This article reports that DWA1 and DWA2 may be the substrate receptors for a CULLIN E3 ligase and that they interact with themselves and each other. Heterodimeric cooperation between DWAs is a previously unknown regulatory mechanism for the action of CULLIN E3 ligases.
Abstract
To elucidate potential roles of CUL4-DDB1-DWD (for Cullin 4-Damaged DNA Binding1-DDB1 binding WD40) E3 ligases in abscisic acid (ABA) signaling, we examined ABA sensitivities of T-DNA mutants of a number of Arabidopsis thaliana DWD genes, which encode substrate receptors for CUL4 E3 ligases. Mutants in two DWD genes, DWA1 and DWA2 (DWD hypersensitive to ABA1 and 2), had ABA-hypersensitive phenotypes. Both proteins interacted with DDB1 in yeast two-hybrid assays and associated with DDB1 and CUL4 in vivo, implying they could form CUL4-based complexes. Several ABA-responsive genes were hyperinduced in both mutants, and the ABA-responsive transcription factors ABA INSENSITIVE 5 (ABI5) and MYC2 accumulated to high levels in the mutants after ABA treatment. Moreover, ABI5 interacted with DWA1 and DWA2 in vivo. Cell-free degradation assays showed ABI5 was degraded more slowly in dwa1 and dwa2 than in wild-type cell extracts. Therefore, DWA1 and/or DWA2 may be the substrate receptors for a CUL4 E3 ligase that targets ABI5 for degradation. Our data indicate that DWA1 and DWA2 can directly interact with each other, and their double mutants exhibited enhanced ABA and NaCl hypersensitivities, implying they can act together. This report thus describes a previously unknown heterodimeric cooperation between two independent substrate receptors for CUL4-based E3 ligases.
INTRODUCTION
Throughout their life cycles, plants encounter various abiotic stresses, such as drought, salinity, heavy metals, and low temperatures. Plants induce various biochemical and physiological responses to overcome these unfavorable conditions. Several abscisic acid (ABA)-inducible genes have been shown to be induced by drought and high-salinity stress, indicating that ABA plays an important role in these types of stress signal transduction pathways (Seki et al., 2002). Moreover, plants respond to ABA in many ways, including closing stomata under drought stress, maintaining seed dormancy, and inhibiting vegetative growth (Finkelstein et al., 2002). There are two main types of ABA-dependent regulatory pathways in plants (Agarwal et al., 2006). The first is mediated by the basic leucine zipper/ABA-responsive element (bZIP/ABRE) system (Uno et al., 2000). ABRE is a cis-acting element for ABA-responsive genes that is bound by several leucine zipper transcription factors, such as ABRE binding (AREB) proteins/ABRE binding factors (ABFs) and ABA INSENSITIVE5 (ABI5) in Arabidopsis thaliana (Choi et al., 2000; Uno et al., 2000). The second is mediated by ABA via MYC/MYB transcription factors, such as MYC2 and MYB2, to induce the drought-responsive RD22 gene in Arabidopsis (Abe et al., 1997; Shinozaki and Yamaguchi-Shinozaki, 2000).
Prior to this report, genetic studies identified a number of important downstream components of ABA signaling (McCourt and Creelman, 2008). ABI1 and ABI2 were reported as group A type 2C protein phosphatases, indicating protein phosphorylation is involved in ABA signal transduction. These proteins negatively regulate SNF1-related protein kinase 2 proteins, which phosphorylate downstream targets such as various AREB/ABFs (Kobayashi et al., 2005; Umezawa et al., 2009). Genetic studies showed that both ABI1 and ABI2 affect various aspects of plant development, such as seed dormancy, root growth inhibition, and stomatal closure (Leung et al., 1994, 1997; Meyer et al., 1994). ABI3, ABI4, and ABI5 have been identified as transcription factors that share overlapping functions for ABA signaling during seed germination and early seedling development (Parcy et al., 1994; Finkelstein et al., 1998; Finkelstein and Lynch, 2000). ABI5 was reported to act downstream of ABI3 (Lopez-Molina et al., 2002) and to be involved in postgerminational developmental arrest and repression of germination (Lopez-Molina et al., 2001; Piskurewicz et al., 2008). Since the stability of ABI5 was shown to be regulated by ubiquitin-mediated degradation and ABA inhibited ABI5 degradation by this pathway (Lopez-Molina et al., 2001), several studies have attempted to identify proteins targeting ABI5 for degradation. Prior to this report, two related proteins were found. AFP, an ABI5-interacting protein, was reported to negatively regulate ABA signaling by facilitating ABI5 degradation through the 26S proteasome-dependent pathway (Lopez-Molina et al., 2003). KEEP ON GOING (KEG), a new Really Interesting New Gene (RING) finger E3 ligase, was also identified as a negative regulator of ABI5, confirming the direct regulation of ABI5 by the ubiquitin-dependent system (Stone et al., 2006).
Eukaryotic cells have evolved distinct superfamilies of E3 ubiquitin ligases that target proteins for selective degradation. The most abundant family uses cullins as scaffold proteins to form a framework for assembling the Cullin-RING ubiquitin E3 ligase (CRL), the ubiquitination machinery (Petroski and Deshaies, 2005; Thomann et al., 2005). All cullin family members commonly possess two essential modules assembled on the cullin protein. One is a RING finger domain protein, Regulator of Cullins1/RING-BOX1 (ROC1/RBX1), which recruits the E2 enzyme; the other is a substrate recognition complex. CULLIN3 uses BTB domain-containing proteins to recognize a number of different substrates and target them to the CUL3-ROC1 catalytic core without additional linkers (Furukawa et al., 2003; Geyer et al., 2003; Pintard et al., 2003; Xu et al., 2003). CULLIN1, CULLIN2/5, and CULLIN4 have evolved separate linkers and substrate receptors. The N-terminal domain of CUL1 binds a linker protein, S-phase Kinase-associated Protein 1 (SKP1), that binds various F-box proteins that specify substrates for ubiquitination (Feldman et al., 1997; Skowyra et al., 1997; Zheng et al., 2002). CUL2 and CUL5 use a heterodimeric linker complex containing elongins B and C to bind VHL-box or SOCS-box proteins, respectively (Kamura et al., 1998, 2004; Stebbins et al., 1999; Zhang et al., 1999).
For CUL4-based E3 ligases, DDB1 has been identified as an adaptor that links the CUL4-ROC1/RBX1 catalytic core to substrate receptors. Recently, there have been several reports of CUL4-based E3 ligase substrate receptors (Angers et al., 2006; He et al., 2006; Higa et al., 2006; Jin et al., 2006). Most proteins associated with CUL4-DDB1 in animals are WD40 repeat–containing proteins that share a conserved motif, called the DWD box, within the WD40 repeats. The DWD box is necessary for the interaction with CUL4-DDB1 and consists of 16 amino acids, of which four are highly conserved: Asp (or Glu) 7, Trp (or Tyr) 13, Asp (or Glu) 14, and Arg (or Lys) 16. There are also several positions within this box that are occupied by residues with similar properties (i.e., there are hydrophobic residues at positions 1, 2, 10, 12, and 15 and small residues at positions 3, 4, and 5). Among them, Arg-16 is most important for DWD protein function, since substitution of Arg-16 with another residue results in the inability to bind DDB1 (Angers et al., 2006; He et al., 2006). In plants, similar machinery for CUL4-based E3 ligases was found. Using the conserved amino acids in the DWD motif as probe, we found Arabidopsis and rice (Oryza sativa) had 85 and 78 DWD candidates, respectively (Lee et al., 2008). Some of the Arabidopsis DWD proteins bound DDB1 protein in vitro and in vivo, implying that these proteins may act as substrate receptors in plants via a similar mechanism as in animals. One, PLEIOTROPIC REGULATORY LOCUS1 (PRL1) was shown to be associated with the CUL4 complex and responsible for the degradation of AKIN10 (Arabidopsis SNF1 Kinase Homolog 10) (Lee et al., 2008).
In some cases, not all 16 amino acids of the DWD box seem necessary for the function of DWD proteins. For example, Zhang et al. (2008) reported that Arabidopsis DCAF1 can act as the substrate receptor for a CUL4-based E3 ligase. However, DDB1-CUL4 ASSOCIATED FACTOR1 (DCAF1) possesses only a WDxR motif in the WD40 region rather than an entire DWD box, implying that other proteins in addition to the 85 DWD proteins may also act as substrate receptors for CUL4 E3 ligases. In addition to the 85 DWD proteins, Arabidopsis also has 34 WDxR proteins that may be substrate receptors for CUL4-based E3 ligases (Zhang et al., 2008).
Although E3 ubiquitin ligases have been shown to be involved in ABA signal transduction (Stone et al., 2006; Zhang et al., 2007), little is known about the contribution of CRLs to ABA responses. Therefore, we have been interested in elucidating functional relationships between CUL4-based E3 ligases and ABA signal transduction. Here, we report the characterization of two Arabidopsis DWD proteins, DWA1 (DWD hypersensitive to ABA1) and DWA2, and their roles as substrate receptors for CUL4 E3 ligase complexes in ABA signaling in Arabidopsis. We also suggest that these two proteins are responsible for negative regulation of the ABA-responsive transcription factors ABI5 and MYC2. Finally, we present evidence that they can physically act together to target shared substrates for degradation.
RESULTS
Identification of Two Previously Unknown DWD Genes Involved in ABA Responses
To investigate whether some DWD proteins may be involved in ABA signaling, we screened the ABA and NaCl sensitivities of homozygous T-DNA–tagged lines of 30 DWD genes (out of the 119 DWD and WDxR genes identified in Arabidopsis). Four showed hypersensitivities to both treatments during our initial screening (see Supplemental Table 1 online). After repeated assays, two genes, At2g19430 and At1g76260, were selected for further study and named DWA1 and DWA2, respectively. DWA1 is 1382 bp long and encodes a 367–amino acid protein. DWA2 is 1416 bp long and encodes a protein of 350 amino acids (see Supplemental Figure 1A online). Both proteins have a single DWD motif, and additional annotated domains other than the WD40 region were not detected (Marchler-Bauer et al., 2005). To check if the two proteins are structurally related, they were analyzed pairwise with EMBOSS Pairwise Alignment Algorithms at EMBL-EBI (Rice et al., 2000). No significant alignments were found, even within the WD40 region (which has limited amino acid conservation), indicating that they are not homologous.
