Abstract
Rationale
Little is known about mechanisms underlying female rodent aggression during the late postpartum period with no pups present. Studies of aggression, dominance, and oxytocin (OT) response in cocaine-treated females are sparse.
Objectives
This study was designed to examine dominance (drinking success) and aggression in a limited-access drinking model of water competition. Acute OT level measures were made on postpartum day (PPD) 36 in several brain regions of interest. Chronic and intermittent cocaine- and saline-treated and untreated rats 10 days post-weaning were tested (without pups) over PPDs 31–35 following cessation of cocaine treatment 10–30 days before testing.
Methods
Subjects were water-deprived overnight, and triads consisting of an untreated control (UN), a chronic continuous saline-treated (CS), and chronic continuous cocaine-treated (CC; 30 mg/kg/day throughout gestation) or a UN, an intermittent saline-treated (IS), and an intermittent cocaine-treated (IC; 30 mg/kg two consecutive days every 4 days throughout gestation until PPD 20) female were tested for aggression and drinking behavior during 5 min sessions on five consecutive days. The amygdala, medial preoptic area (MPOA), and ventral tegmental area were assayed for OT levels.
Results
CC and IC females were more aggressive than controls, but only IC females drank more often than controls. OT levels were lower in the MPOA of IC and CC females than in controls.
Conclusions
Findings demonstrate that long after cessation of treatment, CC- and IC-treated non-lactating females (no pups present) had higher rates of aggression, altered drinking behavior, and acutely lower MPOA OT levels.
Keywords: Cocaine, Aggression, Oxytocin, Dominance, Competition
Introduction
Acute and chronic cocaine abuse is associated with increased aggression in humans (Brody 1990; Miller et al. 1991; Licata et al. 1993; Fals-Stewart et al. 2003) with most clinical studies primarily reporting male aggressive behavior. Studies that have investigated the incidence of increased aggression in women, specifically those using cocaine, have largely focused on postpartum infant- or child-directed maternal abuse or neglect (Wasserman and Leventhal 1993; Hawley et al. 1995; Tyler et al. 1997; Feerick et al. 2002).
Much like the human research, there are numerous preclinical reports of aggression in cocaine-treated subjects, usually male, with mixed reports of increased or decreased levels of aggression depending on various experimental factors, some of which include dose, timing, age of subject, test, etc. (Hutchinson et al. 1977; Miczek and O’Donnell 1978; Hadfield et al. 1982; Hadfield 1982; Darmani et al. 1990; Blanchard and Blanchard 1999). The majority of female rodent aggression studies in the last decade (with or without drug treatment) emphasize maternal aggression. Naturally occurring maternal aggression during lactation in rodents is thought to have evolved to protect offspring from harm and usually occurs near the nest (Paul et al. 1981; Giordano et al. 1984; Wolff and Peterson 1998; Parmigiani et al. 1999). It differs primarily from territorial aggression (Blanchard and Blanchard 1990) in that it is highly conserved across species and under the control of numerous cues and hormonal factors, which play a significant role in its expression (Stern 1989; Numan 1994; Bridges 1996; Lonstein and Gammie 2002; Numan and Insel 2003).
A number of reports on the effects of various gestational or postpartum cocaine treatment regimens on postpartum maternal aggression have been published over the last two decades. Briefly, acute cocaine treatment immediately following parturition and given 30 min before testing results in reduced maternal aggressive behavior towards male or female intruders (Johns et al. 1994b, 1998a; Vernotica et al. 1996), whereas chronic continuous gestational cocaine treatment ending before parturition, significantly increased maternal aggression towards live intruders by postpartum day (PPD) six with repeated violent attacks on intruder males (Heyser et al. 1992; Johns et al. 1994b, 1998b; Lubin et al. 2001a; McMurray et al. 2008). In a later study, intermittent gestational and postpartum cocaine treatment had either little effect on aggression (PPD 12), or if cocaine was given just prior to testing (PPD 8), a decrease in aggression similar to the effects of acute single injections (McMurray et al. 2008). A study of virgin chronic gestational cocaine-treated females determined that rates of resident intruder territorial aggression towards a live male or female rat were slightly but not significantly increased compared to controls at a 30-mg/kg dose over 20 days (Lubin et al. 2001b). This study used the same testing paradigm as the postpartum studies, suggesting that rather than simply dose and duration of treatment, cocaine’s effects on aggression were likely heightened by the endocrine state during early to mid-lactation with pup presence perhaps also important. Few reports of other forms of female aggression following cocaine treatment exist.
The underlying biological mechanisms mediating cocaine-induced increases in female aggressive behavior are not fully understood. However, many studies have observed a relationship between the neuropeptide oxytocin (OT) and the regulation of aggression (Ferris et al. 1992; Winslow et al. 1993; Johns et al. 1994b; Giovenardi et al. 1998; Elliott et al. 2001; Neumann et al. 2001; Lonstein and Gammie 2002; Lubin et al. 2003b; Light et al. 2004; Consiglio et al. 2005; Bosch et al. 2005, 2007; McMurray et al. 2008; Neumann 2008; Veenema and Neumann 2008). A number of studies indicate that cocaine’s effects on aggressive behaviors in lactating rat mothers is concurrent with alterations in the oxytocinergic system as decreased levels of OT in the amygdala (AMY) were correlated with significantly increased levels of maternal or postpartum aggression, defined as increased lateral and front threats, fight attacks, aggressive posture, and rough grooming of an intruder (Johns et al. 1994b, 1998b; Elliott et al. 2001; Lubin et al. 2003b; Light et al. 2004; McMurray et al. 2008). Oxytocin levels in the medial preoptic area (MPOA), ventral tegmental area (VTA), or hippocampus (HIPP) have not been associated with increased maternal aggression in our gestational cocaine treatment model (Johns et al. 1994b). These regions are important for maternal behavior, reward circuitry, and social memory and thus remain areas of interest for drug-induced changes in dominance or non-maternal forms of aggression. Given previous results of heightened maternal aggression in lactating cocaine-treated females with pups present and OT regional changes correlated with these differences, we wanted to determine if after long-term cessation of treatment, other forms of aggression would be altered in postweaning females when no pups were present and females were not lactating. We suspected that specific regional OT differences would be correlated with aggression in a task involving social memory and dominance behavior. To our knowledge, no previous studies have examined dominance or related OT changes in a cocaine-treated female rat model.
