Abstract
Objective To examine the relationships of demographic, maltreatment, neurostructural and neuropsychological measures with total posttraumatic stress disorder (PTSD) symptoms. Methods Participants included 216 children with maltreatment histories (N = 49), maltreatment and PTSD (N = 49), or no maltreatment (N = 118). Participants received diagnostic interviews, brain imaging, and neuropsychological evaluations. Results We examined a hierarchical regression model comprised of independent variables including demographics, trauma and maltreatment-related variables, and hippocampal volumes and neuropsychological measures to model PTSD symptoms. Important independent contributors to this model were SES, and General Maltreatment and Sexual Abuse Factors. Although hippocampal volumes were not significant, Visual Memory was a significant contributor to this model. Conclusions Similar to adult PTSD, pediatric PTSD symptoms are associated with lower Visual Memory performance. It is an important correlate of PTSD beyond established predictors of PTSD symptoms. These results support models of developmental traumatology and suggest that treatments which enhance visual memory may decrease symptoms of PTSD.
Keywords: childhood maltreatment, hippocampus, posttraumatic stress disorder
Interpersonal life-threatening traumatic events to self or others are common in the lives of children (Copeland, Keeler, Angold, & Costello, 2007). The diagnosis of posttraumatic stress disorder (PTSD) is defined as the occurrence of symptoms after an individual experiences a life-threatening traumatic event or events (i.e., Type A trauma), such as child abuse or witnessing domestic violence; and reacts with fear or disorganized behavior, followed by three types of symptom clusters for at least one month: Cluster B—intrusive re-experiencing of the trauma(s); Cluster C—persistent avoidance of stimuli associated with the trauma(s) or numbing of responsiveness; and Cluster D—persistent symptoms of increased physiological arousal (American Psychiatric Association, 2000). Although PTSD was originally intended for adult combat veterans, child maltreatment is associated with PTSD symptoms (De Bellis, 2003). The current diagnostic threshold of DSM-IV PTSD may not be developmentally sensitive, as children with threshold versus subthreshold PTSD have similar functional impairment due to their PTSD symptoms (Carrion, Weems, Ray, & Reiss, 2001).
In the developmental traumatology model, maltreatment is a trauma leading to PTSD symptoms through its effects on the developing biological stress systems and their effects on brain and cognitive development (De Bellis, 2001). Measures of trauma (e.g., type, age of onset) and potential mediating factors [e.g., age, socioeconomic status (SES), gender] are regarded as independent variables or risk factors while behavioral, cognitive, emotional (e.g., PTSD symptoms), and biological measures are conceptualized as dependent variables. An important mission for the field of developmental traumatology research is to unravel the associations and interactions between these dependent variables.
Several neurotransmitter and neuroendocrine systems are activated during maltreatment stress (De Bellis & Putnam, 1994). During stress, the hypothalamus releases corticotropin-releasing hormone (CRH). CRH activates the hypothalamic–pituitary–adrenal (HPA) axis by stimulating the pituitary to secrete adrenocorticotropin (ACTH). ACTH promotes cortisol release from the adrenal gland, stimulates the sympathetic nervous system, and leads to intense arousal and behavioral activation (Chrousos & Gold, 1992). Although baseline and challenge neuroendocrine studies of the HPA axis show that cortisol secretion is dysregulated in child and adult PTSD, the nature of this dysregulation is complex (De Bellis, 2003). Baseline 24 hr cortisol levels are higher in both male and female children with maltreatment-related PTSD (De Bellis, Baum et al., 1999) or subthreshold PTSD (Carrion et al., 2002). These elevated levels of cortisol may damage glucocorticoid-sensitive brain structures leading to cognitive deficits.
The hippocampus is the brain structure most studied in PTSD due to its high density of glucocorticoid receptors (Watanabe, Gould, & McEwen, 1992). Preclinical studies showed that stress and exogenous cortisol are associated with hippocampal atrophy (Sapolsky, 2000; Tanapat, Galea, & Gould, 1998). In a longitudinal pediatric study, stress, defined as PTSD symptoms and baseline 24 hr cortisol levels, predicted smaller hippocampal volumes (Carrion, Weems, & Reiss, 2007). The hippocampus is involved in attention (Jensen et al., 2009) and memory (Diamond, Fleshner, Ingersoll, & Rose, 1996). Studies in adults (Bremner et al., 1995, 2003) demonstrated memory and hippocampal dysfunction in individuals with PTSD. In children, this is an understudied area that produced conflicting findings. Studies showed no memory deficits in maltreated children, who were not assessed for PTSD (Nolin & Ethier, 2007) and who had maltreatment related PTSD (Beers & De Bellis, 2002). Two studies showed memory deficits in children with PTSD (Moradi, Doost, Taghavi, Yule, & Dalgleish, 1999; Moradi, Taghavi, Neshat-Doost, Yule, & Dalgleish, 2000).