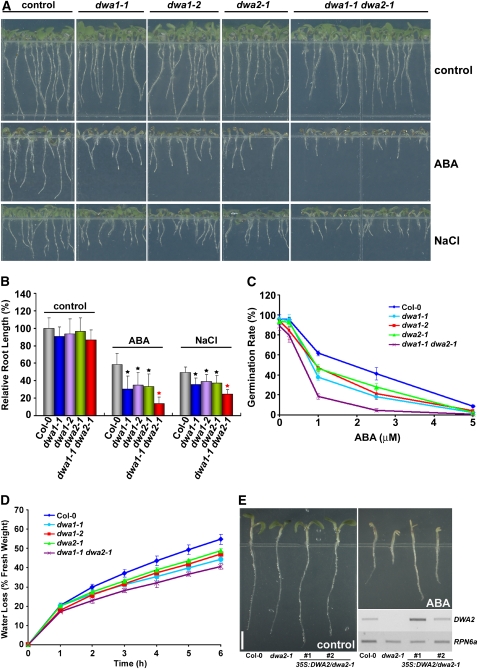
Two independent T-DNA insertion lines, dwa1-1 and dwa1-2, were obtained and analyzed for DWA1. RT-PCR analysis showed that DWA1 expression was abolished in dwa1-1 and markedly decreased in dwa1-2 (see Supplemental Figure 1B online). A T-DNA insertion line dwa2-1, which contained a single T-DNA, was analyzed for DWA2 (see Supplemental Figure 1C online). All three mutants (dwa1-1, dwa1-2, and dwa2-1) were significantly more sensitive to 0.5 μM ABA than was the wild type (Figures 1A and 1B), and all three exhibited even more extreme responses to 1 μM ABA (see Supplemental Figures 2A and 2B online). All three mutants also showed more sensitivity to 100 mM NaCl than did the wild type, although the differences were not as dramatic. Interestingly, the dwa1-1 dwa2-1 double mutant exhibited more severe sensitivity to 0.5 μM ABA than did either single mutant alone. Significant enhancement of NaCl sensitivity was also detected in the double mutant, although it was not as strong as the response to ABA (Figures 1A and 1B).
Figure 1.
Increased ABA and NaCl Sensitivities of dwa1 and dwa2 Mutants, and Enhanced Hypersensitivity in the dwa1 dwa2 Double Mutant.
(A) ABA and NaCl hypersensitivity phenotypes of dwa1-1, dwa1-2, dwa2-1, and dwa1-1 dwa2-1 mutants. All seedlings were grown vertically on germination medium (GM) plates with or without 0.5 μM ABA or 100 mM NaCl for 5 d after stratification.
(B) Root lengths of the wild type, dwa1-1, dwa1-2, dwa2-1, and dwa1-1 dwa2-1 grown on GM with or without 0.5 μM ABA or 100 mM NaCl. Relative root lengths compared with those of Columbia-0 grown on GM plates are indicated. Values are means ± sd (n = 20). Statistically significant differences between the wild type and single mutants (P < 0.01) are indicated by black asterisks. Statistically significant differences between single dwa mutants and the double mutant (P < 0.01) are indicated by the red asterisk.
(C) Germination of the wild type, dwa1-1, dwa1-2, dwa2-1, and dwa1-1 dwa2-1 under various concentrations of ABA. Seeds were germinated and grown on GM plates with or without ABA for 3 d after stratification. Germination was determined for an average of >80 seeds from three independent experiments. Values are means ± sd.
(D) Water loss from detached leaves of the wild type, dwa1-1, dwa1-2, dwa2-1, and dwa1-1 dwa2-1. Results are from three replicates, and values are means ± sd (n = 18).
(E) Complementation of DWA2 in dwa2-1. The entire open reading frame of DWA2 under the control of the cauliflower mosaic virus 35S promoter was introduced into dwa2-1. Two independent transgenic lines were selected, and the expression level of DWA2 in the wild-type, dwa2-1, and both transgenic lines was checked by RT-PCR (bottom right). Seeds were germinated and grown vertically on GM plates with or without 1 μM ABA for 5 d after stratification. RPN6a was used as internal control for the assay. Bar = 2 mm.
To check whether the shorter roots observed in dwa mutants resulted from delayed germination or the inhibition of primary root growth, we compared germination of wild-type and dwa mutant seeds under various ABA concentrations. As shown in Figure 1C, the germination rate of both single dwa mutants was lower than that of the wild type at all tested ABA concentrations, and the germination rate of the double mutant was even more depressed. However, although a slight decrease in primary root length of double mutants compared with the wild type was observed after transfer to media containing 5 μM ABA (see Supplemental Figure 3 online), this difference could not explain the difference in root length between the double mutant and wild-type plants that were germinated on media containing ABA (Figures 1A and 1B). Moreover, we could not detect any differences in root length between the wild-type, dwa1, and dwa2 after transfer to media containing ABA. Therefore, the short root lengths of dwa mutants shown in Figures 1A and 1B appear to be predominantly due to delayed germination rather than inhibition of root growth.
Next, to examine if dwa1 and dwa2 exhibited dehydration tolerance, one of the effects of ABA, water loss assays were performed. Results showed that dwa1 and dwa2 lost water more slowly than did wild-type plants, while the double mutant lost even less water than either single mutant, indicating that the reduced levels of DWA proteins retarded water loss (Figure 1D). Moreover, dwa1 dwa2 showed enhanced tolerance to drought (see Supplemental Figure 4 online). Thus, taken together, dwa1 and dwa2 mutants indeed exhibit the characteristics of ABA hypersensitivity, and this hypersensitivity was further enhanced in the double mutant.
Even though we confirmed that dwa2-1 had a single T-DNA insertion, to ascertain that ABA hypersensitivity of dwa2-1 resulted from the loss of DWA2, we generated transgenic lines complementing dwa2-1. Figure 1E shows that two independent complementation lines (35S:DWA2/dwa2-1) exhibited similar or even less ABA sensitivity than did the wild type, indicating that the loss of the DWA2 gene is responsible for the ABA sensitivity.
DWA1 and DWA2 Interact with DDB1
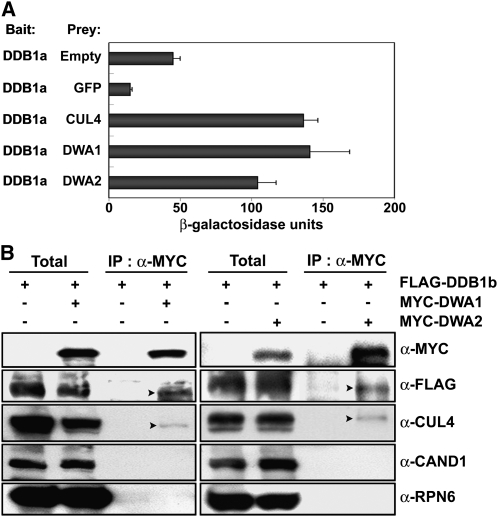
To test whether DWA1 and DWA2 are substrate receptors for CUL4-DDB1 E3 ligase complexes, we first checked if they could bind DDB1. In yeast two-hybrid assays using DDB1a as bait, β-galactosidase activities with DWA1 and DWA2 were at least twofold greater than with the empty vector control and more than sevenfold greater than with another negative control, green fluorescent protein (GFP), used as prey (Figure 2A; see Supplemental Figure 5 online). In a positive control, strong β-galactosidase activity was observed when CUL4, which interacts with DDB1, was used as prey (Figure 2A). To test whether DWA1 and DWA2 are CUL4 E3 complex components in planta, we generated constructs for MYC-tagged DWA1 and DWA2 proteins and transformed them into FLAG-DDB1b/ddb1a lines. Coimmunoprecipitation (co-IP) assays were then performed to test for interactions between these components and DWA proteins. As shown in Figure 2B, both DWA proteins were effectively coimmunoprecipitated with FLAG-DDB1b and CUL4 proteins. Binding of DWA1 and DWA2 to cullin-associated and neddylation-dissociated 1 (CAND1) was not detected in our co-IP experiments. This is consistent with previous findings that CAND1 inhibits the activity of CULLIN-based E3 ligases by binding to unmodified CULLIN and blocking associations between the CULLIN and its adaptor proteins (Feng et al., 2004; Petroski and Deshaies, 2005). Taken together, these results confirmed that DWA1 and DWA2 proteins could act as substrate receptors for CUL4 E3 ligases in Arabidopsis.
Figure 2.
Interactions of Various Proteins with DWA1 and DWA2.
(A) Interactions between DDB1a and DWA proteins checked by yeast two-hybrid assays. Assays were performed with DWA proteins as prey and DDB1a as bait for monitoring their interactions. CUL4 was used as a positive control known to bind DDB1. Empty vector and GFP proteins were used as negative controls. β-Galactosidase activities were quantified after growing yeast strains in liquid culture using o-nitrophenyl-β-d-galactopyranoside as substrate. Values are means ± sd (n = 4).
(B) Co-IP of DWA1 and DWA2 with various proteins. Arabidopsis transgenic plants overexpressing FLAG-tagged DDB1b alone or with MYC-tagged DWA1 or DWA2 were used for co-IP assays to detect interactions between DWA proteins and various other proteins. The immunoblot used anti-RPN6 as internal control. The arrowheads on the α-FLAG and α-CUL4 panels represent the positions of FLAG-DDB1 and CUL4, respectively. Total, 5% of the crude extracts used for co-IP assays.
