Pharmacological and genetic impairments of phospholipase A2 (PLA2) caused anatomical alterations of the trans-Golgi side and defects in trafficking of auxin-transporting PIN proteins to the plasma membrane in Arabidopsis root epidermal cells. The results implicate PLA2-mediated lipid hydrolysis in PIN trafficking.
Abstract
Phospholipase A2 (PLA2), which hydrolyzes a fatty acyl chain of membrane phospholipids, has been implicated in several biological processes in plants. However, its role in intracellular trafficking in plants has yet to be studied. Here, using pharmacological and genetic approaches, the root hair bioassay system, and PIN-FORMED (PIN) auxin efflux transporters as molecular markers, we demonstrate that plant PLA2s are required for PIN protein trafficking to the plasma membrane (PM) in the Arabidopsis thaliana root. PLA2α, a PLA2 isoform, colocalized with the Golgi marker. Impairments of PLA2 function by PLA2α mutation, PLA2-RNA interference (RNAi), or PLA2 inhibitor treatments significantly disrupted the PM localization of PINs, causing internal PIN compartments to form. Conversely, supplementation with lysophosphatidylethanolamine (the PLA2 hydrolytic product) restored the PM localization of PINs in the pla2α mutant and the ONO-RS-082–treated seedling. Suppression of PLA2 activity by the inhibitor promoted accumulation of trans-Golgi network vesicles. Root hair–specific PIN overexpression (PINox) lines grew very short root hairs, most likely due to reduced auxin levels in root hair cells, but PLA2 inhibitor treatments, PLA2α mutation, or PLA2-RNAi restored the root hair growth of PINox lines by disrupting the PM localization of PINs, thus reducing auxin efflux. These results suggest that PLA2, likely acting in Golgi-related compartments, modulates the trafficking of PIN proteins.
INTRODUCTION
Phospholipases are classified into four functional groups, PLA (PLA1 and PLA2), PLB, PLC, and PLD, depending on the phospholipid site they hydrolyze, and they have long been known to play key roles in diverse cellular signaling processes by producing second messengers (Brown et al., 2003). In particular, PLC and PLD enzymes have been intensively studied for their roles in intracellular membrane trafficking in mammalian cells. Recently, Arabidopsis thaliana PLDζ2, a PLD isoform, was shown to be implicated in the trafficking of the PIN-FORMED2 (PIN2) auxin-transporting plasma membrane (PM) protein (Li and Xue 2007). The role of phospholipase A2 (PLA2; EC 3.1.1.4; catalyzes the hydrolysis of acyl chains at the sn-2 position) proteins in membrane trafficking has been studied in animal cells, although this role is not well understood (Brown et al., 2003). On the other hand, plant PLA2s have scarcely been investigated with respect to membrane trafficking.
The superfamily of PLA2 enzymes consists of 15 groups and many subgroups, and they are divided into five distinct classes: secreted PLA2s, cytosolic PLA2s, Ca2+-independent PLA2s, platelet-activating factor acetylhydrolases, and lysosomal PLA2s (Schaloske and Dennis, 2006). Plant PLA2s are classified into two groups: low molecular weight PLA2s (PLA2α, β, γ, and δ) and patatin-like PLAs that have both PLA1 (catalyzes hydrolysis at the sn-1 position) and PLA2 activities (Ryu, 2004; Lee et al., 2005). Plant PLA2s play roles in cell elongation, auxin response, gravitropism, guard cell movement, and defense (Ryu, 2004; Seo et al., 2008). In this study, we present a novel function of plant PLA2s in the trafficking of auxin efflux transporter PIN proteins.
Three groups of auxin-transporting proteins have been characterized: AUXIN-RESISTANT1 (AUX1)/LAX (LIKE-AUX1) for influx, and PIN and the P-glycoprotein of ABCB (ATP binding cassette-type transporter subfamily B) for efflux (for recent review, Vanneste and Friml, 2009). PINs localize asymmetrically to the PM and play key roles in establishing local auxin gradients and thus in modulating organogenesis and tropisms. PIN proteins not only traffic to the PM after de novo synthesis, but also undergo endocytosis from and are recycled to the PM (Vanneste and Friml, 2009). Genetic and physiological studies revealed that PIN trafficking is modulated by protein phosphorylation/dephosphorylation (Friml et al., 2004; Lee and Cho, 2006; Michniewicz et al., 2007) and adenosyl ribosylation factor-guanine nucleotide exchange factors (ARF-GEFs; Geldner et al., 2001, 2003). In addition, pharmacological approaches using brefeldin A (BFA, an ARF-GEF inhibitor; Steinmann et al., 1999; Geldner et al., 2001, 2003), wortmannin (a phosphatidylinositol-3-kinase inhibitor; Jaillais et al., 2006), staurosporine (a protein kinase inhibitor; Lee and Cho, 2006), and endosidin 1 (an endocytosis inhibitor; Robert et al., 2008) have been useful in dissecting PIN trafficking pathways.
The PLA2 inhibitors ONO-RS-082 (2-[p-amylcinnamoyl] amino-4-chlorobenzoic acid, C21H22ClNO3; hereafter ONO; Figure 1A, inset) and bromoenol lactones (haloenol lactone suicide substrate [HELSS], bromoenol lactone) can induce Golgi fragmentation and inhibit membrane trafficking and BFA-induced endosome tubulation in mammalian cells (de Figueiredo et al., 1998, 1999). There is only one report of ONO being used to analyze vesicle trafficking in plants. The endoplasmic reticulum (ER), the Golgi body, and prevacuolar compartments/multivesicular compartments aggregate in large compartments (so-called BFA compartments) at high BFA concentrations in tobacco Bright Yellow-2 cells, and pretreatment with ONO before BFA specifically blocks the aggregation of prevacuolar/multivesicular compartments in BFA compartments (Tse et al., 2006), which is reminiscent of the inhibitory function of ONO against the BFA effect in mammalian cells (de Figueiredo et al., 1998, 1999). Because ONO seems to interfere with the BFA effect and PIN trafficking is affected by BFA, we were prompted to use ONO to further explore the role of PLA2 in the intracellular trafficking of PIN proteins.
Figure 1.
ONO Suppresses the PINox-Mediated Inhibition of Root Hair Growth.
(A) Root hair restoration of PINox lines by ONO. Data represent means ± se for each transformant (n = 192). The inset depicts the molecular structure of ONO. The ProE7:YFP transformant was used as a control (Cont). Bar = 100 μm for all.
(B) Root hair phenotypes of PINox lines (ProE7:PINs-GFP), untreated (−ONO; DMSO) or treated with 10 μM ONO (+ONO).
In this study, we examined the subcellular localization of PLA2 proteins and the effects of ONO and/or PLA2 defects on organelle distribution and PIN trafficking. To determine the physiological relevance of PLA2 in PIN trafficking, we used the root hair bioassay system (Lee and Cho, 2006, 2008), in which the mislocalization of PINs induced by ONO or by PLA2 defects suppressed the PIN-mediated inhibition of root hair growth. Our results suggest that PLA2 proteins are required for PIN trafficking.
RESULTS
PLA2 Inhibitors Suppress the PIN-Mediated Inhibition of Root Hair Growth
Based on the knowledge that root hair growth is stimulated by auxin (Okada and Shimura, 1994; Pitts et al., 1998; Schiefelbein, 2000), we previously demonstrated that a root hair system can be used to assay the activity of auxin transporters. For example, auxin efflux activity lowers cellular auxin levels and shortens root hairs, and auxin influx activity has the opposite effect (Lee and Cho, 2006, 2008; Cho et al., 2007a, 2007b). Changes in auxin transporter activities often result from the intracellular mislocalization of transporter proteins, and these changes are also reflected in root hair growth. Here, we used PLA2 inhibitors and the root hair system to test whether PLA2 activity is implicated in PIN-mediated auxin transport.
To express PIN1, PIN2, or PIN3 specifically in root hairs, we used the root hair–specific EXPANSIN A7 (EXPA7) promoter (ProE7; Cho and Cosgrove, 2002; Kim et al., 2006). PINs were translationally fused to the green fluorescent protein (GFP) gene to monitor their intracellular dynamics. These root hair–specific PIN overexpressors (PINoxs) greatly inhibited root hair growth (Figure 1), most likely due to their enhancement of auxin efflux activity in root hair cells, which lowers cellular auxin levels (Lee and Cho, 2006; Cho et al., 2007a). Quantification of PIN-GFP protein levels in the transgenic roots showed that PIN1- and PIN3-GFP levels were similar and that the PIN2-GFP level was ∼25% lower than those of other two PIN-GFP lines (see Supplemental Table 1 online). We reasoned that if PLA2 acts as a positive modulator of PIN activity, inhibition of PLA2 would in turn inhibit PIN activity and restore root hair growth in PINox lines.
