Abstract
Objective Youth who experience interpersonal trauma and have posttraumatic stress symptoms (PTSS) develop cognitive deficits that impact their development. Our goal is to investigate the function of the hippocampus in adolescents with PTSS during a memory processing task. Methods Twenty-seven adolescents between the ages of 10–17 years (16 with PTSS and 11 healthy controls) encoded and retrieved visually presented nouns (Verbal Declarative Memory Task) while undergoing fMRI scanning. Results The PTSS group demonstrated reduced activation of the right hippocampus during the retrieval component of the task. Further, severity of symptoms of avoidance and numbing correlated with reduced left hippocampal activation during retrieval. Conclusions Decreased activity of the hippocampus during a verbal memory task may be a neurofunctional marker of PTSS in youth with history of interpersonal trauma. The results of this study may facilitate the development of focused treatments and may be of utility when assessing treatment outcome for PTSS.
Introduction
Youth who experience child maltreatment and develop posttraumatic stress symptoms (PTSS) develop cognitive deficits that impact their academic, social and emotional development (Perry, Pollard, Blakley, Baker, & Vigilante, 1995; Pynoos, Steinberg, & Wraith, 1995). Some of these children develop full DSM-IV criteria for posttraumatic stress disorder (PTSD), however many youth develop symptoms that do not meet full criteria. Research shows that this partial symptomatic response to stress can still significantly impact children’s functioning (Aaron, Zaglud, & Emergy, 1999; Carrión, Weems, Ray, & Reiss, 2002; Terr et al., 1999).
Cognitive impairments in PTSS range from difficulty concentrating or remembering aspects of the traumatic event to more general explicit (declarative) memory difficulties (Brewin 2001; Buckley, Blanchard, & Neill, 2000; Elzinga & Bremner, 2002; Moradi, Taghavi, Doost, Neshat, Yule, & Dalgleish 1999; Yasik, Saigh, Oberfield, & Halamandaris, 2007). Adult women with PTSS secondary to interpersonal trauma, for example, have deficits in verbal declarative memory that correlate with increased posttraumatic symptoms and with severity of trauma (Bremner, Vermetten, Afzal, & Vythilingam, 2004a). The youth literature is more limited. Our goal is to identify neurofunctional correlates of memory processing in adolescents with PTSS.
Preclinical studies have investigated the neuroanatomy of memory processing and have identified the hippocampus, a medial temporal structure in the limbic system, as a primary structure involved in memory processing and contextual learning (Corcoran & Maren, 2001; Eichenbaum, 2000; Squire & Zola-Morgan, 1991). The availability of high-resolution magnetic resonance imaging (MRI) has made it possible to obtain accurate volumetric measurements of the hippocampus in clinical studies of adults and children with maltreatment-related PTSS.
There is evidence of smaller hippocampal volume in adults who experienced child maltreatment and developed PTSS (Bremner & Narayan, 1998; Bremner et al., 2003; Stein, Koverola, Hanna, Torchia, & McClarty, 1997). This finding, however, has not been replicated in pediatric studies (Carrion et al., 2001; De Bellis et al., 1999; De Bellis, Hall, Boring, Frustaci, & Moritz, 2002), suggesting that hippocampal atrophy associated with early life stress may manifest later in life. Supporting this notion are preclinical studies demonstrating that rats exposed to early life stress show differences in synaptic density of their hippocampus only post-pubertally as a consequence of an attenuated synaptogenesis (Roceri, Hendriks, Racagni, Ellenbroek, & Riva, 2002).
Although, not universal, most studies of adults with PTSD secondary to childhood maltreatment-related PTSD have demonstrated reduced hippocampal volume (see Woon & Hedges, 2008 for a review), suggesting that the hippocampus may be particularly vulnerable to the neuroendocrine response to trauma. Various mechanisms may operate in altering the structure of the hippocampus after the experience of early life stress (Brunson, Aghbal-Ahmadi, Bender, Chen, & Baram, 2001; Moghaddam, 2002; Sapolsky, 2000; Sapolsky, Uno, Rebert, & Finch, 1990). A study on combat-related PTSD, however, suggests that a smaller hippocampus may be a risk factor for PTSD (Gilbertson et al., 2002).
Most studies of youth with PTSS and PTSD have not identified hippocampal volumetric differences (Carrión et al., 2001; DeBellis et al., 1999, 2002). These studies, however, as in the case of adult research, have been cross-sectional in design. One longitudinal developmental study (DeBellis et al., 2001) found no evidence of hippocampal atrophy when comparing children with maltreatment-related PTSD and controls. Another longitudinal study from our laboratory, however, found hippocampal volume reductions developing after 12–18 months to be associated with high levels of cortisol within the maltreatment-related PTSS group (Carrión, Weems, & Reiss, 2007).
