Abstract
Objective:
To examine the effect of antipsychotic medication on neuromotor abnormalities in a sample of psychotic patients never exposed to antipsychotic drugs.
Method:
One hundred psychotic patients were assessed (from January 1998 to December 2002) using DSM-IV criteria for parkinsonism, dyskinesia, akathisia, catatonia, and dystonia at baseline and after 4 weeks of treatment with haloperidol (n = 23), risperidone (n = 52), or olanzapine (n = 25). We examined change scores in neuromotor ratings over the treatment period across treatment groups and rates of drug-responsive and drug-emergent neuromotor syndromes in patients with and without preexisting neuromotor abnormalities.
Results:
Overall time effects revealed a worsening of parkinsonism (P = .002) and akathisia (P = .002) ratings and an improvement of dyskinesia (P = .001) and catatonia (P < .001) ratings. Main treatment effects revealed that patients taking haloperidol had a significant mean increase in akathisia scores compared with those of patients taking risperidone (P = .002) or olanzapine (P < .001). A significantly greater percentage of olanzapine-treated patients experienced remission of preexisting parkinsonism than did the other treatment groups (P = .047). Patients without preexisting motor abnormalities were more likely to experience drug-emergent parkinsonism if they were treated with haloperidol or risperidone than with olanzapine (P = .001) and were more likely to experience drug-emergent dystonia (P = .014) and akathisia (P = .013) if they were treated with haloperidol than with risperidone or olanzapine.
Conclusions:
The relationship between antipsychotic medication and neurologic abnormalities is more complex than previously acknowledged since antipsychotic drugs may both improve preexisting abnormalities and cause “de novo” neurologic syndromes. Overall, olanzapine has a more favorable neuromotor profile than risperidone, which in turn has a more favorable profile than haloperidol.
Numerous historical accounts from the preneuroleptic era clearly showed that a broad range of neuromotor abnormalities may be an indigenous feature of the psychotic illness.1–4 Besides the core symptoms of catatonia (ie, stupor, negativism, or catalepsy), classical authors also described less dramatic motor abnormalities such as choreic- and athetoid-like movements, dystonia, tics, tremor, motor restlessness, muscular tone abnormalities, and hypokinesia, all of which are now thought to be extrapyramidal symptoms. After the introduction of antipsychotic drugs, most of the neuromotor abnormalities described in the preneuroleptic era came to be equated with the neurologic side effects of antipsychotic medication.5–7 More recently, a number of studies conducted in drug-naive psychotic patients came to the conclusion that a significant proportion of schizophrenia patients do have motor abnormalities in at least one domain.8–10 For example, Honer et al9 reported that 45% of drug-naive schizophrenia patients endorsed at least 1 extrapyramidal symptom and that 28% had at least 1 mild sign of an extrapyramidal disorder. Although the prevalence rates of primary motor abnormalities highly vary across studies, specific prevalence in most studies ranges 15%–20% for parkinsonism, 10%–15% for dyskinesia, and 5%–10% for akathisia. This converging evidence clearly suggests that abnormal movements may be related to the illness itself rather than just the result of antipsychotic medication.
There is no doubt that antipsychotic drugs of any type (ie, typical or atypical) may cause neurologic side effects.11 However, a number of studies have reported that antipsychotic medication may ameliorate a broad range of neuromotor symptoms such as catatonia,12,13 parkinsonism,14,15 dyskinesia,16,17 and akathisia.15 Furthermore, the introduction of antipsychotic drugs seems to be at least in part responsible for the marked decline in the incidence of the most severe forms of catatonia.18 All these data converge to indicate that drug-induced movement disorders may have been overemphasized in that antipsychotic medications are not the sole cause of neurologic abnormalities in psychotic patients. This issue is further complicated by the fact that primary and drug-induced motor abnormalities are difficult to differentiate on a purely phenomenological basis.19,20 Furthermore, the different domains of neuromotor abnormalities tend to cluster together, this irrespective of their primary or secondary nature.16,21–23
Clinical Points
♦ Preexisting motor abnormalities in psychotic disorders are the manifestation of a disease process reflecting dysfunction in basal ganglia-cortical circuitry.
♦ Antipsychotic drugs interact with or modify the disease-based motor disorders.
♦ Clinicians should carefully monitorize neuromotor abnormalities in patients with a first episode of psychosis before and after starting antipsychotic medication.
Earlier controlled studies suggested that atypical antipsychotics were superior to the typical ones in producing less neurologic side effects. However, this widely accepted view has been challenged by recent studies using intermediate-potency typical antipsychotics at modest doses.24 Most of these studies, notwithstanding, have been conducted in chronic schizophrenia patients with long-lasting antipsychotic treatment, and conflicting results may be due to the extent of prestudy washout periods, carryover effects of prior drug treatment, or utilizing haloperidol at high doses as the comparator drug.25 To overcome these confounds, a new generation of studies conducted on first-episode patients have begun to appear and while most of them found that typical antipsychotic drugs tend to develop more extrapyramidal symptoms than the atypical ones,26–28 others have produced ambiguous results29 or did not find differences at all,30 it despite these studies tended to use relatively low doses of typical drugs (ie, haloperidol).
A number of confounders persist in first episode studies that may account for conflicting results. First, most studies include a varied proportion of patients previously exposed to antipsychotic drugs, which may affect neurologic ratings at baseline. Second, preexisting motor abnormalities are not usually considered or controlled for when examining the incidence rate of neurologic side effects. Third, and most importantly, most studies do not take into account that antipsychotic drugs may both improve and worsen extrapyramidal symptoms. More specifically, previous studies usually treated baseline neuromotor scores as a whole without differentiating between patients with and without preexisting motor abnormalities. For these reasons, it remains difficult to draw definite conclusions about the expected prevalence rate of drug-emergent and drug-responsive neuromotor disorders in patients not previously exposed to antipsychotic drugs.
We reasoned that examining neurologic abnormalities in first-episode, medication-naive patients before and after starting antipsychotic treatment would allow a more precise differentiation of the effects (either beneficial or harmful) of antipsychotic drugs on neurologic ratings. Preexisting motor abnormalities would be disease-related, whereas emergent neurologic syndromes appearing after starting antipsychotic treatment in patients without preexisting abnormalities would be drug-related. We report here on the results from a naturalistic and non–commercially funded study conducted on neuroleptic-naive psychotic subjects, who were assessed for a broad range of neurologic abnormalities including parkinsonism, akathisia, catatonia, dyskinesia, and dystonia at the drug-naive state and after 4 weeks of treatment with haloperidol, risperidone, or olanzapine.
