This work examines the interactions among MOTHER OF FT AND TFL1 (MFT) and the genes of the abscisic acid (ABA) signaling pathways in the initiation of germination, finding that MFT expression is regulated by key ABA- and gibberellin-responsive factors and MFT in turn also regulates ABA signaling, providing a feedback loop.
Abstract
Abscisic acid (ABA) and gibberellin (GA) are two antagonistic phytohormones that regulate seed germination in response to biotic and abiotic environmental stresses. We demonstrate here that MOTHER OF FT AND TFL1 (MFT), which encodes a phosphatidylethanolamine-binding protein, regulates seed germination via the ABA and GA signaling pathways in Arabidopsis thaliana. MFT is specifically induced in the radical-hypocotyl transition zone of the embryo in response to ABA, and mft loss-of-function mutants show hypersensitivity to ABA in seed germination. In germinating seeds, MFT expression is directly regulated by ABA-INSENSITIVE3 (ABI3) and ABI5, two key transcription factors in ABA signaling pathway. MFT is also upregulated by DELLA proteins in the GA signaling pathway. MFT in turn provides negative feedback regulation of ABA signaling by directly repressing ABI5. We conclude that during seed germination, MFT promotes embryo growth by constituting a negative feedback loop in the ABA signaling pathway.
INTRODUCTION
The first phase transition in the life cycle of higher plants is the switch from embryonic to germinative growth. Seed dormancy blocks the germination process and can be considered an adaptive trait that optimizes the distribution of germination over time (Bewley, 1997). The breakdown of seed dormancy is influenced by both environmental and intrinsic signals (Koornneef et al., 2002), which are mainly mediated by two phytohormones, abscisic acid (ABA) and gibberellin (GA) (Gubler et al., 2005).
ABA is a sesquiterpene hormone that is well known for its physiological role in the processes of seed development and germination as well as plant adaptation to abiotic environmental stresses (Leung and Giraudat, 1998; Finkelstein et al., 2002; Gubler et al., 2005). It has been suggested that ABA inhibits water uptake by preventing cell wall loosening of the embryo during seed germination, indicating that ABA is able to reduce embryo growth potential (Schopfer and Plachy, 1985). In addition, ABA has also been found to specifically inhibit endosperm rupture rather than testa (i.e., seed coat) rupture (Muller et al., 2006). After extensive efforts made toward understanding the ABA signal transduction, ABA-insensitive (ABI) alleles, ABI1 to ABI5, have been identified through screening mutant seeds that could germinate even in the presence of high concentrations of ABA (Koornneef et al., 1984; Finkelstein, 1994). Among these identified ABIs, ABI3 encoding a B3 domain transcription factor and ABI5 encoding a basic region leucine-zipper (bZIP) transcription factor are necessary for achieving growth arrest when germinating seeds encounter unfavorable conditions (Giraudat et al., 1992; Finkelstein and Lynch, 2000). ABI5 functions downstream of ABI3 and is essential to execute the ABA-dependent growth arrest, which occurs after the breakage of seed dormancy but prior to autotrophic growth (Lopez-Molina et al., 2001, 2002). Such growth arrest occurs through recruiting of de novo late embryogenesis programs and confers osmotic tolerance to harsh environmental conditions.
GA is a well-known hormone that promotes seed germination and growth. GA biosynthesis and responses are well coordinated during seed germination (Ogawa et al., 2003). Complex regulatory events in the GA signaling pathway include the crosstalk of GA with other hormones and regulation of genes involved in promoting cell elongation and division (Ogawa et al., 2003). Accumulation of GA is accompanied by reduction of ABA during seed germination, suggesting that GA and ABA play antagonistic roles in this process (Olszewski et al., 2002; Nambara and Marion-Poll, 2005). GA signaling is regulated by a group of repressors collectively called DELLA proteins, including REPRESSOR OF ga1-3 (RGA), GA-INSENSITIVE (GAI), and RGA-LIKE1-3 (RGL1-3) (Peng and Harberd, 1997; Dill and Sun, 2001; Lee et al., 2002; Wen and Chang, 2002; Tyler et al., 2004), among which RGL2 appears as the major DELLA factor involved in repressing seed germination (Lee et al., 2002; Tyler et al., 2004). Recent studies have shown that RGL2 stimulates ABA biosynthesis and ABI5 activity, while ABA enhances the RGL2 expression (Ko et al., 2006; Zentella et al., 2007; Piskurewicz et al., 2008), indicating that RGL2 plays a role in mediating the interaction of GA and ABA during seed germination.
MOTHER OF FT AND TFL1 (MFT) is a homolog of FLOWERING LOCUS T (FT) and TERMINAL FLOWER 1 (TFL1), two regulators with opposite functions in the control of flowering time (Bradley et al., 1997; Kardailsky et al., 1999; Kobayashi et al., 1999). These three genes, together with three additional homologs in Arabidopsis thaliana, form the phosphatidylethanolamine-binding protein (PEBP) family, which is evolutionarily conserved in a wide range of species. As loss of MFT function does not exhibit observable defects under normal growth conditions (Yoo et al., 2004), its exact biological function is so far unknown. Here, we show that MFT is regulated by both ABA and GA pathways during seed germination. In germinating seeds treated with exogenous ABA, MFT is upregulated throughout the embryo, most strongly in the cortex and epidermis layers of the radical-hypocotyl transition zone. Seeds of mft loss-of-function mutants are hypersensitive to exogenous ABA and exhibit lower germination rate than those of wild-type plants. MFT transcription is directly repressed or promoted by ABI3 or ABI5, respectively, while ABI5 transcription is directly repressed by MFT. Furthermore, the DELLA protein RGL2 directly promotes MFT transcription. These results suggest that MFT responds to both ABA and GA signals to control seed germination through mediating negative feedback regulation of ABA signaling.
RESULTS
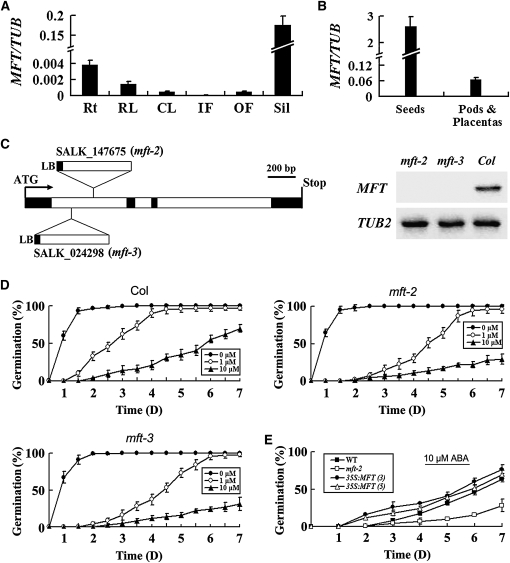
Phenotypic Characterization of mft Mutants in Arabidopsis
To examine the expression pattern of MFT in Arabidopsis, real-time RT-PCR analysis was performed with total RNA extracted from various tissues. MFT was expressed highly in developing siliques, moderately in roots and rosette leaves, but weakly in other tissues (Figure 1A). We further dissected siliques and found that MFT was preferentially expressed in the developing seeds (Figure 1B), implying a possible role of MFT in seed development. To investigate the biological function of MFT, two T-DNA insertion alleles of MFT in the Columbia-0 (Col-0) background were isolated and designated as mft-2 and mft-3, respectively (Figure 1C). There was no detectable expression of MFT in these two homozygous mutant lines, suggesting that mft-2 and mft-3 are null alleles (Figure 1C). Under normal growth conditions, homozygous mutants did not show obvious defects. However, in the presence of exogenous ABA, the germination rate of both mft-2 and mft-3 seeds was much lower than that of wild-type seeds (Figure 1D), indicating that MFT is involved in seed germination in response to ABA. To further assess the role of MFT in seed germination, we generated 35S:MFT transgenic plants. Among 23 lines generated, 16 lines showed higher germination rates, particularly at early stages of seed germination when exogenous ABA was applied (Figure 1E). These results suggest that MFT regulates seed germination in response to exogenous ABA. As endogenous ABA levels in mft-2 seeds after imbibition were comparable to those in the wild type (see Supplemental Figure 1 online), MFT may not be directly involved in regulating ABA levels during seed germination.
Figure 1.
Phenotypic Characterization of mft Mutants in Arabidopsis.
(A) Quantitative real-time PCR analysis of MFT expression in various tissues. Results were normalized against the expression of TUB2. Rt, roots; RL, rosette leaves; CL, cauline leaves; IF, inflorescences without open flowers; OF, open flowers; Sil, siliques. Error bars denote sd.
(B) MFT expression determined by quantitative real-time PCR in developing siliques dissected into seeds and pods plus placentas. Error bars denote sd.
(C) Schematic diagram indicating the T-DNA insertions in two mft loss-of-function mutants, mft-2 (SALK_147675) and mft-3 (SALK_024298). Black and white boxes indicate exons and introns of MFT, respectively. RT-PCR analysis using a pair of primers flanking the T-DNA insertion sites did not detect MFT expression in mft-2 and mft-3, indicating that both of them are null alleles.