In the current study, we examined dominance (operationally defined as significantly greater access to and drinking of a limited resource (water)) and aggression (fights, threats, pushing) in water-deprived groups of control and cocaine-treated post-lactational rat dams during a competition task. Gravid females treated with cocaine either chronically and continuously during gestation (CC), or intermittently throughout gestation and the postpartum period (IC), and who reared untreated (UN) surrogate pups (as did all females in the study) were tested in triads (a cocaine-treated, a UN and a saline-treated female) over PPDs 31–35 using a modified water competition task (Wood and Spear 1998). Aggression (frequency and duration of fighting, threats, and pushes) and dominance, defined as having the highest drinking success (highest frequency and duration of drinking water), were measured. Dominant rats were expected to display the highest initial rates of aggressive behaviors until a pattern of relatively unopposed access to the drinking spout was established. OT levels were measured in brain regions previously associated with cocaine-induced aggressive behavior in lactating or estrogen-treated rats given an OT antagonist (Ferris et al. 1992; Caldwell et al. 1994; Giovenardi et al. 1997; Elliott et al. 2001; Lubin et al. 2003a; Johns et al. 2004; Jarrett et al. 2006; McMurray et al. 2008). We hypothesized that both cocaine treatment regimens would result in acute changes in both dominance and aggressive behavior towards control conspecifics, resulting in the more dominant females having at least initially greater access to and drinking more from the waterspout. We also expected to see a reduction in OT levels in the AMY and possibly the MPOA of the most aggressive cocaine-treated females compared to controls. CC-treated females were expected to be the most aggressive although their dominance across the testing period was thought to be less certain.
Methods
Subjects
Following a 2-week habituation period, virgin female (200–240 g) Sprague–Dawley rats (Charles River, Raleigh, NC) were placed singly with males on a breeding rack until a sperm plug was found, which was designated as gestation day (GD) zero. Subjects were randomly assigned to one of five treatment or control groups (detailed below), singly housed, and maintained on a 12:12 h reverse light cycle (lights off at 0900 hours) for 7 days. They were then transferred to a room with a regular light cycle (lights on at 0700 hours) for the remainder of the experiment, a procedure that generally results in the majority of females delivering their litters during daylight hours (Mayer and Rosenblatt 1998). Within an hour of delivery, litters were removed from all females and replaced with eight UN Sprague–Dawley surrogate pups (four male and four female) born within 24 h of the treatment dam’s delivery. Surrogate pups remained with females until weaned on PPD 21 prior to competition testing. Postpartum females were housed singly for the duration of the study. All procedures were conducted under federal and institutional animal care and use committee guidelines for humane treatment of laboratory subjects.
Treatment
Treatment groups and regimens, which have been previously described (Johns et al. 2005), included: CC, IC, chronic continuous saline (CS), chronic intermittent saline (IS), and UN females. CC and CS females received subcutaneous injections twice daily on GDs one through 20 of 15 mg/kg cocaine HCL (dose calculated as the free base; Sigma Chemical Company, St. Louis, MO) dissolved in 0.9% normal saline (total volume 2 ml/kg) or the same volume of normal saline (0.9%), respectively, at approximately 0800 and 1600 hours. Daily injections were done on alternating flanks to reduce cocaine-induced skin lesions, but once apparent, lesions were treated daily with betadine and an antibiotic ointment (Neomycin, Polymycin B Sulfate, and Bacitracin Zinc Ointment, Fougera, Melville, NY) to encourage rapid healing and prevent infection. These methods have significantly reduced the number and severity of lesions in our lab. These treatment regimens were primarily chosen for the scientific information previously gathered from use of these paradigms in previous studies (Johns et al. 1994b; Elliott et al. 2001; Lubin et al. 2003b; Light et al. 2004; McMurray et al. 2008). Intermittently treated females (IC and IS) received the same doses of their respective drugs as the chronically treated females. Injections for intermittent treatment groups occurred only on two consecutive days, every 5 days during gestation (GDs 2, 3, 8, 9, 14, 15, 20) continuing throughout the postpartum period (PPD 2, 3, 8, 9, 14, 15, 20), a model used previously in a study of maternal behavior and aggression in females and offspring (Johns et al. 1992a,b, 2005; McMurray et al. 2008). UN rats were weighed and handled daily but received no drug treatment. CC and UN rats had free access to water and food (rat chow), while CS females were yoke-fed to CC rats to control for the anorexic effects of cocaine, as previously described (Johns et al. 1994b). All intermittent groups had free access to water and food (rat chow), except on injection days when intermittently treated (IC and IS) groups received 50 g of food (more than any rats consumed in a day) with food consumption measured daily.
Water competition testing
The water competition apparatus consisted of a 30 × 40 × 20 cm Plexiglas cage as a chamber with a 4 × 6-cm polycarbonate tube affixed to the side. A single spout was connected to the chamber with the opening of the tube inside the cage such that only one rat at a time could access the spout to drink. Water tubes were cleaned and filled with fresh tap water (100 ml) between tests. The water competition task was adapted from a method used in a previous study of competition behavior in adolescent and adult rat offspring prenatally exposed to cocaine (Wood and Spear 1998). The overall testing procedure lasted 8 days beginning with room habituation on PPD 28. One CC, CS, and UN rat dam were randomly assigned to each of nine chronic triad (CC, CS, UN) groups and one IC, IS, and UN rat dam were assigned to each of nine intermittent triad (IC, IS, UN) groups. The same chronic and intermittent triads were tested together over all test sessions on five consecutive days (PPD 31–35). On PPDs 28 and 29, each subject habituated to the test room in their home cages over a 5-min period. During habituation on PPD 29, all subjects were weighed for a baseline body weight and to habituate them to subsequent weighing which occurred before testing each day. When they were returned to the colony room daily, they were given access to their home cage waterspout for an hour, after which they were water deprived until chamber habituation or later testing (approximately 22 h deprivation).
On the morning of PPD 30, each subject was placed into the testing apparatus alone and given a 30-min period with water to ensure all animals would drink from the spout. On PPD 31, the water competition testing began at 0800 hours until all groups finished (about 1300 hours). Testing times and water deprivation times were balanced and controlled across triads and groups so that all subjects received the same amount of deprivation, and equal numbers of chronic and intermittent triad groups were tested across time. The test chamber was cleaned with soap and water and fresh bedding placed in the cage between subjects. All subjects were weighed daily and marked on their back with an indelible marker (Sharpie) for group identity visualization prior to tests.
