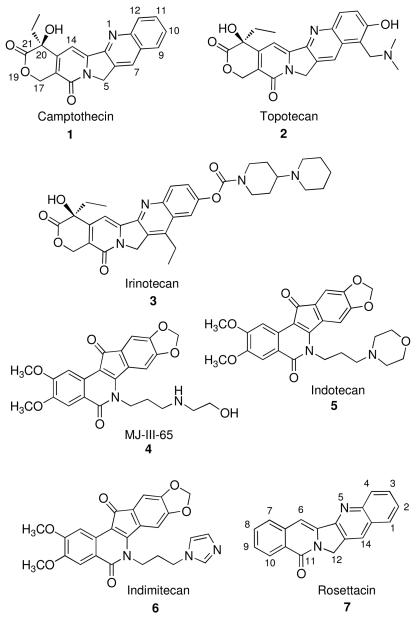
Abstract
Aromathecins are inhibitors of human topoisomerase I (Top1). These compounds are composites of several heteroaromatic systems, namely the camptothecins and indenoisoquinolines, and they possess notable Top1 inhibition and cytotoxicity when substituted at position 14. The SAR of these compounds overlaps with indenoisoquinolines, suggesting that they may intercalate into the Top1-DNA complex similarly. Nonetheless, the proposed binding mode for aromathecins is purely hypothetical, as an X-ray structure is unavailable. In the present communication, we have synthesized eight novel series of A-ring-substituted (positions 1-3) aromathecins, through a simple, modular route, as part of a comprehensive SAR study. Certain groups (such as 2,3-ethylenedioxy) moderately improve Top1 inhibition, and, often, antiproliferative activity, whereas other groups (2,3-dimethoxy and 3-substituents) attenuate bioactivity. Strikingly, these trends are very similar to those previously observed for the A-ring of camptothecins, and this considerable SAR overlap lends further support (in the absence of crystallographic data) to the hypothesis that aromathecins bind in the Top1 cleavage complex as interfacial inhibitors in a “camptothecin-like” pose.
Keywords: topoisomerase 1, aromathecins, anticancer, camptothecin-like
1. Introduction
Topoisomerase I (Top1) is an enzyme that is critical for efficient DNA replication and cell division. As DNA is highly supercoiled, it must also be relaxed prior to cellular processes such as replication and transcription. The enzyme acts by binding to and nicking double-stranded DNA through the action of a nucleophilic tyrosine residue (Tyr723). Within the covalent Top1-DNA cleavage complex, the scissile DNA strand undergoes “controlled rotation”1-3 around the nonscissile strand, relieving the supercoils. The hydroxyl group of the scissile strand's 5′ end then re-ligates the broken strand and the enzyme is released.1 As it plays a pivotal role in cellular proliferation, Top1 is often overexpressed in human tumors. High levels of this enzyme have been found in lung, colorectal, and ovarian cancers4-6 and elevated Top1 levels in breast cancers are also associated with poor patient prognosis.7
The only FDA-approved Top1 inhibitors are topotecan (2) and irinotecan (3),8 drugs based on the pentacyclic antitumor alkaloid camptothecin (1), (Figure 1) which was originally isolated from the Chinese tree Camptotheca acuminata.8-9 Camptothecin binds at the interface of Top1-DNA cleavage complexes in an intercalative mode10-12, where it stacks between the base pairs of the Top1-DNA cleavage complex and, stabilized chiefly by pi-pi stacking13, sterically prevents the re-ligation reaction. It also forms key hydrogen bonds with Top1 amino acid residues.1,10-12 The resulting covalent Top1-DNA adduct then produces collisions with advancing replication forks and transcription complexes, which triggers irreversible DNA damage and apoptosis.14,15
Figure 1.
Representative Top1 poisons.
Although the camptothecins are potent and possess high cytotoxicity, they also suffer from well-identified drawbacks, including short duration of action, poor solubility, resistance mutants,16 and high toxicity.17,18 Additionally, the E-ring lactone of camptothecin is readily opened to its hydroxycarboxylate form in vivo.19 This form is less active and binds strongly to human blood proteins.20
One promising class of noncamptothecin Top1 poisons is the indenoisoquinolines, such as MJ-III-65 (4).21,12 These compounds possess high anti-Top1 activity, are cytotoxic, and are more stable because they lack the hydroxylactone. Through comprehensive SAR studies,21-24 two clinical candidates, indotecan (5) and indimitecan (6) were developed and have begun Phase 1 clinical trials at the National Cancer Institute.25-26
We described in two previous communications27-28 the design, synthesis, and biological evaluation of substituted 12H-5,11a-diazadibenzo[b,h]fluoren-11-ones, called “aromathecins.”27-30 These compounds can be thought of as composites of the camptothecins and indenoisoquinolines, in which the E-ring of the former has been “aromatized” (replaced by a benzene ring). The majority of these compounds, when substituted at position 14, possess greater Top1 inhibitory and antiproliferative activity than the unsubstituted core compound, rosettacin (7).31 Molecular models indicate that these 14-substituents overlap spatially with the lactam substituents of indenoisoquinolines, which are both proposed to project into the major groove of the DNA-Top1 complex and hydrogen-bond to Top1 amino acids and water in the DNA major groove.27,28 Because of the high degree of SAR overlap at these positions (down to specific substituents), it was proposed that “common” SAR elements are shared between the indenoisoquinolines and aromathecins. The SAR studies also support the hypothesis that aromathecins intercalate in a fashion similar to indenoisoquinolines.10-12 Due to the overall similarity in shape and structure to camptothecins, it also was suggested that the aromathecins may bind in a distinctively “camptothecin-like” pose.13,15 These models are purely hypothetical, however, and because aromathecins are difficult to crystallize with the enzyme, no X-ray structure of any aromathecin in ternary complex with DNA and Top1 is currently available.