(D) Germination phenotype of the wild type, mft-2, and mft-3 treated with different concentrations of ABA (0, 1, and 10 μM). Error bars denote sd.
(E) Germination phenotype of two representative 35S:MFT lines (3 and 5) in response to 10 μM ABA. Error bars denote sd.
As ABA induces growth retardation and stomata closure, we further studied whether MFT is also involved in regulating other ABA-related physiological responses. After seed germination, both mft-2 and wild-type seedlings showed similar degrees of growth retardation when exogenous ABA was applied (see Supplemental Figure 2 online). In addition, there was no significant difference in ABA-induced drought tolerance among wild-type plants and the plants with altered MFT expression (see Supplemental Figure 3 online), implying that MFT might have a specific function in seed germination.
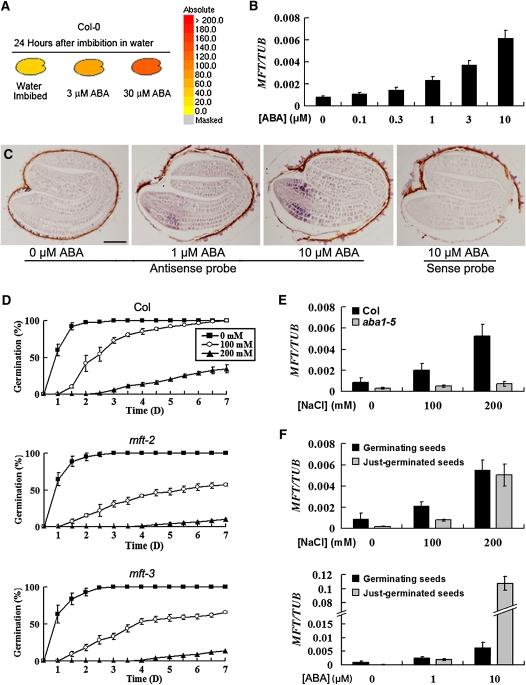
MFT Expression Is Upregulated in Response to ABA
Because the germination of mft mutant seeds was hypersensitive to exogenously applied ABA, we examined MFT expression levels in response to ABA. Information retrieved from the public Arabidopsis microarray database (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) showed that MFT was greatly upregulated by ABA (Figure 2A), which is in agreement with our observation that MFT expression levels in germinating seeds were gradually elevated along with increased concentrations of ABA (Figure 2B). In situ hybridization revealed that MFT was not detectable in the embryo and endosperm of seeds collected 6 h after stratification (see Supplemental Figure 4 online), whereas its upregulation by ABA occurred strongly in the epidermis and cortex and weakly in the provasculature of the radical-hypocotyl transition zone of seeds collected at a later germination stage (Figure 2C). Furthermore, we checked the time-course expression of MFT during seed imbibition in two cyp707a mutants with high levels of endogenous ABA (Okamoto et al., 2006). Our results demonstrate that MFT is also upregulated by endogenous ABA (see Supplemental Figure 5 online).
Figure 2.
MFT Expression Is Upregulated in Response to ABA.
(A) Public microarray data showing upregulation of MFT by ABA in germinating seeds (http://www.bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi). The colors from yellow to red indicate the increased absolute signal values of MFT expression retrieved from microarray data.
(B) MFT expression determined by quantitative real-time PCR in germinating seeds treated with different concentrations of ABA. All seeds were collected 16 h after stratification. Error bars denote sd.
(C) In situ localization of MFT in germinating seeds treated with different concentrations of ABA. Seeds without ABA treatment were collected 12 h after stratification, while other seeds treated with 1 and 10 μM ABA were collected 24 h after stratification. These seeds at the same developmental stage were hybridized with the antisense or sense MFT probe as indicated. Bar = 100 μm.
(D) Germination phenotype of the wild type, mft-2, and mft-3 treated with different concentrations of NaCl. Error bars denote sd.
(E) MFT expression determined by quantitative real-time PCR in wild-type and aba1-5 germinating seeds treated with different concentrations of NaCl. All seeds were collected 16 h after stratification. Error bars denote sd.
(F) MFT expression determined by quantitative real-time PCR in germinating and just-germinated seeds treated with NaCl and ABA. All germinating seeds were collected 16 h after stratification. Seeds with visible protrusion of the radicle tip through all the covering layers were collected as just-germinated seeds. Error bars denote sd.
As high salinity prevents seed germination and stimulates the biosynthesis and accumulation of ABA by activating genes encoding ABA biosynthetic enzymes (Xiong et al., 2002; Xiong and Zhu, 2003), we next investigated whether MFT could affect seed germination in response to high salinity. The germination of mft seeds was also hypersensitive to NaCl treatment (Figure 2D). In addition, high salt concentrations caused dramatic upregulation of MFT expression, which is similar to the ABA effect (Figure 2E). To test if upregulation of MFT by high salinity was mediated via the ABA pathway, we treated seeds of aba1-5 mutants, in which the ABA biosynthesis was severely impaired (Leon-Kloosterziel et al., 1996), with high salinity. Upregulation of MFT by high salinity was significantly attenuated in aba1-5 seeds compared with the wild type (Figure 2E), suggesting that high salinity induces MFT expression mainly through the ABA signaling pathway. Furthermore, MFT was markedly upregulated in just-germinated seeds compared with geminating seeds treated with high concentrations of ABA (e.g., 10 μM), whereas high salinity upregulated MFT in just-germinated seeds to the same level as in geminating seeds (Figure 2F). This demonstrates a cumulative effect of ABA on promoting MFT expression during seed germination. Taken together, these results show that MFT is upregulated by ABA during seed germination, but loss of MFT function results in ABA hypersensitivity, suggesting an antagonistic function of MFT against the inhibitory effect of ABA on seed germination.
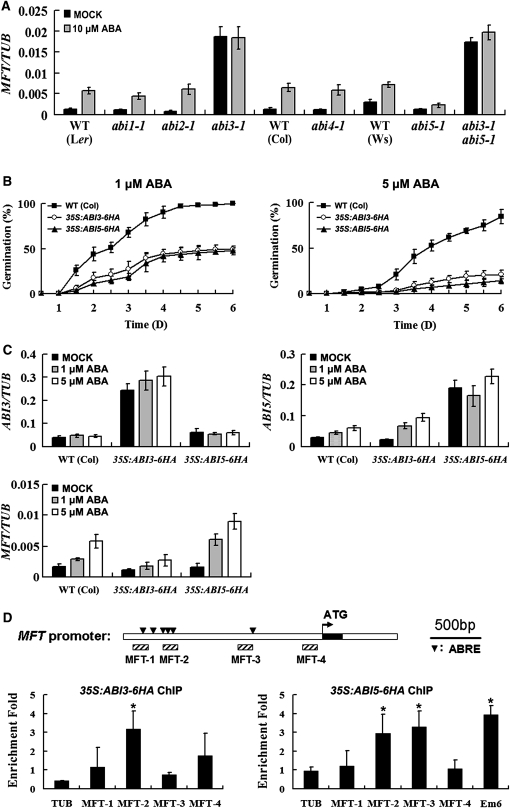
The Response of MFT to ABA Is Directly Mediated by ABI3 and ABI5
Next, we sought to understand the molecular mechanism by which ABA regulates MFT expression. ABI loci (ABI1 to ABI5) have been identified as essential regulators in the ABA signaling network (Koornneef et al., 1984; Finkelstein, 1994). The expression of ABI3-5 had a similar trend as that of MFT in wild-type seeds during imbibition under normal growth conditions (see Supplemental Figure 6 online). We further tested whether these ABI genes are involved in the regulation of MFT by ABA by examining MFT transcript levels in various abi mutant seeds in the absence or presence of exogenous ABA. Upregulation of MFT by ABA was completely abolished in abi3-1 and attenuated in abi5-1 (Figure 3A), suggesting that ABI3 and ABI5 mediate the expression of MFT in response to ABA. It is noteworthy that with or without ABA treatment, MFT expression in abi3-1 mutant was strikingly higher than that in the wild type (Figure 3A), indicating that ABI3 negatively regulates MFT. On the contrary, MFT expression in abi5-1 was the lowest among all the mutants treated with high concentrations of ABA (Figure 3A), indicating that ABI5 promotes MFT expression in response to ABA. As the expression levels of MFT in abi3-1 abi5-1 were similar to those in abi3-1 (Figure 3A), abi3-1 is epistatic to abi5-1 in terms of regulating MFT expression.
Figure 3.
Regulation of MFT by ABA Is Mediated by ABI3 and ABI5.
(A) MFT expression determined by quantitative real-time PCR in wild-type (WT) and various abi mutants mock treated or treated with 10 μM ABA. Because abi3-1 seeds germinated around 14 h after stratification, we collected all germinating seeds 12 h after stratification for comparing MFT expression. Error bars denote sd.