A video camera (Panasonic VHS recorder AG 188) was started just prior to subjects within a triad being simultaneously placed in the testing apparatus, and the water tube was inserted into the spout. Subjects competed for 5 min, the water tube removed, and total water consumption recorded. Behaviors of interest included: push (subject uses its flank or forepaws to push either of the two females in the test triad, most often occurred in order to gain access to the drinking spout; frequency and latency measured only); drink (subject’s snout was in the water access tube and they were actively licking the spout); threat (subject approaches another rat with her body in a lateral position [lateral threat] or [front threat] faces the intruder with her snout close to another subject’s face and holds a still position often accompanied by teeth chattering); fight (dam makes a quick lunge usually followed by rolling, biting, and fur pulling directed towards the neck and back regions of another subject). Threat, push, and fight were operationally defined as offensive or aggressive behaviors for the purpose of this study. Following testing, subjects were returned to their home cage (singly housed) and returned to the colony room for 1-h access to water. After the final test day (PPD 35), subjects were allowed free access to water to rehydrate until the following morning (PPD 36) when they were killed for brain region dissection.
Brain dissection
Following live decapitation, the whole MPOA, HIPP, AMY, and VTA were dissected (see Fig. 1) on ice, weighed, rapidly frozen, and stored at −80°C for later radioimmunoassay Brain dissection procedures have previously been described (Johns et al. 1997). More than half of the HIPP samples were lost to a technical error; thus, results are not included.
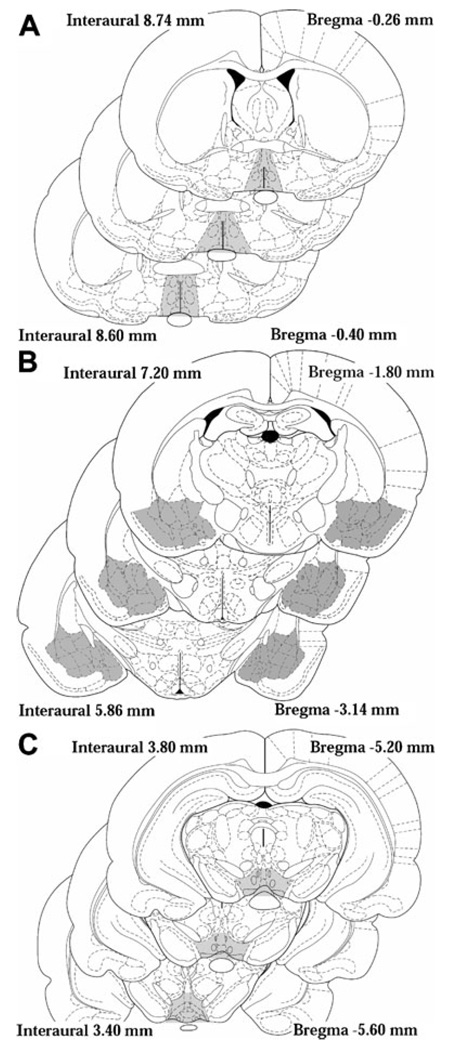
Fig. 1.
Diagram of anatomical brain regions dissected for oxytocin level assessment on PPD 3. Brains were coronally sectioned from the ventral side rostral to the optic chiasm and just caudal to the optic chiasm to define the preoptic-anterior hypothalamic area. Vertical cuts inferior to the lines of lateral ventricles and a horizontal cut ventral to the anterior commissure were made to produce a block section of the medial preoptic area (a). Brains were sectioned once again just caudal to the tuber cinereum and slightly above the cerebellum and the amygdala was removed in this section (b). The ventral tegmental area was dissected from the caudal section by making dorsoventral cuts medial to the optic tracts with a dorsal cut at the ventral extent of the central gray (c)
Oxytocin radioimmunoassay
Oxytocin assay methods for these brain regions have been previously described (Johns et al. 2005). Briefly, brain samples were first homogenized in cold buffer and centrifuged at 3,000 × g for 30 min. OT immunoreactive content was then assayed in the supernatant according to a protocol from Peninsula Labs (Belmont, CA). Samples and standards (1.0–128.0 ρg) were then incubated in duplicate for 16–24 h at 4°C with rabbit anti-OT serum and incubated for 16–24 h at 4°C with 125I-OT. Normal rabbit serum and goat anti-rabbit IgG serum were added and incubated 90 min at room temperature, and the 125I-OT bound to the antibody complex was separated from by centrifugation at 4°C. Radioactivity was measured using a LKB Clini-Gamma counter. The intra-assay coefficient of variance (CV) was 4.05% and inter-assay CV was 8.95% with sensitivity of the assay approximately 0.5 ρg/tube.
Statistical analysis
Videotaped behavioral sessions were scored by two independent observers, blind to treatment condition, with inter- and intra-reliability set at 90–100% concurrence for frequency and latency, and 80% or better for duration of behaviors displayed by the dam (unless otherwise specified, as for push). All behaviors were relative to the behavior of other females within the specific triads such that a push by the CC dam means they pushed either a CS or a UN dam within their triad. Behaviors not displayed by the females were assigned the highest possible latency (300 s for 5-min test).
All gestational, OT, and behavioral data were analyzed using generalized estimating equations (GEE) (Zeger and Liang 1986). Due to the longitudinal design of study, the non-normality of the data (count data is described by a Poisson distribution), and the clustering of our females into triads, which meant the performance of each animal in the triad was not independent, simple analyses of variance could not be used to characterize our data. Thus, GEE was used (for application review, see Hanley et al. 2003), resulting in a series of test statistics with a Chi-squared (χ2) distribution. GEE tests using log linear models were used to analyze the behavioral frequency, gestational, and OT datasets, while weighted additive models were used for the behavioral duration dataset (with weights inversely proportional to the within cell variance). A Cox Proportional Hazard model was used for the behavioral latency dataset. All tests examined behavioral performance for each individual day as well as across repeated days of testing. Contrasts were performed to examine all relevant group differences in each of the above models. Behavioral data are presented in the results section separately for the chronic and intermittent triads, focusing on differences between cocaine-treated and control groups. All data in tables and figures are Mean ± SEM, and alpha levels were set to p≤0.05. Symbols denoting significant differences include an asterisk for differences between CC/IC and UN; a § for differences between CC/IC and CS or IS respectively; and a † for differences between CS/IS and UN. Exact p values are in the text and figure captions.
