically significant between the control transwell and differentiated hBMSC transwell co-cultures, the 1.5-2.5 fold increase in concentration suggests that increased mineralization occurs when hESCs are exposed to soluble factors derived from differentiated hBMSCs. Additionally, the expression of bone-specific transcription factors, runx2 and osterix, as well as collagen type I, further confirmed that we successfully obtained hESC-derived osteoblasts. Gene expression of runx2, osterix, and collagen type I was found to be upregulated by co-culture conditions with differentiated hBMSCs, which is consistent with a mature osteoblast phenotype. There were low levels of bone specific gene expression within the control transwell co-culture with undifferentiated hBMSCS, and this is likely due to the fact that spontaneous differentiation occurs, thus yielding a small population of cells undergoing osteogenic differentiation. When the cells were cultured in osteogenic supplements for 28 days there was significantly stronger induction of bone specific gene expression as compared to the transwell co-cultures. This suggests that the growth factors secreted by the hBMSCs are osteo-inductive, however, further investigation regarding the identification and temporal expression of these secreted factors is needed to optimize this co-culture system. Lastly, immunostaining of condensed bone nodules within the differentiated hBMSC transwell co-cultures verified the presence of osteocalcin, a late marker of osteogenesis that corresponds with induction of mineralization [2]. Such differentiation was not observed in hESCs co-cultured with undifferentiated hBMSCs (Figure 4C).
Human embryonic stem cells hold promise for future regenerative medicine strategies. They were first derived from the inner cell mass of blastocyst stage embryos and have since been studied to determine their capacity to differentiate into all cell types [26, 27]. Osteoblastic differentiation of mouse, human, and monkey embryonic stem cells in vitro has previously been shown using the traditional osteogenic supplements dexamethasone, ascorbic acid and β-glycerophosphate [2, 28, 29]. Additionally, co-culture systems have been used to direct differentiation of both murine and human ES cells. Murine ES cells were shown to differentiate into osteoblasts using fetal murine osteoblasts in transwell co-culture, and hESCs were shown to differentiate into osteoblasts using direct co-culture with primary bone derived cells [28, 30]. Also, hESCs have been proven to have the capacity to differentiate into cartilage both in vitro and in vivo when exposed to primary chondrocytes in a transwell co-culture system [31].
This study differs from previous reports on the osteogenic differentiation of hESCs in two important ways: 1) Using an indirect transwell co-culture system; and 2) Controlling the state of differentiation of hBMSCs to induce hESC differentiation. The osteogenic response of the hESCs to the co-culture system may be explained by the fact that differentiated hBMSCs provide necessary osteo-inductive signals. Collectively, these data strongly support the hypothesis that the lineage progression of hESCs to osteoblasts can be directed by soluble signaling factors secreted by differentiated hBMSCs within the local cell environment. This co-culture system indicates the importance of cellular communication and coordination; therefore, determining the role of pro-osteogenic growth factors derived from hBMSCs and the influence of the microenvironment on osteogenic hESC differentiation is essential. Understanding the regulatory mechanisms that control osteoblastic differentiation will provide segue towards developing a useful cell source for bone tissue engineering repair. Future studies include performing proteomic analyses to identify and define the secreted factors, as well as systematically altering the microenvironment to further investigate the co-culture system we have developed and how it contributes to the osteogenic lineage progression of hESCs.
Acknowledgments
The authors would like to thank the University of Michigan Human Embryonic Stem Cell Core. This work was funded by the NIH National Institute for Dental and Craniofacial Research (DE016530) and (DE016530-01S1).
