Abstract
Objective To present a case study using multisystemic therapy (MST), an intensive family focused psychotherapy. For the clinical trial from which this case was drawn, MST was adapted to address multiple human immunodeficiency virus (HIV) transmission risk behaviors in HIV-infected youth. Targeted behaviors included medication nonadherence, risky sexual behaviors, and substance use. Method One young woman's transmission risk behaviors are described, followed by a description of the MST procedures used to identify and treat the primary drivers of these risk behaviors. Outcome measures were self-report, urine screens, and blood draws. Results At discharge, the young woman showed significant improvements in medication adherence and related health status (e.g., reduced HIV viral load), healthier sexual behaviors, and reduced substance use. Importantly, neither her boyfriend nor her newborn tested positive for HIV. Conclusions Findings from this case study suggest that MST has the potential to reduce transmission risk behaviors among teens with HIV.
Keywords: Adolescence, HIV, multisystemic therapy.
This case study examines the feasibility of using multisystemic therapy (MST; Henggeler, Schoenwald, Borduin, Rowland, & Cunningham, 1998) to address transmission risk behaviors of adolescents with human immunodeficiency virus (HIV). The most common risk factor for HIV transmission in adolescents is risky sexual behavior (Futterman, Chabon, & Hoffman, 2000). A second significant risk factor for transmission is drug use which increases the risk of unprotected or other risky sexual behaviors and, consequently, for acquiring and transmitting HIV (Strunin & Hingson, 1992). Medication non-adherence represents a third transmission risk for HIV. HIV medication regimens can be complex, requiring high pill burdens and complicated dosing regimens (Guarinieri & ICoNA Community Advisory Board, 2002). Even less complex regimens are unrelenting in requiring daily medications. Strict adherence (i.e., <5% missed doses) is required to achieve and maintain virologic control, yet few young people reach this level of adherence (Murphy et al., 2003). Consequences of poor adherence include increased risk of HIV transmission in the event of unprotected sex (Hosseinipour, Cohen, Vernazza, & Kashuba, 2002). Development of comprehensive programs that can simultaneously address these common transmission risk behaviors is a research and clinical priority (NIH, 2004).
Youth HIV transmission risk behaviors tend to have common drivers that coincide with Bronfenbrenner's (1979) theory of social ecology. This theory posits that human behavior is influenced directly or indirectly by the individual, family, peer, and community systems in which an individual is embedded. For example, at the “individual level,” unsafe sexual behaviors and drug use behaviors have been associated with depression and anxiety (Murphy et al., 2001) while low medication adherence has been associated with an unwillingness to disclose HIV status to others (Mellins et al., 2004). At the “family level,” all three transmission risk behaviors have been associated with caregiver substance use (Naar-King et al., 2006) and low parental monitoring (Duncan, Duncan, & Stryker, 2000; Donenberg, Wilson, Emerson, & Bryant, 2002; Murphy et al., 2003; Mellins et al., 2004). At the “peer level,” associating with risk-taking and deviant peers is one of the strongest correlates of sexual risk taking behavior and drug use in youth (Madison, McKay, Paikoff, & Bell, 2000; Bachanas et al., 2002; Voisin, 2002), while low medication adherence is associated with negative censure from peers (Dodds et al., 2003). At the “community level,” unsafe sexual behavior has been associated with community level violence (Voisin, 2002); drug use has been associated with high mobility and disorganization, low community support, and presence of a criminal subculture (for reviews, see Henggeler, 1997); and low medication adherence has been associated with poor relationships between health care providers and caregivers and youth (Ingersoll & Heckman, 2005). While drivers co-occur across behaviors, the specific pattern presents differently for individual youth. Thus, effective strategies for reducing youth transmission risk behaviors require attention to the complex interplay of multiple, interacting causal risk factors and benefit from an individualized intervention focus (Wechsler et al., 1998; Chesney et al., 1999; Barnett et al., 2001; Steele & Grauer, 2003). Yet few interventions attempt to comprehensively reduce HIV transmission risk behaviors by targeting multiple risk factors across ecological levels on an individualized basis. Rather, available interventions for pediatric transmission risk behaviors are typically standardized across patients and frequently target just one or two risk behaviors.