Plants were incubated for 24 h on half-strength Murashige and Skoog (MS) medium containing one of four PLA2 inhibitors, ONO, HELSS, trifluoromethyl ketone (AACOCF3), or palmitoyl trifluoromethyl ketone (PACOCF3). ONO gave rise to the most conspicuous root hair restoration of PINox lines (Figure 1; see Supplemental Figure 1 online). AACOCF3 and PACOCF3 slightly restored the root hair growth of PINox lines, but HELSS did not (see Supplemental Figure 1 online). ONO (at 5 or 10 μM) restored the root hair growth of PINox lines by 5- to 8-fold compared with the control (0 μM ONO). Several concentrations of ONO (0.1, 1, 5, 10, and 20 μM) were tested, and 10 μM resulted in the greatest root hair restoration. In control plants (ProE7:YFP; YFP for yellow fluorescent protein), 10 μM ONO did not have a significant effect, but 5 μM ONO slightly enhanced root hair growth. These results indicate that ONO is the most effective suppressor of PINox-mediated root hair inhibition. Whereas 10 μM of ONO restored root hair growth in the PINox lines during the long-term (over 24 h) incubation, a higher concentration of ONO, between 20 and 50 μM, was needed to produce an effect in the short term. This is similar to previous findings with BFA (Geldner et al., 2001, 2003; Lee and Cho, 2006; Cho et al., 2007a; Robinson et al., 2008), in which 5 μM BFA was used for long-term treatments and 10 to 178 μM (mostly 50 μM) BFA was used for short-term treatments. In addition to root hair growth, ONO enhanced primary root growth, particularly at higher concentrations (see Supplemental Figure 2 online). Furthermore, ONO does not inhibit elongation of root epidermal cells (see Supplemental Figure 3 online).
ONO Interferes with PIN Trafficking to the PM
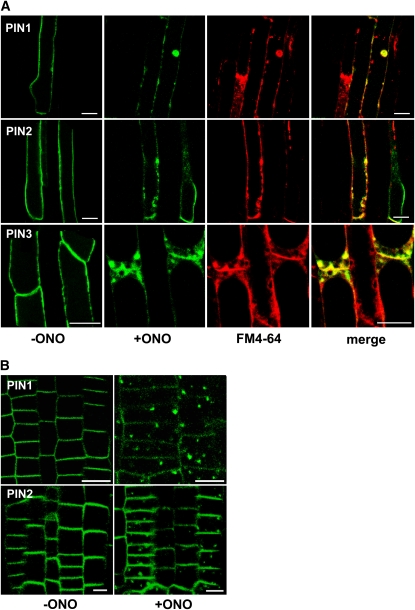
To determine whether ONO restored root hair growth in PINox lines by affecting PIN trafficking, we investigated the subcellular localization of PIN-GFP fusion proteins. PIN1-, PIN2-, and PIN3-GFP localized to the PM of root hair cells, as previously reported (Figure 2A; Lee and Cho, 2006; Cho et al., 2007a) and merged with the FM4-64 dye (the endocytic tracer; stained for <3 min) signal, which stains the PM (see Supplemental Figure 4 online). To test the effect of ONO on the subcellular localization of PIN-GFP over short time periods (2 to 3 h), we used 20 μM ONO, a concentration that has root hair restoration capacity in PINox lines. When ONO was applied to roots, PIN-GFP signals in the PM were weakened and internal PIN-GFP bodies were formed in all three PIN-GFP lines (Figure 2A). Hereafter, we refer to these ONO-induced internal bodies as ONO bodies. A lower concentration ONO (10 μM) also induced ONO body formation when the incubation period was prolonged (see Supplemental Figure 5 online). The ONO bodies overlapped with FM4-64 signals, suggesting that they were at least partly derived from the PM by the endocytic pathway. These results support the notion that reduced targeting of PIN-GFP proteins to the PM or their mislocalization by ONO treatment caused the ONO-induced restoration of root hair growth in PINox transformants.
Figure 2.
ONO Causes PIN Proteins to Form Internal Aggregates.
(A) ONO treatment (20 μM, 2 h) reduces the presence of PIN1, PIN2, and PIN3 (green color from ProE7:PIN1/2/3-GFP) in the PM of root hair–forming cells and induces the formation of internal compartments that overlap (yellow; merge) with the FM4-64 signal (red). Bars = 20 μm.
(B) PIN-including internal ONO bodies also form in meristematic root epidermal cells. PIN1 and PIN2 were expressed under the PIN2 promoter. The transgenic seedlings were treated with ONO (20 μM) for 2 h. Bars = 20 μm.
Next, we further investigated the effects of ONO on the intracellular trafficking of PIN proteins. Because root hair cells are mature cells in which large vacuoles occupy most of the cytosolic space, it was possible for us to miss subtle aspects of the intracellular dynamics of PIN trafficking. To better trace PIN trafficking during ONO treatment, we expressed PIN1- and PIN2-GFP using the PIN2 promoter, whose expression domain is the cytoplasm-rich meristematic zone. As in root hair cells, ONO caused intracellular PIN-GFP–containing ONO bodies to form in the epidermal cells of the meristematic zone (Figure 2B). These ONO bodies are morphologically different from BFA compartments. While BFA causes one or two distinctive internal compartments to form (Figures 3Aa and 3Ad), ONO causes smaller, more numerous aggregates to form (Figures 2B and 3Bb).
Figure 3.
ONO Appears to Block the PIN Recycling Pathway.
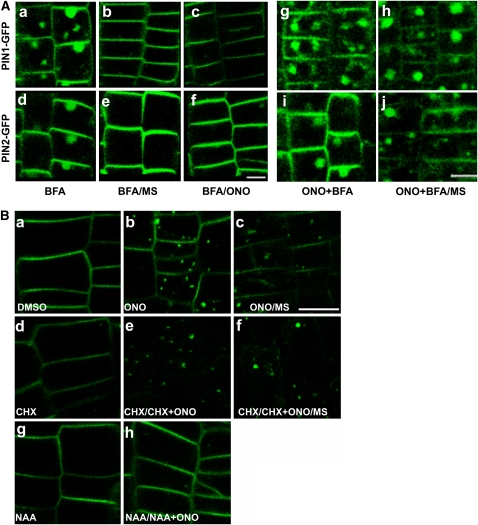
(A) The effect of ONO on BFA compartment formation. PIN1- and PIN2-GFP signals (ProPIN2:PIN1/2-GFP) in root meristematic epidermal cells that were treated with BFA (50 μM) for 1 h ([a] and [d]) and washed out with half-strength MS solution ([b] and [e]) or with ONO (20 μM) ([c] and [f]) for 1 h or that were pretreated with ONO (20 μM) and BFA (50 μM) for 3 h ([g] and [i]) and washed out with half-strength MS for 1 h ([h] and [j]). Bars =10 μm.
(B) The effects of cycloheximide and auxin on PIN2-including ONO body formation. PIN2-GFP signals (ProPIN2:PIN2-GFP) in root meristematic epidermal cells that were pretreated with DMSO only (a), CHX (50 μM [d]), or auxin (NAA, 5 μM; [g]) for 30 min, pretreated as in (a), (d), and (g), respectively, and treated with ONO (50 μM [b]), CHX+ONO (50 μM for each [e]), or NAA+ONO (5 and 50 μM for each [h]) for 3 h, or pretreated as in (a) and (d), treated as in (b) and (e), and washed out with half-strength MS for 1 h ([c] and [f], respectively). Bars = 10 μm.
[See online article for color version of this figure.]