(B) Germination phenotype of the wild type, 35S:ABI3-6HA, and 35S:ABI5-6HA treated with 1 or 5 μM ABA. Error bars denote sd.
(C) Expression of ABI3, ABI5, and MFT determined by quantitative real-time PCR in germinating seeds of the wild type, 35S:ABI3-6HA, and 35S:ABI5-6HA mock treated or treated with 1 or 5 μM ABA. All germinating seeds were collected 16 h after stratification. Error bars denote sd.
(D) ChIP enrichment test showing the binding of ABI3-6HA and ABI5-6HA to the MFT promoter. The upstream region and the first intron of MFT are represented by white boxes, while the first exon is represented by a black box. The arrowheads in the top panel indicate the sites containing putative ABREs on the MFT promoter. Hatched boxes represent the DNA fragments amplified in ChIP assays. ChIP assay results of 35S:ABI3-6HA and 35S:ABI5-6HA are shown in the bottom panels. Seeds were sown on Murashige and Skoog (MS) medium supplemented with 10 μM ABA and harvested 16 h after stratification for ChIP assays. Em6, which has been identified as a direct target of ABI5 (Lopez-Molina et al., 2002), is used as a positive control for ABI5-6HA ChIP assay. Significant differences in comparison with the enrichment of a TUB2 fragment are indicated with asterisks (P < 0.05, Student's t test). Error bars denote sd.
To elucidate the relationship between ABI3, ABI5, and MFT, we created 35S:ABI3-6HA and 35S:ABI5-6HA transgenic lines. Low concentrations of ABA significantly lowered the germination rates of these two transgenic plants, which is in agreement with previous reports on 35S:ABI3 and 35S:ABI5 (Lopez-Molina et al., 2001; Zhang et al., 2005), suggesting that both ABI3-6HA and ABI5-6HA fusion proteins are biologically functional (Figure 3B). When ABI3 was overexpressed, ABI5 expression was upregulated, whereas MFT expression was downregulated in the presence of ABA (Figure 3C). Overexpression of ABI5 did not affect ABI3 expression, but did lead to upregulated MFT expression in the presence of ABA. As ABA prevents the degradation of ABI3 and ABI5 proteins (Lopez-Molina et al., 2001; Zhang et al., 2005), these results, together with the observation on MFT expression in abi3-1 and abi5-1, suggest that ABI3 activity inhibits MFT expression, whereas ABI5 has an opposite effect.
We further investigated whether ABI3 and ABI5 directly regulate MFT by chromatin immunoprecipitation (ChIP) assays. Promoter analysis identified several putative ABA-responsive elements (ABREs) in the MFT promoter. There is a single ABRE and a separate cluster of five ABREs located ∼700 bp and 1.7 kb upstream of the initiation codon, respectively (Figure 3D). Since both ABI3 and ABI5 have been shown to regulate ABRE-containing genes (Kim et al., 1997; Ezcurra et al., 1999, 2000; Finkelstein and Lynch, 2000; Kim et al., 2002; Lopez-Molina et al., 2002), the presence of these ABREs in MFT promoter implies that ABI3 and ABI5 may directly regulate MFT expression. Hence, 35S:ABI3-6HA and 35S:ABI5-6HA tagging lines were applied for ChIP assays using four pairs of the primers designed in the MFT promoter (MFT-1 to MFT-4). ChIP enrichment revealed that ABI3-6HA was mainly associated with the genomic region near MFT-2, while ABI5-6HA bound to the regions near both MFT-2 and MFT-3 (Figure 3D), suggesting that both ABI3 and ABI5 directly bind to the MFT promoter in vivo.
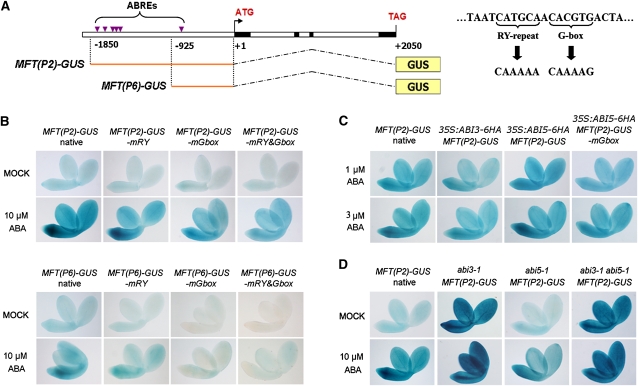
A G-Box Motif Mediates Spatial Regulation of MFT in Response to ABA
To further understand how ABA regulates MFT through ABI3 and ABI5 during seed germination, we generated two MFT:β-glucuronidase (GUS) constructs, MFT(P2)-GUS and MFT(P6)-GUS, in which 1.8-kb and 900-bp promoter sequences with six and one ABREs upstream of the translational start site, respectively, were fused with the GUS reporter gene (Figure 4A, left panel). The MFT coding sequence driven by the 1.8-kb promoter was able to rescue the low germination phenotype of mft-2 in response to ABA (see Supplemental Figure 7 online), implying that this promoter region contains essential cis-elements required for the regulation of MFT expression by ABA. For both MFT(P2)-GUS and MFT(P6)-GUS, moderate GUS signals were detected in embryos without ABA treatment (Figure 4B). In agreement with MFT gene expression profiles (Figures 2A to 2C), when exogenous ABA was applied, GUS staining in these two reporter lines was enhanced throughout the embryos, especially in the radical-hypocotyl transition zone (Figure 4B). While GUS staining was also observed in the seed coat with the endosperm, it was not affected by ABA treatment (data not shown).
Figure 4.
A G-Box Motif in MFT Promoter Mediates Spatial Regulation of MFT in Response to ABA.
(A) Schematic diagram of MFT(P2)-GUS and MFT(P6)-GUS constructs where MFT 5′ upstream sequences containing ABREs were transcriptionally fused with the GUS gene (left panel). The right panel shows the mutagenesis of the RY repeat and the G-box in the ABRE that is located 700 bp upstream of the ATG start codon.
(B) GUS staining in germinating seeds of the transformants containing MFT(P2)-GUS, MFT(P6)-GUS, and their derived constructs with the mutated RY repeat (mRY) and G-box motif (mGbox). Seeds from T3 homozygous plants with a single insertion of the transgene for each construct were analyzed, and representative images are shown. Germinating seeds mock treated or treated with 10 μM ABA, which were at the same developmental stage, were stained 12 or 24 h after stratification, respectively.
(C) GUS staining in germinating seeds of MFT(P2)-GUS, 35S:ABI3-6HA MFT(P2)-GUS, 35S:ABI5-6HA MFT(P2)-GUS, and 35S:ABI5-6HA MFT(P2)-GUS-mGbox. Germinating seeds treated with 1 and 3 μM ABA, which were at the same developmental stage, were stained 24 h after stratification.
(D) GUS staining in germinating seeds of MFT(P2)-GUS, abi3-1 MFT(P2)-GUS, abi5-1 MFT(P2)-GUS, and abi3-1 abi5-1 MFT(P2)-GUS. To examine GUS expression in seeds at the same developmental stage, MFT(P2)-GUS and abi5-1 MFT(P2)-GUS treated with 10 μM ABA were stained 24 h after stratification, while other germinating seeds mock treated or treated with 10 μM ABA were stained 12 h after stratification.
Although MFT(P6)-GUS showed weaker staining in whole embryos than MFT(P2)-GUS in response to ABA, increased GUS staining was specifically observable in the radical-hypocotyl transition zone of MFT(P6)-GUS (Figure 4B). This implies that the fived clustered ABREs at ∼1.7 kb upstream of the start codon may modulate the extent of MFT upregulation throughout the embryo, while the single ABRE close to the start codon may confer the spatial upregulation of MFT in the radical-hypocotyl transition zone in response to ABA. We further mutagenized the single ABRE to evaluate its function in response to ABA (Figure 4A, right panel). This ABRE contains one RY repeat that is recognized by ABI3 and one G-box that is recognized by both ABI3 and ABI5 (Kim et al., 1997; Ezcurra et al., 2000). Mutation of the RY motif had no obvious effect on the GUS staining of either MFT(P2)-GUS or MFT(P6)-GUS in response to ABA, whereas mutation of the G-box motif notably attenuated the responses to ABA (Figure 4B). In particular, upregulation of GUS staining in the radical-hypocotyl transition zone was significantly abolished when the G-box was mutated (Figure 4B). Therefore, the G-box in this single ABRE is essential for upregulating MFT expression particularly in the radical-hypocotyl transition zone in response to ABA.