Two ABA-Responsive Transcription Factors, ABI5 and MYC2, Accumulate to High Levels in dwa1 and dwa2 and to Even Higher Levels in dwa1 dwa2 after ABA or NaCl Application
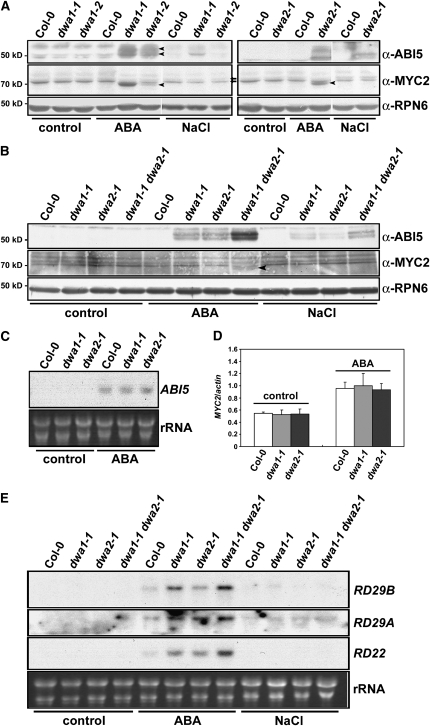
Since dwa1 and dwa2 exhibited ABA hypersensitivity, we checked the protein levels of ABA-responsive transcription factors, such as ABI5 and MYC2, in wild-type and dwa mutants after ABA and NaCl treatments. Wild-type and the mutants were germinated 5 d on Murashige and Skoog (MS) plates supplemented with 1 μM ABA or 100 mM NaCl, then the levels of ABI5 and MYC2 proteins were examined. Stone et al. (2006) reported detecting ABI5 proteins of three different sizes (52.5, 51, and 50 kD) in immunoblot assays using ABI5 antibody, with the 52.5- and 50-kD bands predominating. In our experiments, two bands were also detected with some variation according to gel running conditions, whereas they could not be found in abi5-1 (Finkelstein, 1994) (Figures 3A and 3B; see Supplemental Figure 6 online). As shown in Figure 3A and Supplemental Figure 7 online, the levels of both ABI5 and MYC2 proteins were higher in dwa1 and dwa2 mutants than in the wild type after ABA treatment. This indicates that reducing the level of either DWA protein resulted in the accumulation of ABA-responsive transcription factors in the presence of ABA, consistent with the ABA hypersensitivity of the dwa mutants (Figure 1A). On the other hand, although ABI5 protein levels were slightly increased by NaCl treatment in both mutants, the levels were not as high as those induced by ABA treatment. Also, MYC2 was not increased by NaCl treatment in either mutant. Therefore, the effect of NaCl on accumulation of ABA-responsive transcription factors seems less than that of ABA in spite of the NaCl hypersensitivity of both mutants. Even though 1 μM ABA and 100 mM NaCl treatments inhibited root growth in the wild-type (Figures 1A and 1B; see Supplemental Figure 2 online), we could not consistently detect increased levels of ABI5 and MYC2 proteins in the wild type under these conditions (Figure 3A), indicating that these conditions may not be sufficient to enhance accumulation of these proteins in the wild type. ABI5 was also upregulated by 0.5 μM ABA in both mutants, although to lower levels than in 1 μM ABA, whereas MYC2 was not increased by the lower concentration of ABA. Consistent with the enhanced hypersensitivity to ABA, even higher levels of ABI5 and MYC2 were detected in the double mutant in response to ABA (Figure 3B). To check whether the reduced levels of DWA1 and DWA2 proteins also affected the expression of ABI5 and MYC2 at the mRNA level, we monitored the levels of ABI5 and MYC2 transcripts in the wild type and dwa1 and dwa2 mutants (Figures 3C and 3D). Although ABA treatment induced increased expression of ABI5 and MYC2 in all lines, no significant differences were found between the wild type and dwa mutants. This result indicates that the hyperaccumulation of ABI5 and MYC2 proteins in dwa mutants resulted from posttranscriptional regulation.
Figure 3.
ABI5 and MYC2 Proteins Accumulate to Elevated Levels in dwa1, dwa2, and dwa1 dwa2 Mutants after ABA and NaCl Treatments.
(A) Levels of ABI5 and MYC2 proteins in the wild type, dwa1-1, dwa1-2, and dwa2-1 mutants after ABA and NaCl applications. Wild-type and dwa seedlings were grown on GM plates with or without 1 μM ABA or 100 mM NaCl for 5 d after stratification. Proteins were extracted and the indicated protein levels were determined by immunoblot assays. The arrowheads on the anti-ABI5 and anti-MYC2 panels represent the positions of endogenous ABI5 (two predominant forms) and MYC2, respectively. The arrows on the anti-MYC2 panel indicate nonspecific bands that reacted with this antibody.
(B) Enhanced accumulation of ABI5 and MYC2 proteins in dwa1-1 dwa2-1 after 0.5 μM ABA or 100 mM NaCl application. The arrowhead on the anti-MYC2 panel indicates endogenous MYC2.
(C) ABI5 mRNA level in the wild type, dwa1-1, and dwa2-1 mutants after ABA treatment. Wild-type and dwa seedlings were grown with or without 1 μM ABA for 5 d after stratification. RNA gel blots were then performed to check ABI5 mRNA expression levels. rRNA staining with ethidium bromide was used to demonstrate equal loading of the wells. The samples used for RNA gel blots were confirmed to exhibit protein expression consistent with that in (A).
(D) MYC2 mRNA levels in the wild type, dwa1-1, and dwa2-1 mutants after ABA treatment. mRNA levels were determined by quantitative real-time PCR Analysis. Wild-type and dwa seedlings were grown with or without 1 μM ABA for 5 d after stratification. Relative amounts of MYC2 transcripts were normalized to the levels of ACTIN2 in the same sample. The highest level of MYC2 mRNA was set to 1 arbitrary unit. The same samples used in (A) were used for this assay. Values are means ± sd (n = 3).
(E) Enhanced expression of various ABA-responsive genes in dwa1-1, dwa2-1, and dwa1-1 dwa2-1 mutants after ABA application. Five-day-old wild-type and dwa seedlings grown in the presence/absence of 0.5 μM ABA or 100 mM NaCl were used to measure mRNA levels of RD29B, RD29A, and RD22 on RNA gel blots. rRNA staining with ethidium bromide was used to demonstrate equal loading of the wells.
ABA-Responsive Genes Are Induced to High Levels in dwa1 and dwa2 and Even Higher Levels in dwa1 dwa2 by ABA Treatment
It has been reported that ABI5 transactivates RD29A and RD29B genes, whereas MYC2 activates RD22 by binding its promoter (Abe et al., 2003; Nakashima et al., 2006). We therefore checked the expression of RD29A, RD29B, and RD22 in the wild type and dwa mutants after treatment with ABA or NaCl (Figure 3E). RD29B and RD29A were hyperinduced by 0.5 μM ABA in dwa1 and dwa2 mutants. Moreover, RD22 was also hyperinduced in both mutants treated with 0.5 μM ABA even though MYC2 was not detected under these conditions (Figure 3B). In addition, those RD genes were induced to even higher levels by ABA in the double mutants. Taken together, these data indicate that the elevated levels of ABI5 and MYC2 in dwa mutants contributed to the increased ABA sensitivity via upregulation of downstream ABA-responsive genes. Although ABI5 levels were slightly increased in all mutants by NaCl (Figure 3B), we could not detect any marked differences in mRNA levels of RD29A and RD29B genes between the wild type and the mutants (Figure 3E). These results are consistent with the weaker growth responses to 100 mM NaCl (Figures 1A and 1B).
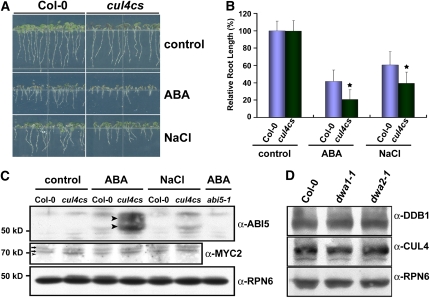
A Reduced-Function Line of CUL4 Is Hypersensitive to ABA
Since at least two DWD proteins were involved in ABA signal transduction, we anticipated that a decrease in CUL4 protein might also affect ABA sensitivity in Arabidopsis. We therefore tested the ABA and NaCl sensitivities of the cul4cs line, which has reduced levels of CUL4 protein (Chen et al., 2006). As shown in Figures 4A and 4B, cul4cs exhibits significant hypersensitivity to ABA and NaCl. Moreover, ABI5 levels were higher in cul4cs than in the wild type after ABA or NaCl application (Figure 4C). Because cul4cs is hypersensitive to ABA, we tested whether the reduced amounts of DWA proteins changed the levels of CUL4 complex proteins in dwa1 and dwa2 after ABA treatment. As shown in Figure 4D, no differences in the levels of DDB1 and CUL4 proteins were detected between the wild type, dwa1, and dwa2. Therefore, the ABA hypersensitivity of dwa1 and dwa2 does not result from altered levels of CUL4 and DDB1 proteins.
Figure 4.
ABA and NaCl Hypersensitivities and Enhanced ABI5 Stability in cul4cs.
Seedlings were grown on GM plates with or without 1 μM ABA ([A] to [D]) or 100 mM NaCl ([A] to [C]) for 5 d after stratification.
(A) ABA and NaCl hypersensitivities of cul4cs.
(B) Comparative analysis of root lengths of wild-type and cul4cs seedlings after ABA and NaCl treatments. Relative root lengths compared with that of Col-0 grown on GM plates are indicated. Values are means ± sd (n = 20). Statistically significant differences between the wild type and cul4cs (P < 0.01) are indicated by asterisks.
(C) Levels of ABI5 and MYC2 proteins in the wild type and cul4cs after both treatments. The arrowheads on the anti-ABI5 panel represent the positions of two predominant forms of ABI5 protein. The arrows on the anti-MYC2 panel indicate nonspecific bands reacting with anti-MYC2, and the arrowhead shows where endogenous MYC2 protein should have run.
(D) Levels of CUL4-based E3 ligase protein components in the wild type, dwa1-1, and dwa2-1 mutants. Wild-type, dwa1-1, and dwa2-1 seedlings were grown in the presence of 1 μM ABA for 5 d after stratification. DDB1 and CUL4 protein levels from each line were checked with the corresponding antibodies.