ONO May Target the Recycling Pathway of PIN Proteins
BFA reversibly inhibits the endosome-to-PM trafficking of PIN proteins and causes endosomes and the Golgi apparatus to aggregate into plant-specific BFA compartments (Grebe et al., 2002; Geldner et al., 2003; Kleine-Vehn et al., 2006). Since BFA inhibits PIN trafficking to the PM, a specific concentration range of BFA (1 to 10 μM) can restore the root hair growth of PIN3ox transformants (Lee and Cho, 2006). ONO inhibits BFA-stimulated Golgi membrane tubulation and the retrograde transport of Golgi membranes to the ER in mammalian cells (de Figueiredo et al., 1998, 1999). ONO pretreatment and subsequent ONO+BFA cotreatment block the BFA effect (de Figueiredo et al., 1998), and a washout of BFA-treated cells with ONO restores untubulated Golgi complexes, although the ONO washout inhibits the reassembly of Golgi complexes around the nucleus (de Figueiredo et al., 1999). Kuroiwa et al. (2001) also observed that ONO causes fragmentation of the Golgi structure but that it does not inhibit the BFA effect in normal rat kidney epithelial cells. These previous studies suggest that ONO generally alters the Golgi structure but that its inhibition of the BFA effect is cell line dependent.
We tested whether ONO has an inhibitory effect on BFA action in plant cells. BFA (50 μM) treatment of transformant seedlings induced the formation of typical BFA compartments that contained the PIN1- or PIN2-GFP signal in the root meristematic epidermal cells of plants transformed with PIN1- or PIN2-GFP, respectively, and these compartments disappeared when the BFA-treated transgenic seedlings were washed out with a half-strength MS solution (Figures 3Aa, 3Ab, 3Ad, and 3Ae). A washout with 10 μM ONO also reversed the BFA effect (Figures 3Ac and 3Af). However, similar to the results of an experiment using animal cells, where ONO did not inhibit the BFA effect (Kuroiwa et al., 2001), ONO pretreatment did not inhibit the BFA effect in root hairs or in meristematic epidermal cells (see Supplemental Figure 6 online). ONO did not block BFA compartment formation when the two agents were simultaneously applied (Figures 3Ag and 3Ai). Intriguingly, the BFA compartments formed by ONO+BFA cotreatment did not disappear after a MS washout (Figures 3Ah and 3Aj). These results indicate that ONO may not antagonize the BFA effect on PIN trafficking in plant cells and that it instead blocks the unidentified endosome-to-PM recycling pathway, even after BFA removal.
To further test the possibility that ONO is implicated in endosome-to-PM recycling, we investigated the effects of cycloheximide (CHX, a protein synthesis inhibitor) and auxin during the recycling of PIN2-GFP. Unlike the reversible BFA effect, ONO-induced internal bodies did not disappear after a MS washout (Figure 3Bc). CHX (50 μM, for 30 min) slightly reduced PIN2-GFP signals in the PM (Figure 3Bd), and subsequent treatment with CHX+ONO removed most PIN2-GFP signals in the PM and caused ONO body formation (Figure 3Be). An MS washout still did not restore these signals in the PM and allowed ONO bodies to persist (Figure 3Bf). This result indicates that the inhibition of PIN2-GFP de novo synthesis by CHX weakened PIN2-GFP signals in the PM and that blocking the endosome-to-PM recycling of PIN2-GFP by ONO led to the internal accumulation of PIN2-GFP, thereby further reducing its signal in the PM. The irreversible formation of ONO bodies (Figures 3Bc and 3Bf) suggests that ONO irreversibly acts on the machinery that is required for endosome-to-PM recycling.
Auxin inhibits the BFA-induced internal accumulation of PINs, most likely by inhibiting the endocytic pathway (Paciorek et al., 2005; Cho et al., 2007a; Robert et al., 2008). We were interested in determining whether auxin also inhibits ONO body formation. When root cells were pretreated with 1-naphthyl acetic acid (NAA; 5 μM) and then cotreated with ONO and NAA, ONO body formation was almost completely blocked (Figure 3Bh). This result further suggests that PIN2-including ONO bodies originate at least partially from the PM and that ONO acts on the exocytic recycling pathway of PIN trafficking rather than on the endocytic pathway.
ONO Affects the Golgi Structure
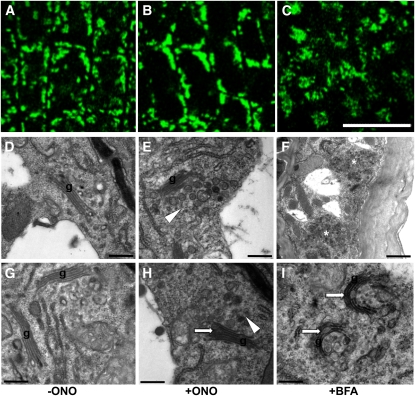
In mammalian cells, Golgi apparatuses are gathered around the nucleus to form a large complex, and ONO causes fragmentation of this complex, although typical Golgi cisternae stacks are retained (de Figueiredo et al., 1998). We examined the effect of ONO on the Golgi structure of Arabidopsis meristematic root epidermal cells by visualizing the Golgi marker sialyl transferase (ST)-GFP after a 3-h incubation of transformants in 20 μM ONO. In controls treated with DMSO, ST-GFP–marked Golgi stacks in root epidermal cells were typically dispersed throughout the cytoplasm (Figure 4A). On the other hand, a 3-h ONO treatment caused ST-GFP signals to form larger aggregates (Figure 4B). BFA (50 μM) treatment did not further expand the ST-GFP signal, but induced a circular distribution pattern around presumptive endosomal vesicles (Figure 4C). These results suggest that, although the target organelle is the same, the cytological effect of ONO appears to be different in animal and plant cells, most likely due to different Golgi distribution patterns and different cytological behaviors during vesicle trafficking (Nebenführ and Staehelin, 2001).
Figure 4.
The Effect of ONO on the Golgi Structure of Root Epidermal Cells.
(A) to (C) Localization pattern of the Golgi marker protein sialyl transferase (ST-GFP) in the DMSO control (A) or in tissues treated with ONO (20 μM for 3 h [B]) or BFA (50 μM for 2 h [C]). Bars = 20 μm.
(D) to (I) Golgi structures visualized by electron microscopy. Root tissues were treated with DMSO ([D] and [G]), ONO (20 μM for 3 h; [E] and [H]), or BFA (50 μM for 2 h; [F] and [I]). Arrowheads point to accumulated TGN vesicles, and arrows indicate deformed Golgi structures. Asterisks in [F] indicate BFA compartments. g, Golgi. Bars =0.5 μm for all except (F) (2 μm).
[See online article for color version of this figure.]
To obtain more detailed information of the effect of ONO on Golgi structure, we conducted electron microscopy analysis of Arabidopsis meristematic root epidermal cells. With the control DMSO treatment, Golgi cisternae were flat and the trans-face included the ordinary trans-Golgi network (TGN) (Figures 4D and 4G). BFA (50 μM) treatment caused the formation of several large compartments consisting of numerous small vesicles (Figure 4F), as previously observed (Grebe et al., 2002; Hause et al., 2006; Robinson et al., 2008), and led to the vesiculation and bending of Golgi cisternae and to the formation of larger vesicles in the trans-Golgi side (Figure 4I). ONO (20 μM) also induced some disintegration of Golgi stacks and the formation of more numerous and larger vesicles in the trans-Golgi face, but unlike BFA did not cause bending of Golgi cisternae (Figures 4E and 4H). On the other hand, ONO did not influence the distribution of molecular markers for the PM (H+-ATPase 2 [AHA2]), the ER (ER-yk; Nelson et al., 2007), endosomes (GFP-RABF2b; Jaillais et al., 2006), vacuoles (vac-yk; Nelson et al., 2007), or actin microfilaments in root epidermal cells (see Supplemental Figure 7 online). These results suggest that ONO inhibits PLA2 in the region of the Golgi apparatus.
PLA2α Is Likely to Localize to the Golgi
Four Arabidopsis PLA2 isoforms (PLA2α, β, γ, and δ) have been identified and biochemically characterized (Lee et al., 2003; Bahn et al., 2003; Ryu, 2004; Ryu et al., 2005). While the PLA2α and PLA2β genes are actively expressed in the root, PLA2γ is expressed at relatively low levels in the root, and PLA2δ is expressed exclusively in floral tissues (Ryu et al., 2005). In this study, we analyzed the involvement of PLA2α and PLA2β in the intracellular trafficking of PIN proteins.