One evidenced-based intervention that targets the diverse drivers of youth problem behaviors within youths’ ecological contexts is MST. MST is an intensive, home- and community-based intervention that has been identified as an evidenced-based intervention for juvenile delinquency (US Department of Health and Human Services, 2001) and juvenile substance abuse (NIDA, 1999). MST therapists are bachelors or masters-level professionals who work on teams composed of two to three therapists and a supervisor. Services are delivered in youths’ homes and other community (e.g., clinic, school) settings at times convenient to families and therapists are available to respond to crises 24 hrs per day. Therapists carry small caseloads of 4–6 families as a result of the intensive and individualized nature of MST. Rather than providing session-by-session protocols for clinical procedures, nine “treatment principles” are used to guide therapists’ case conceptualizations, prioritization of interventions, and implementation of intervention strategies. The overriding goals of MST are to empower parents with the skills and resources needed to address the inevitable difficulties that arise in raising adolescents and to empower adolescents to cope with familial and extrafamilial problems.
MST is conceptually an excellent fit for intervening with transmission risk behaviors due to its focus upon multiple systems that influence youth behavior. Recently, MST was successfully adapted to address medication nonadherence in youth with poorly controlled type 1 diabetes (Ellis et al., 2005) and in youth with HIV (Ellis, Naar-King, Cunningham, & Secord, 2006). Case studies that emerged from these projects identified MST's focus on behavioral drivers across ecological systems as key to improved adherence outcomes (Ellis, Naar-King, Frey, Rowland, Gregor, 2003; Cunningham, Naar-King, Ellis, Pejuan, & Secord, 2006). The present “explanatory” case study (see Drotar, La Greca, Lemanek, & Kazak, 1995, p. 551) is the first to present MST as adapted to address medication nonadherence as well as other transmission risk behaviors including substance use, and sexual risk behaviors.
Patient Presentation
Presenting Information
“Sara,” a 17-year-old Caucasian young woman diagnosed with perinatally acquired HIV, was recruited into a randomized clinical trial piloting the use of MST to address HIV transmission risk behaviors. Sara's case was selected for presentation because she presented with all three risk behaviors targeted by the MST intervention. At study entry, Sara was 2 months pregnant (per HIV clinic and self report), on an extended clinic-ordered medication break due to medication nonadherence (per clinic report), reported frequent marijuana use (per self-report and confirmed by a urine drug test), and had stopped attending school (per caregiver and youth report and confirmed by youth's school). She also was on probation for a prior truancy charge. In the previous year, Sara's viral load varied from 1,033–39,000 copies/ml (per clinic chart review). The clinic-ordered medication break predated Sara's pregnancy and clinic staff were anxious for Sara to resume antiretroviral medications. The goal of antiretroviral treatment (ART) for Sara, especially during pregnancy, was to suppress the viral copy number to as low a level as possible, preferably to one that was undetectable. Current ART guidelines reflect the knowledge that high viral loads are associated with progression of disease, and with both horizontal and vertical transmission (Perinatal HIV Guidelines Working Group, 2009). The MST intake assessment indicated that, in addition to these behavior problems, Sara had a volatile relationship with her mother and had struggled with depression since her preteen years. While not actively suicidal at study entry, Sara had been admitted to inpatient settings for threatening or attempting suicide on three occasions in the preceding 3 years. Sara had minimal contact with her father, who was drug dependent and had been violent with her mother when they still were married.
MST intervention goals were developed based on initial intake information, family priorities, and clinic goals. These “overarching goals” included (a) improved medication adherence as evidenced by undetectable viral load, (b) decreased sexual risk behaviors as evidenced by demonstrated knowledge of condom usage and an effective postnatal birth control plan, (c) decreased substance use as evidenced by clean urine screens, and (4) improved parenting (of Sara by her mother) as evidence by increased parental supervision and monitoring and consistent application of rewards and consequences linked to these intervention goals. As is typical for MST, the intervention was provided primarily in Sara's home although the therapist also attended Sara's clinic appointments and met with other stakeholders (e.g., school officials, probation officer) as needed throughout MST.