MFT Is Promoted by ABI5 but Suppressed by ABI3
As ChIP assays demonstrated that ABI3 and ABI5 physically bound to the MFT promoter, we crossed MFT:GUS lines into plants with overexpression or loss of function of ABI3 and ABI5 to monitor how they control MFT expression in response to ABA during seed germination. Since the germination of 35S:ABI3 and 35S:ABI5 seeds was hypersensitive to low ABA concentrations (Figure 3B) (Lopez-Molina et al., 2001; Zhang et al., 2005), GUS staining of MFT:GUS lines in these backgrounds was examined under the treatment of 1 or 3 μM ABA. MFT(P2)-GUS exhibited enhanced staining specifically in the radical-hypocotyl transition zone in 35S:ABI5-6HA in response to ABA, while mutation of the G-box in MFT(P2)-GUS did not show any change of GUS staining in 35S:ABI5-6HA (Figure 4C). This finding, together with the ChIP result showing the binding of ABI5 to the promoter region near MFT-3 (Figure 3D), strongly suggests that ABI5 specifically regulates MFT expression in the radical-hypocotyl transition zone through the G-box in the ABRE near the start codon. This is confirmed by the observation that MFT(P2)-GUS showed decreased GUS staining in the radical elongation zone in abi5-1 compared with that in wild-type background in response to ABA (Figure 4D).
MFT(P2)-GUS showed slightly reduced GUS staining in 35S:ABI3-6HA in response to ABA (Figure 4C) but exhibited extraordinarily strong GUS staining in the whole embryo of abi3-1 independent of ABA treatment (Figure 4D). Further loss of ABI5 in abi3-1 had little effect on the intensity of GUS staining in MFT(P2)-GUS (Figure 4D). These GUS staining patterns are in agreement with the MFT expression in the corresponding transgenic plants or mutants (Figures 3A and 3C), suggesting that ABI3 plays a dominant role in suppressing MFT expression in the whole embryo, while ABI5 recognizes the G-box motif to promote MFT expression particularly in the radical-hypocotyl transition zone.
MFT Is Regulated by DELLA Proteins
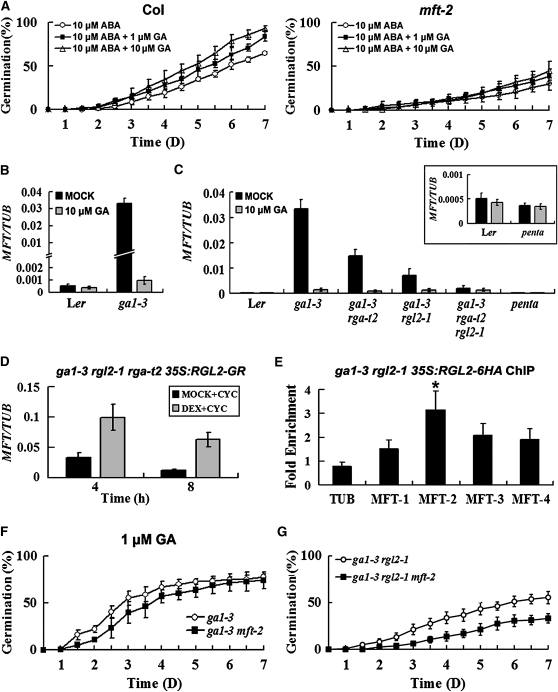
Since GA functions as a major counteracting hormone against ABA to promote both embryo growth potential and endosperm weakening during seed germination (Ogawa et al., 2003; Muller et al., 2006), we asked whether MFT is involved in the crosstalk of ABA and GA signaling. We first examined if GA could rescue the low germination rate of mft-2 caused by ABA. In the presence of 10 μM ABA, increasing the concentration of exogenously applied GA from 1 to 10 μM clearly elevated the germination rate of wild-type seeds but had little effect on the germination of mft-2 (Figure 5A), implying that MFT plays a role in mediating the interaction between ABA and GA signals during seed germination.
Figure 5.
Regulation of MFT by GA Is Mediated by DELLA Proteins.
(A) Germination phenotype of the wild type and mft-2 treated with 10 μM ABA plus different concentrations of GA. Error bars denote sd.
(B) MFT expression determined by quantitative real-time PCR in wild-type and ga1-3 seeds mock treated or treated with 10 μM GA. All germinating seeds were collected 16 h after stratification. Error bars denote sd.
(C) MFT expression determined by quantitative real-time PCR in wild-type and various DELLA mutant seeds in ga1-3 background mock treated or treated with 10 μM GA. All germinating seeds were collected 16 h after stratification. penta indicates the ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1 mutant. Inset shows the comparison of MFT expression between the wild type and penta mutants. Error bars denote sd.
(D) MFT expression determined by quantitative real-time PCR in ga1-3 rgl2-1 rga-t2 35S:RGL2-GR seeds. Seeds were treated with 30 μM DEX plus 30 μM cycloheximide (CYC) or MOCK (0.09% ethanol) plus cycloheximide under vacuum for 1 h. They were subsequently washed three times and collected 4 and 8 h after sowing on MS medium. Error bars denote sd.
(E) ChIP results showing the binding of RGL2-6HA to the MFT promoter. Primers used for the enrichment test are described in Figure 3D. Seeds were sown on MS medium and harvested 16 h after stratification for ChIP assays. A significant difference in comparison with the enrichment of a TUB2 fragment is indicated with an asterisk (P < 0.05, Student's t test). Error bars denote sd.
(F) Germination phenotype of ga1-3 and ga1-3 mft-2 treated with 1 μM GA. Error bars denote sd.
(G) Germination phenotype of ga1-3 rgl2-1 and ga1-3 rgl2-1 mft-2 in the absence of exogenous GA. Error bars denote sd.
We next examined MFT expression in the GA-deficient mutant ga1-3, which blocks GA biosynthesis (Wilson et al., 1992). MFT was highly expressed in ga1-3 seeds, and exogenously applied GA downregulated MFT (Figure 5B), suggesting that GA represses MFT expression in seeds. As DELLA proteins are a major family of growth-restricting nuclear proteins mediating the GA effect on growth and RGA and RGL2 are predominant DELLAs involved in seed germination (Lee et al., 2002; Tyler et al., 2004), we tested if DELLA proteins mediate GA regulation of MFT by checking MFT expression in various DELLA mutants in the ga1-3 background. Loss of RGL2 or RGA activity noticeably reduced MFT expression in ga1-3 with a stronger effect exerted by RGL2 (Figure 5C). In ga1-3 rga-t2 rgl2-1, MFT expression was much reduced (Figure 5C). Further loss of the activity of other two DELLA proteins, GAI and RGL1, reduced MFT expression to a level comparable to that in the wild type (Figure 5C). GA treatment that resulted in the degradation of DELLA proteins considerably downregulated MFT expression in almost all the mutants tested except the penta mutant ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1. These results suggest that all DELLA proteins tested contribute to the upregulation of MFT in ga1-3 and that RGL2 and RGA are two major regulators controlling MFT expression with the former as the most important regulator.
To examine if DELLA proteins directly modulate MFT transcription, we created ga1-3 rgl2-1 rga-t2 35S:RGL2-GR inducible lines, in which glucocorticoid receptor (GR) fusion protein could be activated by its ligand dexamethasone (DEX). Application of DEX delayed the germination rate of ga1-3 rgl2-1 rga-t2 35S:RGL2-GR (see Supplemental Figure 8 online), suggesting that RGL2-GR is biologically functional in inhibiting seed germination. Combined treatment of ga1-3 rgl2-1 rga-t2 35S:RGL2-GR by DEX and cycloheximide, an inhibitor of protein synthesis, resulted in an increase in MFT expression. This demonstrates that RGL2 modulates MFT expression independently of protein synthesis, indicating that MFT may be an immediate target of RGL2 (Figure 5D). To test whether RGL2 could be associated with the MFT promoter, we created 35S:RGL2-6HA lines for ChIP analysis. 35S:RGL2-6HA was introduced into ga1-3 rgl2-1, and the functional lines that mimicked ga1-3 phenotypes were chosen for further ChIP assays. ChIP enrichment test showed that RGL2-6HA was associated with the region near the MFT-2 fragment (Figure 5E), indicating that RGL2 is directly involved in the regulation of MFT expression.
We found that both ABI3 and ABI5 expression was elevated in ga1-3 seeds and that their expression levels in ga1-3 lacking DELLA proteins (particularly RGL2 and RGA) decreased to the same levels as in wild-type seeds (see Supplemental Figure 9 online). GA treatment of these mutant seeds downregulated ABI3 and ABI5 expression to the levels comparable to those of wild-type seeds (see Supplemental Figure 9 online). These results suggest that DELLA proteins promote ABA signaling, which is consistent with the previous findings showing that DELLA proteins stimulate endogenous ABA synthesis (Ko et al., 2006; Zentella et al., 2007; Piskurewicz et al., 2008). Thus, it is likely that in addition to direct regulation of MFT expression, RGL2 also indirectly affects MFT expression through the ABA signaling pathway.