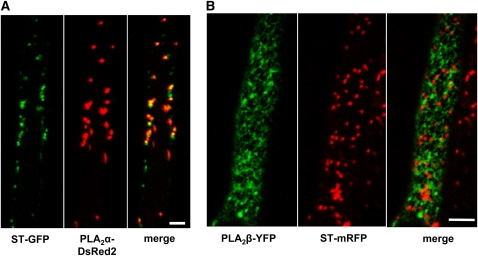
To determine the subcellular localization of these two PLA2 isoforms, we produce translational reporter fusion constructs, Pro35S:PLA2α-DsRed2 (Pro35S: cauliflower mosaic virus 35S promoter) and ProE7:PLA2β-YFP, with the markers fused to each C-terminal end. These constructs were introduced into Arabidopsis marker lines expressing ST-GFP/mRFP (Golgi marker) using Agrobacterium tumefaciens. In root hair epidermal cells, PLA2α-DsRed2 signals mostly overlapped with the Golgi marker (ST-GFP) (Figure 5A). The distribution pattern of DsRED2-tagged PLA2α appeared to be similar with cyan fluorescent protein (CFP)- or YFP-tagged versions of PLA2α (see Supplemental Figure 8 online). By contrast, PLA2β-YFP signals did not associate with the Golgi marker (ST-mRFP) (Figure 5B). This result is consistent with the previous observation that PLA2β-GFP colocalizes with an ER-marker (BiP-RFP) in the Vicia faba guard cell (Seo et al., 2008). These cytological data indicate that PLA2α may play a role in the Golgi, whereas PLA2β functions in the ER.
Figure 5.
PLA2α and PLA2β Colocalize with Golgi and ER Markers, Respectively
(A) Fluorescent signals of PLA2α–DsRed2 (Pro35S:PLA2α–DsRed2 in the ST-GFP [Golgi marker] transgenic background) overlap with ST-GFP foci.
(B) Fluorescent signals of PLA2β–YFP (ProE7:PLA2β–YFP in the ST-mRFP [Golgi marker] transgenic background) do not overlap with ST-mRFP signals. Bars = 20 μm.
ONO Inhibits the Enzymatic Activity of PLA2s
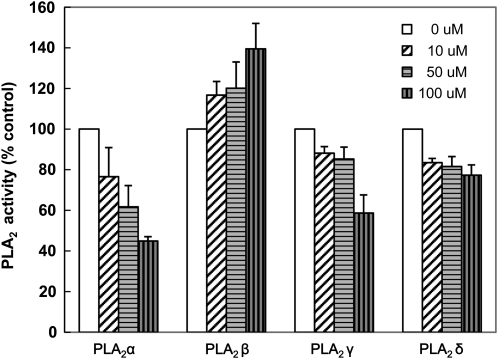
We tested whether ONO directly inhibits the enzymatic activity of PLA2 proteins. PLA2α, β, γ, and δ were heterologously expressed in Escherichia coli, and their hydrolytic activities, which convert [14C]-phosphatidylethanolamine into [14C]- lysophosphatidylethanolamine (LPE), were examined. ONO (50 μM) inhibited PLA2α activity by 40% and PLA2γ and δ activities by ∼15% (Figure 6). However, PLA2β activity tended to be increased by ONO under the pH condition tested (pH 4 to ∼11; Lee et al., 2005). PLA2β seems to be biochemically distinct from the other PLA2 isoforms. For example, it has a different optimal pH range (∼pH 6 for PLA2β, but pH 8 to 9 for the other PLA2s; Lee et al., 2005), which might account for its different response to ONO under in vitro assay conditions. To measure the inhibition of PLA2β by ONO, native proteins expressed and purified from Arabidopsis would be ideal, although these may be difficult to obtain in sufficient quantities. Our in vitro enzyme assay showed that ONO inhibited at least three of the four Arabidopsis PLA2 isoforms, although it failed to inhibit PLA2β.
Figure 6.
The Effect of ONO on the Enzymatic Activity of Heterologously Expressed Arabidopsis PLA2s.
The enzymatic activities of four PLA2 isoforms produced in E. coli were measured in the presence or absence of ONO with palmitoyl-2-linoleoyl-[14C]-phosphatidylethanolamine as a substrate. pH conditions for the assay were 9.0 (PLA2-α), 5.8 (PLA2-β), and 8.0 (PLA2-γ and δ). Data represent means ± sd (n = 4).
PLA2 Is Required for the PM Localization of PIN Proteins
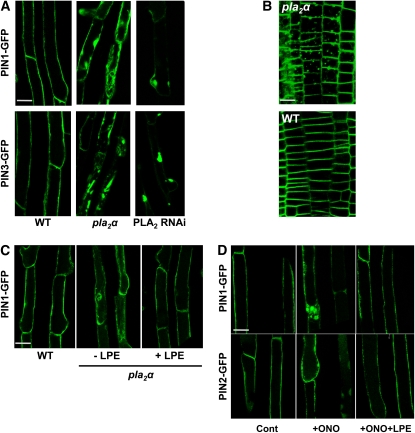
The ONO-mediated disruption of PIN trafficking caused by altered Golgi structure, and the inhibitory effect of ONO on PLA2 isoform activities, suggest that Golgi-localized PLA2s play a role in PIN trafficking, possibly by involvement in Golgi-mediated trafficking events. To obtain direct evidence for the modulation of PIN trafficking by PLA2, we examined the subcellular localization of PIN-GFP proteins in a PLA2α knockout mutant (pla2α [SALK_099415]; Seo et al., 2008) in which ProE7:PIN1 (or PIN3)-GFP constructs were introduced. The subcellular localization of both PIN1- and PIN3-GFP was clearly disrupted in pla2α root hair cells. PIN-GFP proteins aggregated internally, and their localization to the PM was largely disrupted, in contrast with their clear PM localization pattern in wild-type root hair cells (Figure 7A). When PIN1-GFP was expressed in the PIN2-expressing meristematic zone (ProPIN2:PIN1-GFP) of the pla2α mutant, it also formed internal aggregates (Figure 7B). On the other hand, in the PLA2α overexpression (Pro35S:PLA2α-DsRed2) transformant, PIN1 and PIN2 proteins exhibited their habitual subcellular localization patterns (see Supplemental Figure 9 online).
Figure 7.
PLA2s Are Required for Intracellular PIN Trafficking.
(A) The intracellular distribution of PIN1 and PIN3 (ProE7:PIN1/3-GFP) is disrupted in root hair cells of the pla2α mutant or PLA2 RNAi line.
(B) The intracellular distribution of PIN1 (ProPIN2:PIN1-GFP) is disrupted in root meristematic epidermal cells of the pla2α mutant.
(C) Supplementation with LPE, the hydrolytic product of PLA2, rescues the subcellular localization of PIN1-GFP in the pla2α mutant background. ProE7:PIN1-GFP seedlings were treated with LPE (100 μM) for 16 h.
(D) Supplementation with LPE rescues ONO-mediated disruption of PIN trafficking. ProE7:PIN-GFP seedlings were treated with DMSO (Cont) or ONO (50 μM; +ONO), or simultaneously with ONO (50 μM) and LPE (100 μM) (+ONO+LPE) for 3 h each.
Bars = 20 μm.
[See online article for color version of this figure.]
To determine whether other PLA2 isoforms, in addition to PLA2α, are also involved in the trafficking of PIN proteins, the distributions of PIN1- and PIN3-GFP (ProE7:PIN1/3-GFP) were observed in a PLA2-RNAi (RNA interference) line (Lee et al., 2003). As previously described, the PLA2-RNAi construct targets the conserved Ca2+ binding loop and active site motif found in Arabidopsis PLA2 isoforms (Lee et al., 2003). This previous study showed that the RNAi considerably reduces the PLA2γ transcript level and slightly reduces the PLA2β transcript level, whereas it does not seem to affect the transcript levels of PLA2α and PLA2δ (Lee et al., 2003). In this RNAi line, the PIN1- and PIN3-GFP signals were greatly decreased in the PM of the root hair cell and instead were observed in several large internal compartments (Figure 7A). The RNAi and pla2α single mutant lines do not seem to considerably affect seedling growth and root hair growth (see Supplemental Figure 10 online). PLA2β is also targeted by the RNAi (Seo et al., 2008), implying that the β-form could be involved in PIN trafficking. However, how PLA2β mediates PIN trafficking remains to be determined because its subcellular localization differs from that of PLA2α (Figure 5; Seo et al., 2008).