Understanding the “Fit” of Sara's Behavior Problems
At the start of the intervention MST therapists conduct an “assessment of fit” to identify factors that directly or indirectly influence the problem behavior (Henggeler et al., 1998, p. 24). As a staring point, the therapist assesses the influence of factors identified in the empirical literature as causative for the problem behavior (e.g., low parental supervision and monitoring is known to influence medication adherence) and also identifies idiosyncratic factors relevant to the specific individual. When conducting fit assessments, MST therapists rely primarily on interviews with key informants, and behavioral observations. In Sara's case, semi-structured interviews were conducted with Sara and her mother, Sara's boyfriend, and members of the HIV clinic team responsible for Sara's care. Therapists also gather data based on direct observation of behavior and the interactions among family members. Based on these sources, Sara's MST therapist identified the specific combination of behavioral “fit factors” that were maintaining her nonadherence, sexual risk, and substance use behaviors. This is in contrast to more traditional assessment methods (e.g., standardized questionnaires) that might assess for common drivers of behavior (e.g., lack of consistent schedule for medication taking) but miss less common or idiosyncratic factors that influence the behavior of a specific youth (e.g., presence of a boyfriend who supplies marijuana, use of which can interfere with medication taking as discussed next).
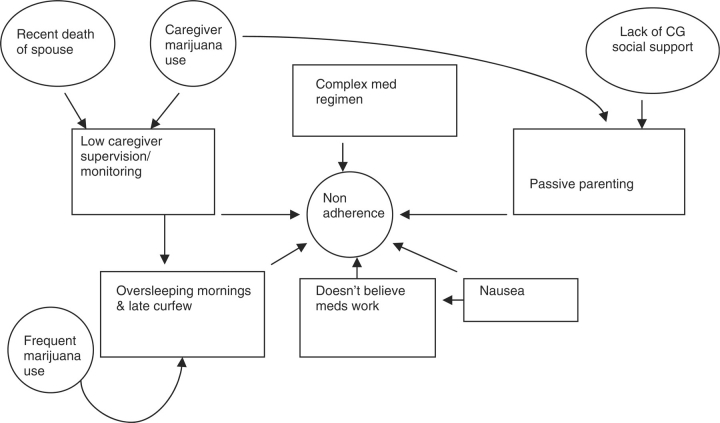
The MST assessment of medication nonadherence identified several fit factors at the individual level including Sara's medication-related nausea, her pattern of going to bed late and sleeping in most mornings (which interfered with routine medication dosings), and her belief that medications were ineffective. Family level factors included low caregiver supervision and monitoring of medication taking and a permissive parenting style which contributed to a lack of limit setting with regard to medication adherence. Sara's mother's permissive parenting was due, in part, to her feelings of guilt surrounding transmitting HIV to her daughter and also because she had been advised by physicians that Sara would die as an infant due to AIDS. Sara's mother's own HIV status and medication adherence (which had improved in recent years) did not otherwise contribute to drivers for Sara's poor adherence. Additionally, while her boyfriend knew Sara's HIV status, Sara had not disclosed her status to his parents and grandparents with whom they often spent the night, making it difficult for Sara to take her medications due to concealment. Previous regimen complexity was also found to be a contributing factor. As depicted in Figure 1, these more proximal drivers (indicated by boxes) were influenced by factors that indirectly influenced Sara's medication adherence (indicated by circles). For example, Sara's marijuana use (indirect driver) influenced her sleeping habits (direct driver) while Sara's mother's depression (indirect driver) influenced parental supervision and monitoring (direct drivers).
Figure 1.
Fit for poor adherence. Notes. “med” and “meds” refer to medications; “CG” refers to caregiver.
Several drivers also were identified for Sara's sexual risk behaviors. At the individual level, Sara's sexual risk taking was influenced by her marijuana use and her unease with and lack of knowledge about the proper use of condoms. At the family level, Sara was permitted a significant amount of unsupervised time with her boyfriend. At the family and peer levels, teen pregnancy was modeled by several family members and friends. For example, Sara's mother was 19-years-old when she gave birth to Sara's older brother; Sara's older brother also had a child at the age of 19 years. An indirect driver of Sara's sexual risk taking was her boyfriend's marijuana use.
