Abstract
Importance of the field
Gene therapy has the potential to treat a wide variety of diseases including genetic diseases and cancer.
Areas covered in this review
This review introduces biomaterials used for gene delivery and then focuses on the use of electrostatic surface modifications to improve gene delivery materials. These modifications have been used to stabilize therapeutics in vivo, add cell-specific targeting ligands, and promote controlled release. Coatings of nanoparticles and microparticles as well as non-particulate surface coatings are covered in this review. Electrostatic principles are crucial for the development of multilayer delivery structures fabricated by the layer-by-layer method.
What the reader will gain
The reader will gain knowledge about the composition of biomaterials used for surface modifications and how these coatings and multilayers can be utilized to improve spatial control and efficiency of delivery. Examples are shown for the delivery of nucleic acids, including DNA and siRNA, to in vitro and in vivo systems.
Take home message
The versatile and powerful approach of electrostatic coatings and multilayers will lead to the development of enhanced gene therapies.
Keywords: gene delivery, coating, electrostatic, multilayer, surface, nanoparticle, targeting, polymer, biomaterials
Highlight Box.
Biomaterials have been developed for various applications and, through electrostatic interactions, are particularly well-suited to enhance the delivery of genes.
While nucleic acid delivery holds promise, there are many barriers that need to be overcome in order to improve delivery efficiency.
Electrostatic surface modifications of particles have been used to overcome specific barriers to gene delivery including improved serum resistance, cell targeting, cellular uptake, endosomal escape, controlled release, and reduced toxicity.
A major strength of the electrostatic coating approach is its suitability for a wide range of biomaterials including peptides, sugars, polymers, and nucleic acids.
Sequential coatings of polyelectrolytes create complex multilayer assemblies that improve spatial and temporal delivery.
Electrostatic coating techniques can be used to facilitate triggered release either from environmental cues (pH) or an external source (electric field).
Electrostatic coating techniques can enable exciting applications in stem cell engineering and regenerative medicine.
1. Introduction
Biomaterials have been developed for use in many fields including tissue engineering and drug delivery. There are many types of biomaterials, including macromolecules, synthetic polymers, and lipid systems [1, 2]. Biomaterials have been designed to respond to and interact with biological systems in a variety of ways. For example, in tissue engineering, which includes the regeneration and replacement of damaged and diseased tissues, biomaterials support a specific function of the target tissue. In drug delivery, biomaterials need to have the required encapsulation and release profiles necessary to treat the particular disease with a particular drug. A hydrophobic biomaterial may be preferred for delivery of a small molecule hydrophobic drug for cancer therapy, whereas a hydrophilic cationic biomaterial may be preferred for gene delivery. Gene delivery is particularly challenging as the drug cargos (nucleic acids) are large, highly charged, and degradable. Fortunately, the charged, polyvalent nature of nucleic acids enables various strategies for forming electrostatically associated coatings, complexes, and films. This review highlights how biomaterials can be used for particle-based and surface-based gene delivery and how electrostatic coatings can further enhance efficacy.
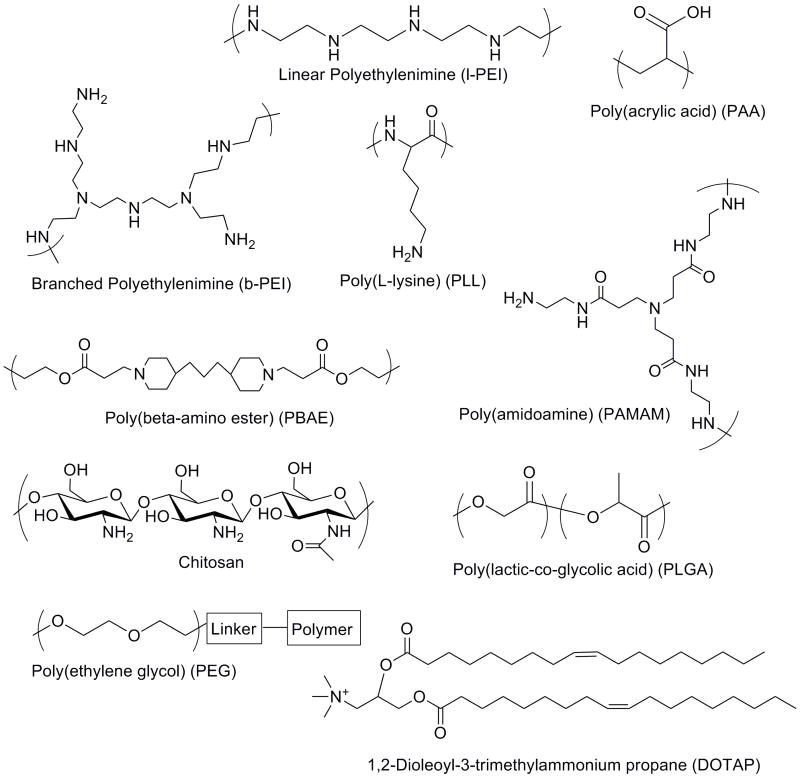
2. Biomaterials
Synthetic polymers are a popular biomaterial choice for drug and gene delivery. The ability to control the properties of synthetic polymers facilitates rational design. For example, polymers that contain positive charges are made so that they can electrostatically bind to negatively charged nucleic acids such as DNA molecules. These materials can be used directly to encapsulate cargos or as coatings for particles and devices. Examples of some of the commonly used biomaterials investigated for gene delivery are shown in Figure 1. Polyethylenimine (PEI) [3] is a polymer that is composed of multiple units of ethylenimine in both linear and branched arrangements. The branched PEI polymer contains primary, secondary and tertiary amines at a ratio of 1:2:1, while the linear polymer is composed of all secondary amines except for primary amines at the end groups. These amines are responsible for the positive charges that are necessary to bind to DNA. PEI is an off-the-shelf polymer for gene delivery that can condense DNA into nano-sized complexes and facilitate some in vitro and in vivo gene transfer [4]. Further modifications to PEI have been made to increase its performance at various points of the gene delivery process [5-7]. Polyamidoamine (PAMAM) [8] is a dendrimer system composed of amidoamine bonds. The dendrimer is built by serial addition of acrylates and amines to different core molecules that have amine functionalities.
Figure 1.
Biomaterials used to form particles and coatings for gene delivery.
Many types of degradable biomaterials have been created including hydrolytically degradable esters such as polylactic acid (PLA) [9, 10], polyglycolic acid (PGA) and copolymers [11], polycaprolactone (PCL) [12], and others [13]. The physical properties of the materials tune its degradation and rates of drug release. Poly(beta-amino ester)s (PBAE) [14-16] are a class of polymers that are both positively charged and hydrolytically degradable. They are synthesized from the conjugate addition of amine to diacrylates. The type of monomer diacrylates and amines can be varied to achieve different polymer properties. Poly(ester-anhydrides) [17] are often used to form microparticles that can encapsulate drugs or genetic material and degrade through surface erosion rather than the bulk erosion exhibited by PLA/PGA microspheres. Lipid based systems such as 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP) can also form liposomes or lipoplexes that encapsulate drugs or nucleic acids [18]. The cationic head, linker region and hydrophobic tails can be modified to alter delivery properties.
Natural polymer based systems have also been studied as they are often biocompatible and degradable [19]. For example, poly-L-Lysine (PLL) [20] is a linear polymer made from the amino acid lysine and it is a highly positively charged macromolecule due to the basic primary amine at the end of the lysine residue. Other natural-based biomaterials include sugars such as chitosan [21, 22], dextran [23], and cyclodextrins [24]. Combining natural polymers with synthetic polymers can improve drug delivery or tissue engineering functionality.
3. Nucleic Acid Delivery
Some particularly promising, but challenging drug delivery cargos are nucleic acids such as DNA and various types of RNA including antisense RNA, siRNA, isRNA, and miRNA [25, 26]. Genetic material is relatively large compared to most therapeutics. This makes it more difficult to transport within the body, into a cell, within a cell and into the nucleus. Viral vectors such as adenovirus [27] or lentivirus [28] have been developed for therapeutic gene delivery as they have evolved to do this very efficiently. However, there are continuing safety concerns with their use such as the potential for tumor induction [29] and the generation of immune responses [30]. Once the nucleic acid-containing particle is formed, it must remain stable and enable internalization within target cells. Common pathways for nano-formulations to enter the cell are via clathrin and caveolae-mediated endocytosis [31]. If the DNA/delivery particle is too large it will not be able to enter the cell. As the cell surface is negatively charged, particles need to be neutral or positively charged in order to promote cell interactions and efficient internalization.