The major hydrolytic product of the four Arabidopsis PLA2 isoforms is LPE (Lee et al., 2005). It is conceivable that if PLA2 acts on PIN trafficking via this catalytic product, exogenous LPE may rescue the disrupted PIN localization in the pla2α mutant background. When the pla2α seedlings expressing PIN1-GFP were treated with LPE (100 μM, for 16 h), the PM localization of PIN1-GFP was restored in root hair cells (Figure 7C). This restoration effect by LPE on PIN trafficking was also reproducible in ONO-treated seedlings. Supplementation with LPE (100 μM) almost completely inhibited ONO-mediated internal body formation of PIN1- and PIN2-GFP (Figure 7D).
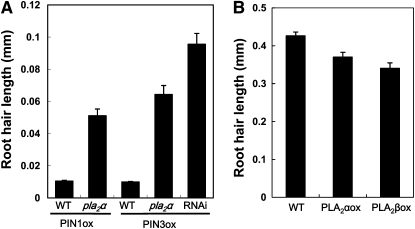
Next, we tested whether the mislocalization of PIN-GFP in the pla2α mutant or in the PLA2-RNAi line affects root hair growth of the PINox transformants. The root hairs of PIN1ox and PIN3ox transformants were as short as 0.01 mm. However, PINox constructs in the pla2α mutant background or in the PLA2-RNAi line resulted in root hairs that were 5 to 10 times longer than those in the wild-type background (Figure 8A). The disruption of PIN protein localization in the PM by the pla2α mutation or by PLA2-RNAi was able to reduce auxin efflux from root hair cells, causing them to retain more auxin, thereby restoring root hair growth. Consistent with these results, root hair–specific PLA2 overexpression inhibited root hair growth (Figure 8B), probably because it facilitated the trafficking of PINs to the PM and thus increased auxin efflux from root hair cells. The 35S promoter–driven overexpression of PLA2α also slightly inhibited root hair growth (see Supplemental Figure 11 online) but considerably inhibited primary root growth (see Supplemental Figure 12 online).
Figure 8.
Effects of PLA2 Suppression and Overexpression on the PINox-Mediated Inhibition of Root Hair Growth.
(A) Root hair growth in PIN1- and PIN3ox transformants (ProE7:PIN1/3-GFP) is enhanced by the pla2α mutation or by PLA2-RNAi. Data represent means ± se (n = 256).
(B) Reduced root hair length of PLA2ox transformants (ProE7:PLA2α- or β-YFP). Data represent means ± se (n = 320).
DISCUSSION
Although lipid-modifying PLA2 enzymes have been implicated in intracellular membrane trafficking processes in animal cells (Brown et al., 2003), their equivalent function has not yet been studied in plants. In this study, we used pharmacological and genetic approaches to investigate the function of PLA2s in the intracellular trafficking of PIN-containing vesicles. We selected PIN auxin efflux transporters as a model molecular system because their intracellular trafficking behavior has been well studied (Klein-Vehn and Friml, 2008) and also because the effects of PLA2 on PIN trafficking can be quantitatively estimated by a biological assay system, the root hair bioassay (Lee and Cho, 2008).
Pharmacological Results Implicate PLA2s in PIN-Containing Vesicle Trafficking
The polar localization of PIN proteins in the PM is critical for the formation of local auxin gradients and thus for organogenesis and asymmetric growth in plants. This observation has led to a plethora of cell biological studies to analyze the intracellular vesicle trafficking of PIN proteins. In particular, several pharmacological approaches have been very useful for dissecting the PIN trafficking pathway. The use of the ARF-GEF inhibitor BFA revealed the recycling route of GNOM-dependent PIN trafficking (Steinmann et al., 1999; Geldner et al., 2003; Richter et al., 2007). Wortmannin, an inhibitor of PI-3K, has been a successful tool for identifying plant retromer complexes, such as SNXs and VPSs, which play important roles in PIN trafficking (Jaillais et al., 2006, 2007). More recently, the use of endosidin 1 demonstrated that PIN2 travels through SYP61/VHA-a1–labeled endosomal compartments that are not shared by PIN1 and PIN7 (Robert et al., 2008).
In this study, we used PLA2 inhibitors to implicate PLA2-mediated lipid metabolism in PIN trafficking. Among three tested PLA2 inhibitors, ONO, AACOCF3, and PACOCF3 (Brown et al., 2003), ONO conferred the greatest suppression of PIN-mediated inhibition of root hair growth (Figure 1; see Supplemental Figure 1 online). Root hair restoration by ONO in PINox lines was likely to result from the inhibition of PIN targeting to the PM by ONO, which in turn led to an elevation of auxin concentration in root hair cells and thus to the restoration of root hair growth. Similarly, we previously showed that BFA-mediated blocking of PIN trafficking restored root hair growth in PIN3ox transformants (Lee and Cho, 2006). The ONO-induced mislocalization of PINs was demonstrated by reduced levels of PIN proteins in the PM and the formation of internal ONO bodies in root hair cells (Figure 2A). We selected ONO to further dissect the role of PLA2 in PIN trafficking because it was considerably effective in biological (root hair restoration), cytological (inhibition of PIN trafficking), and biochemical (inhibition of at least three Arabidopsis PLA2 enzymes) assays.
Our cell biological analyses suggest that ONO acts on the Golgi complex or at least on Golgi-related compartments. ONO caused an accumulation of PIN proteins in specific internal compartments in Arabidopsis root epidermal cells in both meristem/elongation and mature hair-forming regions (Figures 2 and 3). The formation of ONO bodies is unlikely to be general to all PM proteins because the GFP fusion of PM H+-ATPase 2 did not aggregate in internal compartments in root hair cells following ONO treatment (see Supplemental Figure 7 online). This suggests that the trafficking of PINs, and probably of other PM proteins, selectively responds to ONO. ONO altered the Golgi structure so as to disintegrate Golgi cisternae and induce the formation of large vesicles at the trans-Golgi side (Figures 4E and 4H). BFA also caused the formation of numerous vesicles from the trans-Golgi face, which generates so-called BFA compartments (Figures 4F and 4I). However, the number of ONO-induced trans-Golgi vesicles was much smaller than that induced by BFA, and these ONO vesicles were confined to the TGN, as observed by electron microscopy. The Golgi marker ST-GFP aggregated more extensively in ONO-treated root epidermal cells than in control cells (Figures 4A and 4B). The ST protein localizes predominantly to trans-Golgi stacks and Golgi-associated membranes at the trans face (Wee et al., 1998). Therefore, the altered ST-GFP signals in ONO-treated root epidermal cells may reflect the accumulation of ST-containing membrane structures at the trans-Golgi side.
Since PIN-labeled ONO bodies are much smaller than PIN-labeled BFA compartments, it was difficult to cytologically identify ONO bodies by electron microscopy, unlike BFA compartments. However, several lines of evidence indicate that PIN-containing ONO bodies may represent the accumulation of TGN vesicles, and ONO may act on the trafficking machinery in and/or near the Golgi apparatus. First, as we mentioned above, some deformation of Golgi structure and the accumulation of TGN vesicles are distinctive fine cytological changes caused by ONO treatment. Second, ONO-induced TGN vesicles are smaller than BFA compartments. Third, certain PIN proteins travel through SYP61/VHA-a1–marked TGN/endosome compartments (Robert et al., 2008). The TGN is known to serve an endosome compartment that recycles endocytosed materials back to the PM (Dettmer et al., 2006). Fourth, the effect of ONO on vesicle trafficking somewhat resembles that of concanamycin A (ConcA), a specific inhibitor of vacuolar H+-ATPase, which induces the accumulation of secretory and endocytic vesicles in TGN components (Dettmer et al., 2006). ConcA also alters the Golgi cisternae structure and causes the formation of BRI1-containing internal compartments. However, while ConcA inhibits the formation of BFA compartments, ONO did not. Fifth, the inhibition of PIN de novo synthesis by CHX, accompanied with the reduction of PINs in the PM, did not block the formation of ONO bodies (Figures 3Be and 3Bf). This implies that PIN proteins in ONO bodies are derived partly from the PM by endocytosis. Sixth, PIN-containing ONO bodies overlapped partly with endocytosed FM4-64 dye (Figure 2), indicating that some portion of endocytosed PM materials travels in the ONO-sensitive trafficking pathway. Seventh, auxin, which inhibits PIN endocytosis, blocked the formation of ONO bodies. Finally, ONO-inhibitable PLA2α-DsRed2 colocalized with the Golgi marker ST-GFP (Figure 5). Collectively, these results suggest that ONO likely blocks specific recycling at the TGN or Golgi. However, to further understand the effect of ONO on recycling at the Golgi-related compartments, a more detailed molecular marker study should be conducted.