We tested the developmental traumatology model with regard to the hippocampus and neuropsychological measures associated with hippocampal function (i.e., memory and attention). We examined the developmental traumatology model in relationship to hippocampal volumes and cognitive function and its association to total PTSD symptoms as an important dependent variable. Using hierarchical regression, we examined a model comprised of blocks of independent variables including (a) demographic variables, (b) trauma and maltreatment-related, and (c) structural and neuropsychological variables (specifically, hippocampal volumes, IQ, verbal and visual memory, attention regulation, inhibitory control) to determine their relationship with the total number of PTSD symptoms as an important health outcome. It was hypothesized that more exposure to maltreatment, smaller hippocampal volumes, and lower neurocognitive abilities would be associated with a greater number of PTSD symptoms.
Methods
Participants
The sample included 216 children and adolescents who participated in multiple studies examining Department of Social Services defined maltreatment and PTSD symptoms. Participants were included in this study if they had a history of Maltreatment with PTSD (n = 49) or without PTSD (n = 49), or had no history of maltreatment (Controls, n = 118). The sample ranged in age from 3.6 to 17.9 years (Mean = 10.8, SD = 3.5), fell within the middle SES using the Hollingshead Two Factor Index of Social Status (Mean = 41.5, SD = 14.02), and was 58.8% female and 49.5% Caucasian. Approximately 74% were living with their biological parents, with caregiver intellectual functioning falling within the average range. Nearly 89% of the sample was right handed and intellectual functioning fell within the average range. To reduce bias, the study was advertised to child protective services in the State of North Carolina on a Statewide level. To reduce selection biases, participants who lived more than 75 miles from the Research Program were given overnight accommodations. Controls were recruited from the same surrounding communities through IRB approved advertisement at schools and pediatric clinics.
Exclusion criteria were: (a) Full Scale Intelligence Quotient (FSIQ) < 70; (b) disability that made a comprehensive interview of the child difficult; (c) significant medical illness, head injury, or neurological disorder; (d) autism or pervasive developmental disorder; (e) birth weight under 5 lbs or severe prenatal compromise with NICU stay; (e) current or lifetime alcohol or substance use disorder (defined as DSM-IV abuse or dependence). The local university hospital IRB committee approved the study. Legal guardians gave informed consent and children assented prior to participation.
Measures
Trauma-related Variables
Kiddie Schedule for Affective Disorders and Schizophrenia- Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997)
This semi-structured interview was administered with caregivers and subjects. We also used archival records as sources of information. The KSADS-PL was modified to include information about: (a) life event questions, including traumatic events from the Child and Adolescent Psychiatric Assessment (Angold et al., 1995); (b) disorders not present in the KSADS-PL; (c) a structured scale was added to quantify symptom frequency with a minimum score of 0 = no history of a symptom and maximum score of 10 = symptoms present several times a day; and (d) algorithms were created to determine Axis I psychiatric disorders based on DSM-IV criteria. Disorders were assigned a severity score of mild, moderate or severe. This modified version is available upon request. Interviewers were individually trained to obtain 80% agreement for PTSD and over 90% agreement for the presence of any lifetime major Axis I disorder with a board certified child and adolescent psychiatrist and experienced child trauma interviewer (MDDB). Discrepancies were resolved by reviewing archival information (e.g., child protection reports, medical records) or by re-interviewing the child or caregiver. If diagnostic disagreements were not resolved with this method, consensus diagnoses were reached among a child psychiatrist (MDDB) and child psychologist (SRH).