The present study was undertaken in order to explore the hypothesis that the biological activity of the aromathecin system could be modulated and eventually improved through substitution at positions other than 14. To this date, no A-ring (positions 1-4)-substituted aromathecins have been evaluated. Using some of the rationale provided by existing camptothecin and indenoisoquinoline SARs and the “overlapping” SAR hypothesis, we prepared 8 novel series of aromathecins (series 27 through 34) substituted on both the A-ring and position 14. The A-ring substituents encompass a variety of steric, electronic, and H-bonding properties, allowing for maximum exploration of chemical space and elucidation of those elements required for binding and optimal bioactivity.
2. Chemistry
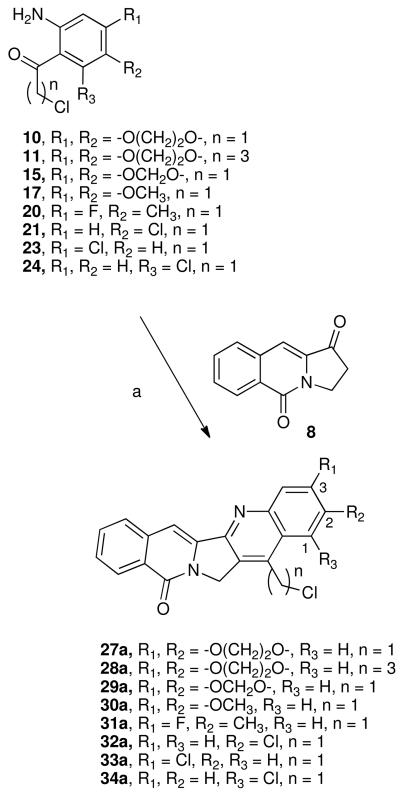
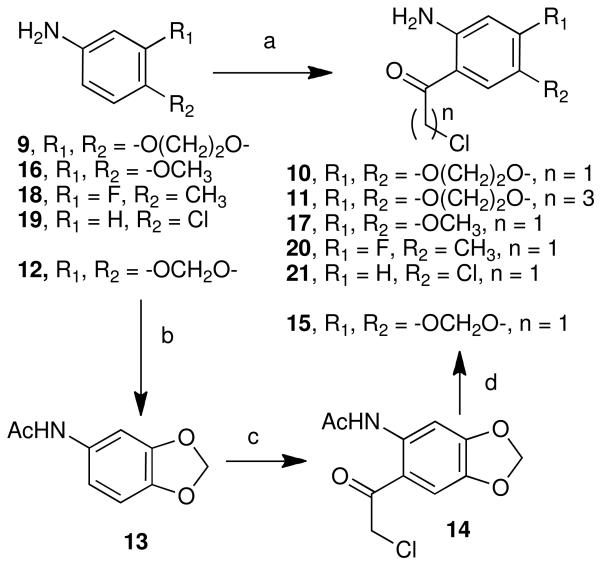
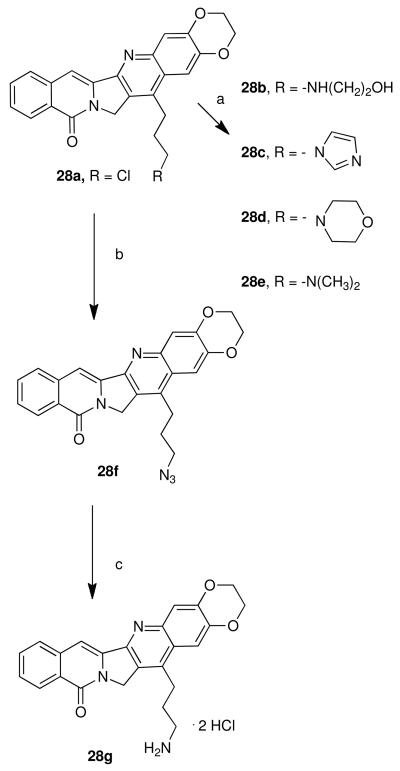
Although there are several routes to rosettacin and aromathecin derivatives,32,33 our route proceeds through tricyclic synthon 8.34 The previously developed route to 828 (See Scheme 3 for structure) proceeds in good yield from commercially available starting materials and can be scaled up readily. This ketone can then be condensed with substituted o-aminochloroaceto- or chlorobutyrophenones to provide versatile aromathecin cores that can be readily functionalized, making the assembly of substituted aromathecins rapid and modular. Following existing camptothecin SARs, a variety of groups (halogens, ethers, the methylenedioxy group, and a methyl group) were chosen for the study. To vary the nature of the A-ring substituent, substituted precursor amino aryl ketones were first prepared.
Scheme 3. Reagents and Conditions.
(a) p-TsOH, benzene or toluene, AcOH (for 11), reflux overnight.
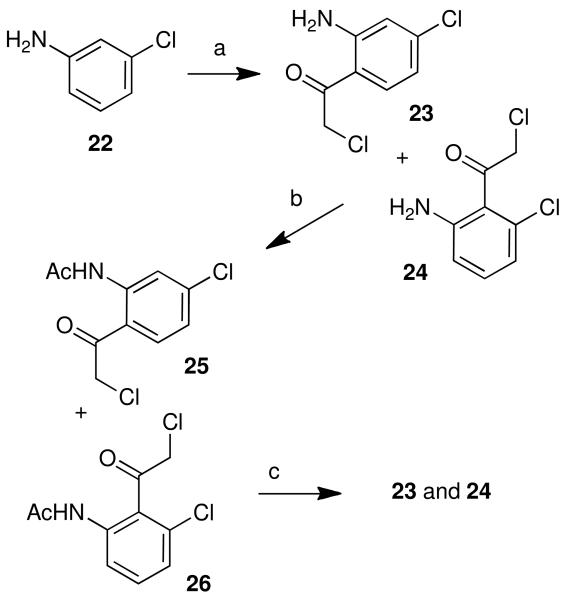
The preparation of some of these ketone coupling partners is described in Scheme 1. To install the 2,3-ethylenedioxy group, 1,4-benzodiozan-6-amine (9) was chloroacetylated19 using Sugasawa's Friedel-Crafts conditions to yield 10, and chlorobutyrated likewise to afford 11.35-37 As the methylenedioxy group (for 2,3-methylenedioxyaromathecins) is not compatible with the strong Lewis acids utilized in the chloroacetylation, the modified zinc-catalyzed procedure of Luzzio et al.19 was employed, and beginning with 3,4-methylenedioxyaniline (12) the ketone 15 was eventually obtained in low yield but high purity. The chloroacetylation of 4-aminoveratrole (16) proceeded in the absence of additional catalyst (due to the electron-donating effects of the methoxy groups) to afford 17. 3-Fluoro-4-methylaniline (18) and 4-chloroaniline (19) were also chloroacetylated to afford their respective ketones 20 and 21, albeit in low yield. The chloroacetylation of 3-chloroaniline (22), however, afforded an inseparable mixture of 23 and 24, which were converted into their respective acetanilides 25 and 26 to aid in purification. These compounds were separated and hydrolyzed to yield 23 and 24, in a ratio consistent with that reported by Sugasawa et al.36 (Scheme 2). Compound 23 was used to prepare the 3-chloroaromathecin series, and 24, the 1-chloro series.