Regarding Sara's marijuana use, individual level drivers included Sara's boredom and her belief that marijuana reduced her morning sickness. At the family level, alcohol and marijuana use were modeled by several family members (including Sara's parents) and tacitly permitted by her mother. At the peer level, Sara's boyfriend used and supplied marijuana. Indirect drivers of these main factors included being out of school (which contributed to boredom) and Sara's pre-natal nausea (relief from which she attributed to marijuana).
Following the identification of behavioral drivers, interventions were identified to target the primary drivers for each transmission risk behavior. Interventions were typically delivered concurrently although the following description presents the interventions sequentially.
Medication Nonadherence
Given the risk of vertical transmission of HIV from Sara to her fetus and horizontal transmission to her boyfriend in the event of additional unprotected intercourse, improved medication adherence was prioritized. High levels of adherence are necessary to achieve the goals of antiretroviral therapy, particularly during pregnancy, when there is the additional goal of preventing transmission of HIV to the infant (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2008). When ART is instituted for prevention of vertical transmission, treatment is generally started by the beginning of the second trimester of pregnancy (Perinatal HIV Guidelines Working Group, 2009).
To address Sara's belief that medication was unhelpful, the MST therapist provided education on the effectiveness of HIV medications and arranged several meetings between Sara, her mother, and external systems (e.g., HIV clinic staff, Sara's obstetrician) to further address the risks of nonadherence to both Sara and her fetus. The therapist used family therapy interventions (e.g., changing discipline strategies) to empower the mother to take appropriate charge of Sara's medication regimen, including directly observing pill taking and tying rewards and consequences to Sara's behavior of taking medications in front of her mother and to viral load test results. The therapist also helped the mother recruit instrumental support from friends and family members specifically around Sara's medication monitoring. Because the mother's depression negatively influenced her ability to actively parent Sara, another intervention addressed maternal depression via empirically supported interventions (cognitive behavior therapy; Whitfield & Williams, 2003). Toward the end of therapy, Sara's status was disclosed to her boyfriend's family (by a cousin, with Sara's consent), making it easier for Sara to maintain adherence when spending time with her boyfriend's family.
Sara initially refused to permit her mother to observe medication taking or the MST therapist to conduct pill counts. Consequently, weekly pill counts were inconclusive for the first month of MST. Sara responded positively, however, to a behavior modification plan that included specific rewards for taking medications when observed by her mother. By the fourth week of MST, pill counts indicated 70–80% adherence and this increased to between 93 and 100% during the final 3 months of MST. This improved adherence quickly translated into reduced viral load, which was undetectable after the first month of MST. Sara's CD4 T-cell count remained >400 prior to and throughout MST. The CD4 cell number is the major marker of immune function in HIV infection, with a count <200 indicating severe immune deficiency and a count ≥500 considered normal in adults (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2008).
Sexual Risk Behaviors
The MST intervention to reduce sexual risk taking included educating Sara, her boyfriend, and her mother about the risks of transmission to others as well as Sara's risk of acquiring sexually transmitted infections (STIs) and new HIV strains (e.g., from new partners). The intervention quickly focused on engaging Sara's boyfriend around the goal of obtaining HIV counseling and testing. Sara and boyfriend and mother participated in condom use skills training, which included practical condom use skills demonstrations, education on HIV/STI prevention, and prevention of unplanned pregnancies. Barriers to obtaining condoms and substance use as a driver of unsafe sex also were addressed. The MST therapist also facilitated an appointment between Sara and her obstetrician to develop a post-natal birth control strategy.
Sara and her family were easily engaged around the goal of reduced sexual risk, due largely to factors associated with Sara's current pregnancy (e.g., severe nausea, anxiety regarding ability to support an infant) and the desire to avoid future unplanned pregnancies. Over the course of MST Sara overcame barriers to obtaining condoms, as evidenced by being able to produce condoms upon request. She was able to demonstrate correct condom use using the condom model and to distinguish between effective disease prevention (i.e., understanding continued need for ongoing condom use) and pregnancy prevention (i.e., understanding continued need for primary birth control methods more effective than condoms). On the day after delivering her baby, Sara began an interim regimen of oral contraceptives, with a plan to switch to an implanted contraceptive when medically indicated.