Results
Gestational variables
There were no significant differences between groups on the number of GDs or individual pup weights (see Table 1). UN females gained more weight during gestation than CC (χ(1)2 = 7.39, p≤0.01) and CS females (χ(1)2 = 11.64, p≤0.01), and UN females had a higher litter birth weight than CC (χ(1)2 = 6.07, p≤0.05) and CS females (χ(1)2 = 16.43, p≤0.01). CC and CS females had slightly fewer pups with CS having significantly fewer than UN females only (χ2 = 15.56, p≤0.01). Untreated culled foster litters had statistically equivalent weights at fostering, and there were no significant differences on postnatal 15 pup weight gain.
Table 1.
Gestational data
| Number of subjects |
Gestational length (Days) |
Gestational weight gain (g) |
Number of Pulps |
Litter weight at birth (g) |
Average pup weight at birth (g) |
||
|---|---|---|---|---|---|---|---|
| Chronic | Cocaine | 9 | 21.4±0.2 | 131.9±6.0** | 13.8±1.1 | 82.1±5.1* | 6.5±0.6 |
| Saline | 9 | 21.3±0.2 | 130.9±4.5** | 12.5±0.8** | 80.5±4.2** | 6.3±0.6 | |
| Untreated | 9 | 21.3±0.2 | 164.7±11.0 | 15.4±0.8 | 101.7±6.2 | 6.6±0.9 | |
| Intermittent | Cocaine | 9 | 21.4±0.2 | 150.3±5.3 | 14.6±0.3 | 90.4±1.3 | 6.4±0.6 |
| Saline | 9 | 21.3±0.2 | 151.2±7.3 | 13.6±0.8 | 85.7±4.8 | 6.3±0.7 | |
| Untreated | 9 | 21.4±0.2 | 158.3±5.6 | 14.7±1.2 | 92.3±6.1 | 6.6±0.9 |
p≤0.01, difference from untreated
p≤0.01, difference from untreated
Water competition
Chronic Triads (CC, CS, UN)
Drink
There were no group differences in water consumption between triads or weight loss resulting from water deprivation during the testing period. As shown in Fig. 2a, all chronic triads drank more across the test period. CC females had lower average rates of drinking on several days than did control groups with no significant group differences across days.
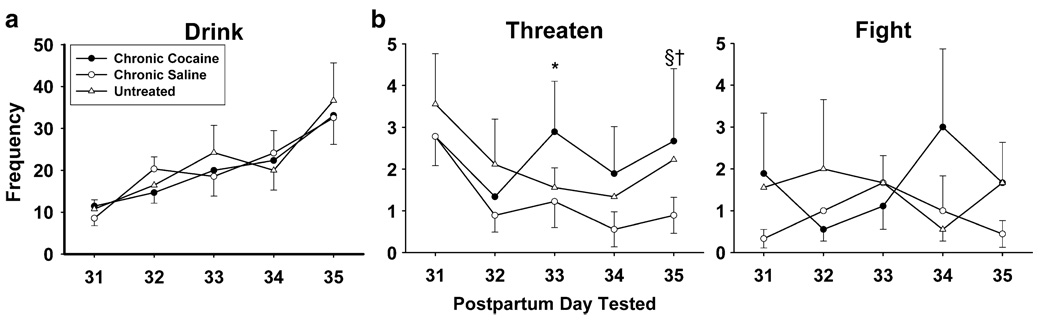
Fig. 2.
Effects of CC treatment compared to CS or UN controls on the frequency of a drinking, b threatening, and c fighting behavior during water competition on PPDs 31–35. All chronic triad subjects had similar drinking patterns with CC females drinking the same or less than controls on most days. CC females threatened more frequently over the final three test days, specifically more than UN on PPD 33 (*p≤0.01) and CS controls on PPD 35 (§p≤0.01). Females in the CS group also threatened more than UN females on the final day (†PPD 35 only p≤0.05). CC females attacked more than control and saline females more often on PPD 31 and 34 as well (nonsignificant)
Push
There were no significant group differences on push in chronic triads (data not shown).
Threat
There were interesting within-group patterns of threats across days specific to each group (Fig. 2b). All females had moderate rates of threats on the first test day (PPD 31) with UN females having slightly higher rates earlier, decreasing over sessions, with a slight rise on the final test day. CS females had a similar pattern to UN except they continued to have the lowest rates across all days. CC females were initially (PPDs 31–32) similar to controls but by test day three had a higher frequency of threats which continued across the final test days compared to UN (PPD 33: χ(1)2 = 6.72, p≤0.01) and CS controls (PPD 35 (χ(1)2 = 13.90, p≤0.01)). CS also threatened less than UN females on PPD 35 (χ(1)2 = 31.98, p≤0.01).
Fight
In general, all chronic triad females had relatively low levels of fighting, with a peak in CC females on PPDs 31 and 34 compared to CS and UN females (see Fig. 2c).
Drink
All test groups increased frequency of drinking across test days. Figure 3a, b indicates that IC females drank significantly more often and for a longer duration across all days than did UN (frequency, χ(1)2 = 19.54, p≤0.01; duration, χ(1)2 = 11.46, p≤0.01) and IS females (frequency only χ(1)2 = 4.20, p≤0.05). UN females also drank for a shorter time than did IS females across days (duration, χ(1)2 = 30.36, p≤0.01).