Functional magnetic resonance imaging (fMRI) allows us to build on this work by investigating the function of the hippocampus apart from putative structural abnormalities. FMRI is of interest to developmental neuroscientists because of its excellent spatial and temporal resolution and lack of requirements for radioactivity. In relation to PTSS, fMRI permits examination of brain function associated with physiological arousal, attention, and memory (Carrión, Garrett, Menon, Weems, & Reiss, 2008). With respect to memory function in particular, fMRI can be utilized to study different memory components such as priming, encoding, and retrieval (Buckner, Wheeler, & Sheridan, 2001). Abnormal hippocampal activity has been identified in fMRI during both encoding (Werner et al., 2008) and retrieval (Geuze, Vermetten, Ruf, deKloet, & Westenberg, 2008) conditions, underscoring the need to explore both of these processes.
Functional neuroimaging studies investigating cognitive activation in adults with PTSD have utilized PET (Shin et al., 2004), [15O] H2O PET (Shaw et al., 2002) and fMRI methods (Astur et al., 2006). Neurocognitive tasks designed to evaluate general cognitive processes that are not specifically trauma-related include the color Stroop task (Bremner et al., 2004b), a word stem completion task and a variant of the n-back task (Shaw et al., 2002). These studies implicate involvement of the anterior cingulate cortex, the middle frontal gyri, medial frontal lobes, right inferior temporal gyrus, and the hippocampus in PTSD. Specifically, the left hippocampus showed decreased regional cerebral blood flow (rCBF) in adults with PTSD when compared to controls while completing a three-letter word stem and contrasting deeply encoded/high recall versus shallow encoded/low recall-words learned during a preceding training session (Shin et al., 2004). These findings suggest an altered function of the matured hippocampus associated with memory processing in PTSD. In the current study, we explore the function of the developing hippocampus during a memory task. We hypothesize that youth with PTSS would demonstrate decreased hippocampal activity when performing a verbal declarative task and compared to a healthy control (HC) group.
Utilizing a task modified from animal studies, the virtual Morris Water task, Astur and colleagues found that adults with PTSD demonstrated reduced hippocampal activity while completing the task when compared to healthy controls. Furthermore, they found a negative correlation between hippocampal activity and symptom severity (Astur et al., 2006). In the current study, we hypothesize that there would be a negative correlation between severity of symptoms and hippocampal activity in adolescents with PTSS. Symptoms of re-experience (Cluster B) and avoidance and numbing (Cluster C) suggest difficulties with memory processing, such as acting or feeling as event was occurring again and avoidance of cues that serve as reminders of the traumatic event.
Methods
Participants
Twenty-seven medication-naïve youth between the ages of 10 and 17 years participated in this study. Sixteen youth with a history of interpersonal trauma and PTSS and 11 youth with similar age and gender as healthy controls (HC) were enrolled. All procedures were approved by Stanford University’s Institutional Review Board (IRB) and parent consent and child assent were obtained in all subjects. Ethnicity for PTSD participants was Hispanic (25%), Caucasian (25%), African American (12.5%), other/multiple (12.5%), Asian/Asian American (6.2%) and not answered (18.7%). Ethnicity for the control group was Caucasian (63.6%), Other/Multiple (12.5%), Asian/Asian American (9%), and not answered (9%). Participants were recruited via referrals from the Santa Clara County Department of Social Services and through announcements/flyers at local mental health clinics and communities within the greater San Francisco Bay Area. For the PTSD group four subjects were recruited from social services, three from mental health clinics and nine from announcements/flyers. For the control group three were recruited from online ads and eight from announcements/flyers.
For the clinical group we recruited youth who had at least one episode of exposure to interpersonal trauma, as defined by DSM-IV criterion A; endorsed PTSS on the Clinician Administered PTSD Scale for Children and Adolescents (CAPS-CA) and scored 10 or higher; lived in a current environment with no exposure to trauma; were not using medications with autonomic or HPA effects; and had no known history of alcohol or drug abuse/dependence, neurological disorder or other major medical conditions, as assessed at intake with a medical history form. Types of trauma included sexual abuse, physical abuse, and witnessing violence. In the experimental group, mean IQ was 101.5 (SD = 15.4).
Eleven healthy control subjects with no history of psychiatric illness or trauma were chosen to have similar age and gender to the clinical group. Their mean IQ was 115.6 (SD = 10.2). Control subjects were screened for a history of trauma and their caretakers completed the Child Behavior Checklist (CBCL) and none scored in the clinical range. Controls had been recruited for this study and for other studies conducted in our lab.
PTSS and HC groups were similar in terms of age; 13.9 (SD = 2.0; range 10.7–17.6) for PTSS and 13.9 (SD = 1.9; range 11.5–16.8) for HC; gender, six males (37.5%) and 10 females (62.5%) in the PTSS group and seven males (58.3%) and four females (41.7%) in the HC group (t = 1.077; p = .291); race (t = 1.805, p = .083); and socioeconomic status (SES) measured as family income (t = 1.802; p = .085).