Substance Use
Several barriers initially presented around the goal of reducing Sara's marijuana use. For example, Sara and her mother believed marijuana to be innocuous and Sara's mother was concerned that her own social life would be negatively impacted if she enforced “no drinking or smoking” rules in her home. The MST therapist was able to establish engagement around this intervention goal by presenting information on state laws that permit removing infants from mothers who test positive for illicit substances during pregnancy, engaging obstetricians and genetic counselors (who already were part of Sara's obstetric team due to genetic risk markers identified for the fetus) who presented information to Sara and her boyfriend regarding possible fetal consequences of marijuana use, and by working with Sara's probation officer to identify possible consequences if she tested positive during a probation-ordered drug screen. After achieving Sara's engagement to reduce substance use, interventions were developed, including (a) engaging Sara's mother around reducing her own drug use and implementing rules that forbade marijuana use in the home; (b) engaging Sara's boyfriend to eliminate his use; (c) utilizing a contingency management plan with Sara in which she could earn points toward rewards for clean urine drug screens; (d) teaching Sara drug refusal skills, and (e) identifying prosocial peers and activities to reduce boredom triggers for use.
Sara's mother made some initial changes to support Sara's reduced use (e.g., implemented new household rules, provided rewards for clean screens) but did not sustain these changes. Despite these set-backs, Sara achieved her first clean drug screen in the fifth month of MST and provided two subsequent (within-therapy) clean screens as did her boyfriend.
MST Outcome Summary
Sara and her mother were involved in MST for 27 weeks and received approximately 3.5 hours of MST services per week. The number of sessions varied by week but occurred more often early in MST, with decreasing frequency as Sara and her mother were empowered to make and maintain intervention gains. Most sessions were conducted in Sara's home. Additionally, the MST therapist conducted sessions with other family members and with other stakeholders (e.g., clinic staff, probation officer) in other settings. Treatment progress was slow at first, with the therapist mediating between frequent family arguments and Sara missing sessions or unwilling to address treatment goals. The MST therapist's persistence and willingness to work with Sara notwithstanding these negative behaviors eventually resulted in strong treatment engagement. Despite the number and complexity of Sara's multiple behavioral problems, she achieved remarkable within-intervention successes that translated to real reductions in transmission risks, as well as other important gains. Sara's mean viral load across four pre-intervention tests was 12,788 copies/ml (mean log10 = 4.11). Each of her four within-treatment viral load tests indicated nondetectible viral loads. Sara's boyfriend tested negative for HIV and her infant was delivered at full term without HIV or other health problems and with significantly less exposure to illicit substances than might have been the case in the absence of MST.
Discussion
A myriad of factors contribute to adolescent HIV transmission risk behaviors and these vary by person, thus requiring a highly flexible and individualized intervention approach. Interventions that focus on only one transmission risk behavior or on a limited subset of behavioral drivers are less likely to reduce a youth's overall transmission risk than a more comprehensive approach. Based on the positive results from this case study, MST appears to warrant additional investigation as a means of averting serious health problems in this high-risk population of young people. Determining whether within-intervention behavioral changes extend beyond the end of MST—a principle goal of MST—is an important aim of the pilot trial and future research.