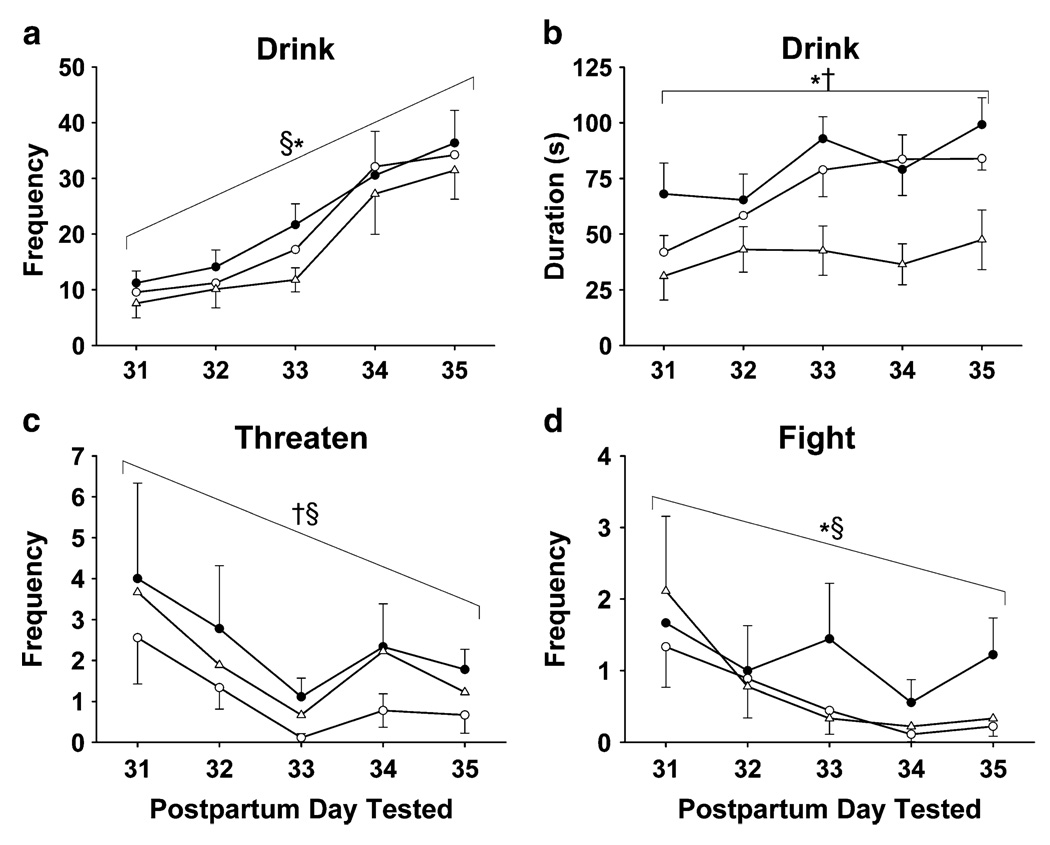
Fig. 3.
Intermittent cocaine treatment resulted in a higher frequency (a) and duration (b) of drinking than both UN (*p≤0.01, frequency and duration) and IS treated females (§p≤0.05, frequency only). Untreated females also drank less than IS females across all test days (†p≤0.01, duration). Females in the IC (§p≤0.01) and UN (†p≤0.02) groups threatened more frequently (c) than IS females across days. Females in the IC group fought more frequently (d) across test days than either UN (*p≤0.01) and IS females (§p≤0.01)
Push
Females in the IC group pushed more often across all days (not shown) than IS (χ(1)2 = 4.71, p≤0.05) and UN females during the final two test days (34: χ(1)2 = 5.63, p≤0.05; PPD 35: χ(1)2 = 4.73, p≤0.05).
Threat
Figure 3c illustrates that threats decreased across the first three test days with a slight rise over the final 2 days in all intermittent groups. Females in the IS threatened less frequently across all test days than did both IC- (χ(1)2 = 13.56, p≤0.01) and UN- (χ(1)2 = 5.80, p≤0.02) treated females.
Fight
Fight attacks decreased across test days for IS females and UN control females. Females in the IC group fought more (see Fig. 3d) frequently over the final 3 days than did IS (χ(1)2 = 11.10, p≤0.01) or UN (χ(1)2 = 10.44, p≤0.01) females.
Oxytocin levels
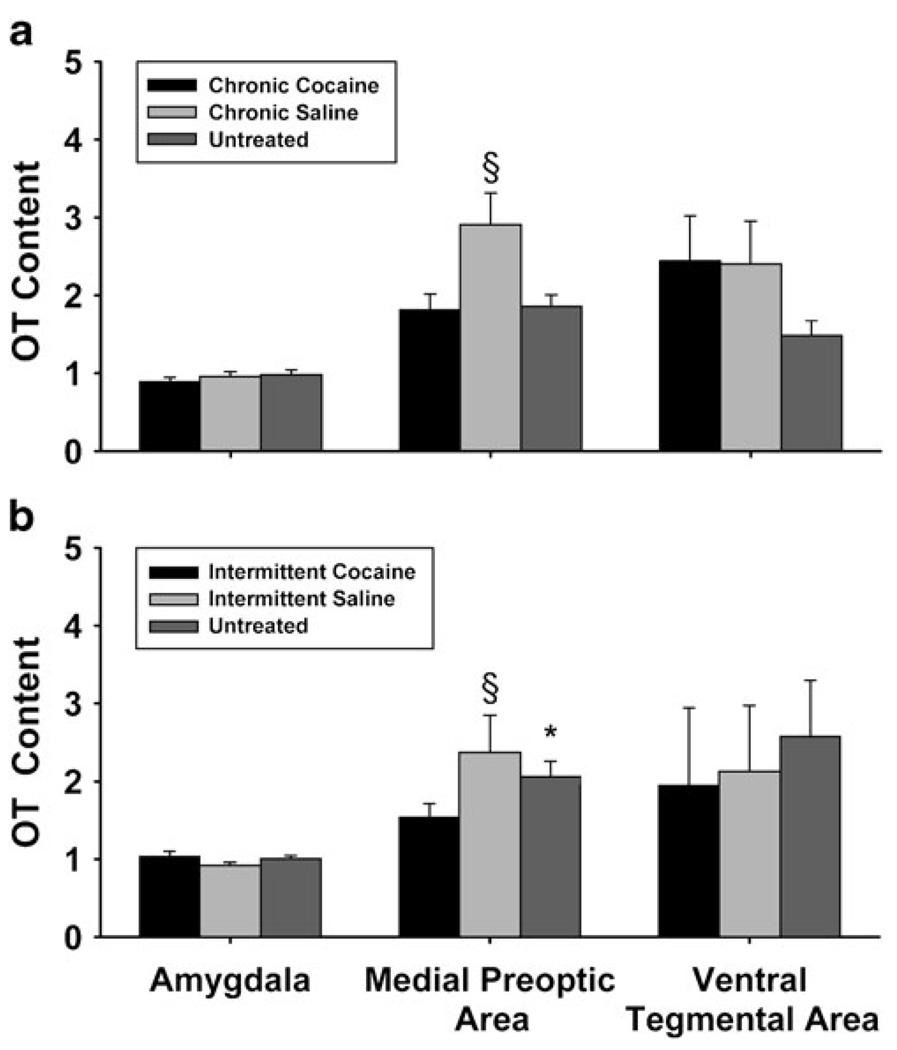
CC-treated females had significantly lower levels of OT in the MPOA (Fig. 4) compared to CS (χ2 = 7.94, p≤0.01) but not UN females and trends (p≤0.07) for lower OT levels in the AMY. There were no differences in the VTA. IC-treated females had lower OT levels in the MPOA (Fig. 4) compared to IS (χ(1)2 = 6.02, p≤0.01) and UN females (χ(1)2 = 4.01, p≤0.05), with no OT differences in the AMY or VTA.