PLA2s Are Necessary for PIN Trafficking
The pharmacological data obtained using PLA2 inhibitors indicate that PLA2s are involved in intracellular PIN trafficking. To substantiate these results, we examined the subcellular localization of PLA2 proteins, alteration of PIN trafficking, and restoration of PINox-inhibited root hair growth in the pla2α loss-of-function mutant and in the PLA2 RNAi line, and we also investigated the effect of PLA2 overexpression on root hair growth (Figures 7 and 8). Currently, there is a paucity of in vivo evidence for the role of PLA2 in membrane trafficking, even in animal cells (Brown et al., 2003). The only reported finding is that a group IV cytosolic PLA2 localizes to the Golgi apparatus, and its overexpression or loss of function affects the trafficking of specific PM proteins in mammalian cells (Choukroun et al., 2000; Downey et al., 2001).
In Arabidopsis root cells, PLA2α and PLA2β proteins were both distributed in a dotted and/or network pattern, and PLA2α colocalized with Golgi, whereas PLA2β did not (Figure 5), consistent with a previous observation in Arabidopsis guard cells (Seo et al., 2008). Our most noteworthy finding for the effect of PLA2 on membrane trafficking is that it is required for the proper targeting of PIN proteins to the PM. In the pla2α mutant background and in the PLA2-RNAi line, in which several PLA2 homologs are likely suppressed, PIN1 and PIN3 did not properly localize to the PM and were retained in internal compartments (Figures 7A and 7B). Golgi-localized PLA2α was significantly inhibited by ONO (Figure 6). These results suggest that PLA2s (at least PLA2α) act in the Golgi to modulate PIN trafficking.
The root hair assay provides further physiological support that PLA2 regulates PIN trafficking to the PM. The disrupted localization of PIN proteins in the PM of the pla2α mutant and PLA2-RNAi root hair cells correlated with the restoration of root hair growth (Figure 8A), which is due to a decrease in auxin efflux from, and thus to a greater retention of auxin in root hair cells. This is consistent with the root hair phenotype observed for root hair–specific PLA2-overexpressing (PLA2ox) transformants, in which root hair growth was decreased compared with that of control plants (Figure 8B). This decrease occurred because of the increased trafficking of PIN proteins to the PM of PLA2ox transformant root hair cells.
The most pronounced molecular function of phospholipases is to produce second messengers for signal transduction. PLA2 hydrolysis products act as signaling molecules for diverse cellular processes in plants (for reviews, see Scherer, 2002; Ryu, 2004). In particular, PLA2 has been reported to function in auxin-mediated phenomena such as gravitropism and cell elongation (Yi et al., 1996; Scherer, 2002; Lee et al., 2003; Ryu, 2004; Scherer et al., 2007). It remains to be determined whether ONO-inhibitable PLA2s regulate vesicle trafficking by affecting signal transduction, which involves their hydrolytic products, or by another mechanism. ONO itself does not seem to affect the canonical auxin signaling process from the F-box receptor to gene activation because auxin-induced DR5:GUS (β-glucuronidase; Ulmasov et al., 1997) was normally expressed, even in the presence of ONO (see Supplemental Figure 13 online). In the current model, vesicle budding is favored by PLA2 activity, in that the lipase hydrolyzes an acyl chain from the phospholipid at a single bilayer leaflet, increases the level of inverted cone-shaped lysophospholipids asymmetrically at the leaflet, and enhances membrane curvature toward that leaflet side (Brown et al., 2003). In accordance with this, exogenous supplementation with LPE, the favored hydrolytic product of PLA2, restored PIN localization to the PM in PLA2α–defective mutant and ONO-treated plants (Figures 7C and 7D). This biophysical model is reminiscent of the PLD-mediated vesicle fusion mechanism by which PLD produces fusogenic lipids, such as phosphatidic acid, which lowers the activation energy for membrane curvature due to its small head group size (Jenkins and Frohman, 2005). In addition to its biophysical contribution, phosphatidic acid also facilitates membrane fusion by acting as a signaling molecule. However, whether PLA2s regulate vesicle trafficking through signal transduction or in a biophysical manner remains to be determined.
METHODS
Chemicals
PLA2 inhibitors and other inhibitors were obtained from the following sources: ONO-RS-082 and BFA from Alexis, PACOCF3 and AACOCF3 from Calbiochem-Novabiochem, HELSS from A.G. Scientific, and SynaptoRed C2 (FM4-64) from Biotium. Stock solutions were as follows: BFA (20 mM), ONO-RS-082 (10 mM), PACOCF3 (20 mM), AACOCF3 (20 mM), and HELSS (10 mM) in DMSO, and SynaptoRed C2 (4 mM) in distilled water. ER and Golgi trackers were obtained from Molecular Probes: ER-tracker Red (E-34250, BODIPY TR glibenclamide) and BODIPY FL C5-ceramide complexed to BSA (B-22650), respectively.
Plant Materials and Growth Conditions
The Columbia ecotype of Arabidopsis thaliana was used as a model plant in this study. The ProPIN2:PIN2-GFP line in the pin2 (eir1-1) background previously described (Xu and Scheres., 2006) was provided by Jiří Friml. Seeds expressing ProE7:YFP and ProE7:PIN3-GFP were previously described (Lee and Cho., 2006). Seeds expressing ProE7:PIN1/2-GFP were as described previously (Ganguly et al., 2010). Seeds for ST-GFP were provided by Inhwan Hwang (Kim et al., 2001), ProE7:AHA2-GFP by Youngsook Lee, GFP-hTalin (Takemoto et al., 2003) by Adrienne R. Hardham, and GFP-RABF2b (Jaillais et al., 2006) by Thierry Gaude. The PLA2-RNAi line was provided by Stephen Beungtae Ryu (Lee et al., 2003). ER -YFP (ER-yk, CS16251; Nelson et al., 2007) and Vac-YFP (vac-yk, CS16257; Nelson et al., 2007) expressing lines and the pla2α (SALK_099415) mutant line were purchased from the Arabidopsis stock center (http://www.Arabidopsis.org/). Seeds were sown on half-strength MS medium (Sigma-Aldrich nutrient mix) containing 1% sucrose, 0.5 g/L MES (pH 5.7, adjusted with KOH), and 0.8% agarose. Three-day-old cold-treated seeds were germinated under long-day conditions (16 h light/8 h dark) at 23°C. Transformants were selected on hygromycin-containing plates (50 μg/mL). For all pharmacological treatments, 3-d-old seedlings of homozygous transformants were transferred to fresh plates with chemical additives and grown for an additional day, and root hairs were observed.
Transgene Constructs
To visualize the subcellular localization patterns of PLA2 (PLA2α and PLA2β) in root hair cells, full genomic sequences of PLA2α and PLA2β were expressed under the EXPA7 promoter (ProE7; Cho and Cosgrove, 2002; Kim et al., 2006). The pCAMBIA1300 vector containing ProE7and YFP was used to generate these constructs (ProE7:YFP; Cho et al., 2007b). The PLA2α genomic fragment was amplified using primers with SalI and BamHI sites (underlined): 5′-TCGTC GACATG GCGGCTCCGATCATA-3′ and 5′-AAGGATCCGGGTTTCTTGAGGACTTT-3′. The PLA2β genomic fragment was amplified using primers with SalI and BamHI sites: 5′-TC GTCGACATGATGTTTCGCACTTCA-3′ and 5′-AAGGATCCTAGCTCTGTTTTCATATC-3′. These PCR products were digested with enzymes and cloned into the ProE7:YFP vector as PLA2α or β-YFP fusion proteins (ProE7:PLA2α- or β-YFP). To express the PLA2α fusion protein with DsRed2 at the C terminus, the PLA2α genomic fragment was amplified using primers containing SalI and EcoRI sites (5′-TCGTCGACATGGCGGCTCCGATCATA-3′ and 5′-cggaattcGGGTTTCTTGAGGACTT-3′) and cloned into the pCAMB1390 vector (Wang et al., 2006), which contains the 35S promoter and DsRed2 sequence (Pro35S:PLA2α–DsRed2). To construct the Pro35S:PLAα2-eCFP construct, the PLA2α fragment (with SalI and EcoRI sites) from Pro35S:PLAα2-DsRed2 was cloned into the ABD2 gene site of Pro35S:ABD2-eCFP/pCAMBIA1390 (Wang et al., 2008). All transgene constructs were confirmed by nucleotide sequencing and transformed into Arabidopsis using Agrobacterium tumefaciens C58C1 (pMP90) (Bechtold and Pelletier, 1998). For the ProPIN2:PIN1-GFP construct, the PIN2 promoter (−1790 to ∼−10 bp from the start codon) was integrated into the PIN1-GFP4 construct (Benková et al., 2003).
