Abstract
Objective The purpose of the present article was to systematically review the literature investigating the long-term physical health consequences of childhood sexual abuse (CSA). Methods Literature searches yielded 31 studies comparing individuals with and without a history of CSA on six health outcomes: general health, gastrointestinal (GI) health, gynecologic or reproductive health, pain, cardiopulmonary symptoms, and obesity. Exploratory subgroup analyses were conducted to identify potential methodological moderators. Results Results suggested that a history of CSA was associated with small to moderate group differences on almost all health outcomes assessed, such that individuals with a history of CSA reported more complaints for each health outcome. Suggestive trends in moderating variables of study design and methodology are presented. Conclusions Results highlight the long-term physical health consequences of CSA and identify potential moderators to aid in the design of future research.
A meta-analytic review previously concluded that in North America, ∼30% of girls and 15% of boys experience some form of sexual abuse during their childhood (Bolen & Scannapieco, 1999). Overall, despite some inconsistencies, research has concluded that childhood sexual abuse (CSA) is associated with increased risk for a variety of adult psychological problems (e.g., depression, anxiety disorders, eating disorders; for reviews, see Neumann, Houskamp, Pollack, & Briere, 1996; Paolucci, Genuis, & Violato, 2001; Rind, Tromovitch, & Bauserman, 1998). In addition, adults who were sexually abused as children tend to use health care services more frequently than non-abused adults, spending, on average, $150–245 more per year than adults without such history (Hulme, 2000; Walker, Unutzer et al., 1999). Further, individuals with a CSA history report more doctor and hospital visits than patients without a history of CSA (Newman et al., 2000; Sickel, Noll, Moore, Putnam, & Trickett, 2002; Walker, Unutzer, et al., 1999). This article presents a meta-analysis of studies examining the long-term physical health consequences of CSA, in order to highlight the associations between CSA and specific somatic complaints as well as to systematically evaluate potential moderators of this relationship. Six categories of physical health outcomes were chosen as the focus of the present review: general health, gastrointestinal (GI) health, gynecologic or reproductive health, pain, cardiopulmonary symptoms, and obesity. These six categories were selected because the relationship between CSA and these health conditions has been well documented in a variety of samples. Further, each physical health outcome included in this review has plausible biological and behavioral mechanisms through which CSA has been proposed to influence physical health (for reviews, see Leserman, 2005; Springer, Sheridan, Kuo, & Carnes. 2003).
CSA and Physical Health Outcomes
General Health
Participants with a history of CSA report higher somatization symptoms and more negative perceptions of overall physical health than participants without such history (Najman, Nguyen, & Boyle, 2007; Springs & Friedrich, 1992; Zlotnick et al., 1996). A meta-analysis of seven population-based samples examining the association between CSA and health perceptions concluded that after controlling for sex, ethnicity and depression, participants with a CSA history held more negative perceptions of their overall physical health than participants without abuse histories (Golding, Cooper, & George, 1997). However, two studies have failed to find significant differences in general health reported by participants with and without CSA histories (Runtz, 2002; Walling, O’Hara, et al., 1994).
Gastrointestinal Health
Research conducted with gastroenterology clinic patients has found that 53% of patients with functional (non-organic) GI disorders have a history of CSA compared with 37% of those with organic disorders (Drossman, 1995). Similarly, Talley and colleagues (Talley, Fett, & Zinsmeister, 1995) found that patients with a history of CSA were 1.7 times more likely to suffer from IBS symptoms than those without abuse history. Several studies have suggested that among primary care patients, individuals with a history of CSA are more likely to report experiencing GI symptoms than patients without CSA history (Felitti, 1991; Hulme, 2000; Jamieson & Steege, 1997; Lechner Vogel, Garcia-Shelton, Leichter, & Steibel, 1993).
Gynecologic Health
Research on CSA and women’s health has identified a strong link between CSA and chronic pelvic pain (CPP). Studies of medical samples have generally confirmed that women with CPP report higher rates of CSA than women without CPP (Harrop-Griffiths et al., 1988; Walker et al., 1988; Walling, Reiter, et al., 1994). Analyses of several community, student and nationally representative groups of women have revealed that individuals with a CSA history report more gynecologic symptoms than non-abused comparison groups (Ernst, Angst, & Foldenyi, 1993; Jamieson & Steege, 1997; Springs & Friedrich, 1992). However, these results have not been consistent across all studies, with a number of well-designed studies finding no significant differences in the gynecologic health of women with and without a history of CSA (Lechner et al., 1993; Runtz, 2002; Sickel et al., 2002).
Pain
Researchers have also investigated the association between CSA and other types of pain and pain disorders. For example, CSA experiences predicted greater risk for later musculoskeletal pain symptoms including headaches (Domino & Haber, 1987; Felitti, 1991), back aches (Golding, 1994; Walker, Gelfand, et al., 1999), muscle aches (Newman et al., 2000), fibromyalgia (Walker et al., 1997), joint pain (Walker, Gelfand, et al., 1999), and general pain symptoms (Golding, 1994; Jamieson & Steege, 1997). In contrast, a number of studies have failed to find differences in general pain measures between individuals with and without a history of CSA (Chartier, Walker, & Naimark, 2007; Lechner et al., 1993; Runtz, 2002). Additionally, sex may moderate these findings as two studies have suggested significant effects of CSA on pain in females but not males (Bendixen, Muus, & Schei, 1994; Najman et al., 2007).
Cardiopulmonary Symptoms
Research in community samples has suggested that individuals with a history of CSA are more likely to report experience chest pain, shortness of breath, irregular heartbeat, and ischemic heart disease (Dong et al., 2004; Hulme, 2000; Walker, Gelfand, et al., 1999) as well as overall poorer cardiopulmonary health (Golding, 1994; Goodwin & Stein, 2004) than individuals without CSA history (see Lechner et al., 1993, for an exception).
Obesity
Finally, obesity has been shown to be associated with both CSA history and a number of psychological and physical health problems (for a review, see Gustafson & Sawrer, 2004). Research in a variety of community samples has demonstrated that individuals with a history of CSA are at increased risk for obesity [Body Mass Index (BMI) ≥30; Aaron & Hughes, 2007; Chartier, Walker, & Naimark, 2009; Felitti, 1991; for an exception, see Johnson, Cohen, Kasen, & Brook, 2002]. Noll and colleagues (Noll, Zeller, Trickett, & Putnam, 2007) conducted a prospective, longitudinal study of girls with and without substantiated CSA to evaluate the developmental changes in BMI from childhood to early adulthood. Results revealed that although abused girls increased body mass at a steeper rate than non-abused girls, the groups did not significantly differ in BMI until early adulthood. Sex may also moderate the relationship between CSA and obesity. For example, Mamun and colleagues (2007) found penetrative CSA was associated with increased BMI in women, but not in men.