METHOD
Patient Population
The study sample comprised 100 nonaffective psychotic patients who had not been exposed to antipsychotic medication and were consecutively admitted for the first time to the psychiatric ward of the Virgen del Camino Hospital between January 1998 and December 2002. The hospital serves an epidemiologic catchment area, predominantly urban population of 250,000 people with no other psychiatric wards in this area.
The criteria for inclusion were (1) a first episode of nonaffective psychosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), (2) no previous exposure to antipsychotic medication as documented by the patient, significant others, medical records, and if necessary, by the primary physician, and (3) age 16–65 years. Exclusion criteria were (1) a history of drug dependence according to DSM-IV criteria (except caffeine and nicotine), (2) evidence of organic brain disorder including mental retardation, epilepsy, brain injury, or neurodegenerative disease, (3) meaningful or unstable somatic disease, (4) change of antipsychotic medication or addition of a second antipsychotic drug during the study period, and (5) premature discharge of the ward not allowing to complete the inpatient treatment period. If the patients met the inclusion criteria, they were asked to participate and provide informed consent after the study was explained. The study protocol was approved by the local ethical committee.
Study Design and Procedures
Once the study procedure was explained to the patients, they underwent a comprehensive neurologic examination before starting antipsychotic medication, usually within a few hours after admission, after which each patient was assigned to a treating psychiatrist. Patients were treated according to clinical choice on the basis of an antipsychotic monotherapy regimen with haloperidol, risperidone, or olanzapine. Overall, patients initially received a low dose of antipsychotic drug, which was gradually titrated up over the course of the episode. All antipsychotics were administered orally. Chlorpromazine equivalence of antipsychotic medications was estimated according to Woods,31 in that 100 mg/d of chlorpromazine equals 2 mg/d of haloperidol and risperidone, and 5 mg/d of olanzapine. Concomitant medications were allowed if necessary and the reason for their use recorded. Anticholinergic medication (biperiden) could be given if extrapyramidal symptoms occurred, but its use as prophylaxis was prohibited. Fourteen patients were excluded from the study because of change of antipsychotic (n = 11) or addition of second antipsychotic drug (n = 3) during the study period. These instances were due to perceived lack of efficacy of the antipsychotic medication, and in no instance was medication changed because of intolerable neurologic side effects. The final study sample was made of 100 patients who fulfilled the inclusion criteria and completed the 1-month treatment period with a single antipsychotic drug.
The subjects were administered the Comprehensive Assessment of Symptoms and History (CASH) schedule,32 which served to assess sociodemographic variables, diagnosis, and clinical symptoms, including the Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS), which were rated at admission and 1 month after starting treatment. On the basis of the SAPS and SAPS subscale ratings, we defined the syndromes as reality-distortion (mean rating of delusions and hallucinations), disorganization (mean rating of positive formal thought disorder, bizarre behavior, and inappropriate affect), and negative (mean rating of affective flattening, alogia, and avolition).33
Outcome Measures
Neurologic abnormalities were rated on the basis of a structured neurologic examination at 2 time points, at the antipsychotic-naive status before starting antipsychotic treatment and 4 weeks later. All the assessments were conducted by the first author (V.P.), who was blind to the medication status of the patients. We defined 5 outcome neuromotor measures, namely dyskinesia, parkinsonism, akathisia, catatonia, and acute dystonia. Parkinsonism was rated according the Simpson-Angus scale.34 The total score was used to determine severity and a score > 3 to determine the presence of parkinsonism.34 Dyskinetic movements were assessed by means of the Abnormal Involuntary Movement Scale.35 We used the global severity rating as a measure of severity, and the Schooler & Kane criteria36 to determine the presence of dyskinesia. Akathisia was rated by means of the Barnes Akathisia Scale37; the global rating was used to determine severity and a score ≥ 2 to determine the presence of akathisia. Catatonia was rated according to the Modified Rogers Scale.19 The total score was used to determine severity and a score ≥ 6 to define the presence of catatonia.38 Acute dystonia was clinically assessed and rated as present if the patient manifested 1 or more acute dystonic reactions over the observation period. Given that dystonia is a typical effect of antipsychotic medication, no one patient had dystonia at baseline.
We also examined neurologic syndromes on the basis of their presence versus absence at baseline and over the treatment period and differentiated between drug-responsive, drug-emergent, and drug-unchanged neurologic syndromes. A drug-responsive syndrome (as categorically defined above) was defined according to its presence at baseline and absence at week 4. A drug-emergent syndrome was defined according to its absence at baseline and presence at week 4. Patients were also considered to meet criteria for a drug-induced syndrome if they had met criteria for any neuromotor event during the observation period. For example, if a patient met criteria for parkinsonism at any time during the study period that was successfully treated with biperiden and thus no longer met criteria for parkinsonism at week 4, the case was counted as drug-induced parkinsonism. A drug-unchanged syndrome was defined as a particular syndrome being present at both assessment points.
Interrater reliability between the 2 authors for the 6 domains of psychopathology included in the CASH (reality-distortion, disorganization, negative, catatonia, mania, and depression) and the 5 neurologic ratings was examined in an independent sample of 30 psychotic patients by means of the intraclass correlation coefficient (ICC) statistic. The mean ICC for the CASH domains was 0.87 (range, 0.78–0.92) and the mean ICC for the neurologic domains was 0.89 (range, 0.79–0.94)
Statistics
Descriptive statistics were obtained on demographic, clinical, and treatment variables. Continuous variables were described using summary statistics such as means and standard deviations. Categorical variables were described using frequencies and percentages. Comparisons between the treatment groups on sociodemographic, clinical, and treatment variables were made using analysis of variance (ANOVA; continuous variables) or χ2 tests (categorical variables). When the overall test for differences across the treatment groups was significant, further pairwise comparisons between treatment groups were performed.