For intracellular delivery, internalization is necessary, but not sufficient. A particle must also have efficient escape from the endosomal compartment to the cytoplasm to be active in the cell [32]. One proposed mechanism by which many of the polymer systems facilitate endosomal escape is through a proton-sponge mechanism. In this model, the basic nature of the polymer buffers the hydrogen ions pumped into the vesicle by the cell. To maintain electroneutrality, chloride ions flux into the endosome as well, creating osmotic pressure. The cell continues to attempt to acidify the vesicle until the vesicle finally ruptures due to the swelling of the endosome with water, releasing the particle [33]. Additional endosome escape mechanisms include the use of viral-based proteins that puncture the lipid bilayer, allowing particle escape.
Once in the cytoplasm, the encapsulated cargo must then be released from the particle in an efficient manner [34]. The delivery system needs to reach a balance between binding and dissociation with DNA. The system needs to bind tightly enough for particle formation, but must be able to unpack to release the DNA when in the cytoplasm or nucleoplasm. One method is for the release of DNA to be triggered by a stimuli that is present inside the cell, but not outside, such as a reducing environment.
An additional requirement for DNA delivery is transport of the DNA into the nucleus following cargo release [35]. It has been reported that the ability of DNA to cross the nuclear pore complex can be enhanced by binding nuclear localization sequence (NLS)-signal peptides to the DNA that allow the DNA to be picked up by the nucleoporin active transport system [36]. Gene expression can occur transiently from an episomal plasmid or targeted integration can be designed [37]. Although longer acting, integration of the exogenous DNA carries the risk of insertional mutagenesis and cancer cell generation.
The delivery of genes holds potential to treat both genetic diseases and acquired diseases such as cancer. While significant progress has been made in the field, non-viral biomaterial-mediated gene delivery is still much less effective than viral gene delivery [38]. New biomaterials continue to be developed to enhance efficacy and safety [39, 40]. Recent approaches to finding new synthetic polymers for gene delivery include polymer library approaches. High-throughput synthesis and screening techniques were used to search over two thousand different polymer structures for gene delivery [16]. Lead structures, poly(beta-amino ester)s, are biodegradable and rival adenovirus for gene transfer to human primary cells in vitro [41].
4. Coated Particles for Delivery
Many different particle systems have been investigated for drug and gene delivery. These systems have ranged from the nanometer to micrometer size, and been fabricated of both inorganic and organic materials. Coating these particles with various functional layers has helped tune the properties of these particles. TABLE 1 summarizes some of the electrostatically modified particle systems discussed below.
4.1 Nanoparticles
Nanoparticles are particles with length scales from 1-1000 nm. They can be “hard” nanoparticles primarily composed of gold [42-44], silver [45], or other inorganic materials [46-48] or they could be “soft” nanoparticles such as polyplexes [49, 50], liposomes [51], lipidoids [52], or other organic molecules. Beyond spheres, they can also be crafted into various shapes such as crystals or prisms [53]. Novel processing methods can enable printing of nanoparticles into more complex shapes such as cylinders, “hex nuts,” or toroids [54]. New kinds of therapies are being packaged into these nanoparticles [55]. The use of nanoparticle systems can allow for improved drug targeting, specific intracellular delivery, and potentially more control over delivery profiles.
Targeting is especially important in cancer drug delivery since the therapeutic is often intentionally cytotoxic. Targeting of cancer cells with nanoparticles is often done by targeting specific cancer cell surface receptors. Specific interactions can be achieved by modifying the delivery vehicle with specific peptides, proteins, or antibodies that bind to the surface receptors [56]. More recently, some groups have used aptamers, which are RNA and DNA molecules with the ability to bind to specific targets with high affinity [57]. Cancer gene therapy can also obtain additional specificity by delivering genes that only a cancer cell would be able to efficiently transcribe via transcriptional targeting [58].
4.2 Microparticles
While microparticles have been typically used as drug reservoirs for controlled release of drugs, peptides, and proteins [59], they can also be used for intracellular delivery. Although most cells cannot take-up microparticles, immune cells such as macrophages can internalize them. Thus, the size of a particle can also affect targeting and a microparticle system can specifically deliver drugs or genes to cells of the immune system. Delivering antigen-coding genes can be useful for vaccine purposes. Cationic PLGA particles have been shown to significantly increase in vivo antibody responses to create better vaccine systems [60]. Positively charged surfactant molecules were added to PLGA during the microparticle fabrication process, resulting in positively charged PLGA particles. The positively charged surface allowed for adsorption of DNA molecules onto the particle surface. In this case, DNA plasmids that code for HIV proteins were used in order to improve vaccination response. Poly(beta-amino ester)s can also be blended with PLGA microparticles to provide pH triggered release and enhanced efficacy [61]. Singh et al., review microparticle systems used for vaccine applications [62].
4.3 Types of Coatings
Various particulate formulations have been used to deliver drugs, whether that cargo is small molecules, protein, sugars, or genetic material [63-66]. The surfaces of these particles have been modified in various ways to improve delivery. Covalent modifications, such as PEGylation and polysaccharide coats, have been used to improve particle stability in serum in vitro and in vivo [67, 68]. These alterations reduce the interactions of the particles with the various serum proteins that can cause aggregation or degradation of the particles. Enhanced stability can also be achieved used electrostatic coatings. For example, Trubetskoy et al. showed that polyacrylic acid can be used to coat polyethylenimine/DNA or cationic lipid/DNA complexes and thereby prevent serum inhibition of the complexes [69]. The polyacrylic acid provides electrostatic shielding from the serum proteins, increasing in vivo gene delivery following systemic injection, and reducing toxicity.
Virus particles have also been coated with polymers to improve their in vivo stability. Adenovirus has been coated with polymers that protect the viral particles from interaction with specific blood components and reduces interaction with the immune system [70]. A hydrophilic polymer, N-(2-hydroxypropyl) methacrylamide (HPMA)-based copolymer, was functionalized in order to bind to the viral surface. The binding included both covalent and electrostatic interactions to further increase the coating stability. The polymer also contained reducible disulfide bonds that would allow the coating to be degraded so that transfection can still occur. Additional modifications have included specific receptor targeting elements [71]. In another electrostatic coating approach, PEI was used to modify the surface of baculoviral vectors [72]. These coating modifications can therefore allow viral gene delivery to overcome some of the limitations mentioned earlier in the review.
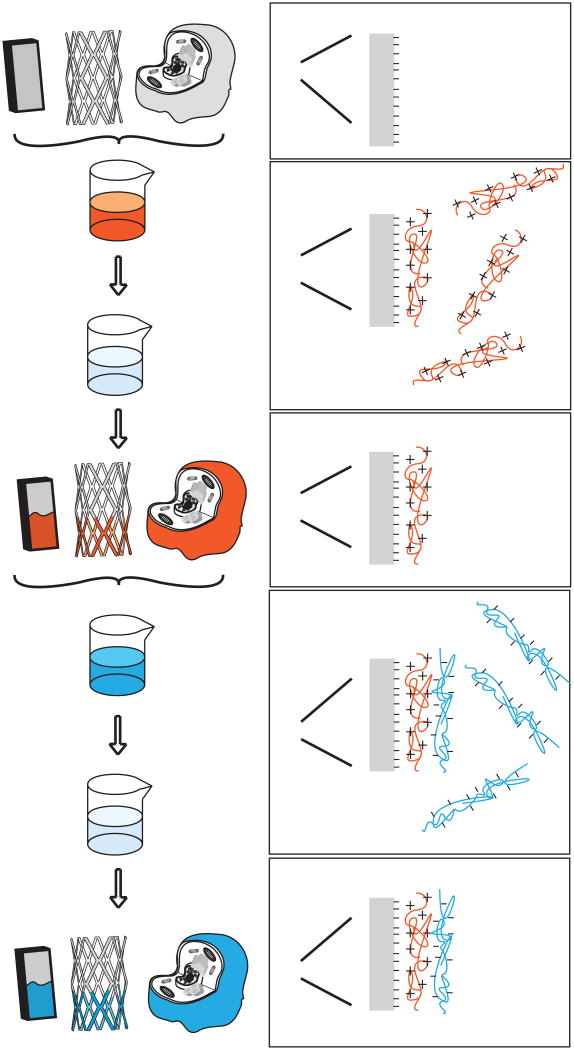
Targeted delivery to cells has been achieved by functionalizing the surfaces of particles so that they can interact more favorably with specific cells. These targeting molecules can be covalently bound to the particles [73] or can be coated to the particles via electrostatic interactions [74]. These targeting molecules are often proteins or protein fragments that bind to a specific cell receptor, such as transferrin, epidermal growth factor (EGF), and Arginine-Glycine-Aspartic Acid (RGD) domains [73, 75-77]. In the case of gene delivery, one problem with covalent modification with targeting ligands is that this changes the functionality of polymer moieties and efficacy can decrease [76, 77]. A coating approach avoids this potential issue. A schematic for multilayer electrostatically coated particles is depicted in Figure 2.