Psychobiological and Behavioral Factors
Given the breadth of health problems that have been linked to CSA, research has consequently examined a wide range of possible mechanisms for this relationship. Severe traumatic stress at an early age may cause disruption and dysregulation in the neuroendocrine and sympathetic nervous systems (SNS), which impact other body systems (for a review, see Shonkoff, Boyce, & McEwen, 2009). The physical manifestations of these alterations include a variety of health problems (including GI, gynecologic and cardiopulmonary symptoms, pain and obesity), although they may not become apparent until adulthood (see Shonkoff et al., 2009).
Additional behavioral risk factors are more common in CSA victims, including substance use, smoking, risky sex behaviors, and lack of regular exercise (e.g., Chartier et al., 2009; Springs & Friedrich, 1992; Walker, Gelfand, et al., 1999). Psychopathology, such as depression or PTSD, is often reported by adult victims of CSA (see Neumann et al., 1996; Paolucci et al., 2001), and may impact physical health symptoms both directly and indirectly through health behaviors. In sum, a wide range of biological and behavioral mechanisms have been proposed to explain the association between CSA and later physical health.
Methodological Moderators
Although findings have consistently linked CSA and physical health outcomes, there have been notable exceptions. The reasons behind these inconsistencies are unclear, but may reflect methodological differences, such as differences in samples or differing operational definitions of abuse. Although qualitative reviews have suggested a number of explanations for varying effect sizes (e.g., Leserman, 2005; Springer et al., 2003), no study to date has evaluated these factors using meta-analysis. Meta-analysis provides the opportunity to determine whether differences between studies’ methodologies account for meaningful differences in results. The present review examined five methodological factors: type of sample, sex of sample, definition of abuse, method of CSA assessment, and type of comparison group.
Clinical samples (e.g., GI clinic patients, psychiatric outpatients) may inflate the relationship between CSA and health and are not generalizable to the population (e.g., Rind et al., 1998). Community samples may provide a more accurate estimate of the increased health risk associated with CSA history. Sex is another sample characteristic that may influence results. Prevalence rates of CSA are twice as high for girls (Bolen & Scannapieco, 1999), which may help explain why many studies of CSA and health often rely on female samples. When males and females are directly compared, results generally report larger effect sizes in females than males (e.g., Bendixen et al., 1994; Najman et al., 2007). These sex differences may be influenced by various factors such as cultural sex norms or differences in types of abuse experienced.
Prevalence rates and effect sizes are also affected by the way in which abuse is defined. Reviews have noted the lack of standardization and wide range of definitions of CSA (for reviews, see Hulme, 2004; Leserman, 2005). A broad definition of CSA includes acts of sexual contact such as penetration, oral sex, and inappropriate touching as well as sexual acts that do not involve actual contact such as exposure to genitals or pornography. Narrow definitions might include only sexual acts that involve contact, or only specific acts such as penetration. Prevalence rates tend to be higher when broad definitions of CSA are employed, which may affect physical health differences between CSA and comparison groups (e.g., Alami & Kadri, 2004; Leserman, 2005).
Furthermore, the way in which abuse is assessed may be an important methodological factor, although recommendations for assessment are mixed (for a review, see Hulme, 2004). In most cases, CSA is assessed by retrospective self-reports of the victims, either via self-administered questionnaire or interview. Questionnaires may allow for anonymity and reduce participants’ embarrassment or discomfort while interviews allow for the development of rapport and reduction of confusion or incomplete data (see Hulme, 2004; Peters, Wyatt, & Finkelhor, 1986). CSA data may also be collected via review of medical or legal records, though this is much less common. Child abuse often goes unreported, which may be problematic when determining CSA and comparison groups based solely on chart review (e.g., Besharov, 1997). Even within a single study, results obtained through one method of assessment are often substantially different than those obtained through a second method of assessment (e.g., Raphael, Widom, & Lange, 2001).
The final methodological concerns differences in comparison groups. The present review has identified four common types of comparison groups: participants who did not report CSA, participants who did not report sexual abuse (SA) at any point during childhood or adulthood, participants who reported that they had never experienced any type of childhood abuse (CA), and participants who reported other types of abuse such as child physical abuse or less severe sexual abuse than the “CSA group.” If the purpose of research is to determine the impact of CSA on long-term health outcomes, it seems important to strictly define a control group with no abuse history. This limitation appears to be one of the most significant, yet unappreciated, flaws in the methodology of the current literature. A more precise definition of comparison groups would help to clarify the relationship between CSA and health, and provide more opportunity to examine underlying mechanisms of this relationship.
While the relative contributions and specific characteristics of hypothesized mechanisms are not yet well understood, the pathway from CSA to long-term physical health is clearly complex. The current meta-analysis aims to standardize and synthesize the broad literature pertaining to CSA and physical health outcomes and to examine several methodological moderators that have been posited to influence such research in order to suggest future areas of research to further clarify the impact of CSA on physical health.
Hypotheses
The following hypotheses were tested: (1) individuals with a history of CSA would report more general, gastrointestinal, pain, gynecologic, and cardiopulmonary symptoms, and would be more likely to be obese than individuals without a history of CSA; (2) studies comprised of clinical samples would demonstrate greater mean differences between CSA and comparison groups than studies comprised of community samples; (3) analyses of studies including only female participants would yield greater differences between CSA and comparison groups than studies that included male participants; (4) when the definition of abuse included only acts involving contact, rather than non-contact sexual acts (e.g., exposure or harassment), the mean effect sizes would be greater; (5) the more the comparison group excluded participants for lifetime abuse history, the larger the mean group difference would be between CSA and comparison groups. In addition, the present analysis sought to explore the potential moderating effect of CSA assessment method, which may help address the inconsistencies in this literature.
Methods
Selection of Studies
Literature searches were conducted in PsycINFO, MEDLINE, and PILOTS databases to identify appropriate articles. Search terms included various combinations of the following: child(hood), sexual abuse, sexual assault, adversity, physical health, health outcomes, gastrointestinal, gynecologic, cardiovascular, cardiopulmonary, pain, symptoms, obesity, BMI. Reference sections of identified articles were also searched. Authors were contacted and asked to provide any non-significant or unpublished findings regarding CSA and physical health. No additional studies were obtained by this method.