As noted, MST is an intensive intervention. Family engagement is enhanced by the availability and persistence of MST therapists in the event of missed sessions or other barriers to intervention participation (Cunningham & Henggeler, 1999). In addition to improved family engagement, the home-based delivery model offers other advantages over office-based sessions, including more thorough and reliable assessment information (e.g., given therapists’ ability to witness behaviors and interactions in natural contexts) and improved maintenance and generalizability of newly acquired skills (e.g., due to practicing new skills in natural contexts). These strengths of the MST model also increase the immediate cost of care, relative to group-based or other less intensive interventions and potentially increase barriers to “uptake” by providers whose practices differ significantly from MST (Schoenwald & Hoagwood, 2001). However, fiscal cost reductions have been supported in trials of MST with other clinical (non-HIV) populations. These include youth with poorly controlled type 1 diabetes, high-risk juvenile offenders, and youth with substance use disorders. Regarding youth with poorly controlled type 1 diabetes, youth in the MST condition had a significant decrease in the number of hospital admissions over a 9-month post-referral period compared to youth in the control condition and this reduction was correlated with improved metabolic control (Ellis et al., 2005). Direct costs for the MST and usual services groups were equivalent prior to study entry, whereas direct costs dropped 68% during the study period for the MST group, and nearly doubled for the usual services group. Regarding high-risk juvenile offenders, a comprehensive economic evaluation of juvenile crime prevention and intervention programs reported that treating high-risk juvenile offenders with MST resulted in a net gain or savings to the state of $21,863 per youth (Aos, Phipps, Barnoski, & Lieb, 1999). Regarding substance abusing or dependent youth, investigators reported that, relative to youth treated by usual services, youth treated in the MST condition evidenced significant reductions in incarceration and inpatient days across a 12-month post-referral time period, resulting in an incremental cost of MST of $877/youth (Schoenwald, Ward, Henggeler, Pickrel, & Patel, 1996). The combined evidence of clinical and cost effectiveness has compelled some payers to fund MST for juvenile delinquency and youth substance abuse (e.g., there is a separate federal Medicaid billing code for MST services). There currently are no funding mechanisms in place for addressing HIV transmission risk behaviors with MST. Future research goals include conducting a larger randomized trial that will include cost effectiveness analyses. If research supports the clinical and cost effectiveness of MST as an intervention for HIV transmission risk behaviors, there is an existing platform (i.e., 450 MST sites in the USA and 11 other countries) upon which to launch the intervention.
Funding
This study was supported by funding from the National Institute of Mental Health, R34 077550. (R34 077550 to E.J.L., PI).
Conflict of interest: Phillippe B. Cunningham, Deborah Ellis, and Sylvie Naar-King are co-owners of Evidence Based Services, Inc., an organization that provides training and consultation in MST.
References
- Aos S, Phipps P, Barnoski R, Lieb R. The comparative costs and benefits of programs to reduce crime: A review of national research findings with implications for Washington State, State Version 3.0. Olympia, WA: Washington State Institute for Public Policy; 1999. [Google Scholar]
- Bachanas PJ, Morris MK, Lewis-Gess JK, Sarett-Causay EJ, Flores AL, Sirl KS, et al. Psychological adjustment, substance use, HIV knowledge, and risky behavior in at-risk minority females: Developmental differences during adolescence. Journal of Pediatric Psychology. 2002;27:373–384. doi: 10.1093/jpepsy/27.4.373. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Monti PM, Wood MD. Motivational interviewing for alcohol-involved adolescents in the emergency room. In: Wagner EF, Waldron HB, editors. Innovations in adolescent substance abuse intervention. Kidlington, Oxford, UK: Elsevier Science; 2001. [Google Scholar]
- Bronfenbrenner U. The ecology of human development: Experiments by nature and design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Chesney MA, Ickovics J, Hecht FM, Sikipa G, Rabkin J. Adherence: a necessity for successful HIV combination therapy. AIDS. 1999;13:271–278. [PubMed] [Google Scholar]