Scheme 1. Reagents and Conditions.
(a) i. BCl3Me2S, 1,2-dichloroethane, 0 °C, ii. chloroacetonitrile (to afford 10), 4-chlorobutyronitrile (to afford 11), AlCl3 (to afford all except 16), reflux, iii. 2 M HCl, reflux; (b) AcO2, Et3N, H2O, r.t.; (c) chloroacetyl chloride, ZnCl2, CH3NO2, reflux; (d) concd. HCl.
Scheme 2. Reagents and Conditions.
(a) i. BCl3Me2S, 1,2-dichloroethane, 0 °C, ii. chloroacetonitrile, AlCl3, reflux. iii. 2 M HCl, reflux, (b) Ac2O, 80 °C; (c) concd. HCl, EtOH, 0 °C - reflux.
The condensation of these ketones with 8 was performed under Friedlander conditions,27 and the chloroalkylated aromathecin cores 27a-34a were obtained in modest to excellent yield. In the majority of cases, a stoichiometric amount (or less) of p-TsOH was sufficient, although several cases did require higher temperatures, longer times, and (in the case of 28a) catalytic amounts of AcOH (Scheme 3).
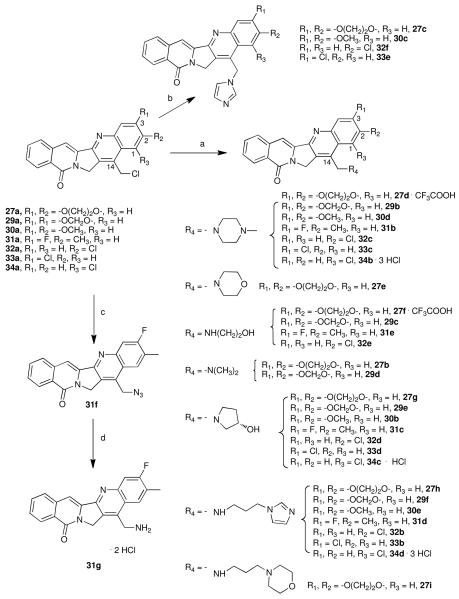
Aromathecin analogues 27b-i (2,3-ethylenedioxy), 29b-f (2,3-methylenedioxy), 30b-e (2,3-dimethoxy), 31b-g (2-methyl-3-fluoro), 32b-f (2-chloro) 33b-e (3-chloro) and 34b-d (1-chloro) (Scheme 4) were prepared by simple SN2 displacement of the unhindered, benzylic chloride by a nucleophile at room or slightly elevated temperature in DMSO or DMF. Many nucleophiles were chosen to explore an assortment of 14-substituents and to search for possible synergistic or antagonistic effects with the A-ring substituents. Nucleophiles available as salts [(S)-pyrrolidin-3-ol38 and N,N-dimethylamine] were used in the presence of excess triethylamine. This displacement reaction proceeded overnight in most cases to yield the substituted aromathecins in modest to excellent yield, although displacement by imidazole (to prepare 27c, 30c, 32f and 33e) was performed at a higher temperature. Some ethylenedioxy analogues (27d and 27f) were converted into trifluoroacetate salts to aid in solubility. Likewise, analogues 34b-d were converted to their hydrochloride salts.
Scheme 4. Reagents and Conditions.
(a) i. Amine or amine salt + Et3N, DMSO or DMF. ii. HCl/MeOH (series 34) or CF3COOH (27d and f); (b) imidazole, DMSO or DMF, 60-100 °C; (c) For 31a, NaN3, DMSO, r.t.; (d) i. (EtO3)P, benzene, reflux, ii. HCl/MeOH, reflux.
Finally, to synthesize the “extended” 3′-substituted propyl ethylenedioxy series 28b-g (Scheme 5), an in situ Finkelstein reaction27 was performed at high temperatures using both anexcess of nucleophile and sodium iodide to compensate for the decreased electrophilicity of theterminal alkyl chloride.
Scheme 5. Reagents and Conditions.
(a) Amine, NaI, DMSO or DMF, 100 °C; (b) NaN3, DMSO, 100 °C (c) i. (EtO3)P, benzene, reflux, ii. 3 M HCl, MeOH, reflux.
3. Results and Discussion
Aromathecin analogues (with the exception of chlorinated compounds 29a, 31a, and 32-34a, and azides 28f and 31f, due to the historically low bioactivities of these compounds) were assayed in the National Cancer Institute's Developmental Therapeutics Assay,39-40 where they were tested against the eight cancer cell line subpanels described in Table 1 as well as a leukemia panel (approximately 60 cell lines total). After an initial one-dose assay (at 10−5 molar), selected compounds were tested at five concentrations ranging from 10−8 to 10−4 molar. Cytotoxicity results are reported as GI50 values for selected cell lines from each subpanel, and overall antiproliferative potency is quantified as a mean-graph midpoint (MGM) in Table 1. The MGM is a measure of the average GI50 against all cell lines tested, where compounds whose GI50 values fall outside the concentration range tested (10−8 to 10−4 M) are assigned GI50 values of either 10−8 M or 10−4 M. For comparative purposes, Top1 and antiproliferative activity data for camptothecin (1),17 indenoisoquinoline 4,21,41 clinical leads 5 and 6,25 and rosettacin (7) are included.