Empirical studies were selected for inclusion based on five criteria. First, articles must have been available in English. Second, participants had to be adults or older adolescents with several years between the abuse and assessment. The average amount of time between abuse and assessment varied greatly among included studies, ranging from approximately seven years (Sickel et al., 2002) to over three decades (Felitti, 1991). Third, acceptable articles must have compared individuals with a history of CSA to an appropriate comparison group. Fourth, studies must have included at least one health outcome variable (either dichotomous or continuous) that fit into one of the following six categories: general health, gastrointestinal health, gynecologic health, musculoskeletal or general pain, cardiopulmonary symptoms, or obesity. Finally, studies must have provided the necessary statistical data for analysis. Based on these criteria, 31 studies were included in the present meta-analysis.
Coding of Studies
For each study, two reviewers coded a number of sample, abuse, group, and statistical characteristics. Type of sample was coded as either clinical or community. Clinical samples included individuals recruited from specialty health facilities (e.g., pain centers), general/primary care facilities, and psychiatric outpatient facilities. Samples derived from health plan members (i.e., HMOs) were coded as community samples unless participants were specifically recruited based on medical condition. In addition to noting sample sizes, studies were coded for whether their samples included both males and females or females only.
Type of assessment was coded as self-report questionnaire, interview (e.g., telephonic or clinical interview), or chart review. One study (Raphael et al., 2001) included CSA data from both self-report and chart review. The self-report data were used in the present review to ensure inclusion of individuals with undocumented CSA. Definition of sexual abuse was coded as either “penetration during childhood”, “sexual contact during childhood” (including penetration, inappropriate touching, etc.), or “any sexual acts during childhood” (including sexual contact and/or non-contact acts such as exposure to pornography or sexual harassment). All abuse definitions included forced or unwanted acts and/or inappropriate age differences between the child and the abuser (generally 5 years). If the definition of abuse was unclear, the study was coded as “any sexual act”. When data were presented separately for multiple types of abuse (e.g., penetration, touching without penetration, no contact) and no aggregate statistics were provided, the most severe form of abuse (generally penetration) was used to define the CSA group.
Comparison groups were considered “No CSA” if group members reported not having a history of CSA. They were coded “No CA” if groups members reported no forms of child abuse (e.g., no CPA or CSA), and “No SA” if comparison group members had no history of SA at any age. In cases where multiple comparison groups were available, the group with the least amount of abuse was selected as the comparison group for meta-analysis.
Additional coded variables included means and standard deviations for continuous measures of each of the six health outcome categories and the number and/or percentage of each group endorsing a dichotomous measure for each of the six health outcome categories. In some studies, statistics were reported separately for a number of symptoms within one health category. In such cases, rather than arbitrarily select one symptom to represent the health outcome, the mean across symptoms was computed (Lipsey & Wilson, 2002). If health outcomes were reported at multiple time points, the time point furthest from the time of abuse was used, in an attempt to examine the most long-term effects possible.
As meta-analytic statistical procedures differ when comparing groups on a continuous versus dichotomous outcome, studies were separated based on the measurement used. Studies included in the meta-analysis are summarized in Table 1. Four studies (Finestone et al., 2000; Newman et al., 2000; Sickel et al., 2002; Walker, Gelfand, et al., 1999) reported both continuous and dichotomous health outcomes, and therefore are included in both sets of analyses. Three studies (Bendixen et al., 1994; Mamun et al., 2007; Najman et al., 2007) reported results separately for males and females, but no aggregate results. As there was no overlap between the samples, we include male and female results as separate studies (Lipsey & Wilson, 2002). Only one study (Runtz 2002) reported continuous data for gynecologic health and no studies reported continuous data for cardiopulmonary health. Therefore, the present analyses were conducted on 10, rather than 12, health outcomes: continuous measures of general health, GI health, pain, and obesity and dichotomous measures of general, GI, gynecologic and cardiopulmonary health, pain, and obesity.
Table 1.
Summary of Studies Included in Meta-Analysis
| Study | Type of sample | Males included | N of CSA group | N of comparison Group | Definition of CSAa | CSA assessment | Type of comparison group |
|---|---|---|---|---|---|---|---|
| Aaron & Hughes (2007) | Community | No | 129 | 287 | Any sexual act before age 18 | Interview | No CSA |
| Bass et al. (1999) | Clinical | Yes | 7 | 41 | Penetration during childhood | Interview | No CSA |
| Bendixen et al. (1994) (female sample) | Community | No | 99 | 411 | Any sexual act before age 18 | Questionnaire | No CSA |
| Bendixen et al. (1994) (male sample) | Community | Yes | 17 | 469 | Any sexual act before age 18 | Questionnaire | No CSA |
| Brown et al. (2005) | Community | Yes | 28 | 572 | Sexual contact before age 18 | Interview | No CA |
| Chartier et al. (2007) | Community | Yes | 798 | 9155 | Any sexual act during childhood | Questionnaire | No CSA |
| Chartier et al. (2009) | Community | Yes | 730 | 7386 | Any sexual act during childhood | Questionnaire | No CSA |
| Dong et al. (2004) | Community | Yes | 3586 | 13,751 | Sexual contact before age 18 | Questionnaire | No CSA |
| Draper et al. (2008) | Clinical | Yes | 1429 | 20,393 | Any sexual act before age 15 | Questionnaire | No CSA |
| Ernst et al. (1993) | Community | No | 25 | 199 | Any sexual act before age 16 | Interview | No CSA |
| Felitti (1991) | Community | Yes | 131 | 100 | Sexual contact during childhood or adolescence | Questionnaire | No CSA |
| Finestone et al. (2000) | Clinical | No | 26 | 54 | Sexual contact before age 16 | Questionnaire | No CSA |
| Goodwin & Stein (2004) | Community | No | 607 | 7556 | Sexual contact before age 18 | Interview | No CSA |
| Grilo et al. (2006) | Clinical | Yes | 44 | 42 | Any sexual act before age 18 | Questionnaire | No CA |
| Hulme (2000) | Clinical | No | 87 | 293 | Sexual contact before age 18 | Questionnaire | No CSA |
| Jamieson & Steege (1997) | Clinical | No | 150 | 354 | Any sexual act before age 14 | Questionnaire | No SA |