Analyses of treatment effects on each neurologic domain were conducted in 2 ways. First, a dimensional analysis of neuromotor ratings was performed by means of repeated-measures ANOVAs with baseline and 4-week scores as dependent variables, time as a within-subject repeated measure (overall treatment effect), and treatment group as a between-subjects fixed factor (specific treatment effect). Each baseline neurologic rating was included in the model as a covariate. Second, categorical analyses were performed for comparing rates of neuromotor syndromes at baseline and over the treatment period (McNemar tests) and across treatment groups in patients with and without preexisting motor abnormalities (χ2 tests). To examine the extent to which baseline syndromes predicted specific syndromes over the treatment period, we used logistic regression analysis in which the dependent variable was each specific syndrome at week 4 and over the treatment period and the predictor variables were the baseline syndromes and medication type. All tests of hypothesis were done at a 2-sided 5% level of significance, and no correction for multiple testing was done. The Statistical Package for the Social Sciences (SPSS) program, version 14.0 (SPSS, Inc, Chicago, Illinois), was used to perform all analyses.
RESULTS
Background Characteristics
Sixty-six of the patients were male and 79 were single. Twenty-one patients had taken some type of psychotropic drug during the previous year, and 11 of them were taking the following drugs at admission: mood stabilizers (n = 1), benzodiazepines (n = 5), selective serotonin reuptake inhibitors (SSRIs) (n = 3), and SSRIs plus benzodiazepines (n = 2). The mean age was 29.6 years (SD = 10.9), and the mean age at onset of the first psychotic symptom was 26.4 years (SD = 9.8). The DSM-IV diagnoses were schizophrenia (n = 49), schizophreniform disorder (n = 23), schizoaffective disorder (n = 7), brief psychotic disorder (n = 12), delusional disorder (n = 7), and psychosis not otherwise specified (n = 2).
Twenty-three patients were treated with haloperidol (mean = 8.1 mg/d, SD = 3.8; range, 2–18), 52 patients were treated with risperidone (mean = 6.6 mg/d, SD = 2.6; range, 2–13), and 25 were treated with olanzapine (mean = 16.7 mg/d, SD = 6.8; range, 5–30). Type of antipsychotic and dose reflected well general treatment practices (ie, availability of second-generation antipsychotics) for first-episode psychotic patients during the study period.
Demographic and clinical characteristics of the patients by treatment group are displayed in Table 1. Treatment groups did not differ in any of the sociodemographic or clinical variables, excepting for the reality-distortion dimension (F = 3.31, df = 2, P = .041). Post hoc analysis showed that patients in the haloperidol group had marginally significant higher baseline SAPS scores than those in the olanzapine group (P = .043).
Table 1.
Background Characteristics of 100 Psychotic Patients by Treatment Groupa
| Characteristic | Haloperidol (n = 23) | Risperidone (n = 52) | Olanzapine (n = 25) | χ22 or F2 | P |
| Age, y | 30.0 ± 12.7 | 29.9 ± 9.5 | 30.8 ± 12.2 | 0.26 | .769 |
| Gender, male, n (%) | 13 (56) | 34 (65) | 19 (76) | 2.04 | .360 |
| Civil status, never married, n (%) | 19 (83) | 40 (77) | 20 (80) | 0.33 | .848 |
| Education, y | 11.3 ± 3.8 | 10.4 ± 3.5 | 9.6 ± 1.9 | 1.64 | .198 |
| Age at first psychotic symptom, y | 26.4 ± 10.2 | 26.1 ± 8.5 | 26.8 ± 11.8 | 0.39 | .962 |
| CASH domain | |||||
| Reality-distortion | 4.21 ± 0.85 | 3.98 ± 0.93 | 3.52 ± 1.12 | 3.31 | .041b |
| Disorganization | 2.08 ± 1.53 | 1.92 ± 1.78 | 2.08 ± 1.32 | 0.12 | .885 |
| Negative | 1.65 ± 1.33 | 1.50 ± 1.56 | 2.24 ± 1.66 | 1.97 | .145 |
| Diagnosis, schizophrenia, n (%) | 11 (48) | 26 (50) | 14 (56) | 0.36 | .834 |
| Any psychotropic drug at admission, n (%) | 3 (13) | 5 (10) | 3 (12) | 0.25 | .961 |
Values are mean ± SD unless otherwise specified.
Haloperidol > olanzapine.
Abbreviation: CASH = Comprehensive Assessment of Symptoms and History.
Because parkinsonism and dyskinesia increase with age, we explored the association of age with these variables. Pearson correlation coefficients of age with parkinsonism and dyskinesia at admission were 0.004 (P = .966) and –0.05 (P = .598), respectively.
Treatment Characteristics
There were no statistically significant differences across treatment groups with respect to the mean daily doses of chlorpromazine equivalents (Table 2). Treatment groups did not differ in rates of previous psychotropic medications nor concurrent medications over the treatment period excepting for treatment with biperiden. Twenty-four patients received biperiden over the 4-week treatment period, 13 because of acute dystonia and 11 because of parkinsonism. There were significant differences among treatment groups in the rates of patients requiring biperiden (P < .001), the mean daily doses (P < .001), and the mean number of days of exposure to biperiden (P = .007).
Table 2.
Treatment Characteristics of the Patients by Treatment Group
| Characteristic | Haloperidol (n = 23) | Risperidone (n = 52) | Olanzapine (n = 25) | χ22 or F2 | P |
| Neuroleptic dosage (mean CPZ equivalents/d ± SD) | 396.8 ± 196.1 | 329.8 ± 128.8 | 365.0 ± 154.2 | 1.87 | .159 |
| Concomitant medication | |||||
| Benzodiazepines, any, n (%) | 7 (30) | 19 (36) | 3 (12) | 4.96 | .083 |
| Antidepressants, any, n (%) | 1 (4) | 4 (8) | 5 (20) | 3.90 | .142 |
| Mood stabilizers, any, n (%) | 1 (4) | 6 (11) | 2 (8) | 1.04 | .592 |
| Biperiden, any, n (%) | 11 (48) | 13 (25) | 0 (0) | 15.08 | < .001a |
| Biperiden, mean mg/d ± SD | 2.00 ± 2.33 | 1.12 ± 2.03 | 0 (0) | 7.10 | < .001a |
| Biperiden, mean no. of days ± SD | 4.95 ± 6.71 | 2.92 ± 5.93 | 0 (0) | 5.24 | .007a |
Haloperidol > risperidone > olanzapine.
Abbreviation: CPZ = chlorpromazine.