An overall maltreatment variable was created that was comprised of positive responses to abuse, neglect, and trauma-related questions. This was done so that these “continuous” variables can be included in the same equation and to avoid dichotomizing groups because most of our maltreated children suffered from several types of abuse and neglect. A principal components factor analysis of six abuse and neglect domains with Varimax rotation produced two reliable factors: (a) General Maltreatment, comprised of Failure to Supervise, Failure to Provide, Physical Abuse, Emotional Abuse, and Witnessing Interpersonal Violence, and (b) Sexual Abuse. A reliability test of the six original maltreatment variables gives a Cronbach’s alpha of .82, indicating consistency in the measurements that make up the two maltreatment factors used in the analysis. The two resulting factors were not correlated (r = .002, p = .98). Those six original maltreatment variables did have a large measure of strong positive correlation indicating their overlap, but the data reduction from the factor analysis was able to tap the underlying interdependencies and separate them into the two factors. Each of the factors contains some aspects of the six original maltreatment variables (though the Sexual Abuse Factor score was dominated by the Sexual Abuse incident count). By creating these two factors, the model was simplified and multicollinearity was reduced. These two factors were derived from the data below and employed in our analyses.
The failure to supervise variable was composed of positive responses to seven questions regarding neglect resulting in (a) serious accidents, (b) not knowing child’s whereabouts, (c) being left home alone, (d) unexplained school absences, (e) witnessing caregiver using drugs or being drunk, and (g) exposure to inappropriate adult sexual activity. Failure to provide was composed of three questions regarding basic physical or medical care. Physical abuse was composed of five questions regarding discipline by a caregiver resulting in bruises or serious injury sustained on one or more occasions, being pushed into objects, shaken, burned, or being threatened with a deadly weapon. Witnessing interpersonal violence was composed of 10 questions regarding witnessing or being told about domestic violence, threats involving violence to important attachment figures, threatening or violent crime where significant injury or death occurred or could have occurred, being the victim of serious threats or violent crime not perpetrated by a caregiver, or witnessing family members’ explosive behaviors resulting in serious property damage or attempts to hurt themselves. Emotional abuse was defined by three questions regarding a caregiver making hurtful comments or swearing at the child or witnessing or hearing about other family members’ physical abuse. Sexual Abuse was defined by questions regarding isolated incidents of genital fondling, oral sex, or vaginal or anal intercourse by a person in a caregiver capacity (i.e., incest).
Hippocampal Volumes
Magnetic resonance imaging (MRI) was performed using a Siemens Trio 3.0 Tesla MRI system (Trio, Siemens Medical Systems) running version VA 24 software located at the Duke University Medical Center (DUMC) Department of Radiology. Hippocampal volumes and total brain volumes were measured at the Duke Neuropsychiatric Imaging Research Laboratory using the Katholieke Universiteit Leuven (KUL) procedure and the GRID Program (MacFall, Byrum, & Parashos, 1994), respectively. Hippocampal volumes were adjusted for total brain volume to correct for differences in the sample due to age and gender.
Neuropsychological
Visual memory was measured using the Face Memory Subtest from the NEPSY (Korkman, Kemp, & Kirk, 2001) or the Rey-Osterrieth Complex Figure Delay Condition score. Verbal memory was measured using the NEPSY Narrative Memory or the California Verbal Learning Test Total T-score. For both verbal and visual memory domains, a composite score was calculated by utilizing the age-based standard scores that were generated by each of the tests. Attention-related functions were assessed using the Conners’ Continuous Performance Test-II (Conners & MHS, 2000). The CPT-II measures the participant’s attention regulation and inhibitory control. We include the T-scores for the errors of commission and variability variables, with each of these scores being reversed such that higher scores reflected better functioning.
Wechsler Intelligence Scale for Children-III (WISC-III) or Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) (Wechsler, 1989, 1991)
Participants received the age-appropriate version of the Wechsler scale. Participants between the ages of 2.5 and 6.0 years of age were administered the WPPSI. Participants between the ages of 6.0 and 16 years 11 months of age were administered the WISC-III. A two-subtest short-form, comprised of Vocabulary and Block Design, generated an IQ score.
PTSD Symptoms
Using the K-SADS-PL, we examined the total number of PTSD symptoms. PTSS counts did not include the maltreatment-related scales described earlier. Because all three PTSD cluster types were highly correlated with one another (r = .79–.84), and with PTSD Total Number of Symptoms (r = .93–.95), these variables were omitted from the hierarchical regression in favor of a single outcome variable, the total number of PTSD Symptoms.