| Lechner et al. (1993) | Clinical | No | 136 | 387 | Any sexual act before age 16 | Questionnaire | No CSA |
| Leserman et al. (1996) | Clinical | No | 15 | 200 | Penetration before age 14 | Interview | No SA |
| Mamun et al. (2007) (female sample) | Community | No | 103 | 933 | Penetration before age 16 | Questionnaire | No CSA |
| Mamun et al. (2007) (male sample) | Community | Yes | 95 | 1045 | Penetration before age 16 | Questionnaire | No CSA |
| Najman et al. (2007) (female sample) | Community | No | 109 | 583 | Penetration before age 16 | Interview | No CSA |
| Najman et al. (2007) (male sample) | Community | Yes | 35 | 720 | Penetration before age 16 | Interview | No CSA |
| Newman et al. (2000) | Clinical | No | 112 | 452 | Sexual contact before age 14 | Questionnaire | No CSA |
| Noll et al. (2007) | Community | No | 75 | 81 | Sexual contact before age 17 | CPS referral | No CSA |
| Raphael et al. (2001) | Community | Yes | 291 | 520 | Any sexual act before age 12 | Interview | No CA |
| Runtz (2002) | Community | No | 143 | 627 | Sexual contact before age 18 | Questionnaire | No CSA |
| Sachs-Ericsson et al. (2005) | Community | Yes | 314 | 5317 | Sexual contact before age 15 | Interview | No CA |
| Sickel et al. (2002) | Community | No | 64 | 84 | Sexual contact before age 16 | Interview | No CSA |
| Springs & Friedrich (1992) | Clinical | No | 112 | 398 | Any sexual act before age 18 | Questionnaire | No CSA |
| Thakkar & McCanne (2000) | Community | No | 18 | 27 | Sexual contact before age 15 | Questionnaire | No SA |
| Walker, Gelfand et al. (1999) | Clinical | No | 201 | 698 | Any sexual act before age 17 | Questionnaire | No CA |
| Williamson et al. (2002) | Community | Yes | 836 | 10,317 | Penetration before age 18 | Questionnaire | No CSA |
| Wilsnack et al. (1997) | Clinical | No | 278 | 691 | Any sexual act before age 18 | Interview | No CSA |
| Zlotnick et al. (1996) | Clinical | No | 74 | 34 | Sexual contact before age 16 | Questionnaire | No CSA |
Note. CSA: childhood sexual abuse; SA: sexual abuse; CA: childhood abuse.
aAll definitions imply some level of force and/or inappropriate age differences between child and abuser.
Data Analysis
Statistical analyses were based on the recommendations of Lipsey and Wilson (2002). A standardized mean difference effect size (d) was calculated for each continuous health outcome in each study by subtracting the mean of the comparison group from the mean of the CSA group and dividing by the pooled standard deviation. The pooled standard deviation was obtained with the formula 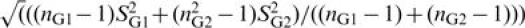 where nG1 is the number of participants in the CSA group, sG1 is the standard deviation of the CSA group, nG2 is the number of participants in the comparison group, and sG2 is the standard deviation of the comparison group. As this estimation of d has been shown to be biased in small samples, estimates of d have been corrected with the formula d = (1 − 3/4N − 9)*(X1 − X2)/sp, where N is the total sample size, X1 is the CSA group mean, X2 is the comparison group mean, and sp is the pooled standard deviation (Hedges, 1981). The standard error (SEd) was calculated for each d with the formula SEd =
where nG1 is the number of participants in the CSA group, sG1 is the standard deviation of the CSA group, nG2 is the number of participants in the comparison group, and sG2 is the standard deviation of the comparison group. As this estimation of d has been shown to be biased in small samples, estimates of d have been corrected with the formula d = (1 − 3/4N − 9)*(X1 − X2)/sp, where N is the total sample size, X1 is the CSA group mean, X2 is the comparison group mean, and sp is the pooled standard deviation (Hedges, 1981). The standard error (SEd) was calculated for each d with the formula SEd = 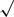 (nG1 + nG2)/nG1*nG2 + d2/2(nG1 + nG2) where nG1 is the number of participants in the CSA group and nG2 is the number of participants in the comparison group.
(nG1 + nG2)/nG1*nG2 + d2/2(nG1 + nG2) where nG1 is the number of participants in the CSA group and nG2 is the number of participants in the comparison group.
A logged odds ratio (LOR) was calculated for each dichotomous health outcome in each study by taking the natural logarithm of a*e/b*c where a is the number of people in the CSA group endorsing the health outcome, b is the number of people in the CSA group not endorsing the health outcome, c is the number of people in the comparison group endorsing the health outcome, and e is the number of people in the comparison group not endorsing the health outcome. The standard error was computed for each LOR (SELOR) using the formula SELOR = 1/a + 1/b + 1/c + 1/e. Weighted effect sizes (d+ and LOR+) were then calculated by multiplying the effect size (d or LOR) by the inverse variance weight (w) for each study.
Mean effect sizes were also calculated by summing the weighted effect sizes for each continuous and dichotomous health outcome and dividing by the sum of the inverse variance weights. The standard error (SE) of this mean effect size is calculated by the formula SE = 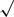 1/∑wi, where wi is the inverse variance weight associated with effect size i with i representing all effect sizes for this outcome (Hedges & Olkin, 1985). The statistical significance of the mean effect size is calculated by dividing the absolute value of the mean effect size by the SE of the effect size, and comparing the value to a critical value in the z table. Homogeneity of the effect size distribution for each mean effect size was calculated using the Q statistic, which follows the chi-square distribution. Homogeneous distributions used the fixed effects model of meta-analysis (Lipsey & Wilson, 2002). Lipsey and Wilson (1993) have provided recommendations for the interpretation of standardized mean difference effect scores in meta-analysis; effect sizes less than .30 are considered small, those equal to .50 are considered medium, and those greater than .67 are considered large. Logged odds ratios were converted back to odd’s ratios for ease of interpretation.
1/∑wi, where wi is the inverse variance weight associated with effect size i with i representing all effect sizes for this outcome (Hedges & Olkin, 1985). The statistical significance of the mean effect size is calculated by dividing the absolute value of the mean effect size by the SE of the effect size, and comparing the value to a critical value in the z table. Homogeneity of the effect size distribution for each mean effect size was calculated using the Q statistic, which follows the chi-square distribution. Homogeneous distributions used the fixed effects model of meta-analysis (Lipsey & Wilson, 2002). Lipsey and Wilson (1993) have provided recommendations for the interpretation of standardized mean difference effect scores in meta-analysis; effect sizes less than .30 are considered small, those equal to .50 are considered medium, and those greater than .67 are considered large. Logged odds ratios were converted back to odd’s ratios for ease of interpretation.