Table 1.
Antiproliferative Potencies and Topoisomerase I Inhibitory Activities of A-Ring- and 14-Substituted Aromathecin Analogue Series 27-32.
| Cytotoxicity (GI50 in μM)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| lung | Colon | CNS | melanoma | Ovarian | renal | prostate | breast | Top 1 | ||
| Compd | HOP-62 | HCT-116 | SF-539 | UACC-62 | OVCAR-3 | SN12C | DU-145 | MCF-7 | MGMb | Cleavagec |
| 1 17 | 0.01 | 0.03 | 0.01 | 0.01 | 0.22 | 0.02 | 0.01 | 0.01 | 0.0405±0.0187f | ++++ |
| 4 17 | 0.02 | 0.10 | 0.04 | 0.03 | 0.5 | <0.01 | <0.01 | <0.01 | 0.21±0.19 | ++++ |
| 5 20 | 1.78 | 1.15 | 0.04 | 0.03 | 74.1 | 0.813 | 0.155 | 0.37 | 4.64±1.25 | ++++ |
| 6 20 | <0.01 | <0.01 | 0.037 | <0.01 | 0.085 | <0.01 | <0.01 | 0.01 | 0.079±0.023 | ++++ |
| 7 | >100 | 57.3 | >100 | >100 | >100 | >100 | >100 | 60.3 | 91.2 | ++ |
| 27a | - | - | - | - | - | - | - | - | - | + |
| 27b | 3.31 | 1.17 | 2.69 | 1.66 | 2.19 | 2.51 | 2.19 | 0.30 | 2.95 | +++ |
| 27c | 19.5 | >100 | 4.37 | 21.4 | 18.6 | 81.3 | >100 | 0.30 | 19.05 | +++ |
| 27d d | - | - | - | - | - | - | - | - | - | ++(+) |
| 27e | 3.80 | 1.33 | 0.81 | 0.99 | 1.70 | 2.57 | 0.93 | 0.30 | 1.78±0.165 | ++(+) |
| 27f | 5.69 | 2.63 | 4.26 | 1.16 | 1.95 | 5.75 | 2.04 | 0.44 | 2.33±0.24 | ++(+) |
| 27g | 1.01 | 0.82 | 0.49 | 0.46 | 1.84 | 0.94 | 1.02 | 0.27 | 1.24±0.21 | ++ |
| 27h d | - | - | - | - | - | - | - | - | - | ++ |
| 27i | - | - | - | - | - | - | - | - | - | ++ |
| 28a | - | - | - | - | - | - | - | - | - | 0 |
| 28b | 1.01 | 0.82 | 0.49 | 0.46 | 1.84 | 0.94 | 1.02 | 0.27 | 2.35±0.215 | 0 |
| 28c | 0.57 | 1.95 | 1.95 | 0.93 | 7.08 | 12.6 | 2.88 | 0.19 | 3.24 | ++(+) |
| 28d | 0.54 | 1.42 | 1.15 | 1.15 | 1.84 | 1.13 | 0.76 | 0.06 | 0.82 ± 0.03 | ++(+) |
| 28e | - | - | - | - | - | - | - | - | - | +++ |
| 28g | 2.14 | 1.95 | 1.86 | 1.62 | 1.95 | 1.86 | 1.82 | 1.05 | 2.14 | +++ |
| 29b | 17.0 | 1.45 | 11.7 | 1.62 | 16.2 | 2.34 | 51.3 | 0.38 | 8.91 | ++ |
| 29c | 0.66 | 0.95 | 1.35 | 0.47 | 1.55 | 1.91 | 1.82 | 0.28 | 2.00 | ++(+) |
| 29d | - | - | - | - | - | - | - | - | - | ++ |
| 29e | 0.48 | 0.85 | 0.60 | 0.35 | >100 | 0.79 | - | 0.07 | 6.91 | ++ |
| 29f | 5.01 | 1.32 | 2.14 | 3.39 | 4.26 | 5.89 | 2.57 | 0.48 | 5.50 | ++ |
| 30a | - | - | - | - | - | - | - | - | - | 0 |
| 30b-e e | - | - | - | - | - | - | - | - | - | 0 or 0/+ |
| 31b | 20.1 | 1.80 | 6.46 | 16.2 | 14.6 | 16.0 | 2.63 | 1.80 | 6.31±0.145 | ++(+) |
| 31c | - | - | - | - | - | - | - | - | - | ++ |
| 31d | 36.7 | 3.05 | 1.10 | >100 | 4.68 | 1.80 | 6.24 | 2.21 | 11.3±0.95 | 0 |
| 31e | 11.2 | 18.4 | 9.23 | 14.2 | 16.8 | 20.2 | 12.2 | 1.62 | 14.4±1.81 | ++(+) |
| 31g | - | - | - | - | - | - | - | - | - | +(+) |
| 32b | - | - | - | - | - | - | - | - | - | ++ |
| 32c | 2.29 | 0.15 | 1.58 | 1.41 | 1.51 | 1.78 | 2.75 | 3.31 | 1.85±0.34 | +(+) |
| 32d | 1.05 | 1.10 | 1.26 | 0.74 | 1.86 | - | 1.66 | 0.37 | 1.44 | +(+) |
| 32e | 7.24 | 2.14 | 4.36 | 2.04 | 6.46 | 10.2 | - | 0.23 | 5.02 | ++ |
| 32f | 0.46 | 0.40 | 0.39 | 0.31 | 0.69 | 1.54 | 0.48 | 0.19 | 1.02 | + |
| 33b | 2.00 | 1.82 | 1.86 | 1.91 | 2.04 | 2.09 | - | 1.66 | 2.51 | + |
| 33c | 18.1 | 13.5 | 15.1 | 17.0 | 15.1 | 19.0 | 23.4 | 10.9 | 14.5 | + |
| 33d | - | - | - | - | - | - | - | - | - | + |
| 33e | - | - | - | - | - | - | - | - | - | 0 |
| 34b | 1.86 | 1.41 | 1.51 | 15.5 | 4.17 | 13.8 | 9.77 | 1.00 | 4.37 | 0 |
| 34c | 1.66 | 1.55 | 1.95 | 3.09 | 2.63 | 3.71 | 3.98 | 0.37 | 2.40 | + |
| 34d | 2.51 | 1.58 | 1.90 | 7.24 | 4.90 | 6.03 | 1.02 | 0.55 | 3.09 | +++ |
The cytotoxicity GI50 values are the concentrations corresponding to 50% growth inhibition.