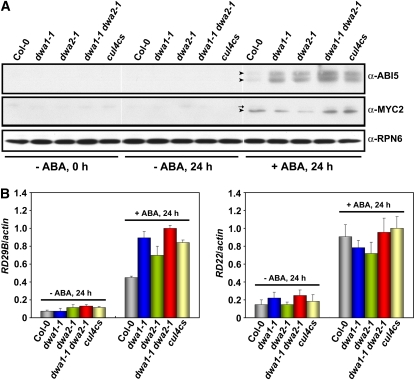
ABI5 Accumulates to High Levels in dwa1 and dwa2 Single and Double Mutants after ABA Treatment at the Postimbibition Stage but before Germination
Since ABI5 and MYC2 accumulated to high levels in 5-d-old dwa and cul4cs seedlings treated with ABA (Figures 3A and 4C), at which stage the mutants exhibited short roots, we examined if the increased levels of ABI5 and MYC2 in the mutants play any role in the reduced elongation or are simply a consequence of the retardation of the mutant seedling's growth. To this end, we examined the levels of ABI5 and MYC2 proteins in the wild type and the mutants after ABA treatment at the postimbibition stage, but before germination. As shown in Figure 5A, ABI5 proteins were not detectable in the absence of ABA, consistent with the previous report (Lopez-Molina et al., 2001), but accumulated in all seeds after 24 h of ABA treatment following 3 d of stratification. Under this condition, the proteins accumulated to high levels in dwa1, dwa2, and cul4cs and even higher levels in dwa1 dwa2, indicating the delayed germination phenotype of dwa mutants is related to the increase in ABI5 proteins at the postimbibition stage. However, although MYC2 accumulated in all seeds treated with ABA, no differences in MYC2 levels were observed between mutants and the wild type. Therefore, MYC2 may not be related to the delayed germination of dwa mutants. The expression levels of RD29B and RD22, the representative genes activated by ABI5 and MYC2, respectively, were consistent with the accumulation patterns of ABI5 and MYC2 shown in Figure 5A (Figure 5B).
Figure 5.
Levels of ABI5 and MYC2 Proteins and Their Target mRNAs at the Postimbibition stage, but before Germination, in Response to ABA.
(A) Levels of ABI5 and MYC2 proteins in the wild type and various mutants at the postimbibition stage in response to ABA. Seeds were stratified in darkness at 4°C for 3 d and then transferred to MS plates with or without ABA (3 μM) for 24 h. The arrowheads on the anti-ABI5 and anti-MYC2 panels represent the positions of endogenous ABI5 and MYC2, respectively. The arrows on the anti-MYC2 panel indicate nonspecific bands that reacted with this antibody.
(B) RD expression levels in the material from (A). mRNA levels of RD29B and RD22 were determined by quantitative real-time PCR Analysis. Relative amounts of RD29B and RD22 transcripts were normalized to the levels of ACTIN2 in the same sample. The highest levels of RD29B and RD22 transcripts were set to 1 arbitrary unit. Values are means ± sd (n = 3).
[See online article for color version of this figure.]
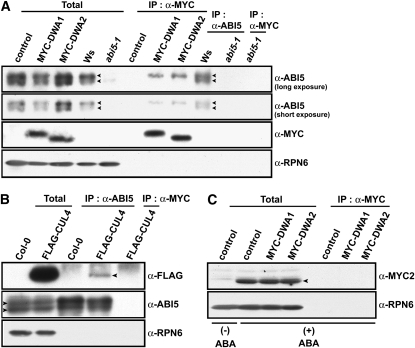
DWA1 and DWA2 Physically Associate with ABI5
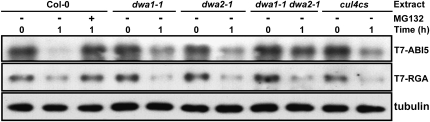
ABI5 and MYC2 proteins were highly induced by ABA treatment (Figure 3A). Also, it has been reported that ABI5 is negatively regulated by the 26S proteasome pathway (Lopez-Molina et al., 2001). Since DWA1 and DWA2 have the characteristics of CUL4 substrate receptors (Figures 2A and 2B) and may therefore target proteins for degradation via the 26S proteasome pathway, we hypothesized that ABI5 and MYC2 may be the substrates of CUL4-based E3 ligases mediated by DWA1 and/or DWA2. To test this, we treated FLAG-DDB1/ddb1a and FLAG-DDB1/MYC-DWA1/2/ddb1a seedlings with ABA and then performed in vivo co-IP assays. Both DWA proteins were successfully coimmunoprecipitated with the slower migrating form of ABI5, but association of either DWA protein with MYC2 was not detected (Figures 6A and 6C). This indicates that ABI5 may be the substrate for DWA1- and DWA2-mediated CUL4 E3 ligases. Moreover, ABI5 associated with CUL4 protein in vivo (Figure 6B), supporting the role of DWAs in mediating ABI5 degradation via a CUL4-based E3 ligase. Next, cell-free degradation assays were performed to test whether ABI5 protein stability was affected by reduced levels of DWA1 or DWA2 (Figure 7). T7- tagged ABI5 recombinant protein was incubated with protein extracts prepared from the wild type, dwa1-1, dwa2-1, dwa1-1 dwa2-1, and cul4cs treated with 0.5 μM ABA, in the presence of ATP. T7-ABI5 was gradually degraded when incubated with protein extracts from wild-type, dwa, and cul4cs plants, and this degradation was blocked by MG132, a proteasome-specific inhibitor (Figure 7; see Supplemental Figure 8 online). This is consistent with the previous observation that ABI5 is destroyed via the 26S proteasome pathway (Lopez-Molina et al., 2001). However, T7-ABI5 proteins were degraded more slowly in dwa1-1 and dwa2-1 extracts than in wild-type extracts and even more slowly in dwa1-1 dwa2-1 double mutant extracts. Moreover, T7-ABI5 degradation was also retarded in cul4cs extracts (Figure 7). Therefore, ABI5 degradation requires DWA1 and DWA2, and this degradation is related to the action of CUL4-DDB1 E3 ligase complexes in which DWA1 and DWA2 function as substrate receptors. Furthermore, the observation that extracts from double mutants degraded ABI5 more slowly than those from either single mutant is consistent with the enhanced ABA sensitivity and much higher accumulation of ABI5 in the double mutant. Conversely, degradation of REPRESSOR OF GA1-3 (RGA), a negative regulator of GA responses, was not retarded in dwa1, dwa2, or dwa1-1 dwa2-1, confirming the specific role of both DWAs in ABI5 degradation.
Figure 6.
Co-IP of ABI5 and Components of CUL4-DDB1-DWAs.
(A) Association of DWAs with ABI5. Seedlings were grown in the presence of 5 μM ABA for 5 d after stratification. After protein extraction, the extracts were coimmunoprecipitated with α-MYC or α-ABI5. The arrowheads indicate two predominant forms of ABI5 protein. Two images from an immunoblot with anti-ABI5 are shown after short or long exposure to film (Fuji). Control, FLAG-tagged DDB1b transgenic plants; MYC-DWA1 or 2, FLAG-tagged DDB1b with MYC-tagged DWA1 or DWA2 transgenic plants; Ws, Wassilewskija. Total, 5% of the crude extracts used for co-IP assays.
(B) Association of FLAG-CUL4 and ABI5. Seedlings were grown in the presence of 5 μM ABA for 5 d after stratification. After protein extraction, the extracts were coimmunoprecipitated with α-ABI5. The extracts of FLAG-CUL4 coimmunoprecipitated with α-MYC were used as a negative control to exclude the possibility of nonspecifically bound FLAG-CUL4. The arrowheads on the anti-FLAG and anti-ABI5 panels represent the positions of FLAG-CUL4 and ABI5 proteins, respectively.
(C) MYC2 does not detectably associate with DWA proteins. Five-day-old seedlings treated with 5 μM ABA were used for co-IP with the anti-MYC antibody (which recognizes the MYC tag but not MYC2). The arrowheads represent endogenous MYC2 protein. To show clearly the existence of MYC2 induced by ABA, the extracts without ABA were loaded on the same gel. Control, FLAG-tagged DDB1b transgenic plants; MYC-DWA1 or 2, FLAG-tagged DDB1b with MYC-tagged DWA1 or DWA2 transgenic plants. Total, 5% of the crude extracts used for co-IP assays.
Figure 7.
Degradation of T7-Tagged ABI5 Protein in Wild-Type, dwa1-1, dwa2-1, dwa1-1 dwa2-1, and cul4cs Extracts.
T7-ABI5 and T7-RGA proteins were incubated with extracts (10 μg) prepared from 5-d-old seedlings, which were grown in the presence of 0.5 μM ABA, with or without 20 μM MG132, for 1 h at 30°C. T7-ABI5 and T7-RGA levels were examined by immunoblotting with anti-T7 antibody. Equal loading of cell extracts was confirmed with antitubulin antibody.
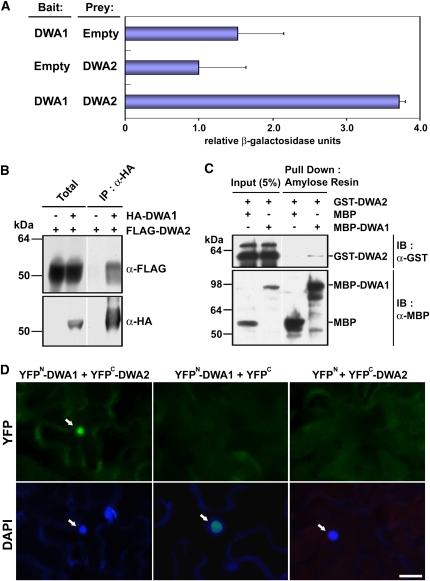
DWA1 and DWA2 Are Also Capable of Directly Interacting with Each Other
Because dwa1 and dwa2 mutants had very similar hypersensitivity to ABA and NaCl and mutation of both DWA1 and DWA2 genes enhanced ABA hypersensitivity, we hypothesized that DWA1 and DWA2 could act together in ABA signal transduction. Toward this end, we first investigated whether the proteins were able to interact directly with each other in yeast two-hybrid assays. When DWA1 and DWA2 were used as bait and prey, respectively, β-galactosidase activity increased at least twofold compared with empty vector negative controls (Figure 8A; see Supplemental Figure 5 online). When we used tobacco transient expression assays, HA-DWA1 was able to coimmunoprecipitate FLAG-DWA2 in planta (Figure 8B). Moreover, the purified recombinant MBP-DWA1 was effectively pulled down with GST-DWA2 in vitro (Figure 8C). Finally, direct interaction between DWA1 and DWA2 was confirmed using bimolecular fluorescence complementation (BiFC) assays (Figure 8D). Taken together, these data strongly indicate that these two proteins directly interact with each other in vivo and in vitro, suggesting they might form heterodimers in playing their roles as substrate receptors for the action of CUL4-based E3 ligases.