Fig. 4.
Oxytocin levels (pg/mg/tissue) in the MPOA of the hypothalamus, AMY, and VTA of treatment and control females in chronic and intermittent triads on PPD 36. Females in the CC group a had lower levels of OT in the MPOA compared to CS females (§p≤0.01), while IC females b had lower levels of OT in the MPOA compared to IS (§p≤0.01) and UN (*p≤0.05) controls
Discussion
As hypothesized, both cocaine treatment regimens were associated with increased aggression and altered drinking patterns though behaviors were differentially expressed in the two groups. CC females were less aggressive than we predicted, as they exhibited primarily higher levels of threatening on several different days, while IC females displayed higher rates of fighting, pushing and threatening over much of the test period. We operationally defined dominance as a greater drinking success (time at the water spout actually drinking), a more traditional definition of dominance (Rowell 1974; Bernstein 1981; Benton 1982; Drews 1993). Usually, once dominance is established, it negates the need for excessive or continued fighting for access to limited biological resources. The continuous pattern of threats and aggression by both groups of cocaine-treated females suggest that they did not establish and maintain dominance using a typical pattern of responses.
CC-treated females were not more dominant, in that they did not drink more than controls, similarly to behavior of CC exposed male offspring in a water competition task (Wood and Spear 1998) who were more aggressive towards a conspecific but not more “dominant.” Whether they drank less because they were simply engaging in more aggressive behavior or were perhaps less motivated to drink is unknown, as this was not tested. Cocaine-induced changes in motivation for salient stimuli have been previously reported (Mattson et al. 2001).
Increased aggression by CC-treated females in this study is consistent with data from several previous reports of maternal aggression in lactating postpartum females (Johns et al. 1994b, 1998a) whereas lower aggression levels were seen in virgin cocaine-treated females in a resident intruder situation or in lactating females towards an inanimate object (Lubin et al. 2001b, 2003a). That rates of aggression were much lower in a competition task as compared to maternal aggressive behavior indicates that differences in various forms of aggressive behavior reflect cocaine’s interaction with the endocrine environment, stimulus salience, and test paradigm. Common to all these studies is a continued indication of cocaine-related patterns of unusual or atypical social or aggressive responses to other conspecifics or novel situations associated with regional OT system differences (Johns et al. 1994a, 1998b; Johns and Noonan 1995; Spear 1996; Wood and Spear 1998; Overstreet et al. 2000).
IC-treated females were both more “dominant” (as we defined it) and more aggressive. In a previous report (McMurray et al. 2008), IC-treated females displayed no effects on maternal aggression 2 days following cocaine treatment and were less aggressive towards an intruder when injected with cocaine 30 min prior to testing. In the present study, there was a longer post-injection period (10 days before testing). Without more data, we cannot determine if time since final cocaine injection was an important factor in aggression levels of IC-treated females. It is quite probable that differences in the testing paradigm (maternal versus non-maternal aggression) had a greater impact on our results in the present study.
The time course and differential display of behavior by CC versus IC females indicate both direct and indirect effects of cocaine treatment are likely. Other differences could include stress related effects, which may have affected IC and CC females differentially. A paradigm of repeated withdrawal, similar to intermittent treatment, has been hypothesized to induce differential effects in the stress response compared to chronic cocaine treatment (Breese et al. 2005). The role of stress may be particularly important given that psychological stress can dramatically alter social aggression and dominance patterns (Lucion and Vogel 1994; Wood et al. 2003; Mikics et al. 2004). Stress hormone levels, particularly corticosterone (CORT), have been associated with dominant animals (Popova and Naumenko 1972; Haller et al. 2000). Acute cocaine increases hypothalamic and extrahypothalamic corticotrophin-releasing factor, a trend reversed following chronic (daily) cocaine exposure. Less is known about the long-term effects of intermittent treatment. OT’s role in the stress response may also play a significant role in cocaine-related indirect effects on aggressive behavior (Uvnas-Moberg 1997; Ludwig 1998; Neumann 2002). Lower brain OT has been found to increase CORT in response to psychogenic stressors in females (Mantella et al. 2004) and in males that were water deprived (Mantella et al. 2005), suggesting multiples mechanisms through which OT may influence CORT responding.
Cocaine’s effects on the OT system continue to be correlated with various forms of aggression; the degree of effect probably dependent on many factors including type of aggression (Ferris et al. 1992; Lubin et al. 2003b; Johns et al. 2004; Bosch et al. 2005; McMurray et al. 2008). Acute, CC, and IC treatment have all been previously shown to alter OT levels in several different brain regions in lactating females, including the MPOA, HIPP, VTA, and AMY (Johns et al. 1993, 1997; Elliott et al. 2001). We predicted lower OT levels OT in the AMY of the most aggressive females but found only trends in the CC females with no effect in IC females. Instead, there were lower levels of OT in the MPOA of both groups of cocaine-treated females (see Fig. 3) suggesting that OT involvement in different forms of aggression may be region-specific. OT is also implicated in social recognition and “bonding”; thus, aggression levels could reflect altered OT signaling or social memory. Lower OT levels were previously found in dominant male rats while higher levels occurred in socially defeated males (Engelmann et al. 1999). Cocaine’s effects on social recognition have not been directly tested in pregnant, lactating, or post-weaning females in any species thus far.