Determination of Root Hair Cell Length
Root hair length was measured as described by Cho et al. (2007b). To estimate root hair length, digital photographs of roots were taken under a stereomicroscope (Leica MZ FLIII) at ×40 to ×50 magnification. Eight consecutive fully grown hairs protruding perpendicularly from each side of the root, representing a total of 16 hairs from both sides, were measured.
Electron Microscopy
Roots of 4-d-old Arabidopsis seedlings were treated with DMSO and ONO for 3 h or with BFA for 2 h. The root tips were excised, fixed for 4 h with 2% paraformaldehyde and 2% glutaraldehyde in 50 mM cacodylate buffer, pH 7.2, rinsed with the same buffer, and postfixed with osmium tetroxide-potassium ferrocyanide in cacodylate buffer for 2 h. After dehydration, specimens were embedded in London Resin White. Ultrathin sections (60- to 80-nm thick) were collected on formvar- and carbon-coated copper grids (150 mesh), stained with 3% uranyl acetate and Reynolds' lead citrate, and examined by transmission electron microscopy (G2 SPIRIT TWIN; FEI) at 120 kV.
Observation of Reporter Gene Expression
The fluorescence from reporter proteins and organelle markers was observed by confocal laser scanning microscopy (LSM 510; Carl Zeiss). GFP, YFP, and FM4-64/ER/Golgi-tracker were detected using 488/505- to 530-nm, 514/>530-nm, and 543/>560-nm excitation/emission filter sets, respectively. Fluorescence images were digitized using the Zeiss LSM image browser.
PLA2 Activity Assay
The cDNAs coding PLA2s were cloned into the vector pET-40b (+) and expressed in Escherichia coli BL21(DE3) pLysS (Novagen). Proteins were purified as previously described (Bahn et al., 2003; Lee et al., 2003; Lee et al., 2005). All PLA2s were dialyzed overnight at 4°C in 50 mM Tris-HCl or 50 mM MES buffer, under the following pH conditions: pH 9.0 to 8.0 (for PLA2α), pH 5.8 to 6.5 (PLA2β), or pH 8.0 (PLA2γ and PLA2δ). Dialyzed PLA2s were treated with enterokinase, preincubated with PLA2 inhibitors, and dissolved in 100% ethanol at 37°C for 60 min. The reaction mixture contained 10 mM Ca2+ (for PLA2α) or 0.5 mM Ca2+ (for PLA2β, γ, and δ) and 0.05% Triton X-100 in a final volume of 300 μL 50 mM Tris-HCl (for PLA2α, γ and δ) or 50 mM MES buffer (for PLA2β). Unlabeled phosphatidyl-ethanolamine (PE; 0.5 μmol) and 35 pmol of radiolabeled l-3-phosphatidyl-[14C]-ethanolamine-1,2-dioleoyl (55 mCi/mmol; GE Healthcare) were used as substrates for each reaction. The substrate solution was prepared as previously described (Seo et al., 2008). Lipid products were extracted, separated by thin layer chromatography (Silica Gel 60; Merck), and developed with chloroform/methanol/acetic acid/water (85:15:12.5:3.5, v/v/v/v). Radioactive bands of 14C-PE and 14C-lysoPE were detected and quantified using the Bio-Imaging Analyzer (FLA7000; Fuji Film).
GUS Histochemical Analysis
Four-day-old DR5:GUS seedlings were treated with auxins (indole-3-acetic acid and NAA) and ONO for 2 h before visualizing GUS activity. Histochemical GUS staining was performed by incubating whole seedlings in the staining buffer containing 1 mM 5-bromo-4-chloro-3-indoyl-β-d-glucuronic acid cyclohexylammonium salt (X-Gluc; Glycosynth), 0.1 M NaH2PO4, 0.01 M EDTA, 0.1% Triton-X, and 0.5 mM potassium ferri- and ferrocyanide at 37°C until the blue color appeared (6 to 24 h). Stained seedlings were cleared in 70% ethanol for 1 h. Seedlings were photographed under a stereomicroscope (Leica MZ FLIII).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At1g12560 (EXPA7), At1g73590 (PIN1) At5g57090 (PIN2), At1g70940 (PIN3), At2g06925 (PLA2α), At2g19690 (PLA2β), At4g29460 (PLA2γ), and At4g29470 (PLA2δ).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. PLA2 Inhibitors Suppress the PINox-Mediated Inhibition of Root Hair Growth.
Supplemental Figure 2. ONO Enhances Primary Root Growth.
Supplemental Figure 3. ONO Does Not Inhibit Root Epidermal Cell Elongation.
Supplemental Figure 4. Colocalization of PIN3-GFP and FM4-64 in the Plasma Membrane.
Supplemental Figure 5. LPE Restored PIN Trafficking in ONO-Treated Seedlings.
Supplemental Figure 6. Pretreatment with ONO Does Not Inhibit BFA-Mediated PIN Aggregation in Root Hair and Meristematic Epidermal Cells.
Supplemental Figure 7. ONO Does Not Affect the Subcellular Distribution Patterns of PM, ER, Endosome, Vacuole, and Actin Microfilament Markers.
Supplemental Figure 8. Distribution Patterns of Three Different Fluorescently Tagged PLA2α Fusion Proteins.
Supplemental Figure 9. Overexpression of PLA2α Does Not Alter the Subcellular Localization of PIN1- and PIN2-GFP in Their Native Expression Domains.
Supplemental Figure 10. Effects of PLA2-RNAi, pla2α Loss of Function, and PIN3ox on Root Hair Growth.
Supplemental Figure 11. Overexpression of PLA2α under the 35S Promoter Slightly Decreases Root Hair Growth.
Supplemental Figure 12. Overexpression of PLA2α Inhibits Primary Root Growth.
Supplemental Figure 13. The Effect of ONO on Auxin-Induced DR5:GUS Expression in Arabidopsis Seedlings.
Supplemental Table 1. Relative Expression Levels of PIN-GFP Proteins.
Acknowledgments
We thank Zee-Won Lee at the Korea Basic Science Institute for assistance with confocal microscopy, Jiří Friml at Ghent University for providing ProPIN2:PIN2-GFP seeds, Youngsook Lee at POSTECH for ProE7:AHA2-GFP seeds, Thierry Gaude at Université Claude Bernard Lyon for GFP-RABF2b seeds, Adrienne R. Hardham for GFP-hTalin lines, and Inwhan Hwang at POSTECH for ST-GFP seeds. This study was supported by grants from the Korea Science and Engineering Foundation (R01-2007-000-10041-0), the BioGreen 21 Program (20070401034022) of the Rural Development Administration, the Crop Functional Genomics Center of the 21st Century Frontier Research Program (CG2151), and the Research Settlement Fund for the new faculty of Seoul National University to H.-T. Cho. S.B. Ryu was partly supported by a grant from the Korea Research Foundation (KRF-C00403).