Mean graph midpoint for growth inhibition of all human cancer cell lines successfully tested, ranging from 10−8 to 10−4 molar.
Compound-induced DNA cleavage due to Top1 inhibition is graded by the following rubric relative to 1 uM camptothecin: 0, no inhibitory activity; +, between 20 and 50% activity; ++, between 50 and 75% activity; +++, between 75% and 95% activity; ++++, equipotent. Compounds that are ranked between two scores are delineated with parentheses [i.e., between ++ and +++ is ++(+)].
Some compounds such as this were not selected for further testing; refer to text for details.
These compounds were declined for testing by the NCI.
For MGM GI50 values in which a standard error appears, the GI50 values for individual cell lines are the average of two determinations; values without standard error are from one determination. The values for 1, 4, 5, and 6 are from many determinations.
Top1 inhibition was measured by a compound's ability to induce enzyme-linked DNA cleavage, and is graded by the following semiquantitative rubric relative to 1 μM camptothecin: 0, no inhibitory activity; +, between 20 and 50% activity; ++, between 50 and 75% activity; +++, between 75% and 95% activity; ++++, equipotent. Compounds that are between two scores are delineated with parentheses [i.e., between ++ and +++ is ++(+)].
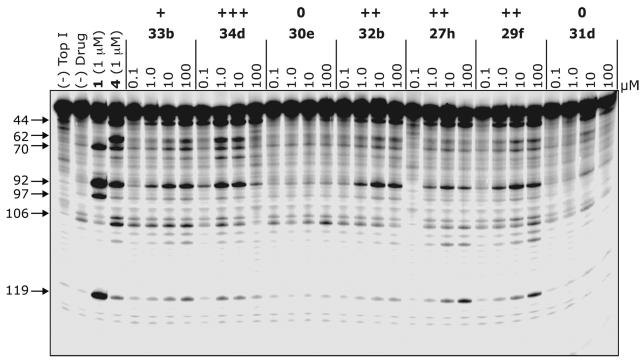
As can be observed in Figure 2, substitution on the A-ring very easily changes a compound's anti-Top1 activity for a given 14-substituent (here, the aminopropylimidazole side chain), although no “synergistic” effect was observed with the 14-substituents. Several important SAR trends can be gleaned from these data. The ethylenedioxy group, in general, is well tolerated on the A-ring, and for all except 27g, anti-Top1 activity is either unchanged or improved over the analogous unsubstituted compounds. For the monomethylene compounds 27b, 27e, and 27i, Top1 inhibitory activity is improved, and for 27a, 27c, 27d, 27f and 27h, the anti-Top1 activity is unchanged. Although they are potent Top1 poisons, more variability (with the same comparison to the unsubstituted series) is seen in the extended (28) series. General variability was also observed for extended unsubstituted compounds alone.27
Figure 2.
Top1-mediated DNA cleavage induced by aromathecins 33b, 34d, 30e, 32b, 27h, 29f, and 31d. Lane 1: DNA alone; lane 2: Top1 alone; lane 3: 1, 1 μM; lane 4: 4, 1 μM; lanes 5-32: lanes 6-29: 33b, 34d, 30e, 32b, 27h, 29f, and 31d at 0.1, 1, 10, and 100 μM, respectively, from left to right. Numbers and arrows on left indicate arbitrary cleavage site positions.
As antiproliferative activity involves factors more complicated than Top1 inhibition alone (as evidenced by low correlation between Top1 inhibition and cytotoxicity27,28,42) the GI50 values are variable in both the monomethylene and extended series, although improvements in potency on a per-analogue basis are observed in several cases, and many are more potent than clinical candidate 5. Examining the antiproliferative activity of this entire series of ethylenedioxy compounds (series 27 and 28), although some were not selected for five-dose testing, all compounds (except for 28a, which was not tested for cytotoxicity) possessed at least some activity. At a concentration of 10 μM, compound 27a inhibited cell growth at a mean 10.3%, 27d at 23%, 27i at 10.8%, 28e at 19%, and 27h at 14.3%.
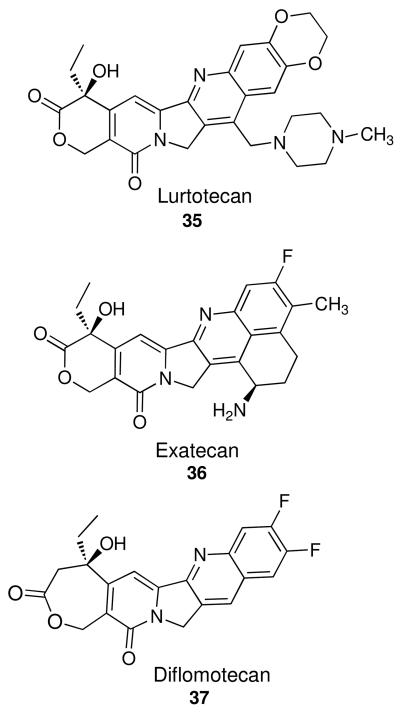
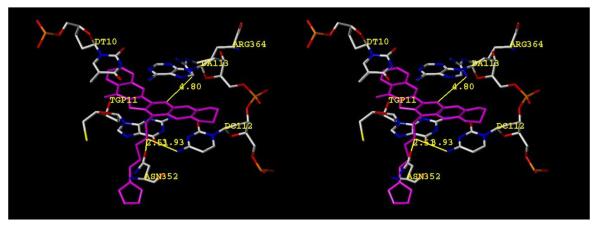
It is unknown exactly how the ethylenedioxy group may enhance potency, although it is reported that this group improves activity for camptothecin derivatives, such as in the clinical candidate lurtotecan (Figure 3, 35)1,19,43-45 For camptothecins, placement of ethylenedioxy (or methylenedioxy) groups at the analogous 10,11 position may improve solubility over the parent compound, although it is clear that improved solubility is not solely responsible for improved potency, as racemic 9,10-methylenedioxycamptothecin is only 20% as active as the 10,11-isomer.45 For hexacyclic camptothecin analogues, hypotheses ranging from “extended coplanarity”42,46 to increased positive charge density47 have been posited. In our earlier proposed binding mode (Figure 4) the aromatic system (here, of 27h, which was chosen due to its initial high potency against top1) intercalates between the base pairs and the A-ring of the aromathecins abuts the nonscissile DNA strand as the ligand in the crystal structures of topotecan and camptothecin does.27-28,48 The E-ring faces the cleaved strand, and the quinoline nitrogen of the B-ring faces the general direction of Arg364, which it may hydrogen bond to (although it is out of distance in Figure 4).