Figure 2.
Fabrication of a multilayer particle. Synthesis begins with a charged colloidal substrate. Oppositely charged polyelectrolytes are added in solution in a cyclic fashion. After the addition of each polyelectrolyte there is a wash and centrifugation step. As a final step, targeting ligands can be electrostatically added (yellow triangles). The colloidal substrate can be left encapsulated for delivery or can be chemically degraded and removed to form a hollow core.
In one example of an electrostatic coating approach, positively charged poly(beta-amino ester)/DNA nanoparticles were coated with negatively charged peptides [74]. Peptides were chosen such that they contained a stretch of anionic amino acids (Glutamic Acid residues), a linker (Glycine residues), and a ligand (Arginine-Glycine-Aspartic Acid). Addition of the peptide coating was found to neutralize the particle charge and promote gene delivery to human umbilical vein endothelial cells. Interestingly, it was only when the surface charge was neutralized, and the non-specific delivery of the nanoparticles reduced, that the presence of the ligand impacted gene delivery. However, once neutralized, nanoparticles coated with polyglutamic acid-polyglycine-RGD peptides had up to an order of magnitude higher gene delivery efficacy than the same particles coated with the scrambled sequence polyglutamic acid-polyglycine-RDG peptides [74]. Thus, the sequence of the ligand and the overall charge of the particle were important for intracellular deliver of the particles.
As a complementary strategy, negatively charged particles can be coated with cationic polymers to improve their delivery properties. For example, Lee et al. [78] showed that gold nanoparticles thiolated with siRNA could subsequently be coated with PBAEs to achieve significantly enhanced cytoplasmic delivery of the siRNA. In this work (depicted in Figure 3), gold-siRNA nanoparticles were unable to mediate gene silencing alone, but when coated with polymer, silencing increased to >95% using the same dose of gold-siRNA nanoparticles. Interestingly, small structural differences to the PBAEs used as coatings, dramatically changed the silencing behavior of the gold-siRNA particles. The most effective polymers were those that had terminal tertiary amine groups. In other work Fuller et al. [79] showed that PEI could be used as a cationic polymer to coat negatively charged fluorescent silica particles to enable enhanced intracellular delivery and endosomal escape. This system enabled the combination of both imaging and gene delivery within the same nanoparticles.
Figure 3.
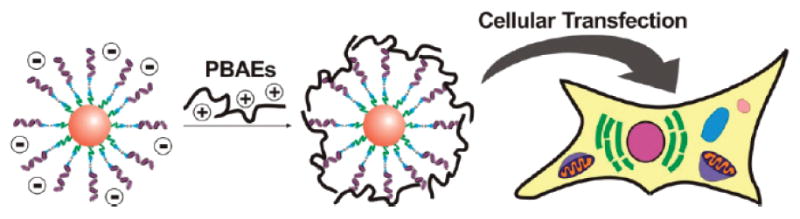
SiRNA modified gold-nanoparticles (orange sphere) are electrostatically coated with cationic polymers (PBAEs) to enhance cell transfection. Reproduced with permission from Nano Letters. Copyright ACS 2009 [78].
In vivo, it has also been shown that tissue targeting can be tuned via electrostatic coating [80]. In this study, cationic polymer/DNA nanoparticles were coated with anionic peptides and following tail-vein injection, gene delivery was directed away from the lungs and to the spleen and bone marrow or alternatively to liver cells depending on the coating amount and type. Following systemic administration, cationic particles can potentially aggregate with blood constituents that embolize in the vascular beds of the lung [69, 81, 82]. These coatings were shown to prevent aggregation with erythrocytes and prevent lethality following injection as compared to uncoated polymeric particles.
Thus the effectiveness of charged nanoparticles, either cationic or anionic, can be greatly improved by single coats of an oppositely charged biomaterial that incorporates new functionality to the nanoparticle. This functionality could consist of improved serum resistance, cell or tissue targeting, efficient internalization, endosomal escape, controlled release, and reduced toxicity.
4.4 Multilayer Particle Coating
Multiple electrostatic layers can be deposited on a particle using a layer-by-layer (LbL) approach (Figure 2). For this method, successive and alternating anionic and cationic layers are added to a particle core [83, 84]. After each layer is added, centrifugation allows for isolation of the particles so that further layers can be added. In some formulations, DNA is added as one of the anionic layers [85]. Using this approach, the layers were observed to disassemble inside the cell, where the DNA molecules then have access to the nucleus. In another system, DNA was added first as an internal layer adsorbed to a core. After multilayers were formed with spermidine and DNA coats, the core substrate was subsequently dissolved away so that the DNA becomes an internal encapsulated layer within a capsule [86]. In other work, DNA has also been encapsulated within chondroitin sulfate/poly(-L-arginine) LbL particles [87]. DNA was first added to the core particle and then alternating layers of chondroitin sulfate and poly(-L-arginine) polyelectrolytes were added subsequently to create a core/shell structure. The core was then dissolved so that only the DNA was left encapsulated and free inside the polyelectrolyte shell. Many of these systems are on the micrometer scale, which can reduce the ability of cells to take up the particles. Reducing the size of these LbL particles would therefore improve delivery of DNA. One such example uses liposomes as the substrate onto which polyelectrolyte layers, such as DNA, can be added [88]. Additionally, polyplexes have been used as a substrate onto which further layers are added electrostatically [89]. In this case, the extra PEI layer increased transfection activity further, presumably because the increased amount of PEI enabled greater endosomal escape. A negatively charged poly(acrylic acid) layer was added in between the PEI layers in order to construct the multilayer particle. Examples of multilayer coatings are summarized in Table 2.
Table 2. Multilayer coatings and films.