- Cunningham PB, Henggeler SW. Engaging multiproblem families in treatment: Lessons learned throughout the development of Multisystemic Therapy. Family Process. 1999;38:265–281. doi: 10.1111/j.1545-5300.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Cunningham PB, Naar-King S, Ellis DA, Pejuan S, Secord E. Achieving adherence to antiretroviral medications for pediatric HIV disease using an empirically supported treatment: A case report. Developmental and Behavioral Pediatrics. 2006;27:44–50. doi: 10.1097/00004703-200602000-00009. [DOI] [PubMed] [Google Scholar]
- Dodds S, Blakley T, Lizzotte JM, Friedman LB, Shaw K, Martinez J, et al. Retention, adherence, and compliance: Special needs of HIV-infected adolescent girls and young women. Journal of Adolescent Health. 2003;33:39–45. doi: 10.1016/s1054-139x(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Donenberg GR, Wilson HW, Emerson E, Bryant FB. Holding the line with a watchful eye: The impact of perceived parental permissiveness and parental monitoring on risky sexual behavior among adolescents in psychiatric care. AIDS Education and Prevention. 2002;14:138–157. doi: 10.1521/aeap.14.2.138.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotar D, La Greca AM, Lemanek K, Kazak A. Case reports in pediatric psychology: Uses and guidelines for authors and reviewers. Journal of Pediatric Psychology. 1995;20:549–565. doi: 10.1093/jpepsy/20.5.549. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Strycker LA. Risk and protective factors influencing adolescent problem behavior: a multivariate latent growth curve analysis. Annals of Behavioral Medicine. 2000;22:103–109. doi: 10.1007/BF02895772. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham PB, Cakan N. The effects of multisystemic therapy on diabetes stress in adolescents with chronically poorly controlled type I diabetes: Findings from a randomized controlled trial. Pediatrics. 2005;116:e826–e832. doi: 10.1542/peds.2005-0638. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Cunningham PB, Secord E. Use of multisystemic therapy to improve antiretroviral adherence and health outcomes in HIV-infected pediatric patients: Evaluation of a pilot program. AIDS, Patient Care, and STD’s. 2006;20:112–121. doi: 10.1089/apc.2006.20.112. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Frey MA, Rowland M, Greger N. Case study: Feasibility of multisystemic therapy as a treatment for urban adolescents with poorly controlled Type 1 diabetes. Journal of Pediatric Psychology. 2003;28:287–293. doi: 10.1093/jpepsy/jsg017. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Naar-King S, Frey M, Templin T, Rowland MD, Cakan N. Multisystemic treatment of poorly controlled Type 1 diabetes: Effects on medical resource utilization. Journal of Pediatric Psychology. 2005;30:656–666. doi: 10.1093/jpepsy/jsi052. [DOI] [PubMed] [Google Scholar]
- Futterman D, Chabon B, Hoffman ND. HIV and AIDS in adolescents. Pediatric Clinics of North America. 2000;47:171–188. doi: 10.1016/s0031-3955(05)70200-5. [DOI] [PubMed] [Google Scholar]
- ICoNA Community Advisory Board. Guarinieri M. Highly active antiretroviral therapy adherence: the patient's point of view. Journal of Acquired Immune Deficiency Syndromes. 2002;31(Suppl 3):S167–S169. doi: 10.1097/00126334-200212153-00017. [DOI] [PubMed] [Google Scholar]
- Henggeler SW. The development of effective drug abuse services for youth. In: Egertson JA, Fox DM, Leshner AI, editors. Treating drug abusers effectively. New York: Blackwell; 1997. pp. 253–279. [Google Scholar]
- Henggeler SW, Schoenwald SK, Borduin CM, Rowland MD, Cunningham PB. Multisystemic treatment of antisocial behavior in children and adolescents. New York: Guilford Press; 1998. [Google Scholar]
- Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD. Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1? Clinical Infectious Diseases. 2002;34:1391–1395. doi: 10.1086/340403. [DOI] [PubMed] [Google Scholar]