Figure 3.
A-ring substituted camptothecins.
Figure 4.
Hypothetical model for binding of 2,3-ethylenedioxyaromathecin 27h in a Top1-DNA complex. The ligand is colored in magenta and all other relevant structures are labeled and colored by atom type. Distances are from heavy atom to heavy atom; the diagram is programmed for wall-eyed (relaxed) viewing.
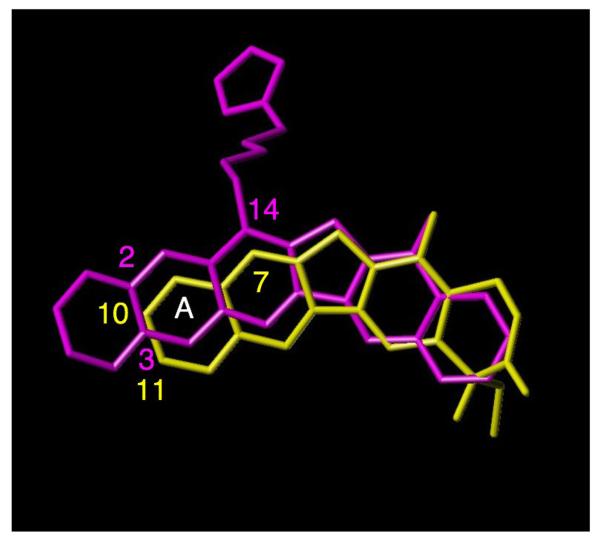
Overlaying hypothetical models of aromathecin 27h with the ligand in a camptothecin crystal structure (Figure 5) roughly superimposes the A-rings of the two systems. These compounds' ethylenedioxy groups could be positioned similarly and thus exert their effect via similar mechanisms, which could lend support to the proposed binding mode shown in Figure 4.
Figure 5.
Ligand overlay of aromathecin 27h (magenta) and camptothecin (1, yellow). Relevant positions are indicated in their respective colors and the A-ring is labeled.
10,11-Methylenedioxycamptothecins are potent cytotoxic Top1 inhibitors,45-46, 49-50 and are usually more so than the corresponding ethylenedioxycamptothecins, possibly due to the dioxolane ring's ability to better hold coplanarity.46 Disappointingly, the same effect was not observed for 2,3-methylenedioxyaromathecins. These compounds still retain notable antiproliferative activity (compound 29d possessed 22.3% inhibition at 10 μM as well) but there is no trend toward improved Top1 inhibition. In our experience, these compounds were very insoluble, and unfavorable chemical properties could hinder obtaining significant SAR data.
Other A-ring substituents lend additional support to a proposed camptothecin-like binding mode. In the elegant studies of Monroe Wall and colleagues, it is reported that methoxy substituents, including 10,11-dimethoxy substitution, attenuate bioactivity when placed on the A-ring of camptothecin.51-52 Remarkably, this trend is also observed for the aromathecins, indicating more SAR overlap. Compounds 30a-30e all possess minimal (all 0 or 0/+) anti-Top1 activity. Aromathecin 30b has only 14.2% mean growth inhibition at 10 μM, and the remainder of the series was declined for testing by the NCI. The bulkier, free-rotating methoxy groups could simply pose a steric liability toward the nonscissile strand or decrease the number of planar conformations available due to repulsions between the groups. Even before crystallography, it was proposed that the inactivity of dimethoxycamptothecins was caused by a steric “blocking” effect.52-53
The 2-Methyl-3-fluoro compounds 31b-g were originally synthesized to mimic fluorinated camptothecins such as exatecan (36) and diflomotecan (37)1,17 (Figure 3). Disappointingly, no increases in Top1 inhibitory activity were seen in this series, and antiproliferative activity generally decreased (neither compound 31c nor 31e induced >25% growth inhibition at 10 μM). This is not entirely surprising, as other 10,11-disubstitution patterns are reported to have deactivating effects for camptothecins as well. A comparison between compounds in this series and the clinical candidates in Figure 3 is perhaps unjustified due to the presence of other structural motifs (e.g. chiral amines, homolactones) that aromathecins lack. The increased antitumor activity observed for these fluorinated camptothecins could also be due to their lipophilicity, which increases partitioning to lipids and slows the rate of lactone hydrolysis in vitro and in vivo.54
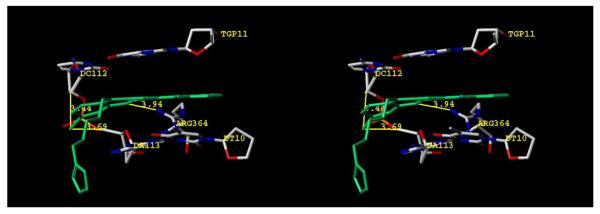
In light of these deactivating disubstitution patterns, it is worth mentioning that substitution at position 11 tends to diminish anti-Top1 activity for camptothecins, regardless of the substituent.55 This effect is identical for aromathecins, observed in the placement of both a methoxy (series 30) and fluorine (series 31) at the analogous 3-position. Placement of chlorine at this position (series 33) also abolishes most of the Top1 inhibitory activity, indicating the same steric constraints that hinder camptothecins at this position exist for the aromathecin system as well. Modeling compound 33b (Figure 6) in ternary complex with Top1 and DNA indicates that when this compound is minimized in our proposed “camptothecin-like” binding mode (Figure 4), the bulky, electronegative chlorine could pose steric and electronic liabilities when projected toward the phosphodiester backbone of the nonscissile strand. Upon minimization, the planar aromatic system of this model distorts to possibly avoid these clashes (Figure 6). Indeed, Staker et al.'s work indicates that this region is also sterically constrained in crystal structures of indenoisoquinolines and indolocarbazoles.10-11 Additionally, prior to these studies, Wang et al. proposed that modifications at this region of camptothecin (positions 9-11) could greatly affect its biochemical activity due to close proximity to flanking nucleotides.56 Nonetheless, cyclic groups (e.g. ethylenedioxy) are more restrained and could theoretically be better accommodated, as cyclic groups are also proposed to face the nonscissile strand for indenoisoquinolines.57 Even if substituted camptothecins bound in an alternative mode to fit these groups, it is likely that aromathecins behave similarly due to the trends in bioactivity. Antiproliferative activity for the 3-chloro series is also variable; (cf. compound 33b with 33d and 33e, the latter two of which did not inhibit cell growth beyond 15% at 10 μM).