There were no statistically significant differences between treatment groups on either continuous or dichotomous measures of baseline neuromotor abnormalities, with one exception: patients taking haloperidol had both a higher mean baseline akathisia score (F = 5.66, df = 2, P = .005) and a higher proportion of a categorically defined baseline akathisia syndrome (χ2 = 13.9, df = 2, P < .001) than those taking risperidone or olanzapine. These differences among treatment groups are explained by the fact that out of the 4 patients with baseline akathisia, 3 were in the haloperidol group and 1 was in the risperidone group.
Overall Treatment Effect
Repeated-measures ANOVA indicated a statistically significant overall worsening (P = .002) for parkinsonism and akathisia ratings from baseline to week 4, together with a statistically significant overall improvement for dyskinesia (P = .001) and catatonia (P ≤ .001) ratings (Table 3).
Table 3.
Mean ± SD Ratings for Each Neuromotor Domain at Baseline and Endpoint in the Whole Sample and by Treatment Group
| Treatment Group |
Overall Treatment Effect |
Specific Treatment Effect |
||||||||||
| Whole Sample |
Haloperidol (n = 23) |
Risperidone (n = 52) |
Olanzapine (n = 25) |
|||||||||
| Neuromotor Domain | Baseline | 4 Wk | Baseline | 4 Wk | Baseline | 4 Wk | Baseline | 4 Wk | F1 | P | F2 | P |
| Parkinsonism | 2.34 ± 3.61 | 3.62 ± 4.10 | 2.39 ± 4.09 | 4.04 ± 4.11 | 1.69 ± 2.93 | 3.81 ± 4.36 | 3.64 ± 4.19 | 2.84 ± 3.55 | 9.87 | .002 | 2.78 | .067 |
| Dyskinesia | 0.37 ± 0.88 | 0.20 ± 0.55 | 0.57 ± 1.16 | 0.30 ± 0.63 | 0.21 ± 0.69 | 0.12 ± 0.47 | 0.52 ± 0.91 | 0.28 ± 0.61 | 4.94 | .001 | 0.29 | .750 |
| Akathisia | 0.12 ± 0.53 | 0.43 ± 0.92 | 0.43 ± 1.03 | 1.13 ± 1.32 | 0.04 ± 0.19 | 0.31 ± 0.75 | 0.00 ± 0.00 | 0.04 ± 0.20 | 9.97 | .002 | 9.32 | < .001a |
| Catatonia | 2.98 ± 5.28 | 0.94 ± 2.32 | 2.61 ± 4.37 | 0.65 ± 1.33 | 3.08 ± 5.87 | 1.12 ± 2.74 | 3.12 ± 4.92 | 0.84 ± 2.11 | 21.36 | < .001 | 0.32 | .723 |
Haloperidol > risperidone, olanzapine.
Specific Treatment Effects
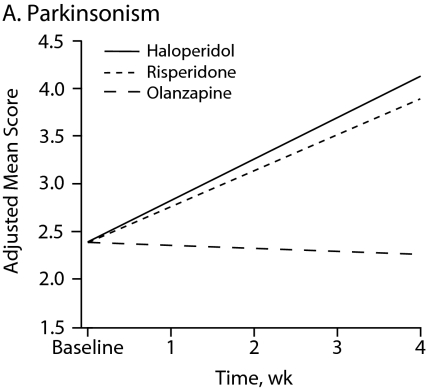
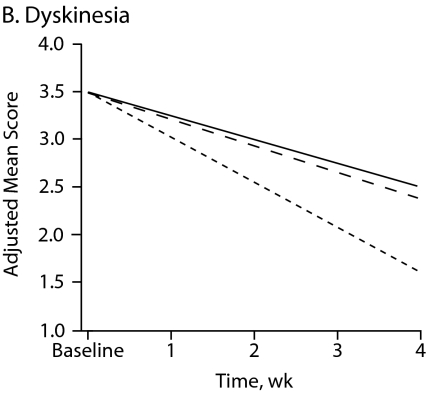
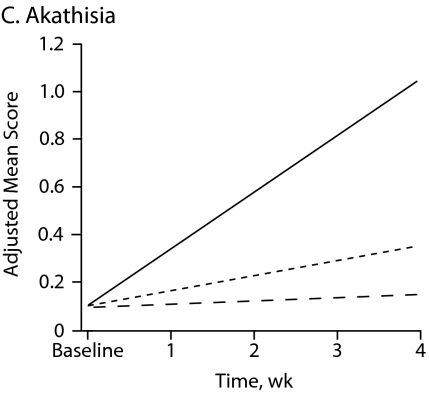
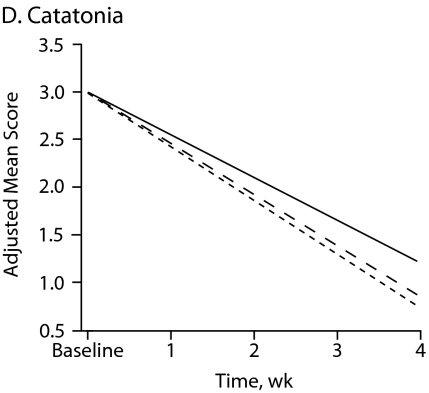
Figure 1 shows symptom change trajectories for each neuromotor domain across treatment groups after controlling for baseline ratings. Between-treatment effects were significant for akathisia (F = 9.32, df = 2, P < .001) and marginally significant for parkinsonism (F = 2.78, df = 2, P = .067). No specific treatment effect was observed for dyskinesia and catatonia change scores (Table 3). Main treatment effects revealed that patients taking haloperidol had a significant mean increase in akathisia scores compared with those of patients taking risperidone (P = .002) or olanzapine (P < .001).
Figure 1.
Change in Neuromotor Scores After 4 Weeks of Treatment With Haloperidol, Risperidone, or Olanzapinea
aFor each motor domain, scores were evaluated among treatment groups using an analysis of covariance model that included baseline score as a covariate.
Given that baseline reality-distortion symptoms appeared to differ among treatment groups, we conducted a series of repeated analyses of covariance to examine the effect of baseline positive symptoms on time and treatment group. Results remained basically unchanged after the baseline SAPS score was included as a covariate in the statistical model. More specifically, there were no statistically significant interactions of baseline SAPS score with time and treatment for each neurologic domain (data not shown).