Behavioral Measures
The CAPS-CA (Nader et al., 1996) is a structured clinical interview that is a developmentally sensitive counterpart to the Clinician Administered PTSD Scale (CAPS) for adults (Blake et al., 1995). It facilitates assessment of exposure to criterion A1 events, including current exposure to trauma and the individuals’ experience of these events (A2), frequency and intensity for each of the 17 symptoms for PTSD clustered in DSM-IV (i.e., criteria B, C, and D) and the 1-month duration requirement (criterion E). Additional features to increase the utility of this instrument with youth include: iconic representations of the behaviorally anchored 5-point frequency and intensity rating scales. A licensed child psychiatrist or child psychologist, who was trained to administer the instrument, conducted the CAPS-CA interview. Training involved observing live interviews and matching diagnoses with one of the originators of the CAPS-CA or with another trained coder using videotaped recordings of the interviews. Our previous research (Carrión et al., 2002) has established interrater agreement for the CAPS-CA by comparing the agreement between two raters, one who observed 10 videotaped interviews given by the primary interviewer and was blind to the rating of the primary interviewer. The intra-class correlation coefficient was .97. A symptom is considered present if it receives scores of at least one in frequency and two in intensity for a total of three in severity. We set an inclusion threshold for a CAPS total score of 10 based on our previous research indicating functional impairment in children with PTSS (Carrión et al., 2002) and research by others underscoring the importance of studying children that fell short of the diagnostic criteria, but still experience severe stress reactions (Deblinger, Lippman, & Steer, 1996; King et al., 2000).
The K-SADS-Present and Lifetime Version, a semi-structured clinical interview designed to identify Axis I DSM-IV disorders (Kaufman et al., 1997), was administered by a licensed child psychiatrist or child psychologist to assess comorbid Axis I psychiatric disorders. The CBCL (Achenbach, 1991) was used to rule out the presence of internalizing or externalizing disorders in the control sample. The Weschler Abbreviated Scales of Intelligence (WASI) was used to assess IQ (The Psychological Corporation, 1999). The Edinburgh Handedness Inventory was used to determine participants’ handedness (Oldfield, 1971). All experimental and control subjects scored in the right-handed dominance range.
fMRI Procedures: The Verbal Declarative Memory Task
The stimuli for the encoding condition were 40 unique visually presented nouns. In the retrieval condition, the stimuli were 32 of the same words plus 16 new words. The stimuli for the control condition consisted of the same two nouns alternating repeatedly.
The encoding task consisted of epochs (group of words presented consecutively as stimuli) of eight words each presented independently for 2.5 s, with a 0.5-s interstimulus interval. Each of the five encoding epochs was followed by a control epoch in which either of two words was presented in a random order over the eight stimulus periods. The same two words were used for all five control epochs. Each epoch was preceded by an instruction screen shown for 4 s. In addition, a 24-s rest period with fixation occurred before the onset of the first cycle, at the midway point (after the third encoding epoch), and after the last control epoch. The task order can be abbreviated as F-E-C-E-C-E-F-C-E-C-E-C-F, where F is fixation, E is encoding, and C is control. To enhance encoding (Craik, Moscovitch, & McDowd, 1994), subjects were instructed to press one of two buttons to make a man-made/not man-made semantic discrimination for each word in the encoding epoch. The words were equally divided between the two semantic categories. The subjects were also instructed to remember the words as they would be asked to identify them later. During the control epoch, they were instructed to alternate pressing buttons 1 and 2 without making a semantic discrimination. This task has been described previously by researchers in our laboratory. For details see Greicius et al. (2003).
During the retrieval phase, six retrieval epochs alternated with six control epochs. During the retrieval epochs, subjects were presented with a series of words, and asked to press one of two buttons to indicate whether the word had been presented during the encoding task. Sixteen novel words and 32 previously presented words were intermixed throughout the six retrieval epochs. Each retrieval epoch was followed by a control epoch in which the same two words were presented alternately, and subjects were alternated pressing buttons 1 and 2 without making a recognition judgment. Each word was presented for 2.5 s with a 0.5-s interstimulus interval. Each epoch began with an instruction screen that lasted 4 s, and a 24-s rest period with fixation cross was presented at the beginning, end, and midpoint of the task.
Behavioral Data Analysis
Accuracy was calculated as the percentage of correct trials during the encoding or retrieval tasks. Response times also were calculated for the encoding and retrieval tasks, including only the correct trials. Behavioral task performance data were analyzed using SPSS software (version 16.0). An independent samples t-test (two-tailed, p = .05, equal variances not assumed) was used to test for group differences in accuracy and response time.
fMRI Data Acquisition
Whole-brain imaging data were acquired on a GE-Signa 3 T MRI scanner (General Electric, Milwaukee, WI). For structural whole brain images, a 3D high resolution spoiled gradient scan (SPGR) (repetition time, 35 ms; echo time, 6 ms; flip angle, 45°; matrix size, 256 × 192; field of view, 24 cm; slice thickness, acquired resolution, 1.5 × 0.9 × 1.2 mm) were conducted. Functional images were collected using a gradient echo spiral T2*-weighted scan and were obtained using a flip angle of 89°, repetition time (TR) = 2.0 s; echo time (TE) = 30 ms; 32 slices; and a field of view (FOV) = 200 × 200 mm2; matrix = 64 × 64. Head motion was restricted by using a custom-built head stabilization system.