There were limitations of the present study. We did not directly compare CC and IC females in this study on behavior given the test paradigm. Early postpartum maternal behavior differences in CC and IC females (Johns et al. 2005) were not expected to directly impact OT levels on PPD 36 as the test was long after cocaine injections and pup interactions (11 days IC, 31 CC), but this cannot be totally ruled out. The role of cocaine-induced changes in stress was also not directly addressed in this study though others have reported effects on these measures (Lucion and Vogel 1994; Wood et al. 2003; Mikics et al. 2004). The water competition task may also arguably be more a model for assessing resource negotiation or sociability but is not without validity and has been used in other dominance studies (Joly and Sanger 1991; Lucion and Vogel 1994; Wood and Spear 1998). The present findings indicate cocaine effects on dominance, aggression, and OT levels in post-lactation females that are both treatment and task dependent. Results highlight the need for further studies of aggression and social behavior in female models of substance abuse.
Acknowledgements
We would like to acknowledge the support of numerous undergraduate students who assisted with behavioral analyses our consultant Dr. Linda Spear and Abigail Jamieson-Drake for assistance in preparation of the manuscript. This study was funded by NIDA grants DA13362, DA13283, and 1PO1DA022446-01A2 to JMJ, and the NIH Federal Child Neglect Consortium.
Contributor Information
Josephine M. Johns, Email: jjohns@med.unc.edu, Department of Psychiatry, University of North Carolina, 430 Taylor Hall, CB# 7096, Chapel Hill, NC 27599, USA; Department of Psychology, University of North Carolina, Chapel Hill, NC, USA; Curriculum in Neurobiology, University of North Carolina, Chapel Hill, NC, USA; School of Medicine, University of North Carolina, Chapel Hill, NC, USA.
Matthew S. McMurray, Department of Psychology, University of North Carolina, Chapel Hill, NC, USA
Paul W. Joyner, Department of Surgery, University of Connecticut, Mansfield, CT, USA
Thomas M. Jarrett, School of Medicine, University of North Carolina, Chapel Hill, NC, USA
Sarah K. Williams, Curriculum in Neurobiology, University of North Carolina, Chapel Hill, NC, USA
Elizabeth T. Cox, Curriculum in Neurobiology, University of North Carolina, Chapel Hill, NC, USA
Mitchell A. Black, Department of Internal Medicine, Duke University, Durham, NC, USA
Christopher L. Middleton, Department of Neurosurgery, Medical College of Georgia, Augusta, GA, USA
Cheryl H. Walker, Department of Psychiatry, University of North Carolina, 430 Taylor Hall, CB# 7096, Chapel Hill, NC 27599, USA
References
- Benton D. Is the concept of dominance useful in understanding rodent behaviour? Aggress Behav. 1982;8:104–107. [Google Scholar]
- Bernstein IS. Dominance: the baby and the bathwater. Behav Brain Sci. 1981;4:419–457. [Google Scholar]
- Blanchard DC, Blanchard RJ. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neurosci Biobehav Rev. 1990;14:455–462. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Sartori SB, Singewald N, Neumann ID. Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress. 2007;10:261–270. doi: 10.1080/10253890701223197. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R. Biochemical basis of parental behavior in the rat. In: Rosenblatt JS, Snowdon CT, editors. Parental care: evolution, mechanisms, and adaptive significance. New York: New York Academic Press; 1996. pp. 215–242. [Google Scholar]
- Brody SL. Violence associated with acute cocaine use in patients admitted to a medical emergency department. NIDA Res Monogr. 1990;103:44–59. [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm Behav. 1994;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA, Lucion AB. Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav. 2005;85:354–362. doi: 10.1016/j.physbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Hadfield MG, Carter WH, Jr, Martin BR. Acute and chronic effects of cocaine on isolation-induced aggression in mice. Psychopharmacology (Berl) 1990;102:37–40. doi: 10.1007/BF02245741. [DOI] [PubMed] [Google Scholar]
- Drews C. The concept and definition of dominance in animal behavior. Behaviour. 1993;125:283–313. [Google Scholar]
- Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35:127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Golden J, Schumacher JA. Intimate partner violence and substance use: a longitudinal day-to-day examination. Addict Behav. 2003;28:1555–1574. doi: 10.1016/j.addbeh.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Feerick MM, Haugaard JJ, Hien DA. Child maltreatment and adulthood violence: the contribution of attachment and drug abuse. Child Maltreat. 2002;7:226–240. doi: 10.1177/1077559502007003005. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR. Oxytocin in the amygdala facilitates maternal aggression. In: Pedersen CA, Caldwell JD, Jirikowski GF, Insel TR, editors. Oxytocin in maternal, sexual, and social behaviors. New York: The New York Academy of Sciences; 1992. pp. 456–457. [DOI] [PubMed] [Google Scholar]
- Giordano AL, Siegel HI, Rosenblatt JS. Effects of mother-litter separation and reunion on maternal aggression and pup mortality in lactating hamsters. Physiol Behav. 1984;33:903–906. doi: 10.1016/0031-9384(84)90226-9. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Ann NY Acad Sci. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav. 1998;63:351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Hadfield MG. Cocaine: peak time of action on isolation-induced fighting. Neuropharmacology. 1982;21:711–713. doi: 10.1016/0028-3908(82)90015-6. [DOI] [PubMed] [Google Scholar]
- Hadfield MG, Nugent EA, Mott DE. Cocaine increases isolation-induced fighting in mice. Pharmacol Biochem Behav. 1982;16:359–360. doi: 10.1016/0091-3057(82)90172-1. [DOI] [PubMed] [Google Scholar]
- Haller J, Millar S, van de Schraaf J, de Kloet RE, Kruk MR. The active phase-related increase in corticosterone and aggression are linked. J Neuroendocrinol. 2000;12:431–436. doi: 10.1046/j.1365-2826.2000.00470.x. [DOI] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Hawley TL, Halle TG, Drasin RE, Thomas NG. Children of addicted mothers: effects of the ‘crack epidemic’ on the caregiving environment and the development of preschoolers. Am J Orthopsychiatry. 1995;65:364–379. doi: 10.1037/h0079693. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicol Teratol. 1992;14:415–421. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- Hutchinson RR, Emley GS, Krasnegor NA. The effects of cocaine on the aggressive behavior of mice, pidgeons and squirrel monkeys. In: Ellinwood EH, Kilby MM, editors. Cocaine and other stimulants. New York: Plenum; 1977. pp. 457–480. [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides. 2006;40:161–167. doi: 10.1016/j.npep.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR. Prenatal cocaine exposure affects social behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1995;17:569–576. doi: 10.1016/0892-0362(95)00017-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Anderson DR, Bass EW, Means LW, McMillen BA. Prenatal exposure to cocaine: II. Effects on open field activity and cognitive behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1992a;14:343–349. doi: 10.1016/0892-0362(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Means LW, McMillen BA. Prenatal exposure to cocaine: I. Effects on gestation, development and activity in Sprague-Dawley rats. Neurotoxicol Teratol. 1992b;14:337–342. doi: 10.1016/0892-0362(92)90040-h. [DOI] [PubMed] [Google Scholar]