Moderation analyses were conducted to assess the impact of the above-mentioned variables on the relationship between CSA and health. Subgroups were created for each moderator, and mean effect sizes, standard errors, Q statistics and confidence intervals were created for each subgroup. A variable was considered to have a moderating effect if the variance was lower in the subgroups than it was in the combined analysis and/or if the mean effect size differed between the subgroups (Hunter & Schmidt, 2004).
Results
Overall Effects
Weighted and unweighted effect sizes for each study are presented in Tables 2 and 3 (insert hyperlink here). Analysis of mean effect sizes (displayed in Table 4) revealed significant small to moderate group differences for all but two analyses. Continuous measures of general health and pain outcomes yielded significant positive group differences, such that victims of CSA experienced significantly more health complaints (e.g., frequency, intensity) than comparison groups. Mean group differences were not significant for continuous measures of GI symptoms or obesity. Mean effect sizes of all dichotomous health outcomes also demonstrated significant group differences. Odds ratios revealed that individuals with a history of CSA are 1.35–2.12 times more likely to endorse health outcomes than individuals from comparison groups. Examination of the Q statistics revealed that the distributions for all 10 health categories were homogeneous, suggesting that there was not substantial between-study variation. Therefore, the fixed effects model was appropriate for the present analyses.
Table 2.
Weighted and Unweighted Continuous Effect Sizes for Each Study
| Study | General d + (d) | GI d + (d) | Pain d + (d) | Obesity d + (d) |
|---|---|---|---|---|
| Bass et al. (1999) | 2.80 (1.19) | – | – | – |
| Finestone et al. (2000) | – | – | 3.62 (.90) | – |
| Grilo et al. (2006) | – | – | – | −.82 (−.18) |
| Leserman et al. (1996) | 1.16 (.31) | – | 1.27 (.34) | – |
| Mamun et al. (2007) (female sample) | – | – | – | 3.50 (.36) |
| Mamun et al. (2007) (male sample) | – | – | – | −.63 (−.07) |
| Najman et al. (2007) (female sample) | 3.78 (.40) | – | 6.18 (.65) | – |
| Najman et al. (2007) (male sample | 2.89 (.50) | – | 0.69 (.12) | – |
| Newman et al. (2000) | 5.09 (.54) | 4.79 (.51) | 4.35 (.46) | – |
| Raphael et al. (2001) | – | – | 5.43 (.40) | – |
| Runtz (2002) | 2.01 (.19) | 1.21 (.11) | 1.70 (.16) | – |
| Sickel et al. (2002) | – | 2.92 (.49) | 0.07 (.01) | 2.38 (.40) |
| Spring & Friedrich (1992) | 2.50 (.27) | – | – | – |
| Thakkar & McCanne (2000) | 0.73 (.22) | – | – | – |
| Walker, Gelfand et al. (1999) | 5.50 (.44) | – | 5.79 (.47) | – |
| Zlotnick et al. (1996) | 2.94 (.62) | – | – | – |
Note. GI: gastrointestinal health.
Table 3.
Weighted and Unweighted Dichotomous Effect Sizes for Each Study
| Study | General LOR + (OR) | GI LOR + (OR) | Gyn LOR + (OR) | Pain LOR + (OR) | CP LOR + (OR) | Obesity LOR + (OR) |
|---|---|---|---|---|---|---|
| Aaron & Hughes (2007) | – | – | – | – | – | 13.56 (2.00) |
| Bendixen et al. (1994) (female sample) | – | – | 9.09 (1.65) | 5.98 (1.59) | – | – |
| Bendixen et al. (1994) (male sample) | – | – | −0.40 (.65) | 0.61 (1.35) | – | – |
| Brown et al. (2005) | – | – | – | 9.28 (4.66) | – | – |
| Chartier et al. (2007) | 73.00 (1.66) | – | – | 15.77 (1.25) | – | – |
| Chartier et al. (2009) | – | – | – | – | – | 43.99 (1.59) |
| Dong et al. (2004 | – | – | – | – | 81.09 (1.31) | – |
| Draper et al. (2008) | 99.14 (1.35) | – | – | – | 38.65 (1.25) | – |
| Ernst et al. (1993) | – | – | 4.64 (2.37) | – | – | – |
| Felitti (1991) | – | 3.72 (2.78) | – | 10.67 (2.45) | – | 16.96 (4.06) |
| Finestone et al. (2000) | – | – | – | 4.24 (2.95) | – | – |
| Goodwin & Stein (2004) | – | 12.83 (2.02) | – | 22.03 (1.60) | 22.53 (1.81) | – |
| Hulme (2000) | – | 7.70 (1.93) | 6.79 (2.11) | 9.51 (1.81) | 11.11 (3.66) | – |
| Jamieson & Steege (1997) | – | 3.49 (2.70) | 17.17 (1.84) | 11.66 (1.64) | – | – |
| Lechner et al. (1993) | – | 5.07 (3.52) | 8.50 (1.78) | 10.09 (1.91) | 2.50 (1.68) | – |
| Newman et al. (2000) | – | 2.35 (1.71) | – | 10.97 (1.66) | – | – |
| Noll et al. (2007) | – | – | – | – | – | 5.29 (1.84) |
| Sachs-Ericsson et al. (2005) | 39.60 (1.82) | – | – | – | – | – |
| Sickel et al. (2002) | – | – | 4.10 (1.79) | – | – | 5.58 (2.33) |
| Walker, Gelfand et al. (1999) | – | 2.03 (1.63) | 22.87 (1.89) | 19.20 (1.92) | 12.87 (2.78) | 24.19 (1.96) |
| Williamson et al. (2002) | – | – | – | – | – | 81.19 (1.61) |
| Wilsnack et al. (1997) | – | – | 25.74 (1.94) | – | – | – |
Note. GI: gastrointestinal health; Gyn: gynecologic health; CP: cardiopulmonary health.
Table 4.
Overall Mean Effect Sizes for Health Outcomes
| Health Outcome | K | ES + | SE | z | 95% CI |
|---|---|---|---|---|---|
| Continuous health outcomesa | |||||
| General health | 10 | .41 | .12 | 3.48** | .18–.64 |
| GI symptoms | 3 | .34 | .20 | 1.74 | −.04–.72 |
| Pain | 9 | .39 | .12 | 3.36** | .15–.61 |
| Obesity | 4 | .15 | .18 | .82 | −.21–.51 |
| Dichotomous health outcomesb | |||||
| General health | 3 | .39 (1.48) | .04 | 9.10** | .31–.47 |
| GI symptoms | 7 | .75 (2.12) | .14 | 5.28** | .47–1.03 |
| Gynecologic symptoms | 9 | .64 (1.90) | .08 | 7.94** | .48–.80 |
| Pain | 12 | .50 (1.65) | .06 | 8.05** | .38–.62 |
| Cardiopulmonary symptoms | 6 | .31 (1.36) | .04 | 7.26** | .23–.39 |
| Obesity | 7 | .55 (1.73) | .05 | 10.23** | .44–.66 |
Note. GI: gastrointestinal; K: number of studies; ES+: weighted effect size.
aES+: d+; bES+: LOR+ (OR).