To understand the biological significance of the upregulation of MFT by DELLA proteins, we crossed mft-2 in Landsberg erecta (Ler) background with ga1-3 to remove MFT activity. As ga1-3 germinates only upon application of exogenous GA, seed germination was examined in the presence of exogenous GA. Under the same growth conditions, ga1-3 mft-2 germinated at a lower rate than ga1-3 in response to GA treatment, especially within 5 d after stratification (Figure 5F). When GA levels are low, DELLA proteins overaccumulate, thus concomitantly promoting MFT expression and ABA signaling. The lower germination rate in ga1-3 mft-2 than in ga1-3 indicates that when GA biosynthesis is impaired and ABA signaling is stimulated, MFT is required to maintain the seed germination potential. In agreement with this, ga1-3 rgl2-1 mft-2 exhibited a lower germination rate than ga1-3 rgl2-1 in the absence of exogenous GA (Figure 5G).
MFT Represses ABI5 Expression during Seed Germination
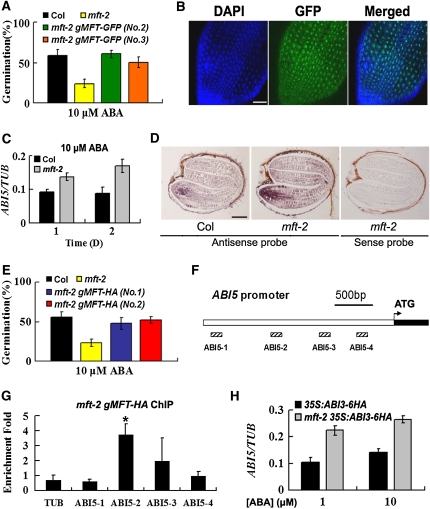
To understand the mechanism by which MFT regulates seed germination, we first checked MFT intracellular localization by examining the localization of gMFT-GFP, in which the MFT coding sequence driven by the 1.8-kb promoter was fused with the green fluorescent protein reporter gene. Among 16 mft-2 gMFT-GFP transgenic lines obtained, 10 lines could rescue the ABA hypersensitivity phenotype of mft-2, indicating that the MFT-GFP fusion protein is biologically functional (Figure 6A). MFT-GFP signals in the cells of the radical-hypocotyl transition zone were located in the nucleus, implying that MFT may function as a transcription coregulator that modulates downstream gene expression (Figure 6B). The similar nuclear localization has also been observed in another MFT homolog, FT, which functions in the nucleus to regulate the expression of other flowering genes (Abe et al., 2005; Wigge et al., 2005).
Figure 6.
MFT Antagonizes ABA Signaling by Directly Repressing ABI5 during Seed Germination.
(A) Two representative mft-2 gMFT-GFP transgenic lines showing rescued germination phenotype in response to 10 μM ABA. The percentage of germination was scored 7 d after stratification. Error bars denote sd.
(B) MFT-GFP localization in the cells of the radical-hypocotyl transition zone. DAPI, fluorescence of 4',6-diamidino-2-phenylindole; Merged, merge of DAPI and GFP. Bar = 50 μm.
(C) Expression of ABI5 determined by quantitative real-time PCR in wild-type and mft-2 seeds treated with 10 μM ABA at 1 or 2 d after stratification. Error bars denote sd.
(D) In situ localization of ABI5 in wild-type and mft-2 seeds treated with 10 μM ABA. Seeds were collected 24 h after stratification. Bar = 100 μm.
(E) Two representative mft-2 gMFT-HA transgenic lines showing rescued germination phenotype in response to 10 μM ABA. The percentage of germination was scored 7 d after stratification. Error bars denote sd.
(F) Schematic diagram of the ABI5 promoter region. White and black boxes represent the upstream region and part of the first exon, respectively. Hatched boxes represent the DNA fragments amplified in ChIP assays.
(G) ChIP enrichment test showing the binding of MFT-HA to the ABI5 promoter. Seeds were sown on MS medium supplemented with 10 μM ABA and harvested 24 h after stratification for ChIP assays. A significant difference in comparison with the enrichment of a TUB2 fragment is indicated with an asterisk (P < 0.05, Student's t test). Error bars denote sd.
(H) ABI5 expression determined by quantitative real-time PCR in germinating seeds of 35S:ABI3-6HA and mft-2 35S:ABI3-6HA treated with 1 and 10 μM ABA. Germinating seeds were collected 24 h after stratification. Error bars denote sd.
To evaluate how MFT affects the expression of other genes during seed germination, we tested a group of ABA marker genes and found that the expression of RD29A, RD29B, and Em6 was higher in mft-2 than in the wild type in response to ABA (see Supplemental Figure 10 online). Since Em6 is a known direct downstream target of ABI5 (Finkelstein and Lynch, 2000; Lopez-Molina et al., 2002) and ABI5 expression in the radicle overlaps with MFT expression in response to ABA (Piskurewicz et al., 2008) (Figure 2C), we asked if MFT regulates ABI5, thus affecting Em6 expression. We found that in germinating seeds, ABI5 expression in mft-2 was higher than in the wild type in response to exogenous ABA (Figure 6C). Furthermore, in situ hybridization revealed higher ABI5 expression levels in the radicle of mft-2 than in the wild type (Figure 6D). This suggests that MFT negatively regulates ABI5 in the radicle of germinating seeds in response to ABA. To test whether MFT is directly associated with the ABI5 promoter, we created mft-2 gMFT-HA transgenic plants that fully rescued the mft-2 phenotype (Figure 6E). ChIP assays showed the enrichment of MFT-HA at the promoter region near the ABI5-2 fragment (Figures 6F and 6G), whereas no binding was observed on the ABI3 promoter (see Supplemental Figure 11 online), suggesting that MFT antagonizes ABA signaling by directly repressing ABI5. This was supported by the expression analysis showing that ABI3 upregulation of ABI5 in 35S:ABI3-6HA was enhanced in mft-2 in response to ABA (Figure 6H). Furthermore, abi5-1 mft-2 double mutants could fully rescue the low germination defects of mft-2 in response to ABA.
DISCUSSION
MFT Expression Is Mediated by ABA and GA Signaling Pathways
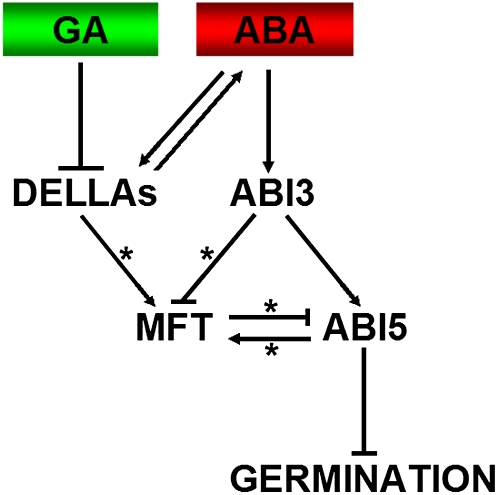
Seed germination starts with the uptake of water by the dry seed and terminates with the visible penetration of the structures surrounding the embryo by the embryonic axis (Bewley, 1997). This process is governed by two major counteracting phytohormones, ABA and GA, in response to various environmental factors. As these two hormones act through a complex crosstalk rather than through independent pathways (Kucera et al., 2005), integration of their mutual interaction is critical for a plant to make a decision on whether it should initiate germination. The DELLA protein RGL2 and ABA biosynthesis are two known factors involved in this integration, in which they promote each other via a positive feedback regulatory loop (Piskurewicz et al., 2008). In this study, we show that MFT responds to ABA and GA signaling to modulate ABI5 expression in the radicle of the embryo, thus specifically regulating the timing of seed germination in response to environmental factors (Figure 7).
Figure 7.
A Proposed Model of Seed Germination Mediated by MFT.
ABA regulates MFT expression via ABI3 and ABI5 with the former acting as a repressor and the latter as a promoter. MFT confers negative feedback regulation of the ABA signaling pathway through directly repressing ABI5. On the other hand, GA downregulates MFT expression and inhibits ABA synthesis via DELLA proteins (i.e., RGL2). Therefore, MFT serves as a mediator in response to ABA and GA signals to promote seed germination through constituting a negative feedback regulation of ABA signaling. Asterisks represent direct transcriptional regulation.
[See online article for color version of this figure.]
Several pieces of evidence suggest that MFT serves as a convergence point of ABA and GA signaling pathways during seed germination. First, ABI5, which represses seed germination in response to ABA and GA (Piskurewicz et al., 2008), directly upregulates MFT in the radical-hypocotyl transition zone of the embryo. In the absence of ABI5, upregulation of MFT in response to ABA is abolished, while overexpression of ABI5 increased MFT expression. ABI5 binds to the MFT promoter region close to the start codon, where there is a single ABRE. Mutation of the G-box in this ABRE abolishes the upregulation of MFT expression in the radical-hypocotyl transition zone by increased ABI5 activity. These results strongly suggest that ABI5 specifically regulates MFT expression in the radical-hypocotyl transition zone through the G-box in the ABRE near the start codon. It is noteworthy that in abi5-1, GUS staining of MFT(P2)-GUS in the whole embryo still increased in response to ABA (Figure 4D). This is consistent with the expression analysis showing slightly upregulated MFT expression in abi5-1 in response to ABA (Figure 3A), implying that some other factor(s) act concomitantly with ABI5 to upregulate MFT in the ABA pathway. In addition to ABI5, there are other four bZIP ABRE binding factors (ABFs) that recognize the G-box motif (Choi et al., 2000). Among them, ABF3 has been shown to function redundantly with ABI5 during seed germination (Finkelstein et al., 2005) and thus might be another potential upstream regulator of MFT.