| Core Substrate |
Anionic Layers |
Cationic Layers |
No. Layers (Range used in papers) |
Zeta- potential (mV) |
Layer thickness (nm) |
Application | Notes | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Silica particles (∼3 μm) | DXS, pDNA-GFP | PAH, PRM | 7-12 | HEK 293T cells (5% FBS) | Increased cellular uptake with cationic outer layer | 85 | Reibentanz, 2006 | ||
| Liposome (∼100 nm) | DSX, DNA | Chitosan | 1-4 | ∼ -90 | a few nm | Increased liposome stability | 88 | Fukui, 2009 | |
| PEI/DNA polyplex (∼100 nm) | PAA | PEI | 4 (including interior PEI/DNA polyplex) | +15.1±6.9 (Quaternary complex) | a few nm | NIH-3T3 cells | Quaternary complexes showed improved transfection with no change in toxicity | 89 | Saul, 2007 |
| E. coli cells | Alginate, Hyaluronic Acid, DNA (ODN) | Chitosan | 4-7 | ∼ -30 (anionic layers); ∼ +30 (cationic layers) | ∼ 24 - 60 (per 2 bilayers) | Cells maintain viability, ability to divide | 93 | Hillberg, 2006 | |
| 316L Stainless steel intravascular stent | pDNA-GFP, SPS | PBAE, l-PEI | 8 bilayers PBAE/pDNA + 10 bilayers l-PEI/SPS core | ∼ 120 total | COS-7 cells | Uniform coating did not crack, peel or delaminate under mechanical stress; Successful transfection of cells | 94 | Jewell, 2006 | |
| Silicon, Zinc Selenide substrate | DXS, Heparin, SPS, PAA | PBAE, l-PEI, PAH | up to 40 bilayers PBAE/DXS or PBAE/Heparin + up to 10 bilayers PAH/PAA + 10 bilayers l-PEI/SPS core | ∼ 100 (20 bilayers PBAE/DXS + 10 l-PEI/SPS | Better controlled release by including covalently cross-linking layers that prevent interlayer diffusion | 101 | Wood, 2006 | ||
| PLA electrospun fibers (∼1-10 μm diameter) | pDNA-Luc | PEI | 1-3 bilayers | a few nm | COS-7 cells | Allows for gene delivery for tissue engineering applications | 106 | Sakai, 2009 | |
| Stainless steel mesh | pDNA | RHB | 15 bilayers | ∼ 70 | NIH-3T3 and SMC cells | Use of bioredicble polycations increases and prolongs transfection activity | 107 | Blacklock, 2009 | |
| ITO glass and silicon substrate | RNA | PABA | 4-8 bilayers | ∼ 10 per bilayer | Potential for electrochemical control of RNA release | 109 | Recksiedler, 2006 |
DXS = dextran sulfate; ODN = oligonucleotide; PAA = poly(acrylic acid); PABA = Poly(anilineboronic acid); PAH = poly(allylamine hydrochloride); PRM = protamine sulfate; RHB = reducible hyperbranched poly(amido amine); SPS = poly(sodium styrene sulfonate)
5. Multilayer Coated Non-Particulate Substrates for Delivery
In addition to particles, other surfaces can also be coated for controlled release and drug delivery. The sequential addition of positively and negatively charged polyelectrolytes to form a multilayered structure is a general approach that can be applied to diverse fields including optics, separations, and drug delivery [90]. Many different materials can be incorporated into these multilayers. These multilayers can then also be used as substrates themselves onto which cells can adhere. Surface mediated gene delivery can be useful as a mechanism for efficient gene transfer and as an enabling technology for tissue engineering [91]. Growing transfected cells on a surface can enable the release of soluble drugs, such as proteins, and has other potential advantages over particulate systems. One such advantage is spatial control of release of the therapeutic molecule. Surface-mediated delivery also allows for more efficient delivery since there is a much higher local concentration of drug available to the cells. The reverse system has also been developed where a live cell can serve as the substrate for multilayer coating [92, 93].
Multilayer electrostatic coatings have been used by researchers to tune the release of various biomolecules from substrates. One common method of fabricating such multilayer surfaces is an electrostatic-based layer-by-layer approach as shown in Figure 4. The first step is to coat a charged substrate with a layer of oppositely charged polyelectrolytes. The substrate can range from an inorganic material of any shape to biological tissue surfaces [94, 95]. The substrate is then washed to remove excess polyelectrolytes. Another layer of polyelectrolytes is added next which has an opposite charge to that of the first layer. Once again the surface is washed to remove the excess. This process cycle can then be repeated until the desired multilayer structure is achieved. Many different polyelectrolytes can be used, which gives flexibility and control over the type of surface and release characteristics that can be attained. Table 2 summarizes some of the multilayer structures discussed in this section.
Figure 4.
Multilayered coatings can be added to structures such as glass slides, stents, and organic tissues. In each case, an oppositely charged polyelectrolyte is added to a charged surface followed by a wash step to remove excess polyelectrolyte. The next layer of oppositely charged polyelectrolytes can then be added and this cycle can be repeated as needed.
5.1 Controlled Delivery via Multilayer Structures
Drug release from the multilayered surfaces has been achieved via different mechanisms. Polyelectrolyte layers that are hydrolytically degradable allow for controlled release in aqueous environments, such as in the body [96]. Therapeutic polysaccharides, such as heparin, have been used for negatively charged layers, along with poly(beta-amino esters) as positively charged layers. PBAE hydrolysis rate is dependent on the pH of the solution and this controls the degradation of PBAE based multilayers. With this system, drugs are released more slowly at lower pH solutions as expected. Other hydrolytically degradable polymers can be used in similar systems. Wood et al. showed that rather than relying on hydrolytic degradation, these multilayer films can have triggered release based on an applied voltage [97]. The key development was using Prussian Blue, a non-toxic FDA-approved material, to assemble the films. Electric current can similarly tune release of encapsulated insulin from hydrogen-bonded gels composed of poly(ethyloxazoline) and poly(acrylic acid) multilayers [98].
Multilayer structures that exhibit pH dependent swelling have also been designed [99]. PH-dependent swelling was achieved by using a combination of polyelectrolyte layers, one of which contains pH responsive functional groups, and the other which has hydrophobic domains. The swelling behavior allows for encapsulation of various drug delivery cargos and release that is highly tuned to environmental stimuli. Polypeptides can also be used to construct similar multilayer films, including those with pH responsive triggered release [100]. Loading of the layers with a drug is a function of solution pH and by varying the polypeptide polyelectrolyte layer composition, one can tune pH triggered response of the multilayer.
These methods can be extended for the controlled release of multiple drugs from one film [101, 102]. In one example, a hydrolytically degradable layer is used to control the overall release profile of a therapeutic polysaccharide and small hydrophobic molecule. In this case, one of the drugs (the polysaccharide) also serves a structural role as a polyelectrolyte that composes the multilayer. The other drug is encapsulated in polymeric micelles that are incorporated within the multilayer. The two delivered drugs can act synergistically to improve therapeutic activity [102]. This approach could be extended for multi-stage gene delivery. For multi-drug release, it is important to control interlayer diffusion. One such strategy is to add covalently cross-linked barriers between the two main polyelectrolyte layers [101]. The cross-linked barriers are also polyelectrolyte layers as well so that they can be seamlessly added during multilayer fabrication. Researchers found that one bilayer of cross-linked barrier is sufficient to block interlayer diffusion. However, the base layers underneath the barrier influence how well the barrier blocked diffusion.
A spray method can be used instead of the dip method to apply each polyelectrolyte layer in order to decrease processing time. Spraying can also be used to introduce asymmetry into multilayers which can be useful for drug delivery and other applications such as purification or biocatalytic membranes [103]. The spraying method is based on electrospinning technology, which involves the use of an applied voltage between the polyelectrolyte solution and surface to create a micrometer or nanometer sized fiber deposited onto the surface.
5.2 Delivery of DNA via Multilayer Structures
These multilayer structures can be useful for gene delivery in particular, where DNA acts as both one of the negatively charged polyelectrolyte structural layers and the drug of interest. For example Zhang et al. showed release of functional DNA from multilayered films [104]. In this study, the addition of an extra gene delivery agent (Lipofectamine) was required to permit intracellular delivery of the released DNA into mammalian cells, but this work demonstrated that the multilayer could serve as a DNA transfection reservoir. Building on this result Jewell et al. showed that PBAE/DNA multilayers could degrade into PBAE/DNA associated complexes that enable gene delivery at areas physically close to the multilayer coatings [105]. Figure 5 demonstrates how this system can enable spatial control of gene expression through quartz slides that were coated with PBAE polymer and enhanced green fluorescent protein (EGFP) DNA. Gene expression is high in areas adjacent to the slide surface, but low in areas further away. In principle, the breakdown products of the multilayer films themselves could serve as in situ gene delivery agents. Combinations of multiple biomaterials, each with specific intracellular gene delivery function (DNA condensation, endosomal escape, nuclear import, etc.), may prove to be the most effective.
Figure 5.