**p < .01.
Moderation Effects
Moderation is generally not tested if the effect size distribution is homogeneous. However, heterogeneity may be masked by the small number of studies included in the present review (Lipsey & Wilson, 2002), and therefore, exploratory moderation analyses were conducted to identify suggestive trends. Analyses were conducted on each of the health outcomes only when at least two studies represented each subgroup. Most moderation analyses were conducted with small subgroups, and should be interpreted with caution.
First, subgroup variance was compared with the variance of the overall group analysis for each health outcome possible. Results did not reveal a meaningful difference in variance between analyses for any of the moderators, suggesting there were no statistically significant moderation effects. However, in exploratory analyses, overall mean effect sizes of the subgroup analyses were examined as these observations may inform future research. With the exception of GI symptoms, mean effect sizes for clinical samples were substantially higher than those for community samples, suggesting that the effect sizes (both standardized mean differences and odds ratios) of health problems between individuals with and without a history of CSA are larger for clinical samples than community samples. Subgroup analyses for sex, abuse definition, method of CSA assessment, and type of comparison group did not yield suggestive findings.
Discussion
Results of the present meta-analysis support the conclusions of many qualitative reviews (e.g., Leserman, 2005; Springer et al., 2003) by demonstrating that CSA was systematically related to higher rates of subsequent physical health symptoms, including general health, GI, gynecologic, pain and cardiopulmonary symptoms, and obesity. The only exceptions to these overall significant results were gastrointestinal symptoms and obesity assessed via continuous measures. Though results were not significant, the effect size for continuous GI symptoms was still small–moderate, and non-significance likely stemmed from lack of power. Furthermore, it is interesting to note the difference in significance between the continuous and dichotomous health outcomes. Although it is not possible to directly compare continuous and dichotomous outcomes in the present analyses, it may be that methodological differences (e.g., continuous versus dichotomous assessment) may account for the observed differences in findings.
The small number of studies that met criteria for inclusion in the present meta-analysis was somewhat surprising considering the large body of literature on CSA and long-term health. While qualitative reviews are able to make broad assessments of a methodologically disparate literature, the present analysis was more constrained by the requirements of meta-analysis. The relatively small number of studies that were appropriate for the present review highlights a potential weakness in the current literature on CSA and long-term health consequences, and suggests a need for more empirical studies, particularly for certain health outcomes such as cardiopulmonary symptoms.
Several meta-analyses (e.g., Neumann et al., 1996; Paolucci et al., 2001; Rind et al., 1998) have been conducted to evaluate the impact of CSA on a broad range of psychological health outcomes while the present review is the first, to our knowledge, to conduct a quantitative review of the impact of CSA on several long-term physical health outcomes. Clearly the cost to both quality of life and health care utilization associated with these physical health problems creates a need to thoroughly examine mechanisms and moderators of this relationship. The present review provides a systematic evaluation of CSA and physical health and demonstrates the potential for growth and improvement in this field.
As a relatively new topic of interest, there are large methodological discrepancies between studies. This lack of standardization creates difficulty in generalizing across studies and interpreting inconsistent findings. Therefore, the consistent homogeneity of the effect size distributions was an unexpected finding. This homogeneity suggests that the variance between studies is due to random error rather than study characteristics or methodological differences.
Moderation analyses are typically recommended when studies are heterogeneous, but may also be appropriate if there are large ranges in effect sizes and when heterogeneity may be masked by small sample sizes. Therefore, exploratory moderation subgroup analyses were conducted. Results did not meet the requirements for significance. However, examination of differences between mean effect sizes in subgroup analyses can identify potential trends and suggest areas of focus for future research.
Subgroup analyses comparing clinical and community samples revealed that the differences in health between CSA and comparison groups were larger in clinical samples than in community samples, suggesting that caution should be taken in generalizing findings from one recruitment setting to the other. Analysis of sex, abuse definition, method of CSA assessment, and type of comparison group did not reveal any clear trends that would improve our understanding of inconsistencies in the literature.
The division and definition of subgroups were quite broad in the present review, which was restricted by available studies. As more empirical evidence of the association between CSA and physical health is collected, it will become possible to form more specific groups to further explore methodological moderators. For example, rather than a simple division between self-administered questionnaire and interview to define method of CSA assessment, it may be more beneficial to examine specific types of assessment (i.e., telephone interview, face-to-face interview, anonymous postal questionnaire, questionnaire self-administered in the presences of a researcher) as these may have very different methodological implications than those observed in the collapsed groups (see Hulme, 2004). In addition, a larger number of studies would allow for examination of interactions between moderators. For example, perhaps method of CSA assessment interacts with the definition of abuse, such that certain methods of assessment are most appropriate for certain definitions or impact of assessment may vary based on the sex. Future analyses could benefit from these more complex considerations of methodological factors which may help explain inconsistencies and improve future research design.
The results of the present analyses clearly demonstrate the greatest limitation; small sample size, particularly with moderation analyses. Although this limits conclusions that may be drawn from the present analyses, it highlights a concern regarding this literature as a whole. Although the present study supports the conclusions of qualitative reviews regarding the long-term health consequences of CSA (e.g., Leserman, 2005; Springer et al., 2003), the present analysis suggests that while there may be a variety of sources available regarding the association between CSA and later health outcomes, the methodologies and statistics are not comparable across studies. Moderation analyses highlighted some potential areas for future exploration, and the literature would benefit from increased attention to these factors. Overall, the present review supports the impact of CSA history on long-term physical health consequences, and identifies methodological factors that should be considered more carefully in future research.
Funding
National Institute of Mental Health (grant R34MH073014, partial) (to D.L.D.).
References
*Indicates studies included in meta-analysis.