We used a commonly used procedure in order to preprocess and statistically analyze the fMRI data (http://www.fil.ion.ucl.ac.uk/spm). Specifically, functional data were preprocessed and statistically analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). The images were temporally realigned to the middle slice and spatially realigned to the first in the time series. The images were then coregistered and spatially normalized into standard stereotactic space (Montreal Neurological Institute template). All images were spatially smoothed with an 8 mm full width-half maximum isotropic Gaussian filter.
fMRI Data Analysis
Fixed-effects models representing encoding and retrieval independently for each participant were used at the individual subject level of analysis and random effects models were used for group-level analyses (SPM5). At the individual level, models were created that represented each experimental (encoding or retrieval) and control (alternating) conditions. Each condition was modeled in blocks. The duration of each block was for 24 s (12 scans). Data were high-pass filtered (a standard method of attenuating discordant amplitudes).
Based on previous studies demonstrating the hippocampus to be engaged during both encoding and retrieval (Greicius et al., 2003) and studies demonstrating atypical hippocampal function and structure to be associated with stress and trauma (Shin, Rauch, & Pitman, 2006) we designated the hippocampus as an a priori region of interest (ROI). The delineation of the left and right hippocampus ROIs were based on Talarairach definitions in standard stereotactic space (http://www.fmri.wfubmc.edu).
Two complementary analyses of hippocampal activation were performed. One analysis procedure was designed to investigate changes in the amplitude of activation, while the other was designed to investigate changes in the spatial extent of activation within the hippocampus ROI. The statistical analysis of amplitude was carried out by using a series of t-tests on a voxel by voxel basis (within the hippocampus) that compared the contrast estimates (betas) between experimental (encoding or retrieval) and the control (alternating) conditions. Within the hippocampus ROI, we used a p < .05 statistical threshold (uncorrected) and a spatial extent threshold of 40 contiguous voxels for this analysis. This combination of an activation threshold and a spatial extent threshold adequately protects against false positives and corresponds to a per-voxel false discovery rate of p = .00011 (Forman et al., 1995).
The statistical analysis of spatial extent was carried out by calculating the percentage of total voxels within the hippocampus ROI that survived a statistical threshold of p < .05 for a given comparison (encoding > control condition or retrieval > control condition). This analysis provides a metric indicative of the proportion of volume within the hippocampus that is responsive during a task. Between-group comparisons of the percentage of voxels activated within the left and right hippocampus ROI were conducted using two-sample t-tests in SPSS with a threshold of p < .05 (two-tailed).
Statistical analyses in SPM5 were initiated by performing a group analysis comparing blood oxygenation level dependent (BOLD) signal during each experimental (encoding or retrieval) condition versus the control (alternating) condition. We initiated our analysis by performing a condition by group (2 × 2) interaction analysis. This was conducted by using a random effects model comparing the difference in BOLD signal during each experimental (encoding or retrieval) condition versus the control (alternating) condition between experimental groups (PTSS vs. HC). The simple effects within each group (PTSS and HC) were then explored by comparing BOLD signal change between the experimental (encoding or retrieval) condition versus the control (alternating) condition. The spatial extent analysis within the hippocampus was used in order to further investigate between-group differences in the volume of ROI activation. In order to rule out the effect of IQ, each between-group comparison was repeated while controlling for each subject’s IQ score.
Lastly, we performed exploratory analyses to test for relationships between hippocampal activation and behavioral measures. Specifically, we examined the relationship between hippocampal activation and retrieval accuracy as well as between hippocampal activation and severity of posttraumatic symptoms. Retrieval accuracy was quantified as the percentage of correct trials during retrieval. Severity of posttraumatic symptoms was quantified using the Clinician Administered PTSD Scale for Children and Adolescents (CAPS) (Nader et al., 1996).
Results
Clinical Characteristics
The CAPS-CA scores ranged from 13 to 70 with a mean score of 44.6 and a standard deviation of 19. Nine participants in the clinical group met DSM-IV diagnostic criteria for PTSD. The other seven demonstrated significant PTSD symptoms on the CAPS-CA, but were sub-threshold for the diagnosis of PTSD. This range reflects the natural continuum of posttraumatic reactions in children. Hence, we refer to the complete group as children with a history of interpersonal trauma and PTSS. The limited understanding and use of empirically derived developmental information regarding the classification of PTSD in youth make the use of PTSS a developmentally viable stance (Carrión et al., 2002; Scheeringa, Peebles, Coook, & Zeanah, 2001; Scheeringa, Zeanah, Drell, & Larrieu, 1995). In terms of comorbidity; three met criteria for depressive disorder not otherwise specified, three for major depressive disorder, one for panic disorder, one for obsessive compulsive disorder, one for enuresis, one subject with ADHD, conduct disorder and social phobia and 10 had no comorbidity.