Second, ABI3, another key transcription factor in the ABA pathway, also directly represses MFT. MFT is downregulated in 35S:ABI3 germinating seeds treated with ABA. Furthermore, ChIP assays have shown that the genomic region near the MFT-2 fragment, where several ABREs are located, is associated with ABI3-6HA (Figure 3D). This suggests that ABI3 plays a role in directly repressing MFT in response to ABA during seed germination. Interestingly, MFT was dramatically upregulated in abi3-1 germinating seeds even without ABA treatment (Figures 3A and 4D). We speculate that upregulation of MFT in abi3-1 may partly result from a failure in establishing seed maturation in abi3-1 (Nambara et al., 1995), which causes a global change in transcription. In agreement with this idea, MFT expression is particularly strong in the whole embryo of abi3-1 seeds (Figure 4D), which resembles its expression pattern in wild-type immature seeds (see Supplemental Figure 12 online). Thus, the significantly elevated MFT expression in abi3-1 may reflect ABI3 regulation of MFT expression in the underdeveloped embryo.
Third, our data suggest that among all the DELLA proteins involved in the regulation of MFT, RGL2 is the main one that directly modulates MFT expression. When GA levels are low, RGL2 promotes ABA biosynthesis through stimulating the expression of XERICO, which encodes a RING-H2 zinc finger factor (Ko et al., 2006; Zentella et al., 2007; Piskurewicz et al., 2008). In turn, an elevation of endogenous ABA levels promotes the mRNA expression of RGL2 and ABI5 and the protein expression and activity of ABI5 (Piskurewicz et al., 2008). Thus, direct upregulation of MFT by RGL2 allows MFT to respond to both GA and ABA pathways when they generate a signal output that does not permit germination under unfavorable environmental conditions.
Negative Feedback Regulation of ABI5
ABI5 has been identified as the final and common downstream repressor of seed germination in response to ABA and GA (Piskurewicz et al., 2008). The function of ABI5 is modulated by phosphorylation mediated by SnRK2-type kinases and sumoylation mediated by SUMO E3 ligase (Lopez-Molina et al., 2001; Fujii et al., 2007; Miura et al., 2009). ABI5 prevents seed germination partly by activating a group of LATE EMBRYOGENESIS ABUNDANT genes, including Em1 and Em6 (Finkelstein and Lynch, 2000; Lopez-Molina et al., 2002), which encode hydrophilic proteins possibly required for desiccation tolerance (Vicient et al., 2000). So far, several upstream regulators of ABI5 have been reported. ABI3 functions as an upstream promoter of ABI5 to regulate the growth arrest of germinating seed (Lopez-Molina et al., 2002). Because 35S:ABI5 could rescue ABA insensitivity of abi3 during germination, ABI5 has been suggested as an essential factor acting downstream of ABI3 and executing an ABA-dependent growth arrest (Lopez-Molina et al., 2002). Genetic analysis has also suggested that ABF1 and ABF3 participate in antagonizing ABI5 expression (Finkelstein et al., 2005). In addition, HY5, a light-signaling mediator, directly activates ABI5 to integrate light and ABA signaling (Chen et al., 2008).
Here, we show that MFT responds to the signals from ABA and GA pathways and generates a negative feedback loop in the ABA pathway by directly repressing ABI5 (Figure 7). When ABA levels are high, ABI5 directly promotes the expression of MFT, which in turn directly represses ABI5 expression in the radicle of the embryo, thus maintaining the embryo growth potential even under high levels of ABA. Upon ABA treatment, mft mutants exhibit enhanced upregulation of ABI5 in the radicle of the embryo, thus resulting in the ABA hypersensitive phenotype. As MFT protein does not contain any DNA binding domain, there are likely to be other transcription factors involved in guiding the MFT protein to the ABI5 promoter. A bZIP transcription factor, FD, has been shown to interact with the MFT homolog FT, and such a protein complex participates in activating a floral homeotic gene APETALLA1 (Abe et al., 2005; Wigge et al., 2005). Identification of the MFT-interacting partner(s) remains as an intriguing subject for future research.
MFT-Like Genes May Have Conserved Function in Plants
The PEBP family is evolutionarily conserved in a wide range of multicellular land plants, and phylogenetic studies of PEBP-like genes in angiosperms have divided them into three main subfamilies, FT-like, TFL1-like, and MFT-like clades (Kobayashi et al., 1999; Carmel-Goren et al., 2003; Chardon and Damerval, 2005; Carmona et al., 2007; Hedman et al., 2009). A recent study on MFT-like genes in a basal plant lineage Physcomitrella patens (bryophyte) suggested that the MFT-like clade is ancestral to the other two clades (Hedman et al., 2009). In Arabidopsis, three PEBP members, FT, TFL1, and TWIN SISTER OF FT, have been shown to regulate flowering time and shoot meristem identity (Bradley et al., 1997; Kardailsky et al., 1999; Kobayashi et al., 1999; Yamaguchi et al., 2005). Although MFT shares sequence similarity with FT and TFL1, its loss-of-function mutant in the Wassilewskija-2 (Ws) ecotype background does not exhibit relevant defects in flowering and meristem development (Yoo et al., 2004), which is consistent with what we have observed in mft-2 and mft-3 in Col. A comparison of protein sequences has revealed a critical amino acid residue (Trp) in MFT that differs from Tyr or His in FT or TFL1, respectively, in which this residue is located in a potential ligand binding pocket and determines the protein function as a flowering inducer or repressor (Hanzawa et al., 2005). Notably, this residue is well conserved in most MFT-like proteins (Hedman et al., 2009). Furthermore, almost all MFT-like proteins share another conserved Pro residue near the C terminus, which is absent in FT-like or TFL1-like proteins (Hedman et al., 2009). These observations imply that MFT may have different roles in plant development compared with FT and TFL1.
In this study, we show that MFT functions as an antagonistic factor of ABA signaling during seed germination. MFT expression is boosted by ABA and inhibited by GA and MFT promotes seed germination particularly when ABA levels are elevated under environmental stresses. Like MFT expression in Arabidopsis (Figures 1A and 1B; see Supplemental Figure 12 online), most of the identified MFT-like genes in various plant species show preferential expression in seeds (Chardon and Damerval, 2005; Danilevskaya et al., 2008), implying a highly conserved function of MFT-like genes in seed development across the plant kingdom. Analysis of the genomic sequences of MFT-like genes in maize (Zea mays) and rice (Oryza sativa) revealed that they all contain several ABREs near their coding regions, which is comparable to the ABREs identified in MFT in Arabidopsis (see Supplemental Figure 13 online). Therefore, it will be interesting and of practical importance to study further whether MFT-like genes in other plants have a conserved function in tuning seed sensitivity to ABA, thus modulating the growth potential of the embryo in response to environmental cues.
METHODS
Plant Materials
Arabidopsis thaliana ecotype Col-0 was used in the generation of all transgenic plants. abi1-1, abi2-1, and abi3-1 are in the Ler background; abi4-1, mft-2, mft-3, aba1-5, cyp707a1-1, and cyp707a2-1 are in Col background; and abi5-1 is in the Wassilewskija background. mft-2 introgressed into Ler was obtained by three backcrosses of mft-2 into Ler. Different combinations of DELLA mutants in ga1-3 background (Ler) have been described previously (Yu et al., 2004b).
Plant Growth Conditions, Seed Germination Assay, and Stress Treatment
Plants were grown at 22°C under long days (16 h of light/8 h of dark). Dry seeds were collected and stored at a dehumidifier cabinet for at least 2 months before the seed germination test was performed. After-ripened seeds were washed with 70% (v/v) ethanol for 30 s, sterilized with 10% (v/v) commercial Clorox bleach for 15 min, and washed three times with sterile water. Sterilized seeds were subsequently plated on MS medium (Sigma-Aldrich) containing 0.8% (w/v) Bacto Agar (Difco/BD) supplemented with ABA, NaCl, or GA3. Stock solution of ABA (mixed isomers; Sigma-Aldrich) was in methanol, while GA3 (Sigma-Aldrich) was in ethanol. Control plates contained equal amounts of the corresponding solvents. Plates were kept at 4°C in darkness for 3 d for stratification and then transferred to a tissue culture room set at 22°C with a 16-h-light/8-h-dark photoperiod. For the germination assay, at least 100 seeds for each genotype were sterilized and sown on MS medium supplemented with or without phytohormones or chemicals. Germination was defined as the first sign of radicle tip emergence and scored daily until the 7th day of the incubation, and the germination results were calculated based on at least three independent experiments. Drought treatment and measurement of transpiration rates were performed as previously described (Kang et al., 2002).