Figure 6.
Hypothetical model for binding of 3-chloroaromathecin 33b in a Top1-DNA complex. The ligand is colored in green and all other relevant structures are labeled and colored by atom type. Distances are from heavy atom to heavy atom. The C, D, and E rings of the aromathecin are coplanar; note the substantial out-of-plane bending to relieve possible steric and/or electronic clashes on the A-ring side. Distances are heavy atom to heavy atom. The diagram is programmed for wall-eyed (relaxed) viewing.
Halogens improve anti-Top1 potency for camptothecins when placed at positions 9 and 10.45 The placement of chlorine at position 2 of the aromathecin system is tolerated in some cases but does not confer any advantages. Interestingly, these compounds possess relatively high antiproliferative activity (even 32b, which was not tested in the 5-dose assay, inhibited cell growth at nearly 30% at 10 μM). Nonetheless, the exact nature of this effect is unknown and several of these compounds may undergo additional testing. Ultimately, action at a second target besides Top1 may be responsible, although preliminary studies with aromathecins indicated that they do not inhibit Top2.28 Unfortunately, the isomeric 1-chloro series (34b-d) possessed less anti-Top1 activity in two out of three cases, but molecular models (not shown) did not indicate any obvious steric or electronic encumbrances. Although 9-chlorocamptothecin was among the most potent analogues synthesized in one study,45 the equivalent 1-chloroaromathecin series cannot be considered an outlier to the overlapping SAR and binding mode hypotheses. Many groups report that, in addition to halogens, a variety of 9-substituents including hydroxyl groups and amines,45,58 oxyimino moieties,59 alkyl chains60 and nitro groups61 can aid in improving Top1 inhibition, antitumor activity, bioavailability, and pharmacokinetics. Perhaps the effects of the 9-substituent could be more general than previously thought.59 Due to synthetic difficulties, no additional 1-substituted aromathecins have been prepared, and the full role 1-substitution plays has yet to be determined.
The idea that aromathecins may bind in a manner similar to camptothecins is not entirely novel. The former's 14 substituents, capable of hydrogen bonding, are calculated to project into the major groove of the ternary complex.27-28 Many 7-substituted camptothecins (Figure 3) also possess similar substituents that would also sit in the major groove region if they bound in the same pose as camptothecin and topotecan.10,15
4. Conclusions
In conclusion, eight novel series of A-ring-substituted aromathecin analogues were prepared by a practical, modular route beginning with commercially available starting materials. These analogues were assayed for Top1 inhibition and antiproliferative activity. Despite the lack of a strong correlation between anti-Top1 activity and cytotoxicity, it has been shown that varying the steric bulk, electronegativity, and hydrogen bonding properties of these A-ring substituents can drastically change the bioactivity of the aromathecin system. The nature of these SAR trends mirror those previously observed for camptothecins (with respect to the ethylenedioxy group, disubstitution, and positions 11 and 3), and these data, coupled with prior knowledge of positions 7 and 14, strongly support the hypothesis that these two systems share many essential SAR elements, which could provide a viable avenue for further optimization of this system. As there is no crystal structure available for aromathecins, these results also provide the only experimental evidence supporting our proposed camptothecin-like binding mode.
5. Experimental
5.1. General Procedures
Reagents and solvents were purchased from commercial vendors and were used without further purification. Melting points were determined in capillary tubes using a Mel-Temp apparatus and are not corrected. Infrared spectra were obtained as films on salt plates using CHCl3 or CDCl3 as the solvent unless otherwise specified, using a Perkin-Elmer Spectrum One FT-IR spectrometer, and are baseline- corrected. 1H NMR spectra were obtained at 300 or 500 MHz, using a Bruker ARX300 or Bruker Avance 500 (QNP probe or TXI 5 mm/BBO probe), respectively. Mass spectral analyses were performed at the Purdue University Campus-Wide Mass Spectrometry Center. ESIMS was performed using a FinniganMAT LCQ Classic mass spectrometer system. EI/CIMS was performed using a Hewlett-Packard Engine or GCQ FinniganMAT mass spectrometer system. APCI-MS was performed using an Agilent 6320 Trap mass spectrometer. Purity of all biologically relevant compounds is ≥95% by combustion microanalysis. Combustion microanalyses were performed by Galbraith Laboratories (Knoxville, TN), Midwest Microlab LLC (Indianapolis, IN), or at the Purdue University Microanalysis Laboratory using a Perkin-Elmer Series II CHNS/O model 2400 analyzer. All reported values are within 0.4% of calculated values. Analytical thin-layer chromatography was performed on Baker-flex silica gel IB2-F plastic-backed TLC plates. Compounds were visualized with both short and long wavelength UV light. Silica gel flash chromatography was performed using 40-63 μm flash silica gel. Precursor compounds 827-28 and 13, 14, and 1519,62 were prepared according to literature procedures and as depicted in schemes 1 and 2.