Behavioral Task Performance
There were no group differences in accuracy or response time during the encoding task. During the retrieval task, the PTSS group had similar response times, but decreased accuracy compared to the control group ( p = .039). See Table I for details.
Table I.
Verbal Declarative Memory Task performance summary
| Variable | PTSS group | Control group | p* |
|---|---|---|---|
| Encoding percent correct | 74.8 ± 20.0 | 84.1 ± 9.2 | .120 |
| Encoding response time (ms) | 1275.4 ± 173.8 | 1274.3 ± 141.5 | .986 |
| Retrieval percent correct | 63.41 ± 22.0 | 76.51 ± 7.3 | .039 |
| Retrieval response time (ms) | 1201.5 ± 187.7 | 1263.8 ± 165.5 | .373 |
fMRI Results
Hippocampal Activation during Encoding
The condition by group (2 × 2: encoding vs. control; PTSS vs. HC) interaction analysis indicated that there were no significant differences in either left or right hippocampus activation, while controlling for IQ. There were no between-group differences in the spatial extent of hippocampal activation during encoding (all p-values >.05).
Hippocampal Activation during Retrieval
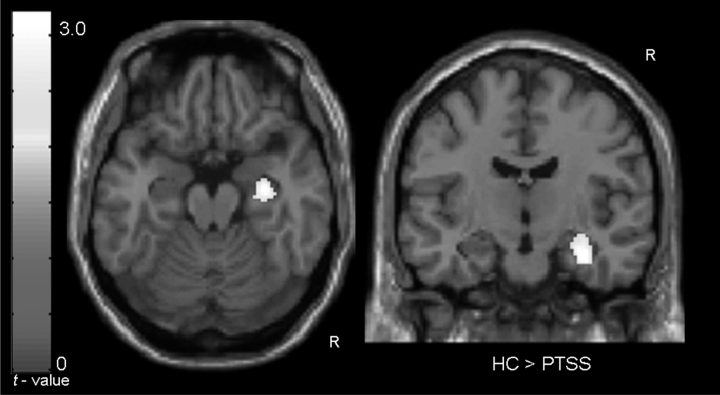
The condition by group (2 × 2: PTSS vs. HC, encoding vs. control) interaction analysis indicated that the HC group had significantly greater right hippocampus activation during retrieval compared to the PTSS group while controlling for IQ (MNI: 32, –12, –22; t = 2.54; p = .009) (Figure 1). The HC group exhibited significant right hippocampal activation during retrieval (MNI: 26, –34, 8; t = 3.67; p = .002), while the PTSS group did not.
Figure 1.
Right hippocampal activation during retrieval found to be greater in the HC group compared to the PTSS group while controlling for IQ. Clusters are overlaid upon a standardized template brain in an axial view (left: z = –18) and a coronal view (right: y = –20). No areas within the left or right hippocampus were found to be display greater activation during retrieval in the PTSS group compared to the HC group, while controlling for IQ.
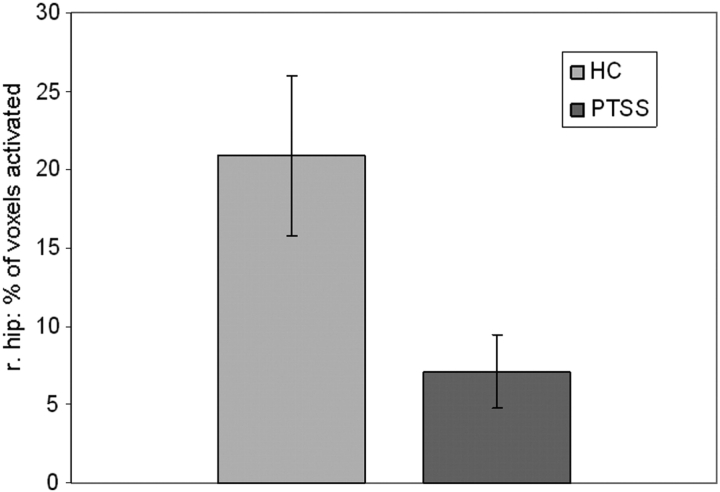
We further investigated between-group differences in the spatial extent of retrieval activation within the left and right hippocampus. This analysis showed that the spatial extent of right hippocampus activation during retrieval was greater in the HC (mean = 20.90%) group compared to the PTSS (7.09%) group while controlling for IQ (t = 2.92, p = .008) (Figure 2).
Figure 2.
Percentage of total voxels within the right hippocampus activated (p < .05) when comparing retrieval versus the control condition in the HC and PTSS group.
Hippocampal Activity Predictive of Retrieval Accuracy
We investigated the relationship between hippocampal activation during encoding and retrieval accuracy by using a Pearson correlation analysis. This analysis demonstrated that there were no significant between-group differences in the relationship between either left or right hippocampus activation, or the spatial extent during encoding and retrieval accuracy, while controlling for IQ.