Data Analyses
Following descriptive analyses, a hierarchical multiple regression was conducted to determine which demographic (age, gender, SES), maltreatment (General Maltreatment, Sexual Abuse), neurostructural (left and right hippocampal volumes), and neurocognitive variables (FS IQ, Visual Memory composite, Verbal Memory composite, CPT-II Variability, CPT-II Errors of Commission) were related to PTSD symptoms. Known correlates and predictors of PTSS (i.e., demographic variables, history of child maltreatment) were entered in earlier steps of the regression. Because the developmental traumatology model specifies neurostructural and neurocognitive variables as factors contributing to PTSD symptoms, these variables were entered into the regression equation after the known predictors were estimated. This approach to data analysis allows for inferences to be made about the unique contributions of neurocognitive and neurostructural factors outlined in the developmental traumatology model above and beyond known predictors of PTSD symptoms.
Results
Hierarchical Modeling
Step 1—Demographic Variables
The demographic variables of age, gender, and SES were entered as a block into the first step of the hierarchical regression analysis to assess the degree of variance accounted for by these variables in relation to total number of PTSD symptoms (PTSS). This block of demographic variables yielded a significant overall model, F(3,135) = 5.81, p < .01, accounting for 11% of the variance in total number of PTSS. As can be seen in Table I, SES was a significant individual contributor to this initial model.
Table I.
Hierarchical Linear Regression Model for Total Number of PTSD Symptoms
| Variable | B | SE | ß | 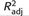 |
R2Δ |
|---|---|---|---|---|---|
| Step 1 | .10 | .11*** | |||
| Age | −.14 | .12 | −.09 | ||
| SES | −.10 | .03 | −.29*** | ||
| Gender | 1.27 | .78 | .13 | ||
| Step 2 | .63 | .53*** | |||
| General Maltreatment Factor | 2.87 | .25 | .65*** | ||
| Sexual Abuse Factor | 2.83 | .35 | .44*** | ||
| Step 3 | .65 | .04* | |||
| Left Hippocampus | .73 | .45 | .11 | ||
| Right Hippocampus | −.44 | .42 | −.07 | ||
| IQ | −.00 | .02 | −.01 | ||
| Visual Memory Composite | −.05 | .02 | −.16** | ||
| Verbal Memory Composite | .03 | .02 | .10† | ||
| CPT-II Variability | −.00 | .04 | −.00 | ||
| CPT-II Errors of Commission | .09 | .05 | .10† |
Note. IQ, Wechsler Intelligent Scale for Children-III Estimated Intelligent Quotient; CPT-II, Conner’s Continuous Performance Test-II.
†p < .10; *p ≤ .05; **p ≤ .01; ***p ≤ .001.
Step 2—Demographic and Maltreatment Variables
A second model that simultaneously added the General Maltreatment and Sexual Abuse factors to the Step 1 model was then conducted to assess whether maltreatment-related variables assessed in this study accounted for significantly more variance in total number of PTSS over demographic variables alone. This second model produced a significant overall model, F(5,133) = 47.91, p < .001, that accounted for an additional 53% of the variance in PTSD Total Symptoms above and beyond the demographic variables. Table I indicates that both the General Maltreatment and Sexual Abuse factors were significant (p < .001) contributors to this second model even after accounting for demographic variables.
Step 3—Demographic, Maltreatment, and Neurostructural and Neurocognitive Variables
A final model that simultaneously entered left and right hippocampal volumes, IQ, Visual and Verbal Memory, CCPT-II Variability and CCPT-II Errors of Commission to the Step 2 model was conducted to assess the relationships of these variables with PTSD symptoms, an important test of the developmental traumatology model. Cumulatively adding all neurostructural and neurocognitive variables to the Step 2 model produced a significant overall model, F(12, 126) = 22.56, p < .001, that accounted for an additional 4% (R2Δ = .04, p < .05) of variance above the Step 2 model with an overall R2 = .68 (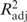 = .65). This change in R2 suggests that adding neurostructural and neurocognitive variables to known correlates of PTSD symptoms, such as demographic and maltreatment-related variables, accounted for a significantly greater amount of variance in total number of PTSS. When examining individual neurostructural and neurocognitive variables, Visual Memory (β = −.16, p < .01) was a significant individual contributor to this model. CCPT-II Errors of Commission (β = .10, p < .06), and Verbal Memory (β = .10, p < .10) achieved marginal significance as contributors to this model.