- Aaron DJ, Hughes TL. Association of childhood sexual abuse with obesity in a community sample of lesbians. Obesity. 2007;15:1023–1028. doi: 10.1038/oby.2007.634. [*] [DOI] [PubMed] [Google Scholar]
- Alami KM, Kadri N. Moroccan women with a history of child sexual abuse and its long-term repercussions: A population-based epidemiological study. Archives of Women’s Mental Health. 2004;7:237–242. doi: 10.1007/s00737-004-0061-9. [DOI] [PubMed] [Google Scholar]
- Bass C, Bond A, Gill D, Sharpe M. Frequent attenders without organic disease in a gastroenterology clinic. General Hospital Psychiatry. 1999;21:30–38. doi: 10.1016/s0163-8343(98)00062-0. [*] [DOI] [PubMed] [Google Scholar]
- Bendixen M, Muus KM, Schei B. The impact of child sexual abuse: A study of a random sample of Norwegian students. Child Abuse and Neglect. 1994;18:837–847. doi: 10.1016/0145-2134(94)90063-9. [*] [DOI] [PubMed] [Google Scholar]
- Besharov DJ. Overreporting and underreporting are twin problems. In: Gelles RJ, Loseke DR, editors. Current controversies in family violence. Newbury Park, CA: Sage Publications; 1997. pp. 257–272. [Google Scholar]
- Bolen RM, Scannapieco M. Prevalence of child sexual abuse: A corrective meta analysis. Social Science Review. 1999;73:281–313. [Google Scholar]
- Brown J, Berenson K, Cohen P. Documented and self-reported child abuse and adult pain in a community sample. Clinical Journal of Pain. 2005;21:374–377. doi: 10.1097/01.ajp.0000149797.16370.dc. [*] [DOI] [PubMed] [Google Scholar]
- Chartier MJ, Walker JR, Naimark B. Childhood abuse, adult health, and health care utilization: Results from a representative community sample. American Journal of Epidemiology. 2007;165:1031–1038. doi: 10.1093/aje/kwk113. [*] [DOI] [PubMed] [Google Scholar]
- Chartier MJ, Walker JR, Naimark B. Health risk behaviors and mental health problems as mediators of the relationship between childhood abuse and adult health. American Journal of Public Health. 2009;99:847–854. doi: 10.2105/AJPH.2007.122408. [*] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino JV, Haber JD. Prior physical and sexual abuse in women with chronic headache: Clinical correlates. Headache. 1987;27:310–314. doi: 10.1111/j.1526-4610.1987.hed2706310.x. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [*] [DOI] [PubMed] [Google Scholar]
- Draper B, Pfaff JJ, Pirkus J, Snowdon J, Lautenschlager NT, Wilson I, et al. Long-term effects of childhood abuse on the quality of life and health of older people: Results from the depression and early prevention of suicide in general practice project. Journal of the American Geriatric Society. 2008;56:262–271. doi: 10.1111/j.1532-5415.2007.01537.x. [*] [DOI] [PubMed] [Google Scholar]
- Drossman DA. Sexual and physical abuse and gastrointestinal illness. Scandinavian Journal of Gastroenterology. 1995;30(Suppl. 208):90–96. doi: 10.3109/00365529509107768. [DOI] [PubMed] [Google Scholar]
- Ernst C, Angst J, Foldenyi M. The Zurich Study XVII. Sexual abuse in childhood. Frequency and relevance for adult morbidity data of a longitudinal epidemiological study. European Archives of Psychiatry and Clinical Neuroscience. 1993;242:293–300. doi: 10.1007/BF02190389. [*] [DOI] [PubMed] [Google Scholar]
- Felitti VJ. Long-term medical consequences of incest, rape and molestation. Southern Medical Journal. 1991;84:328–331. doi: 10.1097/00007611-199103000-00008. [*] [DOI] [PubMed] [Google Scholar]
- Finestone HM, Stenn P, Davies F, Stalker C, Fry R, Koumanis J. Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse & Neglect. 2000;24:547–556. doi: 10.1016/s0145-2134(00)00112-5. [*] [DOI] [PubMed] [Google Scholar]
- Golding JM. Sexual assault history and physical health in randomly selected Los Angeles women. Health Psychology. 1994;13:130–138. doi: 10.1037//0278-6133.13.2.130. [DOI] [PubMed] [Google Scholar]
- Golding JM, Cooper LM, George LK. Sexual assault history and health perceptions: Seven general population studies. Health Psychology. 1997;16:417–425. doi: 10.1037//0278-6133.16.5.417. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychological Medicine. 2004;34:509–520. doi: 10.1017/s003329170300134x. [*] [DOI] [PubMed] [Google Scholar]
- Grilo CM, White MA, Masheb RM, Rothschild BS, Burke-Martindale CH. Relation of childhood sexual abuse and other forms of maltreatment to 12-month postoperative outcomes in extremely obese gastric bypass patients. Obesity Surgery. 2006;16:454–460. doi: 10.1381/096089206776327288. [*] [DOI] [PubMed] [Google Scholar]
- Gustafson TB, Sarwer DB. Childhood sexual abuse and obesity. Obesity Reviews. 2004;5:129–135. doi: 10.1111/j.1467-789X.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- Harrop-Griffiths J, Katon W, Walker E, Holm L, Russo J, Hickok L. The association between chronic pelvic pain, psychiatric diagnoses, and childhood sexual abuse. Obstetrics and Gynecology. 1988;71:589–594. [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107–128. [Google Scholar]
- Hedges LV, Olkin L. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Hulme PA. Symptomatology and health care utilization of women primary care patients who experienced childhood sexual abuse. Child Abuse & Neglect. 2000;24:1471–1484. doi: 10.1016/s0145-2134(00)00200-3. [*] [DOI] [PubMed] [Google Scholar]
- Hulme PA. Retrospective measurement of childhood sexual abuse: A review of instruments. Child Maltreatment. 2004;9:210–217. doi: 10.1177/1077559504264264. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Schmidt FL. Methods of meta-analysis. Thousand Oaks, CA: Sage Publications; 2004. [Google Scholar]
- Jamieson DJ, Steege JF. The association of sexual abuse with pelvic pain complaints in a primary care population. American Journal of Obstetrics and Gynecology. 1997;177:1408–1412. doi: 10.1016/s0002-9378(97)70083-8. [*] [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Kasen S, Brook JS. Childhood adversities associated with risk for eating disorders or weight problems during adolescence or early adulthood. American Journal of Psychiatry. 2002;159:394–400. doi: 10.1176/appi.ajp.159.3.394. [DOI] [PubMed] [Google Scholar]
- Lechner ME, Vogel ME, Garcia-Shelton LM, Leichter JL, Steibel KR. Self reported medical problems of adult female survivors of childhood sexual abuse. Journal of Family Practice. 1993;36:633–638. [*] [PubMed] [Google Scholar]