We next investigated the relationship between hippocampal activation during retrieval and retrieval accuracy by using a Pearson correlation analysis. This analysis demonstrated that there were no significant between-group differences in the relationship between either left or right hippocampus activation or the spatial extent during retrieval and retrieval accuracy, while controlling for IQ.
Severity of Posttraumatic Symptoms and Hippocampal Activity during Retrieval
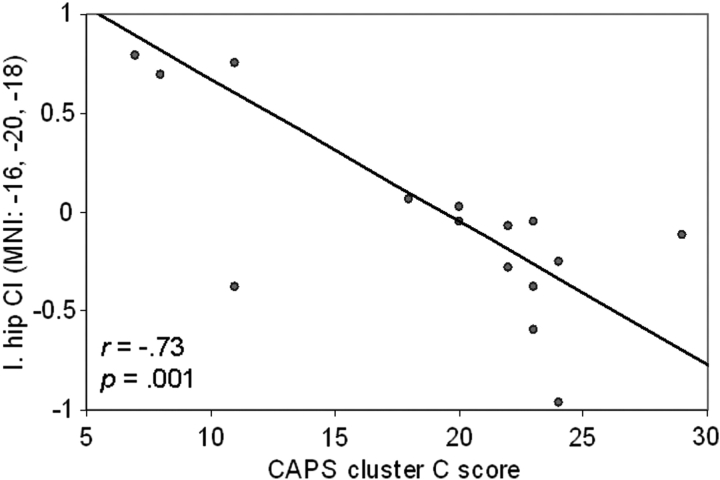
Based on the finding of a significant between-group difference (HC > PTSS) in left and right hippocampus activation during retrieval, we performed a Pearson correlation analysis to determine if greater severity of posttraumatic symptoms was associated with reduced hippocampal activation during retrieval in the PTSS group. This analysis showed that higher scores on CAPS cluster B (re-experience) were associated with less hippocampal activity during retrieval (95 voxels; MNI: –24, –18, –12; t = 2.86; p = .006; 62 voxels; MNI: –22, –36, 2; t = 2.24; p = .012). Higher scores on CAPS cluster C (avoidance/numbing) were also associated with less hippocampal activity during retrieval (221 voxels; MNI: –16, –20, –18; t = 3.95; p = .001; 58 voxels; MNI: –12, –40, 8; t = 2.51; p = .012) (Figure 3). However, the finding that both clusters were significantly associated with hippocampal activity may be, in part, due to the fact that these clusters were significantly associated with one another (r = .53; t = 2.33, p < .05). In order to investigate the specificity of each cluster score predicting hippocampal activity, we conducted a multiple regression analysis where each cluster score (B and C) were simultaneously entered as predictors of left hippocampal activity during retrieval. The results of this analysis indicated that the relationship between CAPS cluster C (avoidance/numbing) and hippocampal activity during retrieval remained significant when controlling for CAPS cluster B (72 voxels; MNI: –16, –20, –18; t = 3.49; p = .002), while the relationship between CAPS cluster B (re-experience) and hippocampal activity during retrieval when controlling for CAPS cluster C (avoidance/numbing) was not significant. No significant relationships were observed between any of the CAPS cluster scores and right hippocampal activity or the spatial extend of activation within either the left or right hippocampus.
Figure 3.
Left hippocampal activation during retrieval associated with trauma-related severity within the PTSS group. Scatterplot with the x-axis representing CAPS cluster C score (avoidance/numbing) and the y-axis representing activation of the peak voxel within the left hippocampus. l.hip: left hippocampus; CI: contrast estimate derived from retrieval > control condition; MNI: Montreal Neurological Institute coordinates.
Discussion
The mean CAPS-CA score of 44.6 in the PTSS group highlights the significant symptomatology in this group. PTSS include intrusive memories, flashbacks and inability to recall important aspects of the traumatic event (APA, 2000). The comorbidity in our PTSS cohort is representative of clinical populations with PTSS where comorbidity for mood, anxiety and behavioral disorders can be as high as 80% (Pfefferbaum, 1997). In this fMRI study we examined hippocampal function in youth with PTSS during a verbal declarative memory task. The hippocampus has been implicated in a number of cognitive operations, perhaps most prominently, those involving memory encoding and retrieval (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; O’Keefe & Nadel, 1978; Squire, 1992; Wheeler & Buckner, 2004).
Our fMRI results for the encoding component of the verbal declarative task indicate that both PTSS and HC groups performed similarly. We also found that during the retrieval component of the task, relative to healthy controls, youth with PTSS demonstrated reduced right hippocampal activation. Furthermore, severity of symptoms of avoidance and numbing (Cluster C) correlated with reduced left hippocampal activation during retrieval.