= .65). This change in R2 suggests that adding neurostructural and neurocognitive variables to known correlates of PTSD symptoms, such as demographic and maltreatment-related variables, accounted for a significantly greater amount of variance in total number of PTSS. When examining individual neurostructural and neurocognitive variables, Visual Memory (β = −.16, p < .01) was a significant individual contributor to this model. CCPT-II Errors of Commission (β = .10, p < .06), and Verbal Memory (β = .10, p < .10) achieved marginal significance as contributors to this model.
Discussion
The purpose of this study was to identify correlates hypothetically linked to PTSS in maltreated children, using the developmental traumatology model. Using hierarchical regression, low SES, the General Maltreatment, and Sexual Abuse Factors were independent correlates of PTSD symptoms. In addition to the variables in the main developmental traumatology model, poorer visual memory was significantly associated with more PTSD symptoms.
Hippocampal volumes did not predict PTSD symptoms in these models. In adults, most studies show smaller hippocampal volumes in individuals with PTSD from a variety of traumas occurring at different developmental time periods (Kitayama, Vaccarino, Kutner, Weiss, & Bremner, 2005). Smaller hippocampal volumes are seen in most adults with PTSD secondary to child abuse (Bremner et al., 2003) and combat (Freeman, Cardwell, Karson, & Komoroski, 1998). However, smaller hippocampal volumes are not seen in children with histories of maltreatment related PTSD (De Bellis, Hall, Boring, Frustaci, & Moritz, 2001; De Bellis, Keshavan, et al., 1999; De Bellis et al., 2002) or who have threshold and subthreshold PTSD (Carrion, Weems, Eliez et al., 2001). Hippocampal volume differences were not seen in survivors of the Nazi Holocaust with and without PTSD, and who were children during the Holocaust, although memory deficits were present (Gollier et al., 2005). It is possible that we were able to demonstrate hippocampal dysfunction (i.e., poorer visual memory) prior to any evidence of smaller MRI measures of the hippocampus for our young population. Smaller hippocampal volumes may be related to a latent effect that manifests in adolescence (Carrion et al., 2007) or adulthood.
Our finding of poorer visual memory may reflect dysfunction of the hippocampus, but not reduced volume. The finding may also reflect dysfunction of other brain regions, such as the right hemisphere (Fjell et al., 2005), left parahippocampal area and the left lingual gyrus (Schmidt et al., 2007) and parietal and prefrontal cortices (Yago & Ishai, 2006). In a developmental study of visual memory of younger and older adults, right cortical volume and hippocampal volume were independent predictors of memory function, leading the authors to conclude that memory function is not uniquely related to the hippocampus (Fjell et al., 2005). Moreover, child maltreatment is a risk for addictions, a frequent lifetime comorbid event in the adults described in most of this existing literature (De Bellis, 2002). It is possible that comorbid alcohol and substance use disorders may play a role through toxicity effects in the outcomes of adult studies of hippocampal volumes and function (De Bellis et al., 2000).
Poorer visual memory was significantly related to more PTSS, which may impair an individual’s ability to focus and maintain a representation of an object or event. This may lead to difficulty processing the traumatic event. Although studies in adult PTSD consistently report poorer visual and verbal memory function in PTSD (Brewin, Kleiner, Vasterling, & Field, 2007; Gollier et al., 2005; Koso & Hansen, 2006) even when controlling for alcohol use disorder history (Samuelson et al., 2006), specific memory indexes are understudied in pediatric PTSD. In one pilot study, children who suffered from maltreatment-related PTSD demonstrated significant deficits in attention and abstract reasoning/executive functions, which involve parietal and prefrontal cortices, when compared with non-maltreated healthy children (Beers & De Bellis, 2002). Long-delay free recall on the California Verbal Learning Test was poorer in these children; however, this finding did not survive after corrections to protect from experiment-wise error. This is similar to our findings here of verbal memory not being predictive of total PTSD symptoms in the hierarchical modeling. In one study of children and adolescents with PTSD, lower verbal memory but no differences in other memory indexes were seen (Yasik, Saigh, Oberfield, & Halamandaris, 2007). In another case–control study of South African adolescents, cognitive deficiencies were seen in attention, visual memory, and nonverbal concept formation that were associated with PTSD symptoms rather than trauma history (Schoeman, Carey, & Seedat, 2009). Our finding of a negative relationship between visual memory and greater number of PTSD symptoms support the adult PTSD literature and most of the limited pediatric PTSD literature to date.