Plasmid Construction
For construct 35S:MFT, the MFT coding region was amplified using primers MFT_P1_PstI (5′-CCCTGCAGATATATATCTCCCTCCCCGC-3′) and MFT_P2_SpeI (5′-CCACTAGTTTTTTGTACTAGCGTCTGCG-3′). The PCR products were digested with PstI and SpeI and inserted into the corresponding sites of the pGreen 0229-35S binary vector (Yu et al., 2004a). To construct MFT(P2)-GUS, the 1.8-kb MFT 5′ upstream sequence was amplified with the primers MFTGUS_P1_HindIII (5′-CCAAGCTTCTACGCGATTGGACGTTGC-3′) and MFTGUS-P5-XmaI (5′-ACCCGGGCGATCAGCGGGGAGGGAGAT-3′). To construct MFT(P6)-GUS, the 900-bp MFT 5′ upstream sequence was amplified with the primers MFTGUS-P4-HindIII (5′-CCAAGCTTCGATGAATATGCGACCGACC-3′) and MFTGUS-P5-XmaI. The digested PCR products were cloned into pHY107 (Liu et al., 2007). These constructs were mutagenized to produce the mutated ABA response elements (Figure 4A) using the QuikChange II XL site-directed mutagenesis kit (Stratagene). Two MFT genomic fragments (gMFT-P2 and gMFT-P6) were used for the complementation test. gMFT-P2 comprised the 1.8-kb upstream sequence and the 2.1-kb coding sequence plus introns and amplified using the primers MFTGUS_P1_HindIII and MFT_P2'_XmaI (5′-ACCCGGGCTAGCGTCTGCGTGAAGCAGGTTCC-3′). gMFT-P6 contained the 900-bp upstream sequence and the 2.1-kb coding sequence plus introns and was amplified by MFTGUS-P4-HindIII and MFT_P2'_XmaI. These fragments were digested and inserted into pHY105 (Liu et al., 2007). To construct gMFT-HA, a single HA tag was incorporated into pHY105-gMFT-P2 construct using the primers MFT-inverse-RC (5′-CAGCGAATTATCTAGAACTAGCTAGCGTCTGCGTGAAGCAGGTCC-3′) and gMFT-HA-R (5′-AGCGTAATCTGGAACGTCATATGGATAGCGTCTGCGTGAAGCAGGTTCC-3′) by the mutagenesis PCR method. To construct 35S:ABI3-6HA, the ABI3 cDNA was amplified with the primers ABI3-6HA_P1_XhoI (5′-CCCTCGAGCC ACTTCAACGATGAAAAGCTTGCATGTGG-3′) and ABI3-6HA_P2_SpeI (5′-GGACTAGTTTTAACAGTTTGAGAAGTTGGTGAAGCGACCAC-3′). The resulting products were digested by SpeI and XhoI and cloned into pGreen-35S-6HA to obtain an in-frame fusion of ABI3-6HA under the control of 35S promoter (Liu et al., 2008). Similarly, 35S:ABI5-6HA and 35S:RGL2-6HA were constructed using the primers ABI5-6HA_P1_XhoI (5′-CCCTCGAGGCAGTTGTTAAATGGTAACTAGAG-3′) and ABI5-6HA_P2_SpeI (5′-GGACTAGTGAGTGGACAACTCGGGTTCCTCATC-3′) and the primers RGL2-EcoRV-F (5′-GGGATATCAACAAGAAAGATGAAGAGAGGATACGGAG-3′) and RGL2-XmaI-R2 (5′-CCCCCCGGGGGCGAGTTTCCACGCCGAGG-3′), respectively.
RNA Extraction and Real-Time PCR
Total RNAs from seeds were extracted using the RNAqueous small-scale phenol-free total RNA isolation kit (Ambion) according to the manufacturer's instructions and reverse transcribed using the SuperScript RT-PCR system (Invitrogen). Quantitative real-time PCR was performed using the Power SYBR Green PCR Master mix (Applied Biosystems) as previously reported (Liu et al., 2007). Gene expression was normalized to the expression of Tubulin (TUB2). Primers used are listed in Supplemental Table 1 online.
ChIP Assays
For ChIP assays, ∼300 mg germinating seeds were fixed for 45 min at 4°C with 1% formaldehyde in MC buffer (10 mM potassium phosphate, pH 7.0, 50 mM NaCl, and 0.1 M sucrose) under vacuum. After adding glycine to a concentration of 150 mM, the fixed seeds were shaken for 20 min at 4°C, washed three times with MC buffer, and homogenized in the reaction buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 0.1% SDS) supplemented with Protease Inhibitor Cocktail tablets (Roche). The mixture was sonicated to produce DNA fragments between 200 and 1 kb. Ten percent solubilized chromatin was saved as an input control, and the remaining was thereafter incubated with anti-HA agarose beads (Sigma-Aldrich) for 1.5 h at 4°C. Beads were washed twice with IP buffer (50 mM HEPES, pH 7.5, 150 mM KCl, 5 mM MgCl2, 10 μM ZnSO4, 1% Triton X-100, and 0.05% SDS), twice with high-salt IP buffer with the concentration of KCl increased to 500 mM, once with LNDET buffer (0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mM EDTA, and 10 mM Tris, pH 8.0), and once with IP buffer again. Beads were then incubated with the elution buffer (50 mM Tris, pH 8.0, 1% SDS, and 10 mM EDTA) for 30 min at 65°C. The eluted supernatant and the input control that was topped up with the elution buffer to a final volume equal to that of the eluted supernatant were added with NaCl to a final concentration of 200 mM. They were incubated at 65°C for 6 h for reverse cross-linking followed by 40 mM proteinase K treatment for 1 h at 45°C. DNA was then recovered using the QIAquick PCR purification kit (Qiagen). For each ChIP assay, three independent experiments were performed using seeds collected separately. DNA enrichment was examined by quantitative real-time PCR in triplicates as previously described (Liu et al., 2008). The enrichment of a TUB2 genomic fragment was used as negative control. Primer pairs used for the ChIP enrichment test are listed in Supplemental Table 1 online.
In Situ Hybridization and GUS Activity Analysis
In situ hybridization was performed as previously reported (Liu et al., 2007). For the synthesis of MFT RNA probe, the gene-specific region was amplified using the primers 5′-CCTCTCTGTTTCTCTCTCTCTC-3′ and 5′-AAGTATCTCTTTTCCTCTTGAG-3′. Full-length ABI5 coding sequence was amplified to synthesize the ABI5 RNA probe. The PCR products were cloned into pGEM-T Easy vector (Promega) to produce the template for in vitro transcription by DIG RNA labeling kit (Roche). For each MFT-GUS reporter construct, we checked at least 15 independent transgenic lines at the T3 generation. A representative line for each MFT-GUS construct was selected for further analysis. GUS staining was performed as previously described (Yu et al., 2002).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: MFT (At1g18100), ABI3 (At3g24650), ABI4 (At2g40220), ABI5 (At2g36270), RGL2 (At3g03450), RD29A (At5g52310), RD29B (At5g52300), At Em6 (At2g40170), CRC (At4g28520), At 2S3 (At4g27160), Os MFT1 (Os06g0498800), Os MFT2 (Os01g0111600), ZCN9 (Eu241925), ZCN10 (Eu241926), and ZCN11 (Eu241927).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Quantification of Endogenous ABA Levels in Wild-Type and mft-2 Seeds after Imbibition.
Supplemental Figure 2. Postgermination Growth of mft Is Not Hypersensitive to ABA Treatment.
Supplemental Figure 3. mft Is Not Hypersensitive to Drought Stress.
Supplemental Figure 4. In Situ Localization of MFT in Seeds Collected 6 h after Stratification Treated without or with 10 μm ABA.
Supplemental Figure 5. Expression of MFT, RGL2, ABI3, and ABI5 in Wild-Type, cyp707a1-1, and cyp707a2-1 Seeds after Imbibition.
Supplemental Figure 6. Expression of MFT, ABI3, ABI4, and ABI5 in Wild-type Seeds after Imbibition.
Supplemental Figure 7. Complementation of mft-2 by Two MFT Genomic Fragments gMFT-P2 and gMFT-P6.
Supplemental Figure 8. A Biologically Active RGL2-GR Fusion.
Supplemental Figure 9. ABI3 and ABI5 Expression in Seeds of ga1-3 Lacking DELLA Proteins That Were Mock Treated or Treated with 10 μM GA.
Supplemental Figure 10. Expression of Several ABA Marker Genes in Wild-Type and mft-2 in Response to ABA.
Supplemental Figure 11. ABI3 Promoter Is Not Directly Bound by MFT-HA.
Supplemental Figure 12. GUS Staining Pattern of MFT(P2)-GUS in Different Tissues.
Supplemental Figure 13. Promoter Analysis of MFT-like Subfamily Genes in Arabidopsis, Rice, and Maize.
Supplemental Table 1. Primers Used in This Study.