Figure 8.
Interactions between DWA1 and DWA2 Proteins.
(A) Interactions between DWA1 and DWA2 proteins detected by yeast two hybrid assays. Values are means ± sd (n = 4).
(B) In vivo interaction between DWA1 and DWA2 shown by transient expression in tobacco plants. Agrobacterium tumefaciens (C58C1) containing HA-DWA1, FLAG-DWA2, or p19 constructs was injected into 3- to 4-week-old tobacco leaves (Nicotiana benthamiana). After total proteins were extracted from the infiltrated leaves, anti-HA affinity matrix was mixed with the extracts, and immunoprecipitation assays were performed.
(C) In vitro interaction between DWA1 and DWA2 detected by pull-down assays. Recombinant MBP, MBP-DWA1, and GST-DWA2 proteins were incubated with amylose resin at 4°C. The eluates were resolved by SDS-PAGE and blotted. The blot was probed with anti-GST or anti-MBP antibody.
(D) BiFC analysis of DWA1 and DWA2. Epifluorescent images of epidermal N. benthamiana cells infiltrated with a mixture of Agrobacterium suspensions harboring constructs YFPN-DWA1+YFPC-DWA2, or negative controls YFPN-DWA1+YFPC and YFPN+YFPC-DWA2, together with the silencing suppressor p19. Green indicates YFP fluorescence, and blue indicates nuclei stained with 4',6-diamidino-2-phenylindole (DAPI). The positions of nuclei are indicated by arrows. Bar = 20 μm.
DWA1 and DWA2 Also Exhibit Self-Interactions
To examine possible molecular bases for explaining why DWA1 and DWA2 may work independently of each other as well as together, we investigated whether DWA1 and DWA2 were able to interact with themselves. As shown in Supplemental Figure 9 online, MBP-DWA1 and GST-DWA2 efficiently pulled down GST-DWA1 and MBP-DWA2, respectively. These results indicate DWA1 and DWA2 are capable of self-interaction in addition to their heterodimeric interaction. Therefore, DWA1 and DWA2 may work together (heterodimeric association) as well as individually (homodimeric association) as substrate receptors for CUL4-DDB1 E3 ligase complexes in mediating ABA responses.
DISCUSSION
Ubiquitin ligases (E3) bind the substrates and thus determine the specificities in many aspects of plant growth and development. It has been reported that signal transduction pathways for many plant hormones, including auxin, gibberellin, ethylene, jasmonic acid, and ABA, use ubiquitin-mediated selective protein degradation for their downstream effects (Smalle and Vierstra, 2004). Although there have been several reports about roles of E3 ligases including CRLs in plant hormonal responses, the connection between CRLs and ABA responses is elusive (Thomann et al., 2005).
To learn more about ABA signaling in plants, we examined functional connections between DWD proteins identified in our previous reports (Lee et al., 2008) and ABA responses. Based on the ABA and NaCl hypersensitivity of dwa1 and dwa2 mutants and the interactions between DWA1 and DWA2 and the CUL4 complexes, we propose that both proteins serve as negative regulators of ABA signaling, possibly as substrate receptors in ubiquitination pathways (Figures 1 and 2). Since salt and drought stress responses are both largely signaled via ABA (Zhu, 2002), the salt hypersensitivity of dwa mutants likely results from the osmotic effects triggering increased ABA levels (Figure 1A). However, since there are ABA-dependent and ABA-independent pathways for salt and drought stress responses in plants (Liu et al., 1998; Kizis et al., 2001), we are not able to exclude the possibility that the salt hypersensitivity of dwa came from ABA-independent salt stress signaling. This might be tested by generating aba dwa mutants and measuring their salt sensitivity.
In the dwa1-1 mutant, T-DNA was inserted into the first intron of DWA1, knocking out DWA1 gene expression, whereas in dwa1-2, the T-DNA was inserted 49 bp upstream from the ATG, which markedly decreased but did not eliminate its expression (see Supplemental Figure 1A online). This is consistent with results shown in Figure 4A (e.g., ABI5 and MYC2 accumulated to lower levels in dwa1-2 than in dwa1-1 in response to ABA and NaCl). Moreover, dwa1-2 exhibits longer root length than that of dwa1-1, and delay of germination was also less sensitive to ABA in dwa1-2 than in dwa1-1 (Figures 1B and 1C; see Supplemental Figure 2B online). Therefore, these results indicate that DWA1 expression level is related to ABA sensitivity and that, at least in part, the resulting hypersensitivity comes from the increased levels of ABI5 and MYC2.
Our results indicate that the absence or reduction of DWA1 and DWA2 is highly correlated with the regulation of ABI5 and MYC2 proteins and expression of their target genes (Figures 3A, 3B, and 3E). In addition, both DWA1 and DWA2 associate with ABI5 and negatively modulate the level of ABI5 (Figures 6A and 7). Moreover, both DWA1 and DWA2 appear to be components of CUL4 E3 ligase complexes based on our yeast two-hybrid and co-IP data (Figure 2). These data lead us to propose that DWA1 and DWA2 are substrate receptors for CUL4-based E3 ligases that mark ABI5 for degradation. At this time, it is unclear how DWA1 and DWA2 negatively regulate the level of MYC2. As shown in Figure 6C, we failed to detect any association between DWAs and MYC2 in our coimmunoprecipitation experiments. Therefore, unlike ABI5, MYC2 protein might not be directly targeted by DWA proteins.
As shown in Figures 1A to 1C, 4A, 4B, and Supplemental Figure 3 online, shorter root lengths in dwa and cul4cs treated with ABA seem to be predominantly due to the delayed germination rather than actual inhibition of root growth. This is supported by the fact that at the postimbibition stage, ABI5 levels in dwa mutants and cul4cs lines were higher than in the wild type treated with ABA (Figure 5A). Since ABI5 plays its main role in repression of germination, postgermination developmental arrest, and seed maturation rather than in inhibition of seedling root growth, it is expected that ABI5 will be most active at the stages preceding root growth. Consistent with this hypothesis, microarray data posted at the AtGenExpress Visualization Tool (http://www.weigelworld.org/resources/microarray/AtGenExpress/) show that DWA1 is abundantly expressed in dry seeds and DWA2 expression increases during seed imbibition, indicating that both DWA proteins can act before germination. Nevertheless, since dwa mutants exhibited mild resistance to dehydration stress, a process that is not normally regulated by ABI5, DWA proteins may possess additional targets, such as ABF/AREBs, that are more highly expressed in mature plants (Uno et al., 2000). Also, this mild tendency may result from reduced effectiveness of DWAs in mature plants compared with during germination. Epistatic analysis by generating abi5 dwa mutants may help us to elucidate the involvement of other factors mediated by DWAs in ABA responses.
Two single-subunit E3 ligases, SALT- AND DROUGHT-INDUCED RING FINGER1 (SDIR1) and KEG, were already reported as positive and negative regulators of ABI5 in ABA responses, respectively (Stone et al., 2006; Zhang et al., 2007). Previous cross-complementation assays showed that ABA insensitivity of sdir1-1 could be rescued by ABRE BINDING FACTOR3 (ABF3), ABF4, and ABI5 genes, whereas SDIR1 was not able to rescue abi5-1, suggesting that SDIR1 positively modulates gene expression upstream of those transcription factors (Zhang et al., 2007). On the other hand, KEG directly interacts with ABI5 in vitro, and keg mutants accumulate high levels of ABI5 even without ABA application, indicating that KEG shares the biological role of negative regulator in ABA signal transduction with DWA1 and DWA2. How KEG, DWA1, and DWA2 recognize the same ABI5 substrate in ABA responses is intriguing because no significantly conserved regions were detected between any of these proteins. It is therefore possible that an unknown component is involved in recruiting ABI5 to some of the substrate receptor proteins. One candidate is AFP, a negative regulator of ABA responses that directly interacted with ABI5 (Lopez-Molina et al., 2003).
As shown in Figure 6A, both DWA proteins efficiently interacted with the slower-migrating of the two predominant forms of ABI5. This slower-migrating ABI5 might be a suitable form for ABI5 protein activity, and both DWAs may possess higher affinity for this form to inhibit more efficiently ABI5 activity. Although it has been reported that the phosphorylated form of ABI5 migrates more slowly on SDS-PAGE, and depends on the presence of ABA (Lopez-Molina et al., 2001), we cannot say this DWA-interacting protein is phosphorylated ABI5 at this point. This is because Stone et al. (2006) reported treatment with protein phosphatase did not modify the pattern of multiple ABI5 proteins observed in keg.
The existence of several E3 ligases that target the same substrate for degradation has been reported in mammals. For example, Synphilin-1, the α-synuclein–interacting protein, has been shown to be ubiquitinated by several E3 ligases including Parkin, Siah-1, and Dorfin in human cells (Chung et al., 2001; Ito et al., 2003; Nagano et al., 2003). Similarly, the degradation of CDT1 (Cdc10-dependent transcript), a licensing factor for DNA replication, is regulated by different CRLs, including SCFSKP2 and CUL4-DDB1-CDT2, according to the stage of the cell cycle (Kim and Kipreos, 2007). Accordingly, the roles of KEG and DWA1/DWA2 in ABI5 degradation may vary according to the tissue and developmental stage. In the presence of 1% sucrose, keg seedlings exhibited strong postgerminative growth in their cotyledons without ABA application, whereas root lengths were less affected, indicating that the absence of KEG has more effect on aerial tissues than on roots. By contrast, we did not observe any tissue-specific differences in dwa mutants in the presence/absence of ABA compared with the wild type. Moreover, KEG is also involved in ethylene signal transduction, whereas DWA1 and DWA2 do not seem to be since ACC treatment did not affect the sensitivity of dwa1 and dwa2 to ethylene compared with the wild type (see Supplemental Figure 10 online). Therefore, KEG may play more pleiotropic roles in hormonal responses than do DWA proteins. Certainly, ABA signaling is much stronger in keg than in dwa1 and dwa2, since ABI5 was strikingly upregulated in keg even without ABA application (Stone et al., 2006), whereas ABI5 was not detectable in dwa1 and dwa2 without this hormone. The elucidation of functional relationships between KEG and DWA1/2 in ABA signaling by biochemical and genetic approaches will improve our understanding of the ABA signal transduction pathway.