Trauma-focused cognitive behavioral therapy (CBT), the evidenced-based and most studied CBT treatment intervention in children with impairing PTSD symptoms, most likely strengthens an individual’s ability to focus through prefrontal inhibition of amygdala fear conditioned processes. Trauma-focused CBT is not enhanced by the use of psychotropics (Cohen, Mannarino, Perel, & Staron, 2007). Our findings suggest that CBT interventions may further benefit from the use of evidence-based visual memory enhancement interventions, particularly in more symptomatic individuals.
Our finding that more CPT-II Errors of Commission was marginally associated with more PTSD symptoms is interesting in that impulsivity is frequently seen in clinical cases of PTSD, but inhibitory control or impulsivity is not a criteria symptom for PTSD. Perhaps inhibitory control should be evaluated as a criteria symptom for PTSD in children and adolescents. Interventions that are aimed at improving inhibitory control may enhance PTSD treatment.
Since our overall rate of PTSD in maltreated children and adolescents was relatively high, these data show that children are more vulnerable than adults to PTSD symptoms (De Bellis, 2001). This finding is consistent with most of the available literature except for an epidemiological study which used the Child and Adolescent Psychiatric Assessment (Copeland et al., 2007), an instrument that was not specifically designed for evaluating PTSD because it does not use a trauma narrative in its approach. In this epidemiological study, cumulative trauma predicted more depression and generalized anxiety symptoms, symptoms which overlap significantly with PTSD. It is important to identify PTSD in individuals with trauma histories and diagnoses of major depression and generalized anxiety disorder because trauma-focused CBT is an appropriate and specific evidenced-based treatment for these individuals, whereas the treatments for depression with comorbid generalized anxiety are relatively non-specific.
In our cross-sectional study of pediatric maltreatment, we cannot establish causal relationships between traumatic events, PTSD symptoms, and psychobiological differences. Our findings suggest the need for larger samples, longitudinal, and multi-site research by developmentally trained investigators that integrate expert clinical diagnostic evaluations with developmental and cognitive neuroscience. This type of work can include path analysis models, where the strength of these relationships and their interactions could be evaluated with respect to risk and resiliency factors for PTSD symptoms. Developmental traumatology model hypotheses can only be tested in a large sample longitudinal study that will clearly pave the way to better clinical treatment of pediatric PTSD in the future.
Funding
This work was supported by funding from several grants from the National Institute of Mental Health (K24MH071434 and RO1-75 MH61744 and RO1-MH63407 to M.D.D.B.) and one grant from the National Institute on Alcohol Abuse and Alcoholism (R01-AA12479 to M.D.D.B.).
Conflict of interest: None declared.
Acknowledgments
The authors thank the staff of the Duke Healthy Childhood Brain Development Developmental Traumatology Research Program and the participants and their families for making this work possible.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR) Washington, DC: 2000. Author. [Google Scholar]
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychological Medicine. 1995;25:739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Capelli S, Scott T, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Research. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP. Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. Journal of Abnormal Psychology. 2007;116(3):448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Reiss AL. Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;41:166–173. doi: 10.1097/00004583-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: A pilot longitudinal study on youth stress PTSD and the hippocampus. Pediatrics. 2007;119:509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Perel JM, Staron V. A pilot randomized controlled trial of combined trauma-focused CBT and Sertraline for childhood PTSD symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:811–819. doi: 10.1097/chi.0b013e3180547105. [DOI] [PubMed] [Google Scholar]
- Conners DK, MHS S. Conners’ Continuous Performance Test-II. Toronto Ontario: Multi-Health Systems Inc; 2000. [Google Scholar]
- Copeland W, Keeler G, Angold A, Costello E. Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:537–561. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: A contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The neurobiology of posttraumatic stress disorder across the life cycle. In: Soares JC, Gershon S, editors. The handbook of medical psychiatry. NY: Marcel Dekker Inc; 2003. pp. 449–466. [Google Scholar]