Acknowledgments
We thank T. Ito, J. Dinneny, and J. Xu for critical reading of the manuscript, the ABRC for the seeds of abi1-1, abi2-1, abi3-1, abi4-1, abi5-1, mft-2, mft-3, and aba1-5, and M. Okamoto and E. Nambara for the seeds of cyp707a1-1 and cyp707a2-1. This work was supported by Academic Research Funds T208B3113 from the Ministry of Education, Singapore, and R-154-000-337-112 from the National University of Singapore and intramural research funds from Temasek Life Sciences Laboratory. C.L. was supported by the Singapore Millennium Foundation.
References
- Abe M., Kobayashi Y., Yamamoto S., Daimon Y., Yamaguchi A., Ikeda Y., Ichinoki H., Notaguchi M., Goto K., Araki T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Bewley J.D. (1997). Seed germination and dormancy. Plant Cell 9: 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Ratcliffe O., Vincent C., Carpenter R., Coen E. (1997). Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83 [DOI] [PubMed] [Google Scholar]
- Carmel-Goren L., Liu Y.S., Lifschitz E., Zamir D. (2003). The SELF-PRUNING gene family in tomato. Plant Mol. Biol. 52: 1215–1222 [DOI] [PubMed] [Google Scholar]
- Carmona M.J., Calonje M., Martinez-Zapater J.M. (2007). The FT/TFL1 gene family in grapevine. Plant Mol. Biol. 63: 637–650 [DOI] [PubMed] [Google Scholar]
- Chardon F., Damerval C. (2005). Phylogenomic analysis of the PEBP gene family in cereals. J. Mol. Evol. 61: 579–590 [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang J., Neff M.M., Hong S.W., Zhang H., Deng X.W., Xiong L. (2008). Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc. Natl. Acad. Sci. USA 105: 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Hong J., Ha J., Kang J., Kim S.Y. (2000). ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Danilevskaya O.N., Meng X., Hou Z., Ananiev E.V., Simmons C.R. (2008). A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol. 146: 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill A., Sun T. (2001). Synergistic derepression of gibberellin signaling by removing RGA and GAI function in Arabidopsis thaliana. Genetics 159: 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra I., Ellerstrom M., Wycliffe P., Stalberg K., Rask L. (1999). Interaction between composite elements in the napA promoter: Both the B-box ABA-responsive complex and the RY/G complex are necessary for seed-specific expression. Plant Mol. Biol. 40: 699–709 [DOI] [PubMed] [Google Scholar]
- Ezcurra I., Wycliffe P., Nehlin L., Ellerstrom M., Rask L. (2000). Transactivation of the Brassica napus napin promoter by ABI3 requires interaction of the conserved B2 and B3 domains of ABI3 with different cis-elements: B2 mediates activation through an ABRE, whereas B3 interacts with an RY/G-box. Plant J. 24: 57–66 [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R. (1994). Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 5: 765–771 [Google Scholar]
- Finkelstein R.R., Gampala S.S., Rock C.D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl): S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R.R., Gampala S.S., Lynch T.J., Thomas T.L., Rock C.D. (2005). Redundant and distinct functions of the ABA response loci ABA-INSENSITIVE(ABI)5 and ABRE-BINDING FACTOR (ABF)3. Plant Mol. Biol. 59: 253–267 [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R., Lynch T.J. (2000). The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Verslues P.E., Zhu J.K. (2007). Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 19: 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Hauge B.M., Valon C., Smalle J., Parcy F., Goodman H.M. (1992). Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4: 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F., Millar A.A., Jacobsen J.V. (2005). Dormancy release, ABA and pre-harvest sprouting. Curr. Opin. Plant Biol. 8: 183–187 [DOI] [PubMed] [Google Scholar]
- Hanzawa Y., Money T., Bradley D. (2005). A single amino acid converts a repressor to an activator of flowering. Proc. Natl. Acad. Sci. USA 102: 7748–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman H., Kallman T., Lagercrantz U. (2009). Early evolution of the MFT-like gene family in plants. Plant Mol. Biol. 70: 359–369 [DOI] [PubMed] [Google Scholar]
- Kang J.Y., Choi H.I., Im M.Y., Kim S.Y. (2002). Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14: 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I., Shukla V.K., Ahn J.H., Dagenais N., Christensen S.K., Nguyen J.T., Chory J., Harrison M.J., Weigel D. (1999). Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Chung H.J., Thomas T.L. (1997). Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 11: 1237–1251 [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Ma J., Perret P., Li Z., Thomas T.L. (2002). Arabidopsis ABI5 subfamily members have distinct DNA-binding and transcriptional activities. Plant Physiol. 130: 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Yang S.H., Han K.H. (2006). Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 47: 343–355 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Koornneef M., Bentsink L., Hilhorst H. (2002). Seed dormancy and germination. Curr. Opin. Plant Biol. 5: 33–36 [DOI] [PubMed] [Google Scholar]
- Koornneef M., Reuling G., Karssen C.M. (1984). The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61: 377–383 [Google Scholar]
- Kucera B., Cohn M.A., Leubner-Metzger G. (2005). Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 15: 281–307 [Google Scholar]
- Lee S., Cheng H., King K.E., Wang W., He Y., Hussain A., Lo J., Harberd N.P., Peng J. (2002). Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 16: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Kloosterziel K.M., Gil M.A., Ruijs G.J., Jacobsen S.E., Olszewski N.E., Schwartz S.H., Zeevaart J.A., Koornneef M. (1996). Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Leung J., Giraudat J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Liu C., Chen H., Er H.L., Soo H.M., Kumar P.P., Han J.H., Liou Y.C., Yu H. (2008). Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135: 1481–1491 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou J., Bracha-Drori K., Yalovsky S., Ito T., Yu H. (2007). Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134: 1901–1910 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., Chua N.H. (2001). A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L., Mongrand S., McLachlin D.T., Chait B.T., Chua N.H. (2002). ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Miura K., Lee J., Jin J.B., Yoo C.Y., Miura T., Hasegawa P.M. (2009). Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 106: 5418–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., Tintelnot S., Leubner-Metzger G. (2006). Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 47: 864–877 [DOI] [PubMed] [Google Scholar]
- Nambara E., Keith K., McCourt P., Naito S. (1995). A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121: 629–636 [Google Scholar]
- Nambara E., Marion-Poll A. (2005). Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Kuwahara A., Seo M., Kushiro T., Asami T., Hirai N., Kamiya Y., Koshiba T., Nambara E. (2006). CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 141: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N., Sun T.P., Gubler F. (2002). Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 14 (suppl.): S61–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Harberd N.P. (1997). Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol. 113: 1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U., Jikumaru Y., Kinoshita N., Nambara E., Kamiya Y., Lopez-Molina L. (2008). The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20: 2729–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P., Plachy C. (1985). Control of seed germination by abscisic acid: III. Effect on embryo growth potential (minimum turgor pressure) and growth coefficient (cell wall extensibility) in Brassica napus L. Plant Physiol. 77: 676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L., Thomas S.G., Hu J., Dill A., Alonso J.M., Ecker J.R., Sun T.P. (2004). DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 135: 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicient C.M., Hull G., Guilleminot J., Devic M., Delseny M. (2000). Differential expression of the Arabidopsis genes coding for Em-like proteins. J. Exp. Bot. 51: 1211–1220 [PubMed] [Google Scholar]
- Wen C.K., Chang C. (2002). Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P.A., Kim M.C., Jaeger K.E., Busch W., Schmid M., Lohmann J.U., Weigel D. (2005). Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wilson R.N., Heckman J.W., Somerville C.R. (1992). Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 100: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Schumaker K.S., Zhu J.K. (2002). Cell signaling during cold, drought, and salt stress. Plant Cell 14 (Suppl): S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Zhu J.K. (2003). Regulation of abscisic acid biosynthesis. Plant Physiol. 133: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Kobayashi Y., Goto K., Abe M., Araki T. (2005). TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 46: 1175–1189 [DOI] [PubMed] [Google Scholar]
- Yoo S.Y., Kardailsky I., Lee J.S., Weigel D., Ahn J.H. (2004). Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1). Mol. Cells 17: 95–101 [PubMed] [Google Scholar]
- Yu H., Ito T., Wellmer F., Meyerowitz E.M. (2004a). Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nat. Genet. 36: 157–161 [DOI] [PubMed] [Google Scholar]
- Yu H., Ito T., Zhao Y., Peng J., Kumar P., Meyerowitz E.M. (2004b). Floral homeotic genes are targets of gibberellin signaling in flower development. Proc. Natl. Acad. Sci. USA 101: 7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Xu Y., Tan E.L., Kumar P.P. (2002). AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc. Natl. Acad. Sci. USA 99: 16336–16341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentella R., Zhang Z.L., Park M., Thomas S.G., Endo A., Murase K., Fleet C.M., Jikumaru Y., Nambara E., Kamiya Y., Sun T.P. (2007). Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 19: 3037–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Garreton V., Chua N.H. (2005). The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 19: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]