References
- Bahn S.C., Lee H.Y., Kim H.J., Ryu S.B., Shin J.S. (2003). Characterization of Arabidopsis secretory phospholipase A2-γ cDNA and its enzymatic properties. FEBS Lett. 553: 113–118 [DOI] [PubMed] [Google Scholar]
- Bechtold N., Pelletier G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Arabidopsis Protocols, Martinez-Zapater J.M., Salinas J., (Totowa, NJ: Humana; ), pp. 259–266 [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Brown W.J., Chambers K., Doody A. (2003). Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 4: 214–221 [DOI] [PubMed] [Google Scholar]
- Cho H.T., Cosgrove D.J. (2002). The regulation of Arabidopsis root hair initiation and expansin gene expression. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Lee O.R., Ganguly A., Cho H.T. (2007a). Auxin-signaling: Short and long. J. Plant Biol. 50: 79–89 [Google Scholar]
- Cho M., Lee S.H., Cho H.T. (2007b). P-Glycotrotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 19: 3930–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun G.J., Marshansky V., Gustafson C., McKee M., Hajjar R.J., Rosenzweig A., Brown D., Bonventre J.V. (2000). Cytosolic phospholipase A2 regulates Golgi structure and modulates intracellular trafficking of membrane proteins. J. Clin. Invest. 106: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P., Drecktrah D., Katzenellenbogen J.A., Strang M., Brown W.J. (1998). Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl. Acad. Sci. USA 95: 8642–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P., Polizotto R.S., Drecktrah D., Brown W.J. (1999). Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol. Biol. Cell 10: 1763–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey P., Sapirstein A., O'Leary E., Sun T.X., Brown D., Bonventre J.V. (2001). Renal concentrating defect in mice lacking group IV cytosolic phospholipase A2. Am. J. Physiol. Renal Physiol. 280: F607–F618 [DOI] [PubMed] [Google Scholar]
- Friml J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865 [DOI] [PubMed] [Google Scholar]
- Ganguly A., Lee S.H., Cho M., Lee O.R., Yoo H., Cho H.-T. (2010). Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.-D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Grebe M., Friml J., Swarup R., Ljung K., Sandberg G., Terlou M., Palme K., Bennett M.J., Scheres B. (2002). Cell polarity signaling in Arabidopsis involves a BFA-sensitive auxin influx pathway. Curr. Biol. 12: 329–334 [DOI] [PubMed] [Google Scholar]
- Hause G., Śamaj J., Menzel D., Baluska F. (2006). Fine structural analysis of brefeldin A-induced compartment formation after high-pressure freeze fixation of maize root epidermis. Plant Signal. Behav. 1: 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miege C., Rollin C., Gaude T. (2006). AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443: 106–109 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Santambrogio M., Rozier F., Fobis-Loisy T., Miége C., Gaude T. (2007). The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130: 1057–1070 [DOI] [PubMed] [Google Scholar]
- Jenkins G.M., Frohman M.A. (2005). Phospholipae D: A lipid centric review. Cell. Mol. Life Sci. 62: 2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Eu Y.-J., Yoo C.M., Kim Y.W., Pih K.T., Jin J.B., Kim S.J., Stenmark H., Hwang I. (2001). Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.W., Lee S.H., Choi S.B., Won S.K., Heo Y.K., Cho M., Park Y.I., Cho H.T. (2006). Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18: 2958–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J., Dhonukshe P., Swarup R., Bennett M., Fiml J. (2006). Subcellular traffricking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18: 3171–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Vehn J., Friml J. (2008). Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 24: 447–473 [DOI] [PubMed] [Google Scholar]
- Kuroiwa N., Nakamura M., Tagaya M., Takatsuki A. (2001). Arachidonyltrifluoromethy ketone, a phospholipase A2 antagonist, induces dispersal of both golgi stack and trans Golgi network-resident proteins throughout the cytoplasm. Biochem. Biophys. Res. Commun. 281: 582–588 [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Bahn S.C., Kang Y.M., Lee K.H., Kim H.J., Noh E.K., Palta J.P., Shin J.S., Ryu S.B. (2003). Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell 15: 1990–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.Y., Banh S.C., Shin S.J., Hwang I., Back K., Doelling J.H., Ryu S.B. (2005). Multiple forms of secretory phospholipase A2 in plants. Prog. Lipid Res. 44: 52–67 [DOI] [PubMed] [Google Scholar]
- Lee S.H., Cho H.T. (2006). PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18: 1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Cho H.T. (2008). Auxin and root hair morphogenesis. Plant Cell Monographs, Emons A.M.C., (Berlin/Heidelberg, Germany: Springer; ), pp. 45–64 [Google Scholar]
- Li G., Xue H.W. (2007). Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell 19: 281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M., et al. (2007). Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Nebenführ A., Staehelin L.A. (2001). Mobile factories: Golgi dynamics in plant cells. Trends Plant Sci. 6: 160–167 [DOI] [PubMed] [Google Scholar]
- Nelson B.K., Cai X., Nebenführ A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Okada K., Shimura Y. (1994). Modulation of root growth by physical stimuli. Arabidopsis, Meyerowitz E.M., Somerville C.R., (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ), pp. 665–684 [Google Scholar]
- Paciorek T., Zazimalová E., Ruthardt N., Petrásek J., Stierhof Y.D., Kleine-Vehn J., Morris D.A., Emans N., Juergens G., Geldner N., Friml J. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Pitts R.J., Cernac A., Estelle M. (1998). Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 16: 553–560 [DOI] [PubMed] [Google Scholar]
- Richter S., Geldner N., Schrader J., Wolters H., Siterhof Y.-D., Rios G., Koncz C., Robinson D.F., Jürgen G. (2007). Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448: 488–492 [DOI] [PubMed] [Google Scholar]
- Robert S., Chary S.N., Drakakaki G., Li S., Yang Z., Raikhel N.V., Hicks G.R. (2008). Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl. Acad. Sci. USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.G., Langhaus M., Saint-Jore-Dupas C., Hawes C. (2008). BFA effects are tissue and not just plant specific. Trends Plant Sci. 13: 405–408 [DOI] [PubMed] [Google Scholar]
- Ryu S.B. (2004). Phospholipid-derived signaling mediated by phospholipae A in plants. Trends Plant Sci. 9: 229–235 [DOI] [PubMed] [Google Scholar]
- Ryu S.B., Lee H.Y., Doellig J.H., Palta J.P. (2005). Charactirization of a cDNA Arabidopsis secretory phospholipase A2-α, an enzyme that generates bioactive lysophospholipids and free fatty acids. Biochim. Biophys. Acta 1736: 144–151 [DOI] [PubMed] [Google Scholar]
- Schaloske R.H., Dennis E.A. (2006). The phospholipae A2 superfamily and its group numbering system. Biochim. Biophys. Acta 1761: 1246–1259 [DOI] [PubMed] [Google Scholar]
- Scherer G.F.E. (2002). Secondary messengers and phospholipase A2 in auxin signal transduction. Plant Mol. Biol. 49: 357–372 [PubMed] [Google Scholar]
- Scherer G.F.E., Zahn M., Callis J., Jones A.M. (2007). A role for phospholipase A in auxin regulated gene expression. FEBS Lett. 581: 4205–4211 [DOI] [PubMed] [Google Scholar]
- Schiefelbein J.W. (2000). Constructing a plant cell: The genetic control of root hair development. Plant Physiol. 124: 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J., Lee H.Y., Choi H., Choi Y., Lee Y., Kim Y.W., Ryu S.B., Lee Y.S. (2008). Phospholipase A2β mediates light-induced stomatal opening in Arabidopsis. J. Exp. Bot. 59: 3587–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgen G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Takemoto D., Jones D.A., Hardham A.R. (2003). GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J. 33: 775–792 [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Lo S.W., Hillmer S., Dupree O., Jiang L. (2006). Dynamic response of prevacuolar compartments to brefeldin A in plant cells. Plant Physiol. 142: 1442–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Friml J. (2009). Auxin: A trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Wang Y.-S., Shrestha R., Kilaru A., Wiant W., Venables B.J., Chapman K.D., Blancaflor E.B. (2006). Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines. Proc. Natl. Acad. Sci. USA 103: 12197–12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-S., Yoo C.-M., Blancaflor E.B. (2008). Improved imaging of actin filaments in transgenic Arabidopsis plants expressing a green fluorescent protein fusion to the C and N termini of the fimbrin actin-binding domain 2. New Phytol. 177: 525–536 [DOI] [PubMed] [Google Scholar]
- Wee E.G.-T., Sherrier D.J., Prime T.A., Dupree P. (1998). Targeting of active sialyltransferase to the plant Golgi apparatus. Plant Cell 10: 1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Scheres B. (2006). Polar auxin transport and patterning: Grow with the flow. Genes Dev. 20: 922–926 [DOI] [PubMed] [Google Scholar]
- Yi J., Park D., Lee Y. (1996). In vivo evidence for the involvement of phospholipae A and protein kinase in the signal transduction pathway for auxin-induced corn coleoptile elongation. Physiol. Plant. 96: 359–368 [Google Scholar]