Several recent reports have described combinatorial interactions between homologous signaling proteins. For example, FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL) have been shown to form homodimers and heterodimers and then act together to regulate downstream events in Phytochrome A (PHYA) signaling, such as nuclear accumulation of PHYA and inhibition of hypocotyl elongation (Zhou et al., 2005; Hiltbrunner et al., 2006; Yang et al., 2009). Similarly, SPAs (for SUPPRESSOR OF PHYA) 1, 2, 3, and 4 are highly homologous proteins that can form homo- or heterodimers. The diverse SPA complexes help repress photomorphogenesis by interacting with CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1), a central regulator in light signaling (Zhu et al., 2008).
By contrast, little is known about interactions between CRL substrate receptors that are not homologous to each other. Therefore, the interaction between DWA1 and DWA2 is intriguing. Furthermore, as shown in Figures 1A to 1D, 3B, and 7, mutation of both DWA1 and DWA2 genes enhanced ABA hypersensitivity, implying DWA1 and DWA2 work together in ABA signaling. One potential explanation for the enhanced effects of dwa1-1 and dwa2-1 on ABA responses is that DWA1 and DWA2 function as E3 substrate receptors separately as well as part of the same complex, and these separate activities of DWA1 and DWA2 additively strengthen their negative effects on ABA signaling. Under this hypothesis, DWA1 and DWA2 might form homodimeric as well as heterodimeric associations in planta, and all three types of dimers may act as substrate receptors for CUL4-based E3 ligases. An alternative possibility is that, even though either DWA1 or DWA2 could play its role by itself as a DWD protein, DWA1-DWA2 formation might enhance the efficiency of the complex. For example, it may enhance its affinity for the substrate or the stability of the complex, thus resulting in more ABI5 degradation. However, since the effects in the double mutant appear to be additive rather than synergistic, the former possibility looks more plausible. Our BiFC assays indicate that the interaction between DWA1 and DWA2 occurred in the nucleus because the interaction signal was exclusively found in the nucleus. Since we failed to detect any significant nuclear localization signals in either protein, the nuclear localization of these proteins may require another protein that interacts with them (Figure 8D).
As shown in Figures 4A to 4C, the reduction of CUL4 significantly affected ABA sensitivity and ABI5 levels, supporting the involvement of DWA1 and DWA2 as components of CUL4 complexes in ABA signaling. Since the decreased level of CUL4 resulted in increased ABA sensitivity, it is apparent that the reduced levels of E3 ligases using DWA1 and DWA2 as substrate receptors contribute to the resulting ABA hypersensitivity. Nevertheless, we cannot simply conclude here that the ABA hypersensitivity of cul4cs is solely due to substrate receptors DWA1 and DWA2. This is supported by our initial screening (see Supplemental Table 1 online), which showed that the previously reported PRL1 (Lee et al., 2008) and two additional DWD proteins are also involved in ABA signaling.
Based on the presence of the DWD and WDxR motifs, Arabidopsis possesses 119 potential substrate receptors related to CUL4-based E3 ligases (Lee et al., 2008; Zhang et al., 2008). Further screening these T-DNA mutants for aberrant responses to other biotic and abiotic signals will enable us to identify additional roles of CUL4-based ubiquitin ligases in plant signal transduction and development.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Columbia and Wassilewskija were used in this study. The dwa1-1 (SALK_051022), dwa1-2 (SALK_021789), and dwa2-1 (SALK_034658) T-DNA insertion mutants were obtained from the Arabidopsis Stock Center (http://www.Arabidopsis.org/; Alonso et al., 2003). Oligonucleotide sequences of the primer pairs used for genotyping analysis of T-DNA insertion mutants are shown in Supplemental Table 2 online. The abi5-1 mutant was described previously (Finkelstein, 1994). FLAG-DDB1b, FLAG-CUL4, and cul4cs transgenic lines were the same ones as those from Chen et al. (2006) and Lee et al. (2008). Arabidopsis seedlings were grown as described previously (Lee et al., 2008). Specifically, for the assays shown in Figures 1A and 1B, all seedlings were grown vertically on GM plates with or without 0.5 μM ABA or 100 mM NaCl for 5 d after stratification under long-day conditions (16 h light/8 h dark) in a controlled-environment chamber at 22°C. For adult plants, 2-week-old seedlings grown on GM plates were transferred to soil and kept under long-day conditions (16 h light/8 h dark) in a controlled-environment chamber at 22°C.
Determination of Water Loss
Water loss was measured by the methods of Cheong et al. (2007). Six rosette leaves per plant were detached from 3-week-old wild-type and dwa mutant plants. The leaves were kept on the laboratory bench for the indicated times, then their fresh weights were measured. Water loss shows the percentage of weight loss at the indicated time versus initial fresh weight. To minimize variation, three independent experiments were performed, and similar results were obtained.
Generation of the 35S:HA-DWA1 and 35S:FLAG-DWA2 Constructs and Transient Expression of DWA Proteins in Tobacco
DWA1 cDNA was amplified using cDNAs made from total mRNAs in Arabidopsis leaves with two primers, DWA1_SpeI_F and DWA1_SpeI_R, which possess SpeI sites at 5′ ends (see Supplemental Table 3 online). Then, it was introduced into the same site of the binary vector pJIM19 (kanamycin) (Lee et al., 2008) under the control of the cauliflower mosaic virus 35S promoter, then XbaI fragments containing three copies of HA were inserted into the corresponding site that was located in frame between the 35S promoter and the first ATG of the DWA1 gene. In the case of DWA2, its cDNA inserts were amplified with DWA2_KpnI_F and DWA2_KpnI_R, which include KpnI sites at the 5′ ends, and introduced into the corresponding site with 3× FLAG-fused pCR-BluntII vector (Invitrogen). Then, SpeI fragments containing the inserted cDNA were inserted into the binary vector pCAMBIA 1390 (hygromycin) (http://www.cambia.org/) under the control of the cauliflower mosaic virus 35S promoter. They were transformed into Agrobacterium tumefaciens strain C58C1 by electroporation, and the presence of transgenes was confirmed by PCR. Transient expression assays in tobacco plants were performed as described by Voinnet et al. (2003). Three- to four-week-old tobacco plants (Nicotiana benthamiana) were used for Agrobacterium infiltration. Agrobacterium strains (C58C1) containing HA-DWA1, FLAG-DWA2, or p19 (Voinnet et al., 2003) constructs were grown at 28°C to stationary phase. Bacteria pelleted by centrifugation were resuspended in 10 mM MgCl2, 10 mM MES, pH 5.6, and 150 μM acetosyringone to OD600 of 1.0. The resuspended bacteria were kept in the dark for 4 h before infiltration. For infiltration, 1 to 2 mL of Agrobacterium culture suspension was infiltrated into the adaxial side of leaves using a syringe. The plants were kept at 25°C for 4 d, and the infiltrated regions were harvested for subsequent protein extraction.
In Vitro Pull-Down Assay
DWA1 cDNA was amplified with two primers, DWA1_EcoRI_F and DWA1_EcoRI_R, which include EcoRI sites at 5′ ends (see Supplemental Table 3 online). Then, it was introduced into the same site of pMAL vectors (New England Biolabs). Amplified DWA2 cDNA with DWA2_XhoI_F and DWA2_XhoI_R (that include XhoI sites at 5′ ends) was introduced into the same site of pGEX-4T-1 (GE Healthcare) (see Supplemental Table 3 online). GST-DWA2 and MBP-DWA1 were expressed in Escherichia coli strain BL21 (DE3) and purified with glutathione sepharose 4B (GE Healthcare) and amylase resin (New England Biolabs), respectively. For the in vitro pull-down assays, 2 μg of purified GST-DWA2 proteins were incubated with 2 μg of either MBP or MBP-DWA1 in binding buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 0.1% Triton X-100) for 4 h at 4°C. After 30 μL of amylose resins were added, the mixtures were incubated for another hour at 4°C. The samples were washed three times with washing buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, and 0.1% Triton X-100) and eluted using 2× SDS sample buffer with boiling for 5 min. The blot was probed with either anti-GST (GE Healthcare) or anti-MBP antibody (New England Biolabs).
RT-PCR and RNA Gel Blot Analysis
Total RNAs were obtained using RNeasy plant mini kits (Qiagen). Five micrograms of RNA per well were resolved by electrophoresis in a 1.0% formaldehyde agarose gel and transferred onto a nylon membrane. Equal loading of the agarose gel was confirmed by ethidium bromide staining of rRNA. The membrane was hybridized to 32P-labeled cDNA probes for RD29B, RD29A, and RD22. The blot was then washed and visualized by autoradiography at −80°C. Oligonucleotide sequences of the primer pairs used to generate the probes used for RNA gel blot analysis are shown in Supplemental Table 4 online.
RT-PCR analysis was performed as described previously (Lee et al., 2008). RT-PCR using specific primers for each gene was performed on total RNA isolated from 5-d-old seedlings. Amplicons produced by RT-PCR with specific primers for RPN6a were used as internal controls in these experiments (Lee et al., 2008). DNA sequences for the primer pairs used in RT-PCR are shown in Supplemental Table 4 online.