Neuromotor Syndromes at Baseline and After 4 Weeks of Treatment
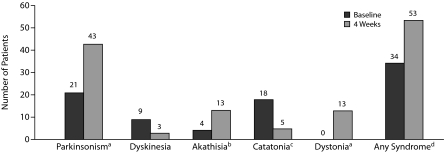
Figure 2 shows the number of patients with specific neuromotor syndromes at baseline and over the treatment period. Out the 34 patients with at least 1 neurologic syndrome at baseline, 21 patients had 1 syndrome, 8 patients had 2 syndromes, and 5 patients had 3 syndromes. Out of the 53 patients with at least 1 neurologic syndrome at week 4 and over the treatment period, 33 patients had 1 neurologic syndrome, 16 patients had 2 syndromes, and 4 patients had 3 syndromes. McNemar tests for comparing proportions of patients with each syndrome at the 2 assessment points revealed a statistically significant rate change for the syndromes of parkinsonism (P < .001), akathisia (P = .02), catatonia (P = .002), dystonia (P < .001), and any syndrome regardless of type (P = .004) but not for dyskinesia (P = .10).
Figure 2.
Number of Patients With Neuromotor Abnormalities at Baseline and Over 4 Weeks of Antipsychotic Treatment in 100 Psychotic Patients
aRate change significant at P < .001.
bRate change significant at P = .02.
cRate change significant at P = .002.
dRate change significant at P = .004.
Parkinsonism was the only baseline syndrome that significantly predicted a motor syndrome at week 4 after adjustment for the other baseline syndromes and type of medication. More specifically, baseline parkinsonism predicted both parkinsonism at week 4 (OR = 7.2, 95% CI, 2.0–26.3, P = .003) and any syndrome at week 4 (OR = 4.0, 95% CI, 1.1–14.3, P = .033).
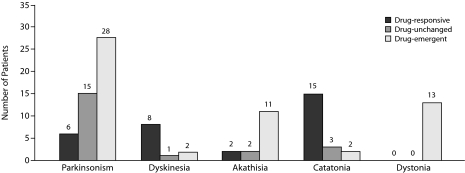
Treatment Response Pattern of Neuromotor Syndromes
Figure 3 shows the number of patients by type of response pattern to antipsychotic medication over the observation period. The number of patients with at least 1 drug-emerging, drug-responsive, or drug-unchanged motor syndrome was 42, 21, and 18, respectively. In line with time effect analysis results, parkinsonism and akathisia were mostly drug-emergent syndromes, and, as expected, dystonia was entirely drug-induced because no one patient had dystonia at admission. On the contrary, dyskinesia and catatonia were mostly drug-responsive syndromes.
Figure 3.
Treatment Response Pattern of Neuromotor Abnormalities in 100 Psychotic Patients
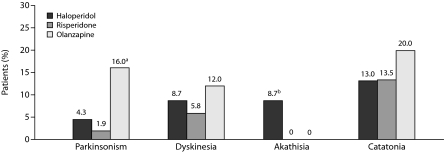
Treatment-Responsive Neuromotor Syndromes
Of the 34 patients with at least 1 neuromotor syndrome at baseline, 21 patients (61.7%) had at least 1 drug-responsive syndrome. Figure 4 shows the distribution of drug-responsive neurologic syndromes across treatment groups. A greater proportion of patients in the olanzapine treatment group (16%) had remitted parkinsonism compared with patients in the haloperidol (4.3%) or risperidone (1.9%) groups (χ2 = 6.07, df = 2, P = .047). Only 2 patients showed an improvement in akathisia, and they were in the haloperidol group (χ2 = 6.83, df = 2, P = .032); this result, however, needs to be understood in the context that 3 of the 4 patients with baseline akathisia were in the haloperidol group. There were no statistically significant differences in the rates of treatment-responsive catatonia (χ2 = 1.88, df = 2, P = .38) and dyskinesia (χ2 = 0.98, df = 2, P = .61) across treatment groups.
Figure 4.
Proportion of Patients in Each Treatment Group With Drug-Responsive Neuromotor Syndromes
aP = .047; olanzapine vs haloperidol and risperidone.
bP = .032; haloperidol vs olanzapine and risperidone.
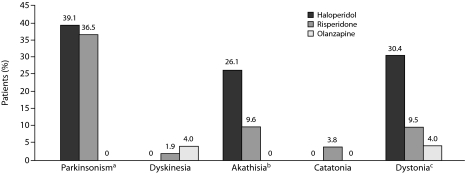
Treatment-Emergent Neurologic Syndromes
Of the 66 patients without neuromotor syndromes at baseline, 41 patients (62.1%) developed at least 1 motor syndrome over the observation period. Figure 5 shows the distribution of drug-emergent neurologic syndromes across treatment groups. Treatment-emergent parkinsonism was observed in a substantial proportion in the haloperidol (39.1%) and risperidone (36.5%) groups, while no emergent parkinsonism was observed in the olanzapine group (χ2 = 13.01, df = 2, P = .001). Treatment-emergent akathisia was significantly more frequent in the haloperidol group (26.1%) than in the risperidone (9.6%) or olanzapine (0%) groups (χ2 = 8.53, df = 2, P = .013). Treatment-emergent acute dystonia was observed in a greater proportion of patients taking haloperidol (30.4%) than in patients taking risperidone (9.5%) or olanzapine (4.0%) (χ2 = 8.49, df = 2, P = .014). There were no statistically significant differences in the rates of treatment-emergent catatonia (χ2 = 1.88, df = 2, P = .38) and dyskinesia (χ2 = 0.98, df = 2, P = .612) across treatment groups.
Figure 5.
Proportion of Patients in Each Treatment Group With Drug-Emergent Neuromotor Syndromes
aP = .001; haloperidol and risperidone vs olanzapine.
bP = .013; haloperidol vs risperidone and olanzapine.
cP = .014; haloperidol vs risperidone and olanzapine.
DISCUSSION
Main Findings
This was an observational prospective study on the short-term effects of antipsychotic medication on neurologic ratings in a sample of patients with a diagnosis of schizophrenia spectrum disorders and no previous exposure to antipsychotic medication. We found that 34 patients at baseline and 53 patients over the treatment period had at least 1 neuromotor syndrome. The only baseline syndrome that significantly predicted its corresponding syndrome over the observation period was parkinsonism. Dimensional analysis of neuromotor ratings showed that, after 4 weeks of antipsychotic treatment, parkinsonism and akathisia ratings worsened while dyskinesia and catatonia ratings improved. Differences among treatment groups were observed for akathisia ratings, with haloperidol producing higher worsening than risperidone or olanzapine, and for parkinsonism ratings, with haloperidol and risperidone producing higher worsening than olanzapine. The overall change pattern of each neuromotor domain revealed that risperidone appears to be intermediate between olanzapine and haloperidol with respect to its potential for producing neurologic symptoms, a finding that was also supported by the percentage of patients who were administered anticholinergic medication, the mean daily dose of anticholinergics, and the number of days of exposure.