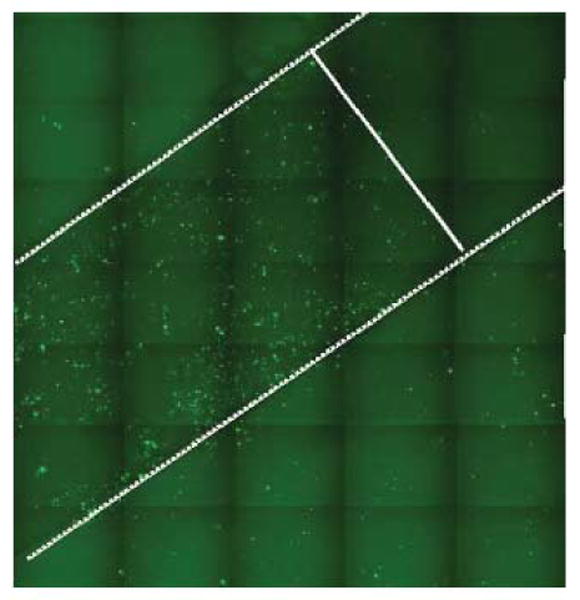
Fluorescence microscopy image showing localized transfection of COS-7 cells. The white lines show the approximate boundary between targeted and non-targeted delivery areas. The targeted areas are the quartz substrate functionalized with multilayered films of a PBAE polymer (seen in Figure 1) and pEGFP. Reproduced with permission from Journal of Controlled Release. Copyright Elsevier 2005 [105].
DNA and PEI have been used to create multilayer structures using an electrospun polymeric mesh as a substrate [106]. Cells were cultured on these multilayered meshes and transfection was observed. Such a system can be useful for drug delivery and tissue engineering applications. Using a bioreducible polyelectrolyte provides another method for controlled delivery [107]. Disulfide bonds within a poly(amido amine) polymer enable degradation in the presence of a reducing environment. Alternating layers of the cationic polymer and plasmid DNA were deposited on a stainless steel mesh, which is a similar system to a stent. Increased and longer lasting transfection was observed as compared to the non-reducible controls. The release and gene expression of this system over 12 days is shown in Figure 6. The authors hypothesized that the plasma membrane might provide a reducing environment, so that polymer degradation can take place upon interaction with the cell surface. Significant gene expression was achieved in both fibroblasts (NIH-3T3) and smooth muscle cells (SMC).
Figure 6.
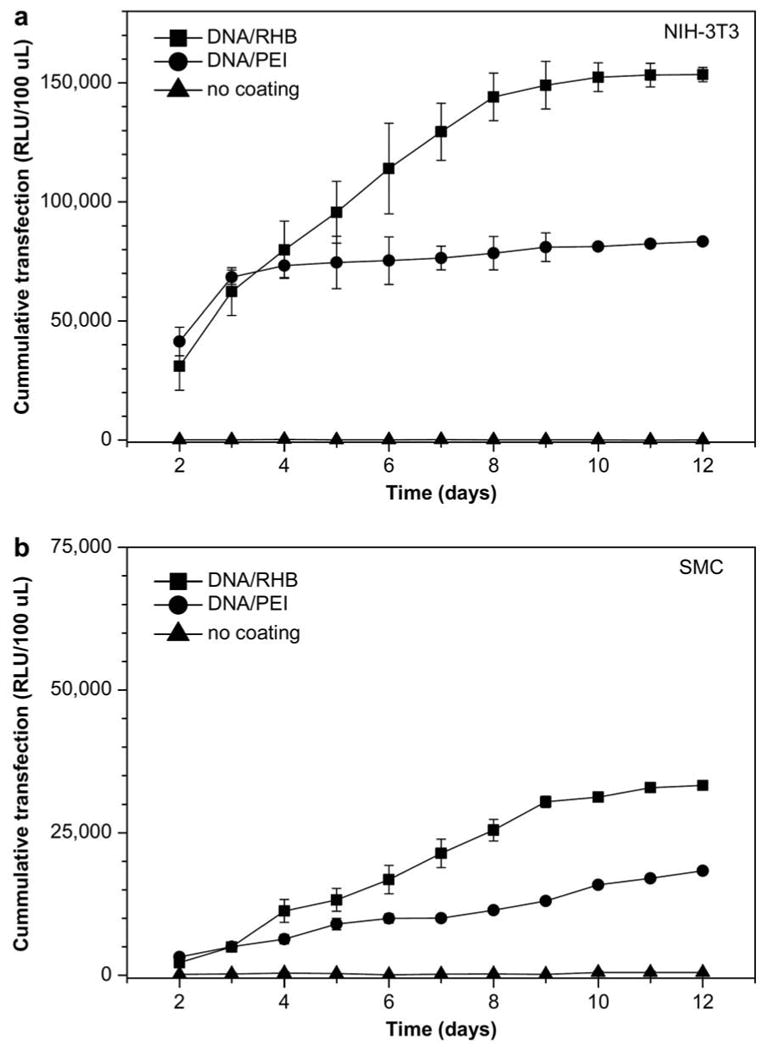
Cumulative transfection of NIH-3T3 (a) and SMC (b) cells with SEAP-DNA (secreted alkaline phosphatase based luminescence). Three surfaces were used: stainless steel mesh coated with DNA/reducible polymer multilayer (■), DNA/PEI multilayer (●), and control non-coated mesh (▲). Reproduced with permission from Biomaterials. Copyright Elsevier 2009 [107].
Another method of delivery is the incorporation of pre-formed DNA nanoparticles embedded into a layer-by-layer structure [108]. In one study, poly(L-lysine) and hyaluronic acid were used as the positively and negatively charged polyelectrolyte layers, respectively. Plasmid DNA was complexed with various formulations of PLL and cyclodextrin. This combination approach, a multilayer structure embedded with DNA complexes, allows for both tunable release of the complexes and improved protection/uptake of the pre-formed nanoparticles.
6. Conclusion
Electrostatic coatings are useful to modify the surfaces of particles and substrates for gene delivery. In the case of particles, these coatings can improve multiple steps of delivery including improved serum resistance, cell targeting, cellular uptake, endosomal escape, controlled release, and reduced toxicity. In the case of substrates, these electrostatic modifications can enable spatial and temporal control of release. These methods can also be used to facilitate triggered release either from environmental cues (pH) or an external source (electric field). Electrostatically adsorbed multilayers permit the creation of more complicated structures such as hollow capsules and surfaces that can release multiple therapeutic components over differing time scales. As these coatings and films can be constructed using a wide array of biomaterials and gentle processing conditions, they can have widespread applications to drug delivery and tissue engineering.
7. Expert Opinion
Physical and chemical properties of particles and substrates can be controlled through the modification of their surfaces with polyelectrolytes. These modifications are especially promising to the fields of drug and gene delivery. A major strength of this approach is its suitability for a wide range of biomaterials including peptides, sugars, polymers, and nucleic acids. Indeed, any macromolecule with a multivalent charge is amenable to this technology.
In some applications, coated nanoparticles were utilized for enhanced intracellular delivery. Specific intracellular delivery bottlenecks improved through electrostatic biomaterial coatings include cell targeting, cellular uptake, and endosomal escape. This work can be extended to include coatings with moieties for active transport through the cell, nuclear uptake, and other intracellular functions. Multilayered particles could be constructed that contain specialized biomaterials for each layer rather than the same two alternating layers throughout the coating. These customized layers could enable enhanced intracellular delivery or more precise multidrug release.
One natural extension of this work, and something that is already appearing in the literature [109, 110], is the use of nucleic acids other than DNA. Small interfering RNA (siRNA), immunostimulatory RNA (isRNA), micro RNA (miRNA), small activating RNA (saRNA), and antigene RNA (agRNA) are all examples of nucleic acids that would be very promising for both encapsulated cargos and coatings for particles and surfaces. There are many applications of this technology including cancer therapy [56] and targeting [111]. In addition, these materials can be used for immunotherapy and as vaccines, enabling controlled delivery of the immune stimulating molecules [112, 113]. In another application, multiple combinations of nucleic acids within the same particle may be especially interesting in creating new tools for efficient non-viral reprogramming of differentiated cells to induced pluripotent stem cells [114].
Encapsulation of cells within polyelectrolytes may enable protection and control of the cells. For example, a cell could be engineered to have a particular affinity for another cell or biomacromolecule through its electrostatic coatings. Such cells could potentially enable increased cell-cell interactions in the formation of engineered tissues, novel mechanisms for the recognition of pathogens, or aid in wound healing. In the case of stem cell engineering, such an approach may enable the controlled differentiation and/or reprogramming of the encapsulated cells within the multilayer coating. This approach could be useful for tissue engineering and regenerative medicine.