Construction of T7-Tagged Recombinant Proteins and Cell-Free Degradation Assay
ABI5 cDNA was amplified with two primers, ABI5_EcoRI_F and ABI5_EcoRI_R, which include EcoRI sites at 5′ ends (see Supplemental Table 3 online). RGA cDNA was amplified with RGA_BamHI_F and RGA_BamHI_R, which include BamHI sites at 5′ ends (see Supplemental Table 3 online). ABI5 and RGA fragments were then inserted into the plasmid pET-28c (+) (Novagen) to generate the genes encoding the T7-tagged recombinant proteins. The constructs were then expressed in vitro using an EasyXpress protein synthesis kit (Qiagen). The resulting proteins were purified by affinity chromatography using Ni+-NTA resin according to the manufacturer's instructions (Invitrogen), since the pET-28c (+) system also fused a run of six His residues into the expressed proteins. Five-day-old seedlings were extracted in a buffer (25 mM Tris, pH 7.5, 10 mM MgCl2, 5 mM DTT, and 10 mM NaCl) (Osterlund et al., 2000). Cell debris was then removed by centrifugation, and the amounts of soluble proteins were determined. Purified T7-ABI5 or T7-RGA (500 ng) were mixed with cell extracts in reaction buffer (25 mM Tris, pH 7.5, 10 mM MgCl2, 5 mM DTT, 10 mM NaCl, and 10 mM ATP) and incubated at 30°C for 1 h with or without 20 μM MG132. The reactions were terminated by adding an equal volume of 2× protein gel loading buffer. The samples were fractionated by SDS-PAGE, transferred onto nitrocellulose membranes, and incubated with anti-T7 antibody (Novagen). To test for equal loading, tubulin levels from the cell extracts used for each reaction were checked with antitubulin antibody (Sigma-Aldrich).
Protein Isolation and Immunoblot Analysis
Protein isolation and quantification were performed as described previously (Lee et al., 2008). Isolated protein extracts were mixed with 2× SDS sample buffer and boiled for 5 min. The boiled samples were then resolved by SDS-PAGE and transferred to nitrocellulose membranes. These were incubated in blocking buffer (1× PBS buffer including 0.1% Tween 20 and 5% dried nonfat milk) for 1 h at room temperature, then with anti-ABI5 (Stone et al., 2006), anti-MYC2 (Yadav et al., 2005), anti-CUL4 (Chen et al., 2006), anti-CAND1 (Feng et al., 2004), anti-RPN6 (Chen et al., 2006), anti-DDB1 (Chen et al., 2010), anti-MYC (Cell Signaling), anti-FLAG (Sigma-Aldrich), and anti-HA (Roche) antibodies overnight at 4°C and additionally incubated for 1 h with secondary antibody with horseradish peroxidase–conjugated anti-IgG.
Yeast Two-Hybrid Assay
DWA1 cDNA was amplified with DWA1_EcoRI_F and DWA1_EcoRI_R (see Supplemental Table 3 online). Then, it was introduced into the same site of pEG202 or pJG4-5 (Clontech). Amplified DWA2 cDNA with DWA2_XhoI_F and DWA2_XhoI_R was introduced into the same site of pJG4-5 (see Supplemental Table 3 online). The EG202-DDB1, JG4-5-GFP, and JG4-5-CUL4 constructs were the same ones as those previously reported (Lee et al., 2008). Yeast two-hybrid interaction assays were performed based on the MATCHMAKER LexA Two-Hybrid System manual (Clontech). All constructs were cotransformed into yeast strain EGY48, which already contained p80p-lacZ. The presence of transgenes was confirmed by growth on SD/-His/-Trp/-Ura plates. Interactions between two proteins were checked by measuring β-galactosidase activity using o-nitrophenyl-β-d-galactopyranoside as substrate.
In Vivo Co-IP Assay
Arabidopsis seedlings or tobacco leaves were homogenized in protein extraction buffer (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5% glycerol, 0.05% Nonidet P-40, 2.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1× complete cocktail of protease inhibitors). After protein extraction, 30 μL of anti-MYC affinity matrix (Covance), anti-HA affinity matrix (Roche), or 10 μL of anti-ABI5 antibody were added to 1 to 5 mg of total proteins, and the mixture was incubated for 4 h at 4°C. The precipitated samples were washed at least four times with the protein extraction buffer and then eluted by the addition of 2× SDS protein loading buffer with boiling for 5 min.
Generation of the 35S:DWA-MYC Constructs and Transformation into FLAG-DDB1b/ddb1a Transgenic Plants
A SacI fragment containing nine copies of MYC was generated with MYC_SacI_F and MYC_SacI_R (see Supplemental Table 3 online) and introduced into the corresponding site of pJIM19 (Gentamycin), generating pMYC- fused pJIM19 (Gentamycin). DWA2 was amplified with DWA2_SpeI_F and DWA2_SpeI_R, which possess SpeI sites at 5′ ends (see Supplemental Table 3 online), and inserted into the corresponding site of pMYC-fused pJIM19 (gentamycin). The resulting construct was transformed into Agrobacterium strain GB3101 by electroporation and introduced into FLAG-DDB1b/ddb1a transgenic plants (Lee et al., 2008) by the floral dip method (Clough and Bent, 1998). The harvested seeds were selected on MS plates containing 1% sucrose and 200 mg L−1 gentamycin to acquire independent T1 transgenic lines. The presence of the transgene was confirmed by PCR with gene-specific primers. FLAG-DDB1/MYC-DWA1/ddb1a transgenic lines were generated as indicated by Lee et al. (2008).
Generation of the 35S:DWA2 Construct and Transformation into dwa2-1 Mutant
A SpeI fragment containing cDNA of DWA2 generated with DWA2_SpeI_F and DWA2_SpeI_R-1 was introduced into the same site of the binary vector pJIM19 (Gentamycin) under the control of the cauliflower mosaic virus 35S promoter. The resulting construct was transformed into Agrobacterium strain GB3101 by electroporation and introduced into FLAG-DDB1b/ddb1a transgenic plants. The harvested seeds were selected on MS plates containing 1% sucrose and 200 mg L−1 gentamycin to acquire independent T1 transgenic lines. The presence of the transgene was confirmed by PCR with gene-specific primers.
BiFC Assay
The entire open reading frames (from start codon to stop codon) of DWA1 and DWA2 were cloned via Gateway reactions into the binary pBiFC vectors (Azimzadeh et al., 2008) containing either N- or C-terminal yellow fluorescent protein (YFP) fragments (YFPN and YFPC). The primers used (DWA1_BiFc_F, DWA1_BiFc_R, DWA2_BiFc_F, and DWA2_BiFc_R) are shown in Supplemental Table 3 online. The resulting constructs were then introduced into Agrobacterium (GV3101) strains. The transformed cells were grown at 28°C in Luria-Bertani liquid medium supplemented with 40 μg mL−1 gentamycin and 100 μg mL−1 spectinomycin to an OD600 of 0.6 to 1. The p19 protein of tomato bushy stunt virus was used to suppress gene silencing (Voinnet et al., 2003). Bacteria were sedimented by centrifugation at 5000g for 15 min at room temperature and resuspended in MS medium containing 10% sucrose, 2.6 mM MES, pH 5.8, and 150 μM acetosyringone. Cells were incubated in this medium at room temperature for at least 3 h and then infiltrated into the abaxial air spaces of 2- to 4-week-old N. benthamiana plants. Coinfiltration of Agrobacterium strains containing the BiFC constructs and the p19 silencing plasmid was performed at OD600 ratios of 0.7:0.7:1.0, respectively. Epidermal cell layers of tobacco leaves were assayed for fluorescence 3 d after infiltration. Cells were observed through a Zeiss Axiophot microscope under fluorescence using the GFP filter. To control for nuclear localization, tissue samples were stained with 4′,6-diamidino-2-phenylindole dihydrochloride.
Quantitative Real-Time PCR Analysis
The assay was performed as described by Chen et al. (2010). Quantitative PCR was performed using the CFX96 real-time PCR detection system (Bio-Rad) and SYBR Green PCR Master Mix (Applied Biosystems). Relative amounts of MYC2 and RD transcripts were calculated using the comparative CT method, which was normalized against ACTIN2 expression from the same sample. Each experiment was repeated three times. Primers used for this assay are listed in Supplemental Table 5 online.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes used in this article are as follows: At2g19430 (DWA1), At1g76260 (DWA2), At5g46210 (CUL4), At1g29150 (RPN6a), At4g21100 (DDB1b), At4g05420 (DDB1a), At5g52310 (RD29A), At5g52300 (RD29B), At5g25610 (RD22), At2g36270 (ABI5), At1g32640 (MYC2), At2g01570 (RGA), and At2g02560 (CAND1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Protein and Gene Structures of DWA1 and DWA2.
Supplemental Figure 2. Increased ABA Sensitivity of dwa1 and dwa2 Mutants.
Supplemental Figure 3. Root Growth Inhibition of the Wild Type, dwa1, dwa2, dwa1 dwa2, and cul4cs after ABA Application.
Supplemental Figure 4. Enhanced Tolerance to Drought in the dwa1 dwa2 Mutant.
Supplemental Figure 5. The Expression Levels of Various Proteins Fused with LexA or B42-HA in Yeast Used in Figures 2A and 8A.
Supplemental Figure 6. Confirmation of Endogenous ABI5 Protein.
Supplemental Figure 7. Level of ABI5 Proteins from the Material in Figure 3C.
Supplemental Figure 8. T7-ABI5 Protein Level in Wild-Type, dwa1-1, dwa2-1, dwa1-1 dwa2-1, and cul4cs Extracts after Treatment with MG132.
Supplemental Figure 9. Self-Interactions of DWA1 and DWA2 Proteins.
Supplemental Figure 10. Hypocotyl Lengths of the Wild Type, dwa1, and dwa2 after Treatment with ACC.
Supplemental Table 1. T-DNA Insertion or Mutant Lines of DWD and Additional DCAF Genes in This Study.
Supplemental Table 2. Oligonucleotide Sequences of the Primer Pairs Used for Genotyping Analysis.
Supplemental Table 3. DNA Sequences of the Primer Pairs Used for Generation of Various Constructs.
Supplemental Table 4. DNA Sequences of the Primer Pairs Used in RNA Gel Blots or RT-PCR Experiments.
Supplemental Table 5. DNA Sequences of the Primer Pairs Used for Quantitative Real-Time PCR Analysis.
Supplemental Methods.
Acknowledgments
We thank Richard Viestra (University of Wisconsin) and Sudip Chattopadhyay (National Centre for Plant Genome Research, India) for providing ABI5 and MYC2 antibodies, respectively. We also thank François Parcy (Commissariat à l'Energie Atomique, Grenoble, France) for providing the BiFC vector system. We are grateful to Rosa Lozano Durán (Universidad de Malaga, Spain) for technical assistance with the tobacco transient expression assay. J.-H.L. was initially supported by the Postdoctoral Fellowship Program of Korea Research Foundation (KRF M01-2004-000-20090-0). H.-J.Y. was supported by the Korean Government Overseas Training Fellowship and by the National Academy of Agricultural Science of the Rural Development Administration, Korea. This research was supported by a National Science Foundation 2010 grant (MCB-0929100) to X.W.D.