- Hutchinson MK, Jemmott JB, Jemmott LS, Braverman P, Fong GT. The role of mother–daughter sexual risk communication in reducing sexual risk behaviors among urban adolescent females: A prospective study. Journal of Adolescent Health. 2003;33:98–107. doi: 10.1016/s1054-139x(03)00183-6. [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, Heckman CJ. Patient–clinician relationships and treatment system effects on HIV medication adherence. AIDS Behavior. 2005;9:89–101. doi: 10.1007/s10461-005-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison SM, McKay MM, Paikoff R, Bell C. Basic research and community collaboration: Necessary ingredients for the development of a family-based HIV prevention program. AIDS Education and Prevention. 2000;12:281–298. [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Dolezal C, Abrams EJ. The role of psychosocial and family factors in adherence to antiretroviral treatment in Human Immunodeficiency Virus-infected children. The Pediatric Infectious Disease Journal. 2004;23:1035–1041. doi: 10.1097/01.inf.0000143646.15240.ac. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Durako SJ, Moscicki A, Vermund SH, Ma Y, Schwarz DF, et al. Adolescent Medicine HIV/AIDS Research Network No change in health risk behaviors over time among HIV infected adolescents in care: Role of psychological distress. Journal of Adolescent Health. 2001;29S:57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- Murphy D, Sarr M, Durako S, Mosciki A, Wilson C, Muenz L. Barriers to HAART adherence among Human Immunodeficiency Virus-infected adolescents. Archives of Pediatric and Adolescent Medicine. 2003;157:249–255. doi: 10.1001/archpedi.157.3.249. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Arfken C, Frey M, Harris M, Secord E, Ellis DA. Psychosocial factors and treatment adherence in pediatric HIV. AIDS Care. 2006;18:621–628. doi: 10.1080/09540120500471895. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. State-of-the-science conference statement, preventing violence and related health-risking social behaviors in adolescents. 2004. Retrieved August 26, 2005, from http://consensus.nih.gov/ta/023/023youthviolencepostconfintro.htm. [Google Scholar]
- National Institute on Drug Abuse. Principles of drug addiction and treatment: A research-based guide. 1999. NIH Publication No. 99-4180, October 1999. [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2008. (pp. 1–139). Retrieved July 20, 2009 from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- Perinatal HIV Guidelines Working Group. Public health service task force recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States (pp. 1–90) 2009. Retrieved July 20, 2009 from http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. [Google Scholar]
- Rotheram-Borus MJ, Lee M, Leonard N, Lin YY, Franzke L, Turner E, et al. Four-year behavioral outcomes of an intervention for parents living with HIV and their adolescent children. AIDS. 2003;17:1217–1225. doi: 10.1097/00002030-200305230-00014. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Lee MB, Murphy DA, Futterman D, Duan N, Birnbaum JM, et al. Efficacy of a preventive intervention for youths living with HIV. American Journal of Public Health. 2001;91:400–405. doi: 10.2105/ajph.91.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwald SK, Hoagwood K. Effectiveness, transportability, and dissemination of interventions: What matters when? Psychiatric Services. 2001;52:1190–1197. doi: 10.1176/appi.ps.52.9.1190. [DOI] [PubMed] [Google Scholar]
- Schoenwald SK, Ward DM, Henggeler SW, Rowland MD. Multisystemic therapy versus hospitalization for crisis stabilization of youth: Placement outcomes 4 months postreferral. Mental Health Services Research. 2000;20:3–12. doi: 10.1023/a:1010187706952. [DOI] [PubMed] [Google Scholar]
- Steele RG, Grauer D. Adherence to antiretroviral therapy for pediatric HIV infection: review of the literature and recommendations for research. Clinical Child & Family Psychology Review. 2003;6:17–30. doi: 10.1023/a:1022261905640. [DOI] [PubMed] [Google Scholar]
- Strunin L, Hingson R. Alcohol, drugs, and adolescent sexual behavior. International Journal of the Addictions. 1992;27:129–46. doi: 10.3109/10826089209068734. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. 2001. Youth Violence: A report of the Surgeon General. Retrieved September 18, 2009, from http://www.surgeongeneral.gov/library/youthviolence/youvioreport.htm. [Google Scholar]
- Voisin DR. Family ecology and HIV sexual risk behaviors among African American and Puerto Rican adolescent males. American Journal of Orthopsychiatry. 2002;72:294–302. doi: 10.1037/0002-9432.72.2.294. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Maenner G, Gledhill-Hoyt J, Lee H. Changes in binge drinking and related problems among American college students between 1993 and 1997. Results of the Harvard School of Public Health College Alcohol Study. Journal of American College Health. 1998;47:57–68. doi: 10.1080/07448489809595621. [DOI] [PubMed] [Google Scholar]
- Whitfield G, Williams C. The evidence base for cognitive-behavioural therapy in depression: Delivery in busy clinical settings. Advances in Psychiatric Treatment. 2003;9:21–30. [Google Scholar]