Categorical analyses showed that 61% of patients with preexisting neuromotor abnormalities responded to antipsychotic medication in at least 1 neuromotor syndrome and that 62% of patients without preexisting neuromotor abnormalities developed at least 1 neuromotor syndrome. These figures illustrate well the dramatic changes that antipsychotic medication produces on neurologic abnormalities. Examination of the specific effects of antipsychotic drugs (remitting vs emerging) revealed interesting results. Regarding parkinsonism, olanzapine had a significant advantage over haloperidol and risperidone in that it produced both a lower rate of drug-emergent parkinsonism and a higher rate of drug-remitting parkinsonism. Regarding akathisia, haloperidol produced a higher incidence rate of drug-emergent akathisia than risperidone and olanzapine.
Comparison With Previous Studies
In line with previous studies,8,9 we found that over one third of patients never treated with antipsychotics had preexisting motor abnormalities. Our finding that antipsychotic medication may improve preexisting neuromotor abnormalities is consistent with previous studies of first-episode antipsychotic-naive patients. In an observational study of 39 drug-naive patients, Kopala et al14 reported that risperidone at low doses (2.9 mg/d) reduced preexisting extrapyramidal symptoms over a 9-week period. A cross-sectional observational study17 of 62 drug-naive psychotic patients who were assessed for dyskinesia after an average treatment period of 17 weeks showed that patients taking atypical antipsychotics experienced significantly less dyskinetic symptoms than those taking typical antipsychotics. In this study, however, baseline assessments were absent, thus findings are difficult to interpret. In a multicenter, double-blind, 6-week study including 83 patients,15 19 of whom had no previous neuroleptic exposure, olanzapine-treated patients (mean modal dose = 11.6, SD = 5.9 mg/d) showed statistically significant improvements in parkinsonism and akathisia ratings, while haloperidol-treated patients (mean modal dose = 10.8, SD = 4.8 mg/d) showed a worsening in both measures. This double dissociation between haloperidol and olanzapine regarding their effect on extrapyramidal symptoms is similar to that reported in our study and clearly suggests that olanzapine has a much more favorable neurologic profile than haloperidol.
In a naturalistic study30 of 350 antipsychotic-naive patients treated with haloperidol (mean dose = 3.7 mg/d) or risperidone (mean dose = 3.2 mg/d), there were no differences among treatments in the incidence rates of parkinsonism, akathisia, dystonia, and dyskinesia after having excluded those patients with preexisting neuromotor abnormalities. Differences in results between our study and this one of similar methodology may be accounted for the higher mean dose of haloperidol relative to that of risperidone employed in our study. However, our findings of drug-emergent neurologic syndromes are in keeping with those from controlled and observational studies of first-episode psychotic patients reporting that olanzapine produces fewer extrapyramidal symptoms than risperidone,27,28,39 which in turn produces fewer extrapyramidal symptoms than haloperidol.27,28,40 The similar relative frequency of concomitant administration of antiparkinsonian drugs across treatments in our and other first-episode studies27,28 supports this conclusion. In summary, our study extends findings from most previous studies to antipsychotic-naive patients without preexisting motor abnormalities. This pattern of drug-induced neurologic syndromes in patients treated with haloperidol, risperidone, and olanzapine fits well to the antidopaminergic (D2 receptor) and antiserotoninergic (5-HT2A receptor) potency that have been reported for these drugs.41 It remains to be explained the mechanism by which antipsychotic drugs may improve preexisting neuromotor symptoms, and future studies of receptor occupancy should focus on this subgroup of patients.
As mentioned above, atypical antipsychotic drugs appears to have a more favorable neurologic profile than typical drugs (ie, haloperidol), however this finding has been questioned by the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), in that no significant differences were observed between perphenazine and atypical drugs in extrapyramidal symptom rates or use of anticholinergic agents.42 How can we reconcile the absence of a difference between typical and atypical antipsychotic drugs in extrapyramidal symptoms liability in CATIE with the preponderance of data suggesting otherwise? It appears that atypical drugs may be less likely to cause neurologic syndromes than typical ones, but this difference is not evident in all populations. For example, it is possible that typical and atypical antipsychotic drugs differ in their liability to produce extrapyramidal symptoms in first-episode patients but not in chronic patients. Furthermore, in the CATIE study, perphenazine was used instead of haloperidol because it produces fewer extrapyramidal symptoms. In any case, there is a need of studies comparing perphenazine with the second generation of antipsychotics in drug-naive patients.
Implications for the Conceptualization of Neuromotor Abnormalities in Schizophrenia Spectrum Disorders
According to our data, the relationship between neuromotor abnormalities and antipsychotic drugs seems to be more complex than previously acknowledged. Antipsychotic medications may improve preexisting (ie, primary) neuromotor abnormalities, cause “de novo” neurologic abnormalities (ie, true side effects), or may lead to unmasking (ie, exacerbating) neuromotor abnormalities already present in schizophrenia, although the later was not specifically addressed in our study. Preexisting motor abnormalities in psychotic disorders indicate that they are an intrinsic feature of the disease process reflecting dysfunction in basal ganglia-cortical circuitry.10 Thus, a more rational view of the relationships between antipsychotics and motor disorders is one of neuroleptic medication interacting with or modifying the disease-based motor disorder.7,10,43
Having in mind that neuromotor abnormalities may be intrinsic to the disease process and that the relationships between neuromotor abnormalities and antipsychotic drugs are rather complex is important for some practical reasons. Firstly, for the clinical psychiatrist, it is essential to carefully examine neuromotor abnormalities in first-episode psychotic patients before starting antipsychotic medication. Without a baseline for comparison, a superficial examination of such a patient soon after starting medication could lead to a misattribute of preexisting motor abnormalities to medication, and a subsequent decision to reduce antipsychotic dose, change antipsychotic, or introduce anticholinergic medication, all of which could have important consequences for the patient. Secondly, it has been reported that first-episode patients are particular sensitive to the pharmacologic effects of antipsychotic drugs44 and that early extrapyramidal symptoms may predict later tardive diskynesia,45–47 tardive dystonia,47,48 and tardive akathisia.47 However, the extent to which a vulnerability to these, often severe, tardive disorders is also shaped by primary neuromotor disorders remains unknown. Future studies should address this relevant question by prospectively examining neuroleptic-naive patients with and without preexisting neuromotor abnormalities in the long run controlling for type, dose, and duration of antipsychotic medication.49
Strengths and Limitations
The main advantages of the study were that it was conducted in a rigorously-defined neuroleptic-naive population, that we examined neurologic abnormalities very comprehensively, and that we examined the differential effect of 3 of the most used antipsychotics on motor disorders. In fact, and to the best of our knowledge, this is one of the few studies examining the prevalence of a broad range of neuromotor abnormalities at the antipsychotic-naive state and the only study differentiating between drug-responsive and drug-emergent neuromotor syndromes in patients with and without preexisting neuromotor abnormalities.