References
- Abe H., Urao T., Ito T., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2003). Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H., Yamaguchi-Shinozaki K., Urao T., Iwasaki T., Hosokawa D., Shinozaki K. (1997). Role of Arabidopsis MYC and MYB homologs in drought- and abscisic-acid-regulated gene expression. Plant Cell 9: 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P.K., Agarwal P., Reddy M.K., Sopory S.K. (2006). Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 25: 1263–1274 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Angers S., Li T., Yi X., MacCoss M.J., Moon R.T., Zheng N. (2006). Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature 443: 590–593 [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Nacry P., Christodoulidou A., Drevensek S., Camilleri C., Amiour N., Parcy F., Pastuglia M., Bouchez D. (2008). Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 20: 2146–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Huang X., Gusmaroli G., Terzaghi W., Lau O.S., Yanagawa Y., Zhang Y., Li J., Lee J.H., Zhu D., Deng X.W. (2010). Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 22: 108–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Shen Y., Tang X., Yu L., Wang J., Guo L., Zhang Y., Zhang H., Feng S., Strickland E., Zheng N., Deng X.W. (2006). Arabidopsis CULLIN4 forms an E3 ubiquitin ligase with RBX1 and the CDD complex in mediating light control of development. Plant Cell 18: 1991–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong Y.H., Pandey G.K., Grant J.J., Batistic O., Li L., Kim B.G., Lee S.C., Kudla J., Luan S. (2007). Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 52: 223–239 [DOI] [PubMed] [Google Scholar]
- Choi H., Hong J.H., Ha J., Kang J.Y., Kim S.Y. (2000). ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Chung K.K., Zhang Y., Lim K.L., Tanaka Y., Huang H., Gao J., Ross C.A., Dawson V.L., Dawson T.M. (2001). Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 7: 1144–1150 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Feldman R.M.R., Correll C.C., Kaplan K.B., Deshaies R.J. (1997). A complex of Cdc4p, Skp1p, and Cdc53p/Cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91: 221–230 [DOI] [PubMed] [Google Scholar]
- Feng S., Shen Y., Sullivan J.A., Rubio V., Xiong Y., Sun T.P., Deng X.W. (2004). Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathwayscontrolled by ubiquitin/proteasome-mediated protein degradation. Plant Cell 16: 1870–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R. (1994). Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 5: 756–771 [Google Scholar]
- Finkelstein R.R., Gampala S.S., Rock C.D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R., Lynch T.J. (2000). The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R., Wang M.L., Lynch T.J., Rao S., Goodman H.M. (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M., He Y.J., Borchers C., Xiong Y. (2003). Targeting of protein ubiquitination by BTB–Cullin 3–Roc1 ubiquitin ligases. Nat. Cell Biol. 5: 1001–1007 [DOI] [PubMed] [Google Scholar]
- Geyer R., Wee S., Anderson S., Yates J., Wolf D.A. (2003). BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol. Cell 12: 783–790 [DOI] [PubMed] [Google Scholar]
- He Y.J., McCall C.M., Hu J., Zeng Y., Xiong Y. (2006). DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4–ROC1 ubiquitin ligases. Genes Dev. 20: 2949–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa L.A., Wu M., Ye T., Kobayashi R., Sun H., Zhang H. (2006). CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat. Cell Biol. 8: 1277–1283 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Tscheuschler A., Viczián A., Kunkel T., Kircher S., Schäfer E. (2006). FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 47: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Ito T., Niwa J., Hishikawa N., Ishigaki S., Doyu M., Sobue G. (2003). Dorfin localizes to Lewy bodies and ubiquitylates synphilin-1. J. Biol. Chem. 278: 29106–29114 [DOI] [PubMed] [Google Scholar]
- Jin J., Arias E.E., Chen J., Harper J.W., Walter J.C. (2006). A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 23: 709–721 [DOI] [PubMed] [Google Scholar]
- Kamura T., Maenaka K., Kotoshiba S., Matsumoto M., Kohda D., Conaway R.C., Conaway J.W., Nakayama K.I. (2004). VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 18: 3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T., Sato S., Haque D., Liu L., Kaelin W.G., Jr., Conaway R.C., Conaway J.W. (1998). The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 12: 3872–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Kipreos E.T. (2007). The Caenorhabditis elegans replication licensing factor CDT-1 is targeted for degradation by the CUL-4/DDB-1 complex. Mol. Cell. Biol. 27: 1394–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizis D., Lumbreras V., Pages M. (2001). Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett. 498: 187–189 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Murata M., Minami H., Yamamoto S., Kagaya Y., Hobo T., Yamamoto A., Hattori T. (2005). Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 44: 939–949 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Terzaghi W., Gusmaroli G., Charron J.B., Yoon H.J., Chen H., He Y.J., Xiong Y., Deng X.W. (2008). Characterization of Arabidopsis and rice DWD proteins and their roles as substrate receptors for CUL4-RING E3 ubiquitin ligases. Plant Cell 20: 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J., Bouvier-Durand M., Morris P.C., Guerrier D., Chefdor F., Giraudat J. (1994). Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264: 1448–1452 [DOI] [PubMed] [Google Scholar]
- Leung J., Merlot S., Giraudat J. (1997). The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., Chua N.H. (2001). A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., Kinoshita N., Chua N.H. (2003). AFP is a novel negative regulator of ABA signaling that promotes ABI5 protein degradation. Genes Dev. 17: 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., McLachlin D.T., Chait B.T., Chua N.H. (2002). ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A., et al. (2005). CDD: A Conserved Domain Database for protein classification. Nucleic Acids Res. 33: D192–D196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P., Creelman R. (2008). The ABA receptors–We report you decide. Curr. Opin. Plant Biol. 11: 474–478 [DOI] [PubMed] [Google Scholar]
- Meyer K., Leube M.P., Grill E. (1994). A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264: 1452–1455 [DOI] [PubMed] [Google Scholar]
- Nagano Y., Yamashita H., Takahashi T., Kishida S., Nakamura T., Iseki E., Hattori N., Mizuno Y., Kikuchi A., Matsumoto M. (2003). Siah-1 facilitates ubiquitination and degradation of synphilin-1. J. Biol. Chem. 278: 51504–51514 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Fujita Y., Katsura K., Maruyama K., Narusaka Y., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. (2006). Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol. Biol. 60: 51–68 [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Parcy F., Valon C., Raynal M., Gaubier-Comella P., Delseny M., Giraudat J. (1994). Regulation of gene expression programs during Arabidopsis seed development: Roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski M.D., Deshaies R.J. (2005). Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6: 9–20 [DOI] [PubMed] [Google Scholar]
- Pintard L., Willis J.H., Willems A., Johnson J.L., Srayko M., Kurz T., Glaser S., Mains P.E., Tyers M., Bowerman B., Peter M. (2003). The BTB protein MEL-26 is a substrate-specific adaptor of the CUL-3 ubiquitin-ligase. Nature 425: 311–316 [DOI] [PubMed] [Google Scholar]
- Piskurewicz U., Jikumaru Y., Kinoshita N., Nambara E., Kamiya Y., Lopez-Molina L. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20: 2729–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. (2000). EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 16: 276–277 [DOI] [PubMed] [Google Scholar]
- Seki M., et al. (2002). Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct. Integr. Genomics 2: 282–291 [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Yamaguchi-Shinozaki K. (2000). Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3: 217–223 [PubMed] [Google Scholar]
- Skowyra D., Craig K., Tyers M., Elledge S.J., Harper J.W. (1997). F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin–ligase complex. Cell 91: 209–219 [DOI] [PubMed] [Google Scholar]
- Smalle J., Vierstra R.D. (2004). The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Stebbins C.E., Kaelin W.G., Jr., Pavletich N.P. (1999). Structure of the VHL-ElonginC-ElonginB complex: Implications for VHL tumor suppressor function. Science 284: 455–461 [DOI] [PubMed] [Google Scholar]
- Stone S.L., Williams L.A., Farmer L.M., Vierstra R.D., Callis J. (2006). KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18: 3415–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann A., Dieterle M., Genschik P. (2005). Plant CULLIN-based E3s: Phytohormones come first. FEBS Lett. 579: 3239–3245 [DOI] [PubMed] [Google Scholar]
- Umezawa T., Sugiyama N., Mizoguchi M., Hayashi S., Myouga F., Yamaguchi-Shinozaki K., Ishihama Y., Hirayama T., Shinozaki K. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y., Furihata T., Abe H., Yoshida R., Shinozaki K., Yamaguchi-Shinozaki K. (2000). Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O., Rivas S., Mestre P., Baulcombe D. (2003). An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33: 949–956 [DOI] [PubMed] [Google Scholar]
- Xu L., Wei Y., Reboul J., Vaglio P., Shin T.H., Vidal M., Elledge S.J., Harper J.W. (2003). BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature 425: 316–321 [DOI] [PubMed] [Google Scholar]
- Yadav V., Mallappa C., Gangappa S.N., Bhatia S., Chattopadhyay S. (2005). A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 17: 1953–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.W., Jang I.C., Henriques R., Chua N.H. (2009). FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell 21: 1341–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.G., et al. (1999). The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc. Natl. Acad. Sci. USA 96: 2071–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Feng S., Chen F., Chen H., Wang J., McCall C., Xiong Y., Deng X.W. (2008). Arabidopsis DDB1-CUL4 ASSOCIATED FACTOR1 forms a nuclear E3 ubiquitin ligase with DDB1 and CUL4 that is involved in multiple plant developmental processes. Plant Cell 20: 1437–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang C., Li Y., Zheng N., Chen H., Zhao Q., Gao T., Guo H., Xie Q. (2007). SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 19: 1912–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., et al. (2002). Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416: 703–709 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Hare P.D., Yang S.W., Zeidler M., Huang L.F., Chua N.H. (2005). FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 43: 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Maier A., Lee J.H., Laubinger S., Saijo Y., Wang H., Qu L.J., Hoecker U., Deng X.W. (2008). Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20: 2307–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]