Our neuroimaging findings showed HC participants to have greater right and left hippocampal activation during retrieval compared to the PTSS group. This between-group difference remained significant for the right hippocampus after controlling for IQ and was supported by convergent evidence from examination of spatial extent of activation. These results suggest that difficulties in memory processing by youth with PTSS may be related to activation deficits of the right hippocampus. The physiological correlates of chronic stress: increased release of glucocorticoids (Sapolksy, 2000) and corticotrophin-releasing hormone (Brunson et al., 2001), increased susceptibility to excitatory amino acids such as glutamate (Moghaddam, 2002) and inhibition of brain-derived neurotrophic factor (BDNF; Duric & McCarson, 2005), may induce atrophy and dendritic loss in the hippocampus and this may lead to altered function.
We also identified a strong correlation between left hippocampal activity during retrieval and the symptoms of Cluster C of PTSD, known as the avoidance and numbing cluster. This cluster includes symptoms such as, inability to recall an important aspect of the trauma, feelings of detachment or estrangement from others, restricted range of affect, and sense of foreshortened future (e.g., does not expect to have a normal life span or career). Our findings implicate functional disturbance of the hippocampus in the manifestation of these symptoms. Interestingly, individuals with temporal lobe epilepsy manifest similar feelings of detachment during the aura phase of a seizure (Bancaud, Brunet-Bourgin, Chauvel, & Halgren, 1994), supporting the involvement of the hippocampus and contiguous regions in these types of symptoms. The link with re-experience (Cluster B) symptoms is also of interest since re-experience symptoms can be conceptualized as spontaneous and involuntary memories (Ferree & Cahill, 2009). This finding may have resulted from the strong association between cluster C and cluster B symptoms. Re-experience symptoms deserve further investigation when conducting studies of hippocampal function in PTSS.
In a study of adults, Moores and colleagues (Moores et al., 2008) found that when compared to non-traumatized controls, subjects with PTSD demonstrated abnormal recruitment of the dorsolateral prefrontal cortex (DLPFC) and the inferior parietal lobe during working memory maintenance. The authors of this study argue that this brain activity may have been an attempt to compensate for decreased activity in the hippocampus, anterior cingulate and pons, when compared to controls. Other frontal regions have been implicated in the processing of memories as well. For example, activation in the ventrolateral prefrontal cortex is consistently increased during encoding of items that are subsequently remembered, compared with items that are later forgotten (Paller & Wagner, 2002).
Although this is the largest sample of youth with PTSS who have been assessed with an fMRI memory task to date, our sample size may have limited our ability to detect additional between-group differences. Replications of this study using larger samples will allow for investigation of other relevant groups, such as a trauma-exposed youth with no posttraumatic symptoms. The lateralization of some of our findings may also speak to the need to replicate this study with larger samples. Alternatively, the right hippocampus may be particularly vulnerable to the effects of chronic stress (Bremner et al., 1995), though most of the literature does not support this notion (Bremner et al., 1997; Gurvits et al., 1996; Stein, Koverola, Hanna, Torchia, & McClarty, 1997). Prenatal and postnatal history need to be included in future evaluations as children exposed to interpersonal violence may experience socio-economic deprivation that may translate into poor nutrition and lack of stimulation and these factors may influence brain development and function. Larger samples would also allow for the study of the effect of comorbidity in these findings.
Clinical Implications
In this study, we identify abnormalities in the function of the hippocampus while children with PTSS engage in memory processing. Investigating the impact of early trauma on learning and memory processing is critical in developmental traumatology since PTSS has been conceptualized as a disorder of failure of extinction. Specifically, learned responses to an unconditioned stimulus (the traumatic event) become the conditioned response to conditioned stimuli (the traumamimetic cues). Difficulties experienced by youth in placing traumatic information in the context of their history may be related to functional abnormalities in their hippocampi during the retrieval of such information.
Identifying brain networks involved in learning and memory processes will help the development of more focused treatments and may be of utility when assessing treatment outcome. In this study we implicate the hippocampus in the memory deficits experienced by youth with PTSS. Treatments targeted at modifying the functional-adaptive activity of the hippocampus may address the memory deficits that characterize this condition. Interventions targeting improvement of hippocampal function in these youth may help alleviate their memory-related symptoms.
Funding
National Institute of Mental Health (MH63893), National Association for Research in Schizophrenia and Affective Disorders (NARSAD) and American Foundation for Suicide Prevention (AFSP) and the Aloha Foundation to Dr Carrion.
Conflict of interest: None declared.