A number of limitations of our study should be noted. First, this was not a randomized clinical trial and thus selection bias could not be accounted for and inherent differences present in treatment groups may not have been fully accommodated by the baseline corrections performed. Second, the relatively small number of patients in the haloperidol and olanzapine groups may create imbalances between the 3 cohorts and leads to a lack of power, all of which limits the generalizability of our findings. Third, we did not correct for multiple testing because the exploratory nature of the study and thus spurious findings cannot be ruled out. Type I error, however, seems rather unlikely because dimensional and categorical analyses of neuromotor ratings produced convergent results. Fourth, while the mean daily doses of the 3 antipsychotics used in this study are in the range of those recommended in the acute phase of schizophrenia,50 and the mean chlorpromazine equivalence doses across the treatment groups were very similar, the doses of haloperidol may appear somewhat higher than those used in current clinical practice or controlled trials. This fact may have favored olanzapine and risperidone over haloperidol, which may have affected the outcomes of our study. Fifth, 13 patients received biperiden for dystonia, which may have secondarily affected parkinsonism and akathisia ratings at 1 month. Sixth, while the neuroleptic-naive status of the patients was exhaustively examined, misreporting from patients or relatives cannot be excluded. Lastly, 4 weeks of follow-up was obviously too short, and our results do not apply beyond this period. Although neuromotor side effects most commonly develop within the first month of treatment, the neurologic effects of antipsychotic drugs may change over time.29
REFERENCES
- 1.Kraepelin E. In: Dementia Praecox and Paraphrenia. Barclay RM, trans. Robertson GM, editor. NY: Huntington; Robert E. Krieger Publishing Co Inc; 1971. [Google Scholar]
- 2.Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin J, editor. New York, NY: International University Press; 1950. trans. [Google Scholar]
- 3.Leonhard K. Afteilung der endogen Psychosen. Berlin, Germany: Akademie Verlag; 1957. [The classification of endogenous psychoses] [Google Scholar]
- 4.Friedman JH. Historical perspective on movement disorders. J Clin Psychiatry. 2004;65(suppl 9):3–8. [PubMed] [Google Scholar]
- 5.Ayd FJ. A survey of drug-induced extrapyramidal reactions. JAMA. 1961;175:1054–1060. doi: 10.1001/jama.1961.03040120016004. [DOI] [PubMed] [Google Scholar]
- 6.Gelenberg AJ, Mandel MR. Catatonic reactions to high-potency neuroleptic drugs. Arch Gen Psychiatry. 1977;34:947–950. doi: 10.1001/archpsyc.1977.01770200085010. [DOI] [PubMed] [Google Scholar]
- 7.Owens DGC. A Guide to Extrapyramidal Side-Effects of Antipsychotic Drugs. Cambridge, United Kingdom: Cambridge University Press; 1999. [Google Scholar]
- 8.Wolff AL, O'Driscoll G. Motor deficits and schizophrenia: the evidence from neuroleptic-naive patients and populations at risk. J Psychiatry Neurosci. 1999;24(4):304–314. [PMC free article] [PubMed] [Google Scholar]
- 9.Honer WG, Kopala LC, Rabinowitz J. Extrapyramidal symptoms and signs in first-episode, antipsychotic exposed and non-exposed patients with schizophrenia or related psychotic illness. J Psychopharmacol. 2005;19(3):277–285. doi: 10.1177/0269881105051539. [DOI] [PubMed] [Google Scholar]
- Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35(2):415–424. doi: 10.1093/schbul/sbn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caroff SN, Mann SC, Campbell EC, et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry. 2002;63(suppl 4):12–19. [PubMed] [Google Scholar]
- 12.Peralta V, Cuesta MJ. Negative, parkinsonian, and catatonic symptoms in schizophrenia: a conflict of paradigms revisited. Schizophr Res. 1999;40(3):245–253. doi: 10.1016/s0920-9964(99)00047-x. [DOI] [PubMed] [Google Scholar]
- 13.Martényi F, Metcalfe S. Schausberger, Dossenbach MRK. An efficacy analysis of olanzapine treatment data in schizophrenia patients with catatonic signs and symptoms. J Clin Psychiatry. 2001;62(suppl 2):25–27. [PubMed] [Google Scholar]
- 14.Kopala LC, Good KP, Fredrikson D, et al. Risperidone in first-episode schizophrenia: improvement in symptoms and pre-existing extrapyramidal signs. Int J Psychiatry Clin Pract. 1998;2(suppl 1):19–25. [Google Scholar]
- 15.Sanger TM, Liebermann JA, Tohen M, et al. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry. 1999;156(1):79–87. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Northoff G, Koch A, Wenke J, et al. Catatonia as a psychomotor syndrome: a rating scale and extrapyramidal motor symptoms. Mov Disord. 1999;14(3):404–416. doi: 10.1002/1531-8257(199905)14:3<404::aid-mds1004>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Boks MPM, Liddle PF, Russo S, et al. Influence of antipsychotic agents on neurological soft signs and dyskinesia in first episode psychosis. Psychiatry Res. 2003;119(1–2):167–170. doi: 10.1016/s0165-1781(03)00126-4. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JR. Changes in subtype diagnosis of schizophrenia: 1920–1966. Am J Psychiatry. 1974;131(6):674–677. doi: 10.1176/ajp.131.6.674. [DOI] [PubMed] [Google Scholar]
- 19.Lund CE, Mortimer AM, Rogers D, et al. Motor, volitional and behavioural disorders in schizophrenia, pt 1: assessment using the Modified Rogers Scale. Br J Psychiatry. 1991;158:323–327. doi: 10.1192/bjp.158.3.323. [DOI] [PubMed] [Google Scholar]