Multilayer films could also be designed not just for how they behave as films, but also how their breakdown products behave. For example, polyelectrolyte layers of the film could be therapeutics themselves or be prodrugs that degrade into therapeutic molecules. They could also form in situ particles or structures that then have functionality even after the multilayer degrades (such as self-assembly into targeted nanoparticles). These coatings and films could also be used to coat virtually any device including stents and stent-like devices.
These biomaterials and approaches can also be utilized to combine drug delivery with imaging to create new theranostics and multifunctional nanomedicines [115]. With such a system, one could visualize the spatial and temporal delivery of the therapeutic agent. It could also be used to better tune ligand coatings or stimuli responsive coatings for improved in vivo function. Putting these two elements together, a triggered diagnostic signal and drug release profile could be engineered to occur in the presence of a target compound or environment.
Although this technology is promising and there are many interesting applications, challenges remain before there can be broad clinical application. Ensuring safety is critical and may be a challenge as the fields of gene therapy and nanotechnology have already had their share of safety-related setbacks. One way that electrostatically coated nanoparticles can improve safety is through specific targeting. By targeting certain tissues or aberrant cells (cancer) with increased specificity compared to uncoated non-viral particles or to viral particles, off-target serious adverse events can be reduced. Proper coatings can also facilitate long circulation times and increased efficacy with a lower dose of active drug, further increasing safety. Biodegradable and biocompatible materials, like many of the polymeric materials discussed, are key to minimizing toxicity. In the case of gene therapy clinical trials, it is important that the DNA itself is also designed with safety in mind so that it is maximally effective and minimally immunogenic. One way to achieve this is by the elimination of toll-like receptor signaling CpG motifs in the DNA vector backbone or through utilization of a minicircle DNA vector free of bacterial backbone elements. Choosing the appropriate biomaterials, animal models, and manufacturing procedures will all be crucial as well. Nonetheless, given the versatile and powerful approach of electrostatic coatings and multilayers, including increasing spatial and temporal precision, many new therapeutics and diagnostics based on this technology will likely appear in the future.
Table 1. Electrostatic surface modifications of particles for gene delivery.
| Base Material |
Modification | Drug | Zeta- potential (mV) |
Size (nm) | Application | Cell Viability (relative to control) |
Activity (relative to control) |
Notes | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| PLGA | CTAB | pDNA-HIV p55 | +36±6 | 1540±200 | Balb/c mice | ∼ ≥ ×100 (PLGA, DNA) | Improved serum antibody and CTL response | 60 | Singh, 2000 | |
| b-PEI | Chondroitin Sulfate | pDNA-Luc | -29.2±0.2 | 77.0±3.2 | B16-F10 cells in vitro (Opti-MEM) | ∼ ×5 (pDNA/PEI) | ∼ ×1 (b-PEI) | Reduces RBC agglutination | 68 | Kurosaki, 2009 |
| b-PEI | Poly(acrylic acid) | pDNA-Luc | (approx. neutral) | ∼ 500 | A549 cells in vitro (100% serum) | ∼ ×100 - ×1000 (b-PEI) | Increased in vivo transfection and reduced in vivo toxicity | 69 | Trubetskoy, 2003 | |
| Baculovirus (BV) | PEI | pDNA-Luc | ∼ +30 | ∼ 350 | Human U87 and HepG2 cells | ∼ 95% up to MOI of 100 | up to ∼ ×1000 (uncoated BV) in serum | Increased in vivo transfection as well | 72 | Yang, 2009 |
| PBAE | E12-RGD peptide | pDNA-GFP | ∼ -5 | ∼ 200 | HUVEC in vitro (12% serum) | ∼ 80-100% | ∼ ×3 (PBAE/E12-RDG); ∼ ×50 (b-PEI); ∼ ×1 (Lipo2000) | Targeted delivery at specific material ratios | 74 | Green, 2007 |
| Gold nanoparticle | PBAE | siRNA | ∼ +13 | ∼ 100 | Luc modified HeLa cells in vitro (10% serum) | ∼ 85-100% | ∼ ×6 (Lipo2000); ∼ ×20 (AuNP) | Greater than 95% knock down | 78 | Lee, 2009 |
| Core-shell fluorescent silica nanoparticles | PEI | pDNA-Luc | +31.2±2.7 | 117±1.1 | COS-7 in vitro (Opti-MEM) | ∼ 100% | ∼ ×1 (PEI) | Particles can be used for imaging and delivery | 79 | Fuller, 2008 |
| PBAE | Poly(glutamic acid) | pDNA-Luc | ∼ -10 | ∼ 200 | Swiss Webster mice | No lethality (mice died with uncoated) | ∼ ×10 liver (PBAE); ∼ ×30-40 spleen, marrow | Varying coating affects tissue specificity | 80 | Harris, 2009 |
CTAB = cetyltrimethylammonium bromide; CTL = cytotoxic T lymphocyte; MOI = multiplicity of infection (number of viral particles per cell); pDNA-GFP = GFP coding plasmid; pDNA-Luc = luciferase coding plasmid
Acknowledgments
Declaration of Interest: The authors thank the TEDCO MSCRF (2009-MSCRFE-0098-00) and the NIH (EB000244 and CA132091) for support.
Bibliography
- 1.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell J. Bioactive biomaterials. Curr Opin Biotechnol. 1999;10(2):123–9. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 3.Godbey WT, Barry MA, Saggau P, et al. Poly(ethylenimine)-mediated transfection: a new paradigm for gene delivery. J Biomed Mater Res. 2000;51(3):321–8. doi: 10.1002/1097-4636(20000905)51:3<321::aid-jbm5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas M, Klibanov AM. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2002;99(23):14640–5. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas M, Lu JJ, Ge Q, et al. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102(16):5679–84. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas M, Ge Q, Lu JJ, et al. Cross-linked small polyethylenimines: While still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22(3):373–80. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang MX, Redemann CT, Szoka FC., Jr In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7(6):703–14. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 9.Luo D, Woodrow-Mumford K, Belcheva N, et al. Controlled DNA delivery systems. Pharm Res. 1999;16(8):1300–8. doi: 10.1023/a:1014870102295. [DOI] [PubMed] [Google Scholar]
- 10.Zeisser-Labouebe M, Lange N, Gurny R, et al. Hypericin-loaded nanoparticles for the photodynamic treatment of ovarian cancer. Int J Pharm. 2006;326(1-2):174–81. doi: 10.1016/j.ijpharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 12.Shuai XT, Merdan T, Unger F, et al. Novel biodegradable ternary copolymers hy-PEI-g-PCL-b-PEG: Synthesis, characterization, and potential as efficient nonviral gene delivery vectors. Macromolecules. 2003;36(15):5751–9. [Google Scholar]
- 13.Wang C, Ge Q, Ting D, et al. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat Mater. 2004;3(3):190–6. doi: 10.1038/nmat1075. [DOI] [PubMed] [Google Scholar]
- 14.Lynn DM, Langer R. Degradable poly(beta-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122:10761–8. [Google Scholar]
- 15.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed Engl. 2003;42:3153–8. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 16.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41(6):749–59. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeifer BA, Burdick JA, Langer R. Formulation and surface modification of poly(ester-anhydride) micro- and nanospheres. Biomaterials. 2005;26(2):117–24. doi: 10.1016/j.biomaterials.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Zhao B, Jiang H, et al. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123(1):1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Numata K, Subramanian B, Currie HA, et al. Bioengineered silk protein-based gene delivery systems. Biomaterials. 2009;30(29):5775–84. doi: 10.1016/j.biomaterials.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lollo CP, Banaszczyk MG, Mullen PM, et al. Poly-L-lysine-based gene delivery systems. Synthesis, purification, and application. Methods Mol Med. 2002;69:1–13. doi: 10.1385/1-59259-141-8:001. [DOI] [PubMed] [Google Scholar]
- 21.Borchard G, Bivas-Benita M. In: Gene delivery using chitosan and chitosan derivatives. Amiji MM, editor. New York: CRC Press; 2005. pp. 301–10. [Google Scholar]