- Johns JM, Caldwell JD, Pedersen CA. Acute cocaine treatment decreases oxytocin levels in the rat hippocampus. Neuropeptides. 1993;24:165–169. doi: 10.1016/0143-4179(93)90081-k. [DOI] [PubMed] [Google Scholar]
- Johns JM, Means MJ, Bass EW, Means LW, Zimmerman LI, McMillen BA. Prenatal exposure to cocaine: effects on aggression in Sprague-Dawley rats. Dev Psychobiol. 1994a;27:227–239. doi: 10.1002/dev.420270405. [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci. 1994b;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides. 1997;31:439–443. doi: 10.1016/s0143-4179(97)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998a;20:525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, McMillen BA, Means LW, Walker CH, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and in their prenatally exposed offspring. Ann NY Acad Sci. 1998b;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Joyner P, Middleton C, Hofler V, McMurray M. Gestational treatment with cocaine and fluoxetine alters oxytocin receptor number and binding affinity in lactating rat dams. Int J Dev Neurosci. 2004;22:321–328. doi: 10.1016/j.ijdevneu.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Elliott DL, Hofler VE, Joyner PW, McMurray MS, Jarrett TM, Haslup AM, Middleton CL, Elliott JC, Walker CH. Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav Neurosci. 2005;119:1605–1618. doi: 10.1037/0735-7044.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly D, Sanger DJ. Social competition in rats: a test sensitive to acutely administered anxiolytics. Behav Pharmacol. 1991;2:205–213. [PubMed] [Google Scholar]
- Licata A, Taylor S, Berman M, Cranston J. Effects of cocaine on human aggression. Pharmacol Biochem Behav. 1993;45:549–552. doi: 10.1016/0091-3057(93)90504-m. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Meter KE, Walker CH, Johns JM. Dose-related effects of chronic gestational cocaine treatment on maternal aggression in rats on postpartum days 2, 3, and 5. Prog Neuropsychopharmacol Biol Psychology. 2001a;25:1403–1420. doi: 10.1016/s0278-5846(01)00197-x. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Meter KE, Walker CH, Johns JM. Effects of chronic cocaine administration on aggressive behavior in virgin rats. Prog Neuropsychopharmacol Biol Psychol. 2001b;25:1421–1433. doi: 10.1016/s0278-5846(01)00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Cannon JB, Black MC, Brown LE, Johns JM. Effects of chronic cocaine on monoamine levels in discrete brain structures of lactating rat dams. Pharmacol Biochem Behav. 2003a;74:449–454. doi: 10.1016/s0091-3057(02)01027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003b;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucion A, Vogel WH. Effects of stress on defensive aggression and dominance in a water competition test. Integr Physiol Behav Sci. 1994;29:415–422. doi: 10.1007/BF02691361. [DOI] [PubMed] [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1494–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Amico JA. Corticosterone release is heightened in food or water deprived oxytocin deficient male mice. Brain Res. 2005;1058:56–61. doi: 10.1016/j.brainres.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in Sprague-Dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32:131–136. [PubMed] [Google Scholar]
- McMurray MS, Joyner PW, Middleton CW, Jarrett TM, Elliott DL, Black MA, Hofler VE, Walker CH, Johns JM. Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress. 2008;11:398–410. doi: 10.1080/10253890701850239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl) 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Mikics E, Kruk MR, Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology. 2004;29:618–635. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- Miller NS, Gold MS, Mahler JC. Violent behaviors associated with cocaine use: possible pharmacological mechanisms. Int J Addict. 1991;26:1077–1088. doi: 10.3109/10826089109058942. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Numan M. Maternal behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven; 1994. pp. 221–301. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Overstreet DH, Moy SS, Lubin DA, Gause LR, Lieberman JA, Johns JM. Enduring effects of prenatal cocaine administration on emotional behavior in rats. Physiol Behav. 2000;70:149–156. doi: 10.1016/s0031-9384(00)00245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani S, Palanza P, Rogers J, Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23:957–969. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Paul L, Gronek J, Politch J. Maternal aggression in mice: protection of young is a by-product of attacks at the home site. Aggress Behav. 1981;6:19–29. [Google Scholar]
- Popova NK, Naumenko EV. Dominance relations and the pituitary-adrenal system in rats. Anim Behav. 1972;20:108–111. doi: 10.1016/s0003-3472(72)80179-9. [DOI] [PubMed] [Google Scholar]
- Rowell TE. The concept of social dominance. Behav Biol. 1974;11:131–154. doi: 10.1016/s0091-6773(74)90289-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of the effects of developmental toxicants: pharmacological and stress vulnerability of offspring. NIDA Res Monogr. 1996;164:125–145. [PubMed] [Google Scholar]
- Stern JM. Maternal behavior: sensory, hormonal, and neural determinants. In: Brush FR, Levine S, editors. Psychoendocrinology. New York: Academic; 1989. pp. 105–226. [Google Scholar]
- Tyler R, Howard J, Espinosa M, Doakes SS. Placement with substance-abusing mothers vs. placement with other relatives: infant outcomes. Child Abuse Negl. 1997;21:337–349. doi: 10.1016/s0145-2134(96)00175-5. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin linked antistress effects—the relaxation and growth response. Acta Physiol Scand Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996;110:315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- Wasserman DR, Leventhal JM. Maltreatment of children born to cocaine-dependent mothers. Am J Dis Child. 1993;147:1324–1328. doi: 10.1001/archpedi.1993.02160360066021. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Shapiro L, Carter CS, Insel TR. Oxytocin and complex social behavior: species comparisons. Psychopharmacol Bull. 1993;29:409–414. [PubMed] [Google Scholar]
- Wolff J, Peterson JA. An offensive-defensive hypothesis for territoriality in female mammals. Ethol Ecol Evol. 1998;10:227–239. [Google Scholar]
- Wood RD, Spear LP. Prenatal cocaine alters social competition of infant, adolescent, and adult rats. Behav Neurosci. 1998;112:419–431. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43:205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]