- 20.Kopala LC. Spontaneous and drug-induced movement disorders in schizophrenia. Acta Psychiatr Scand Suppl. 1996;389:12–17. doi: 10.1111/j.1600-0447.1996.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 21.McKenna PJ, Lund C, Mortimer A, et al. Motor, volitional, and behavioral disorders in schizophrenia. Br J Psychiatry. 1991;158:328–336. doi: 10.1192/bjp.158.3.328. [DOI] [PubMed] [Google Scholar]
- 22.van Harten PN, Hoeck HW, Matroos GE, et al. The inter-relationship of tardive dyskinesia, parkinsonism, akathisia and tardive dystonia: the Curaçao Extrapyramidal Syndromes Study II. Schizophr Res. 1997;26(2–3):235–242. doi: 10.1016/s0920-9964(97)00058-3. [DOI] [PubMed] [Google Scholar]
- 23.Bush G, Petrides G, Francis A. Catatonia and other motor syndromes in a chronically hospitalized psychiatric population. Schizophr Res. 1997;27(1):83–92. doi: 10.1016/S0920-9964(97)00084-4. [DOI] [PubMed] [Google Scholar]
- 24.Millerdel D, Caroff SN, Davis SM, et al. Extrapyramidal side-effects of antipsychotics in a randomized trial. Br J Psychiatry. 2008;193(4):279–288. doi: 10.1192/bjp.bp.108.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hugenholtz GWK, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry. 2006;67(6):897–903. doi: 10.4088/jcp.v67n0606. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 27.Rummel C, Kissling H, Leucht S. New generation antipsychotics for first-episode schizophrenia. Cochrane Database Syst Rev. 2003;(4):CD004410. doi: 10.1002/14651858.CD004410. [DOI] [PubMed] [Google Scholar]
- 28.Crespo-Facorro B, Pérez-Iglesias R, Ramirez-Bonilla M, et al. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first-episode nonaffective psychosis. J Clin Psychiatry. 2006;67:1511–1521. doi: 10.4088/jcp.v67n1004. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52 week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 30.Rosebush PI, Mazurek MF. Neurologic side effects in neuroleptic-naive patients treated with haloperidol or risperidone. Neurology. 1999;52(4):782–785. doi: 10.1212/wnl.52.4.782. [DOI] [PubMed] [Google Scholar]
- 31.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History: an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49(8):615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 33.Peralta V, Cuesta MJ, de Leon J. Are there more than two syndromes in schizophrenia? a critique of the positive-negative dichotomy. Br J Psychiatry. 1992;161:335–343. doi: 10.1192/bjp.161.3.335. [DOI] [PubMed] [Google Scholar]
- 34.Simpson GM, Angus JWS. A rating scale for extrapyramidal side-effects. Acta Psychiatr Scand. 1970;45(S212):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 35.Guy WA. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Dept Health Education and Wellfare; 1976. Abnormal Involuntary Movement Scale (AIMS) pp. 534–537. [Google Scholar]
- 36.Schooler NR, Kane JM. Research diagnosis for tardive dyskinesia. (letter) Arch Gen Psychiatry. 1982;39(4):486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]
- 37.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 38.Peralta V, Cuesta MJ. Motor features in psychotic disorders, pt 1: development of diagnostic criteria for catatonia. Schizophr Res. 2001;47(2–3):117–126. doi: 10.1016/s0920-9964(00)00035-9. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DG, Woerner MG, Napolitano B, et al. Am J Psychiatry Randomized comparison of olanzapine versus risperidone for treatment of first-episode schizophrenia: 4-month outcomes. 2006;163(12):2096–2102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- 40.Emsley RA. Risperidone in the treatment of first-episode psychotic patients: a double blind multicenter study. Schizophr Bull. 1999;25(4):721–729. doi: 10.1093/oxfordjournals.schbul.a033413. [DOI] [PubMed] [Google Scholar]
- 41.Matsui-Sakata A, Ohtani H, Sawada Y. Pharmacokinetic-pharmacodynamic analysis of antipsychotic-induced extrapyramidal symptoms based on receptor occupancy theory incorporating endogenous dopamine release. Drug Metab Pharmacokinet. 2005;20(3):187–199. doi: 10.2133/dmpk.20.187. [DOI] [PubMed] [Google Scholar]
- 42.Rogers D. Motor Disorder in Psychiatry: Towards a Neurological Psychiatry. Chichester, United Kingdom: John Wiley & Sons; 1992. [Google Scholar]
- 43.Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 44.McEvoy JP, Hogarty GE, Steingard S, et al. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry. 1991;48(8):739–745. doi: 10.1001/archpsyc.1991.01810320063009. [DOI] [PubMed] [Google Scholar]
- 45.Kane JM, Woerner M, Borenstein M. Integrating incidence and prevalence of tardive dyskinesia. Psychopharmacol Bull. 1986;22(1):254–258. [PubMed] [Google Scholar]
- 46.Tenback DE, van Harten PN, Slooff CJ, et al. Evidence that early extrapyramidal symptoms predict later tardive dyskinesia: a prospective analysis of 10,000 patients in the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Am J Psychiatry. 2006;163(8):1438–1440. doi: 10.1176/ajp.2006.163.8.1438. [DOI] [PubMed] [Google Scholar]
- 47.Ortí-Pareja M, Jimenez-Jimenez FJ, Vázquez A, et al. Drug-induced tardive syndromes. Parkinsonism Relat Disord. 1999;5(1–2):59–65. doi: 10.1016/s1353-8020(99)00015-2. [DOI] [PubMed] [Google Scholar]
- 48.Sachdev P. Risk factors for tardive dystonia: a case control comparison with tardive dyskinesia. Acta Psychiatr Scand. 1993;88(2):98–103. doi: 10.1111/j.1600-0447.1993.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 49.de Leon J. The effect of atypical versus typical antipsychotics on tardive dyskinesia: a naturalistic study. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):169–172. doi: 10.1007/s00406-006-0705-z. [DOI] [PubMed] [Google Scholar]
- 50.American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia. 2nd ed. Washington, DC: American Psychiatric Association; 2004. [Google Scholar]