- 22.Wang C, Ye S, Dai L, et al. Enzymatic desorption of layer-by-layer assembled multilayer films and effects on the release of encapsulated indomethacin microcrystals. Carbohydr Res. 2007;342(15):2237–43. doi: 10.1016/j.carres.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Chen FM, Wu ZF, Sun HH, et al. Release of bioactive BMP from dextran-derived microspheres: a novel delivery concept. Int J Pharm. 2006;307(1):23–32. doi: 10.1016/j.ijpharm.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–35. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 25.Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12(24):1734–51. doi: 10.1038/sj.gt.3302592. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen DN, Chen SC, Lu J, et al. Drug delivery-mediated control of RNA immunostimulation. Mol Ther. 2009;17(9):1555–62. doi: 10.1038/mt.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H, Koehler DR, Hu J. Adenoviral vectors for gene replacement therapy. Viral Immunol. 2004;17(3):327–33. doi: 10.1089/vim.2004.17.327. [DOI] [PubMed] [Google Scholar]
- 28.Manjunath N, Wu H, Subramanya S, et al. Lentiviral delivery of short hairpin RNAs. Adv Drug Deliv Rev. 2009;61(9):732–45. doi: 10.1016/j.addr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433(7026):561. doi: 10.1038/433561a. [DOI] [PubMed] [Google Scholar]
- 30.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 31.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12(3):468–74. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, May S. Release of cationic polymer-DNA complexes from the endosome: A theoretical investigation of the proton sponge hypothesis. J Chem Phys. 2008;129(18):185105. doi: 10.1063/1.3009263. [DOI] [PubMed] [Google Scholar]
- 33.Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278(45):44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 34.Schaffer DV, Fidelman NA, Dan N, et al. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67(5):598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.van der Aa MA, Mastrobattista E, Oosting RS, et al. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm Res. 2006;23(3):447–59. doi: 10.1007/s11095-005-9445-4. [DOI] [PubMed] [Google Scholar]
- 36.Zanta MA, Belguise-Valladier P, Behr JP. Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci U S A. 1999;96(1):91–6. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrhardt A, Xu H, Huang Z, et al. A direct comparison of two nonviral gene therapy vectors for somatic integration: in vivo evaluation of the bacteriophage integrase phiC31 and the Sleeping Beauty transposase. Mol Ther. 2005;11(5):695–706. doi: 10.1016/j.ymthe.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Delivery Rev. 2006;58(4):467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5(6):439–51. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]; * Excellent review of polymeric gene delivery
- 40.Luten J, van Nostrum CF, De Smedt SC, et al. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release. 2008;126(2):97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Green JJ, Zugates GT, Tedford NC, et al. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv Mater. 2007;19(19):2836–42. [Google Scholar]
- 42.Hill HD, Millstone JE, Banholzer MJ, et al. The role radius of curvature plays in thiolated oligonucleotide loading on gold nanoparticles. ACS Nano. 2009;3(2):418–24. doi: 10.1021/nn800726e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2003;100(16):9138–43. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhar S, Daniel WL, Giljohann DA, et al. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J Am Chem Soc. 2009;131(41):14652–3. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JS, Lytton-Jean AK, Hurst SJ, et al. Silver nanoparticle-oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett. 2007;7(7):2112–5. doi: 10.1021/nl071108g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akerman ME, Chan WC, Laakkonen P, et al. Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A. 2002;99(20):12617–21. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derfus AM, Chen AA, Min DH, et al. Targeted quantum dot conjugates for siRNA delivery. Bioconjug Chem. 2007;18(5):1391–6. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury EH, Akaike T. Bio-functional inorganic materials: an attractive branch of gene-based nano-medicine delivery for 21st century. Curr Gene Ther. 2005;5(6):669–76. doi: 10.2174/156652305774964613. [DOI] [PubMed] [Google Scholar]
- 49.Wagner E. Strategies to improve DNA polyplexes for in vivo gene transfer: Will “artificial viruses” be the answer? Pharm Res. 2004;21(1):8–14. doi: 10.1023/b:pham.0000012146.04068.56. [DOI] [PubMed] [Google Scholar]
- 50.Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. J Control Release. 2001;73(2-3):401–16. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 51.Pedroso de Lima MC, Simoes S, Pires P, et al. Cationic lipid-DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Delivery Rev. 2001;47(2-3):277–94. doi: 10.1016/s0169-409x(01)00110-7. [DOI] [PubMed] [Google Scholar]
- 52.Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–9. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Millstone JE, Hurst SJ, Metraux GS, et al. Colloidal gold and silver triangular nanoprisms. Small. 2009;5(6):646–64. doi: 10.1002/smll.200801480. [DOI] [PubMed] [Google Scholar]
- 54.Gratton SE, Williams SS, Napier ME, et al. The pursuit of a scalable nanofabrication platform for use in material and life science applications. Acc Chem Res. 2008;41(12):1685–95. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Top-down fabrication for patterning nanoparticles with a wide array of features and morphologies
- 55.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 56.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Farokhzad OC, Karp JM, Langer R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin Drug Deliv. 2006;3(3):311–24. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 58.Huang YH, Zugates GT, Peng W, et al. Nanoparticle-delivered suicide gene therapy effectively reduces ovarian tumor burden in mice. Cancer Res. 2009;69(15):6184–91. doi: 10.1158/0008-5472.CAN-09-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21(23):2475–90. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 60.Singh M, Briones M, Ott G, et al. Cationic microparticles: A potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A. 2000;97(2):811–6. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Electrostatic coatings of microparticles to create genetic vaccines
- 61.Little SR, Lynn DM, Ge Q, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004;101(26):9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6(5):797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- 63.Kidane A, Bhatt PP. Recent advances in small molecule drug delivery. Curr Opin Chem Biol. 2005;9(4):347–51. doi: 10.1016/j.cbpa.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Malik DK, Baboota S, Ahuja A, et al. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007;4(2):141–51. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 65.Bae KH, Moon CW, Lee Y, et al. Intracellular delivery of heparin complexed with chitosan-g-poly(ethylene glycol) for inducing apoptosis. Pharm Res. 2009;26(1):93–100. doi: 10.1007/s11095-008-9713-1. [DOI] [PubMed] [Google Scholar]
- 66.Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62(1):27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 67.Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev. 2003;55(10):1261–77. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 68.Kurosaki T, Kitahara T, Kawakami S, et al. The development of a gene vector electrostatically assembled with a polysaccharide capsule. Biomaterials. 2009;30(26):4427–34. doi: 10.1016/j.biomaterials.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 69.Trubetskoy VS, Wong SC, Subbotin V, et al. Recharging cationic DNA complexes with highly charged polyanions for in vitro and in vivo gene delivery. Gene Ther. 2003;10:261–71. doi: 10.1038/sj.gt.3301888. [DOI] [PubMed] [Google Scholar]; * Anionic coating of cationic particles increases gene delivery efficacy in vivo
- 70.Subr V, Kostka L, Selby-Milic T, et al. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J Control Release. 2009;135(2):152–8. doi: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Fisher KD, Stallwood Y, Green NK, et al. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8(5):341–8. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Lo SL, Yang J, et al. Polyethylenimine coating to produce serum-resistant baculoviral vectors for in vivo gene delivery. Biomaterials. 2009;30(29):5767–74. doi: 10.1016/j.biomaterials.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 73.Ogris M, Walker G, Blessing T, et al. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. J Controlled Release. 2003;91(1-2):173–81. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]; * Parallel strategies for shielding and targeting electrostatic polymer/DNA particles