- De Bellis MD, Baum A, Birmaher B, Keshavan M, Eccard CH, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology: Part I: Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff P, Boring AM, Hall J, et al. Hippocampal volume in adolescent onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Clark DB, Casey BJ, Giedd J, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology: Part II: Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related PTSD: A sociodemographically matched study. Biological Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Putnam FW. The psychobiology of childhood maltreatment. Child and Adolescent Psychiatric Clinics of North America. 1994;3:663–677. [Google Scholar]
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behavoral Neurosciences. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Dale AM, Quinn BT, et al. Age does not increase rate of forgetting over weeks—neuroanatomical volumes and visual memory across the adult life-span. Journal of the International Neuropsychological Society. 2005;11(1):2–15. doi: 10.1017/S1355617705050046. [DOI] [PubMed] [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Medicine. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- Gollier JA, Yehuda R, De Santi S, Segal S, Dolan S, De Leon MJ. Absence of hippocampal volume differences in survivors of the Nazi Holocaust with and without posttraumatic stress disorder. Psychiatry Research. 2005;139:53–64. doi: 10.1016/j.pscychresns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Jensen V, Rinholm JE, Johansen TJ, Medin T, Storm-Mathisen J, Sagvolden T, et al. N-methyl-D-aspartate receptor subunit dysfunction at hippocampal glutamatergic synapses in an animal model of attention-deficit/hyperactivity disorder. Neuroscience. 2009;158(1):353–364. doi: 10.1016/j.neuroscience.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of Affective Disorders. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kemp SL, Kirk U. Developmental assessment of neuropsychological function with the aid of the NEPSY. In: Kaufman AS, Kaufman NL, editors. Specific learning disabilities and difficulties in children and adolescents: Psychological assessment and evaluation. New York: Cambridge University Press; 2001. [Google Scholar]
- Koso M, Hansen S. Executive function and memory in posttraumatic stress disorder: A study of Bosnian war veterans. Eur Psychiatry. 2006;21:167–173. doi: 10.1016/j.eurpsy.2005.06.004. [DOI] [PubMed] [Google Scholar]
- MacFall J, Byrum C, Parashos I. Relative accuracy and reproducibility of regional MRI brain volumes for point-counting methods. Psychiatry Research. 1994;55:167–177. doi: 10.1016/0925-4927(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Doost H. TN, Taghavi MR, Yule W, Dalgleish T. Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioral Memory test. Journal of Child Psychology and Psychiatry. 1999;40:357–361. [PubMed] [Google Scholar]
- Moradi AR, Taghavi MR, Neshat-Doost HT, Yule W, Dalgleish T. Memory bias for emotional information in children and adolescents with posttraumatic stress disorder: A preliminary study. Journal of Anxiety Disorders. 2000;14:521–532. doi: 10.1016/s0887-6185(00)00037-2. [DOI] [PubMed] [Google Scholar]
- Nolin P, Ethier L. Using neuropsychological profiles to classify neglected children with or without physical abuse. Child Abuse and Neglect. 2007;31:631–643. doi: 10.1016/j.chiabu.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Samuelson KW, Neylan TC, Metzler TJ, Lenoci M, Rothlind J, Henn-Haase C, et al. Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006;20(6):716–726. doi: 10.1037/0894-4105.20.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Krause BJ, Weiss PH, Fink GR, Shah NJ, Amorim MA, et al. Visuospatial working memory and changes of the point of view in 3D space. Neuroimage. 2007;36(3):955–968. doi: 10.1016/j.neuroimage.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Schoeman R, Carey P, Seedat S. Trauma and posttraumatic stress disorder in South African adolescents: a case-control study of cognitive deficits. Journal of Nervous & Mental Disease. 2009;197(4):244–250. doi: 10.1097/NMD.0b013e31819d9533. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Journal of Developmental Neuroscience. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Rev. Ed. San Antonio: Psychological Corporation; 1989. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Yago E, Ishai A. Recognition memory is modulated by visual similarity. Neuroimage. 2006;31(2):807–817. doi: 10.1016/j.neuroimage.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Yasik AE, Saigh PA, Oberfield RA, Halamandaris PV. Posttraumatic stress disorder: memory and learning performance in children and adolescents. Biological Psychiatry. 2007;61(3):382–388. doi: 10.1016/j.biopsych.2006.06.005. [DOI] [PubMed] [Google Scholar]