References
- 1.Cao T, Heng B, Ye C, et al. Osteogenic differentiation within intact human embryoid bodies result in a marked increase in osteocalcin secretion after 12 days of in vitro culture, and formation of morphologically distinct nodule-like structures. Tissue and Cell. 2005;37:325–334. doi: 10.1016/j.tice.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Karp J, Ferreira L, Khademhosseini A, Kwon A, Yeh J, Langer R. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835–843. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 3.Sotille V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning and Stem Cells. 2003;5:149–155. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Riminucci M, Gronthos S, Robey P. Bone Marrow Stromal Stem Cells: Nature, Biology, and Potential Applications. Stem Cells. 2003;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal N, Haynesworth S, Caplan A, Bruder S. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Jour of Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 6.Kuo C, RS T. Tissue engineering with mesenchymal stem cells. Tissue Eng in Med and Bio. 2003;22:51–56. doi: 10.1109/memb.2003.1256272. [DOI] [PubMed] [Google Scholar]
- 7.Wagers A, Weissman I. Plasticity of adult stem cells. Cells. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 8.Beresford J, Graves S, Smoothy C. Formation of mineralized nodules by bone derived cells in vitro: a model of bone formation? Amer J Med Gen. 1993;45:163–178. doi: 10.1002/ajmg.1320450205. [DOI] [PubMed] [Google Scholar]
- 9.Kalajzic I, Staal A, Yang W, et al. Expression profile of osteoblastic lineage at defined stages of differentiation. J Biol Chem. 2005;280:24618–24626. doi: 10.1074/jbc.M413834200. [DOI] [PubMed] [Google Scholar]
- 10.Krebsbach P, Kuznetov K, Emmons R, Rowe D, Robey P. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 11.Bielby B, Boccaccini A, Polak J, Buttery L. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 12.Brimble S, Zen S, Weiler D, et al. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines derived prior to August 9, 2001. Stem Cells and Dev. 2004;13:585–596. doi: 10.1089/scd.2004.13.585. [DOI] [PubMed] [Google Scholar]
- 13.Miura M, Grothos S, Zhao M, et al. SHED: Stem cells from human exfoliated deciduous teeth. PNAS. 2003;10:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noth U, Osyczka A, Tuli R. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–1069. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 15.Bonewald L, Harris S, Rosser J, et al. Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 16.Wergedal J, Baylink D. Distribution of acid and alkaline phosphate activity in undemineralized sections of the rat tibial diaphysis. Jour of Histochemistry and Cytochemistry. 1969;17:799–806. doi: 10.1177/17.12.799. [DOI] [PubMed] [Google Scholar]
- 17.Ducy P, Zhang R, Geoffroy V, Ridall A, Karsenty G. Osf2/Cbfal: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 19.Olsen B. Collagen it takes and bone it makes. Curr Biol. 1996;6:645–647. doi: 10.1016/s0960-9822(09)00438-2. [DOI] [PubMed] [Google Scholar]
- 20.Purpura K, Aubin J, Zanstra P. Sustained in vitro of bone progenitors is cell density dependent. Stem Cells. 2004;22:39–50. doi: 10.1634/stemcells.22-1-39. [DOI] [PubMed] [Google Scholar]
- 21.Friendenstein A. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–355. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 22.Haynesworth S, Baber M, Caplan A. Characterization of the unique mesenchymal stem cell phenotype in vitro. Trans Ortho Res Soc. 1995;20:7–12. [Google Scholar]
- 23.Johnstone B, Yoo J, Barry F. In vitro chondrogenesis of bone marrow-derived mesenchymal cells. Trans Orthop Res Soc. 1996;21:65. [Google Scholar]
- 24.Pittenger M, Mackay A, Beck S. Human mesenchymal stem cells can be directed into chondrocytes, adipocytes or osteocytes. Mol Biol Cell. 1996;7:305a. [Google Scholar]
- 25.Stein G, Lian J. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocrine Reviews. 1993;4:424–441. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 26.Odorico J, Kaufman D, Thomson J. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 27.Thomson J, Itskovitz-Eldor J, Shapiro S, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Buttery L, Bourne S, Xynos J, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita A, Takada T, Narita J, Yamamoto G, Torii R. Osteoblastic differentiation of monkey embryonic stem cells. Stem Cells and Cloning. 2005;7:232–237. doi: 10.1089/clo.2005.7.232. [DOI] [PubMed] [Google Scholar]
- 30.Ahn S, Kim S, Park K, et al. Primary bone-derived cells induce osteogenic differentiation without exogenous factors in human embryonic stem cells. Biochem Biophys Res Commun. 2006;340:403–408. doi: 10.1016/j.bbrc.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Vats A, Bielby R, Tolley N, et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]