- 74.Green JJ, Chiu E, Leshchiner ES, et al. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007;7(4):874–9. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]; * Anionic coating of cationic particles increases gene delivery specificity
- 75.Kunath K, Merdan T, Hegener O, et al. Integrin targeting using RGD-PEI conjugates for in vitro gene transfer. J Gene Med. 2003;5:588–99. doi: 10.1002/jgm.382. [DOI] [PubMed] [Google Scholar]
- 76.Suh W, Han SO, Yu L, et al. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol Ther. 2002;6:664–72. [PubMed] [Google Scholar]
- 77.Kursa M, Walker GF, Roessler V, et al. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–31. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- 78.Lee JS, Green JJ, Love KT, et al. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9(6):2402–6. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Cationic electrostatic coating of gold/siRNA hybrid nanoparticles
- 79.Fuller JE, Zugates GT, Ferreira LS, et al. Intracellular delivery of core-shell fluorescent silica nanoparticles. Biomaterials. 2008;29(10):1526–32. doi: 10.1016/j.biomaterials.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 80.Harris TJ, Green JJ, Fung PW, et al. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2009;31(5):998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dash PR, Read ML, Barrett LB, et al. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 1999;6(4):643–50. doi: 10.1038/sj.gt.3300843. [DOI] [PubMed] [Google Scholar]
- 82.Zou SM, Erbacher P, Remy JS, et al. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J Gene Med. 2000;2(2):128–34. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<128::AID-JGM95>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 83.Peyratout CS, Dahne L. Tailor-made polyelectrolyte microcapsules: from multilayers to smart containers. Angew Chem Int Ed Engl. 2004;43(29):3762–83. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 84.De Geest BG, Sanders NN, Sukhorukov GB, et al. Release mechanisms for polyelectrolyte capsules. Chem Soc Rev. 2007;36(4):636–49. doi: 10.1039/b600460c. [DOI] [PubMed] [Google Scholar]
- 85.Reibetanz U, Claus C, Typlt E, et al. Defoliation and plasmid delivery with layer-by-layer coated colloids. Macromol Biosci. 2006;6(2):153–60. doi: 10.1002/mabi.200500163. [DOI] [PubMed] [Google Scholar]
- 86.Schuler C, Caruso F. Decomposable hollow biopolymer-based capsules. Biomacromolecules. 2001;2(3):921–6. doi: 10.1021/bm010052w. [DOI] [PubMed] [Google Scholar]
- 87.Shchukin DG, Patel AA, Sukhorukov GB, et al. Nanoassembly of biodegradable microcapsules for DNA encasing. J Am Chem Soc. 2004;126(11):3374–5. doi: 10.1021/ja036952x. [DOI] [PubMed] [Google Scholar]
- 88.Fukui Y, Fujimoto K. The Preparation of Sugar Polymer-Coated Nanocapsules by the Layer-by-Layer Deposition on the Liposome. Langmuir. 2009;25(17):10020–5. doi: 10.1021/la9008834. [DOI] [PubMed] [Google Scholar]
- 89.Saul JM, Wang CHK, Ng CP, et al. Multilayer nanocomplexes of polymer and DNA exhibit enhanced gene delivery. Advanced Materials. 2008;20(1):19–25. [Google Scholar]
- 90.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232–7. [Google Scholar]; ** Classic account of multilayer assembly
- 91.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat Mater. 2004;3(8):569–74. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 92.Diaspro A, Silvano D, Krol S, et al. Single living cell encapsulation in nano-organized polyelectrolyte shells. Langmuir. 2002;18(13):5047–50. [Google Scholar]; * Use of a living cell as the “core” coated by multilayer films
- 93.Hillberg AL, Tabrizian M. Biorecognition through layer-by-layer polyelectrolyte assembly: in-situ hybridization on living cells. Biomacromolecules. 2006;7(10):2742–50. doi: 10.1021/bm060266j. [DOI] [PubMed] [Google Scholar]
- 94.Jewell CM, Zhang J, Fredin NJ, et al. Release of plasmid DNA from intravascular stents coated with ultrathin multilayered polyelectrolyte films. Biomacromolecules. 2006;7(9):2483–91. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Coated stents for gene delivery
- 95.Elbert DL, Herbert CB, Hubbell JA. Thin polymer layers formed by polyelectrolyte multilayer techniques on biological surfaces. Langmuir. 1999;15(16):5355–62. [Google Scholar]
- 96.Wood KC, Boedicker JQ, Lynn DM, et al. Tunable drug release from hydrolytically degradable layer-by-layer thin films. Langmuir. 2005;21(4):1603–9. doi: 10.1021/la0476480. [DOI] [PubMed] [Google Scholar]
- 97.Wood KC, Zacharia NS, Schmidt DJ, et al. Electroactive controlled release thin films. Proc Natl Acad Sci U S A. 2008;105(7):2280–5. doi: 10.1073/pnas.0706994105. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Triggered release from multilayer films
- 98.Kwon IC, Bae YH, Kim SW. Electrically erodible polymer gel for controlled release of drugs. Nature. 1991;354(6351):291–3. doi: 10.1038/354291a0. [DOI] [PubMed] [Google Scholar]
- 99.Hiller J, Rubner MF. Reversible molecular memory and pH-switchable swelling transitions in polyelectrolyte multilayers. Macromolecules. 2003;36(11):4078–83. [Google Scholar]
- 100.Jiang B, Li B. Tunable drug loading and release from polypeptide multilayer nanofilms. Int J Nanomedicine. 2009;4:37–53. doi: 10.2147/ijn.s4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wood KC, Chuang HF, Batten RD, et al. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc Natl Acad Sci U S A. 2006;103(27):10207–12. doi: 10.1073/pnas.0602884103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim BS, Smith RC, Poon Z, et al. MAD (Multiagent Delivery) Nanolayer: Delivering Multiple Therapeutics from Hierarchically Assembled Surface Coatings. Langmuir. 2009;25(24):14086–92. doi: 10.1021/la9017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krogman KC, Lowery JL, Zacharia NS, et al. Spraying asymmetry into functional membranes layer-by-layer. Nat Mater. 2009;8(6):512–8. doi: 10.1038/nmat2430. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Chua LS, Lynn DM. Multilayered thin films that sustain the release of functional DNA under physiological conditions. Langmuir. 2004;20(19):8015–21. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 105.Jewell CM, Zhang J, Fredin NJ, et al. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J Control Release. 2005;106(1-2):214–23. doi: 10.1016/j.jconrel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 106.Sakai S, Yamada Y, Yamaguchi T, et al. Surface immobilization of poly(ethyleneimine) and plasmid DNA on electrospun poly(L-lactic acid) fibrous mats using a layer-by-layer approach for gene delivery. J Biomed Mater Res A. 2009;88(2):281–7. doi: 10.1002/jbm.a.31870. [DOI] [PubMed] [Google Scholar]
- 107.Blacklock J, You YZ, Zhou QH, et al. Gene delivery in vitro and in vivo from bioreducible multilayered polyelectrolyte films of plasmid DNA. Biomaterials. 2009;30(5):939–50. doi: 10.1016/j.biomaterials.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Sharma KK, Boeglin M, et al. Transfection ability and intracellular DNA pathway of nanostructured gene-delivery systems. Nano Lett. 2008;8(8):2432–6. doi: 10.1021/nl801379y. [DOI] [PubMed] [Google Scholar]
- 109.Recksiedler CL, Deore BA, Freund MS. A novel layer-by-layer approach for the fabrication of conducting polymer/RNA multilayer films for controlled release. Langmuir. 2006;22(6):2811–5. doi: 10.1021/la053031m. [DOI] [PubMed] [Google Scholar]
- 110.Zhao X, Pan F, Holt CM, et al. Controlled delivery of antisense oligonucleotides: a brief review of current strategies. Expert Opin Drug Deliv. 2009;6(7):673–86. doi: 10.1517/17425240902992894. [DOI] [PubMed] [Google Scholar]
- 111.Nasongkla N, Bey E, Ren J, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6(11):2427–30. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen DN, Green JJ, Chan JM, et al. Polymeric Materials for Gene Delivery and DNA Vaccination. Adv Mater. 2009;21(8):847–67. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nordly P, Madsen HB, Nielsen HM, et al. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin Drug Deliv. 2009;6(7):657–72. doi: 10.1517/17425240903018863. [DOI] [PubMed] [Google Scholar]
- 114.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khemtong C, Kessinger CW, Gao J. Polymeric nanomedicine for cancer MR imaging and drug delivery. Chem Commun (Camb) 2009;(24):3497–510. doi: 10.1039/b821865j. [DOI] [PMC free article] [PubMed] [Google Scholar]