References
- Aaron J, Zaglud H, Emergy R. Posttraumatic stress in children following acute physical injury. Journal of Pediatric Psychology. 1999;24:335–343. doi: 10.1093/jpepsy/24.4.335. [DOI] [PubMed] [Google Scholar]
- Achenbach T. Manual for the child behavior checklist and revised child behavior profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM- IV-TR) Washington, DC: Author; 2000. [Google Scholar]
- Astur RS, St. Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. CyberPyschology and Behavior. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of déjà vu and vivid ‘memories’ in human temporal lobe epilepsy. Brain. 1994;117:71–94. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. Journal of Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brewin CR. A cognitive neuroscience account of post-traumatic stress disorder. Journal of Abnormal Psychology. 2001;104:537–541. [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JB, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Vermetten E, Staib L, Bronen RA, Charney D. MRI-based measurement of hippocampal volume in PTSD related to childhood physical and sexual abuse: A preliminary report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M. The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Developmental Psychopathology. 1998;10:871–885. doi: 10.1017/s0954579498001916. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib L, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Afzal N, Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 2004a;192:643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, et al. Neural correlates of the classic color and emotional stroop in women with abuse- related posttraumatic stress disorder. Biological Psychiatry. 2004b;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Aghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotrophin- releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD: a review of the empirical literature. Clinical Psychology Review. 2000;28:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. Journal of Cognitive Neuroscience. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Weems CF, Ray RD, Reiss AL. Toward an empirical definition of pediatric PTSD: The phenomenology of PTSD symptoms in youth. Journal of American Academy of Child and Adolescent Psychiatry. 2002;41:166–173. doi: 10.1097/00004583-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Carrión VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Moscovitch M, McDowd JM. Contributions of surface and conceptual information to performance on implicit and explicit memory tasks. Journal of Experimental Psychology Learning Memory and Cognition. 1994;20:864–875. doi: 10.1037//0278-7393.20.4.864. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology part II: Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot study of hippocampal volumes in pediatric maltreatmentrelated posttraumatic stress disorder. Biological Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Lyengar S, Beers SR, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biological Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- Deblinger E, Lippman J, Steer R. Sexually abused children suffering posttraumatic stress symptoms: Initial treatment outcome findings. Child Maltreatment. 1996;1:310–321. [Google Scholar]
- Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133:999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Review Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory of the final common pathway in posttraumatic stress disorder (PTSD) Journal of Affective Disorders. 2002;70:1–70. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree NK, Cahill L. Post-event spontaneous intrusive recollections and strength of memory for emotional events in men and women. Consciousness and Cognition. 2009;18:126–134. doi: 10.1016/j.concog.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, deKloet CS, Westenberg H. GM. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatry Research. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13(1):164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson NW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat related posttraumatic stress disorder. Biological Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King NJ, Tonge BJ, Mullen P, Myerson N, Heyne D, Rollings S, et al. Treating sexually abused children with posttraumatic stress symptoms: A randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1347–1355. doi: 10.1097/00004583-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biological Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Moores KA, Clark C, McFarlane A, Brown G, Puce A, Taylor D. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research. 2008;163:156–170. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Taghavi MR, Doost HT, Neshat HT, Yule W, Dalgleish T. Performance of children and adolescents with PTSD on the Stroop colour-naming task. Psychological Medicine. 1999;29:415–419. doi: 10.1017/s0033291798008009. [DOI] [PubMed] [Google Scholar]
- Nader K, Kriegler J, Blake DD, Pynoos R, Newman E, Weather F. Clinician administered PTSD Scale, Child and Adolescent Version. White River Junction, VT: National Center for PTSD; 1996. [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Science. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Perry BD, Pollard RA, Blakley TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation, and “use-dependent” development of the brain: How “states’ becomes “traits”. Infant Mental Health Journal. 1995;16:271–291. [Google Scholar]
- Pfefferbaum B. Posttraumatic stress disorder in children: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1503–1511. doi: 10.1016/S0890-8567(09)66558-8. [DOI] [PubMed] [Google Scholar]
- Pynoos RS, Steinberg AM, Wraith R. A developmental model of childhood traumatic stress. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. Vol. 2. New York: John Wiley & Sons Inc; 1995. pp. 72–95. [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. Journal of Neuroscience. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Scheeringa M, Zeanah C, Drell M, Larrieu J. Two approaches to the diagnosis of posttraumatic stress disorder in infancy and early childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:191–200. doi: 10.1097/00004583-199502000-00014. [DOI] [PubMed] [Google Scholar]
- Scheeringa M, Peebles CD, Coook CA, Zeanah CH. Toward establishing procedural, criterion and discriminant validity for PTSD in early childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:52–60. doi: 10.1097/00004583-200101000-00016. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Strother SC, Mcfarlane AC, Morris P, Anderson J, Clark CR, et al. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage. 2002;15:661–674. doi: 10.1006/nimg.2001.1024. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Science. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1091–1099. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:1–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Terr LC, Block DA, Michel BA, Shi H, Reinhaardt JA, Metayer S. Children’s symptoms in the wake of Challenger: A field study of distant traumatic effects and an outline of related conditions. American Journal of Psychiatry. 1999;156:1536–1544. doi: 10.1176/ajp.156.10.1536. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace & Company; 1999. [Google Scholar]
- Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, et al. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatry Research. 2008;43:309–318. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: A meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Yasik AE, Saigh PA, Oberfield RA, Halamandaris PV. Posttraumatic stress disorder: memory and learning performance in children and adolescents. Biological Psychiatry. 2007;61:382–388. doi: 10.1016/j.biopsych.2006.06.005. [DOI] [PubMed] [Google Scholar]