- Leserman J. Sexual abuse history: Prevalence, health effects mediators and psychological treatment. Psychosomatic Medicine. 2005;67:906–915. doi: 10.1097/01.psy.0000188405.54425.20. [DOI] [PubMed] [Google Scholar]
- Leserman J, Drossman DA, Li Z, Toomey TC, Naghman G, Glogau L. Sexual and physical abuse history in gastroenterology practice: How types of abuse impact health status. Psychosomatic Medicine. 1996;58:4–15. doi: 10.1097/00006842-199601000-00002. [*] [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. The efficacy of psychological, educational and behavioral treatment: Confirmation from meta-analysis. American Psychologist. 1993;48:1181–1209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DR. Practical meta-analysis. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Mamun AA, Lawlor DA, O’Callaghan MJ, Bor W, Williams GM, Najman JM. Does childhood sexual abuse predict young adult’s BMI? A birth cohort study. Obesity. 2007;15:2103–2110. doi: 10.1038/oby.2007.250. [*] [DOI] [PubMed] [Google Scholar]
- Najman JM, Nguyen M. LT, Boyle FM. Sexual abuse in childhood and physical and mental health in adulthood: An Australian population study. Archives of Sexual Behavior. 2007;36:666–675. doi: 10.1007/s10508-007-9180-5. [*] [DOI] [PubMed] [Google Scholar]
- Neumann DA, Houskamp BM, Pollock VE, Briere J. The long-term sequalae of childhood sexual abuse in women: A meta-analytic review. Child Maltreatment. 1996;1:1–16. [Google Scholar]
- Newman MG, Clayton L, Zuellig A, Cashman L, Arnow B, Dea R, et al. The relationship of childhood sexual abuse and depression with somatic symptoms and medical utilization. Psychological Medicine. 2000;30:1063–1077. doi: 10.1017/s003329179900272x. [*] [DOI] [PubMed] [Google Scholar]
- Noll JG, Zeller MH, Trickett PK, Putnam FW. Obesity risk for female victims of childhood sexual abuse: A prospective study. Pediatrics. 2007;120:e61–e67. doi: 10.1542/peds.2006-3058. [*] [DOI] [PubMed] [Google Scholar]
- Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. Journal of Psychology. 2001;135:17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- Peters SD, Wyatt GE, Finkelhor D. Prevalence. In: Finkelhor D, editor. A sourcebook on child sexual abuse. Beverly Hills, CA: Sage Publications; 1986. pp. 15–59. [Google Scholar]
- Raphael KG, Widom CS, Lange G. Childhood victimization and pain in adulthood: A prospective investigation. Pain. 2001;92:283–293. doi: 10.1016/s0304-3959(01)00270-6. [*] [DOI] [PubMed] [Google Scholar]
- Rind B, Tromovitch P, Bauserman R. A meta-analytic examination of assumed properties of child sexual abuse using college samples. Psychological Bulletin. 1998;124:22–53. doi: 10.1037/0033-2909.124.1.22. [DOI] [PubMed] [Google Scholar]
- Runtz MG. Health concerns of university women with a history of child physical and sexual maltreatment. Child Maltreatment. 2002;7:241–253. doi: 10.1177/1077559502007003006. [*] [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Blazer D, Plant EA, Arnow B. Childhood sexual and physical abuse and the 1-year prevalence of medical problems in the National Comorbidity Survey. Health Psychology. 2005;24:32–40. doi: 10.1037/0278-6133.24.1.32. [*] [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities. Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sickel AE, Noll JG, Moore PJ, Putnam F, Trickett PK. The long-term physical health and healthcare utilization of women who were sexually abused as children. Journal of Health Psychology. 2002;7:583–597. doi: 10.1177/1359105302007005677. [*] [DOI] [PubMed] [Google Scholar]
- Springs FE, Friedrich WN. Health risk behaviors and medical sequalae of childhood sexual abuse. Mayo Clinic Proceedings. 1992;67:527–532. doi: 10.1016/s0025-6196(12)60458-3. [*] [DOI] [PubMed] [Google Scholar]
- Springer KW, Sheridan J, Kuo D, Carnes M. The long-term health outcomes of childhood abuse. An overview and a call to action. Journal of General Internal Medicine. 2003;18:864–870. doi: 10.1046/j.1525-1497.2003.20918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: Association with irritable bowel-type symptoms. American Journal of Gastroenterology. 1995;90:366–371. [PubMed] [Google Scholar]
- Thakkar RR, McCanne TR. The effects of daily stressors on physical health in women with and without a childhood history of sexual abuse. Child Abuse & Neglect. 2000;24:209–221. doi: 10.1016/s0145-2134(99)00129-5. [*] [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, et al. Adult health status of women with histories of childhood abuse and neglect. American Journal of Medicine. 1999;107:332–339. doi: 10.1016/s0002-9343(99)00235-1. [*] [DOI] [PubMed] [Google Scholar]
- Walker EA, Katon W, Harrop-Griffiths J, Holm L, Russo J, Hickok LR. Relationship of chronic pelvic pain to psychiatric diagnoses and childhood sexual abuse. American Journal of Psychiatry. 1988;145:75–80. doi: 10.1176/ajp.145.1.75. [DOI] [PubMed] [Google Scholar]
- Walker EA, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: II. Sexual, physical and emotional abuse and neglect. Psychosomatic Medicine. 1997;59:572–577. doi: 10.1097/00006842-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health cares use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry. 1999;56:609–613. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstetrics & Gynecology. 1994;84:200–206. [PubMed] [Google Scholar]
- Walling MK, Reiter RC, O’Hara MW, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: I. Prevalences of sexual abuse and physical abuse. Obstetrics and Gynecology. 1994;84:193–199. [PubMed] [Google Scholar]
- Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti V. Body weight and obesity in adults and self-reported abuse in childhood. International Journal of Obesity. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [*] [DOI] [PubMed] [Google Scholar]
- Wilsnack SC, Vogeltanz ND, Klassen AD, Harris TR. Childhood sexual abuse and women’s substance abuse: National survey findings. Journal of Studies on Alcohol. 1997;58:264–271. doi: 10.15288/jsa.1997.58.264. [*] [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Zakriski AL, Shea MT, Costello E, Begin A, Pearlstein T, et al. The long-term sequalae of sexual abuse: Support for a complex posttraumatic stress disorder. Journal of Traumatic Stress. 1996;9:195–205. doi: 10.1007/BF02110655. [*] [DOI] [PubMed] [Google Scholar]