5.2 General Procedure for Chloroacylation of Substituted Anilines (10-11, 17, 20-21, and 23-24).35-37
Boron trichloride-methyl sulfide complex (approx. 1.80-5.20 g, 10.0-29.0 mmol, or 1.1-1.5 equiv., based on the aniline) was diluted with 1,2-dichloroethane (30-110 mL) and the mixture was cooled to 0 °C under an argon atmosphere. The aniline (1-4 g, ~10-26 mmol) was added dropwise, or, in the case of solid anilines, as a solution in a minimal amount of dichloroethane. The resulting chunky precipitate was stirred for 15 min, and chloroacetonitrile (for compounds 10, 17, 20-21, and 23-24) or chlorobutyronitrile (for 11) was added. For all but 17, aluminum chloride (approximately 1.2 equiv. based on the aniline) was then added, and the mixture was warmed to room temperature, stirred for approximately 15 min and heated at reflux for between 1.5-4 h (typical time: 3 h). The mixture was cooled, and 2 M HCl (an amount equivalent to the solvent volume of the reaction) was added. The mixture was heated to reflux for 0.5 h, and then cooled and poured into ice water (>100 mL) and the aqueous and organic layers were separated. The aqueous layer was exhaustively extracted with CH2Cl2, and the organic phases were washed with water and sat. NaCl and dried over anhydrous sodium sulfate. Concentration afforded crude products that were purified by precipitation, crystallization, or column chromatography.
5.2.1. 2-Amino-α-chloro-4,5-ethylenedioxyacetophenone (10).19
Boron trichloride-methyl sulfide complex (1.78 g, 9.92 mmol), compound 9 (1.00 g, 6.61 mmol), chloroacetonitrile (0.624 g, 8.27 mmol) and aluminum chloride (1.32 g, 9.92 mmol), in dichloroethane, provided a yellow solid that was isolated by precipitating with EtOAc-hexanes (0.251 g, 17%): mp 110 °C (lit.19 mp 130 °C). 1H NMR (300 MHz, CDCl3) δ 7.13 (s, 1 H), 6.15 (s, 1 H), 4.56 (s, 2 H), 4.31-4.28 (m, 2 H), 4.22-4.19 (m, 2 H).
5.2.2. 1-(7-Amino-2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-chloro-1-butanone (11)
Boron trichloride-methyl sulfide complex (5.23 g, 29.1 mmol), compound 9 (4.00 g, 26.5 mmol), 4-chlorobutyronitrile (3.42 g, 33.1 mmol) and aluminum chloride (3.89 g, 29.1 mmol), in dichloroethane, afforded a residue that was adsorbed onto SiO2 (7.64 g), and purified by flash column chromatography (SiO2, 59.6 g), eluting with CH2Cl2, to yield a clear-yellow oil. Residual 4-chlorobutyronitrile was removed in vacuo by gentle heating in a water bath (50 °C). The crude product was obtained as a sticky red solid, (2.00 g, 29%) contaminated with a small amount of nitrile. This material was used in the next step without further purification (to prepare 26a). 1H NMR (300 MHz, CDCl3) δ 7.34 (s, 1 H), 6.49 (s, 1 H), 4.31-4.23 (m, 4 H), 3.68 (t, J = 6.3 Hz, 2 H), 3.06 (t, J = 7.0 Hz, 2 H), 2.25-2.10 (m, 2 H).
5.2.3. 2-Amino-α-chloro-4,5-(dimethoxy)acetophenone (17).36
Boron trichloride-methyl sulfide complex (3.69 g, 20.6 mmol), compound 16 3.00 g, 19.6 mmol), and chloroacetonitrile (1.78 g, 23.5 mmol), in dichloroethane, afforded a brown semisolid that was dissolved in CH2Cl2 and filtered through a pad of SiO2 to remove black impurities. The pad was rinsed with CH2Cl2 (120 mL) until the filtrate ran clear and colorless. The filtrate was concentrated to yield a brown solid (2.33 g, 52%) after washing with hexanes (40 mL): mp 113-115 °C (lit36 mp 123-124 °C). 1H NMR (300 MHz, CDCl3) δ 7.00 (s, 1 H), 6.50-6.20 (br s, 2 H), 6.12 (s, 1 H), 4.58 (s, 2 H), 3.89 (s, 3 H), 3.84 (s, 3 H).
5.2.4. 2-Amino-4-fluoro-5-methyl-α-chloroacetophenone (20)
Boron trichloride-methyl sulfide complex (5.16 g, 28.8 mmol), aniline 18 (3.00 g, 23.0 mmol), chloroacetonitrile (2.26 g, 30.0 mmol), and aluminum chloride (4.79 g, 36.0 mmol), in dichloroethane, afforded a residue that was adsorbed onto SiO2 (8.84 g) and purified by flash column chromatography (SiO2), eluting with a gradient of 50% hexanes in CH2Cl2 to CH2Cl2 to yield the title compound. Additional column fractions yielded residue that, after recrystallization from boiling hexanes (100 mL), afforded the product as fine yellow-green needles. A total of 0.781 g (16%) was obtained: mp 111-113 °C. IR (film) 3449, 3341, 2946, 1656, 1639, 1592, 1553, 1229, 1139, 867, 849, 781, 619; 1H NMR (300 MHz, CDCl3) δ 7.47 (d, J = 8.3 Hz, 1 H), 6.35 (d, J = 11.5 Hz, 1 H), 6.30 (br s, 2 H), 4.62 (s, 2 H), 2.18 (s, 3 H); CIMS m/z (rel intensity) 202 (MH+, 100).
5.2.5. 5-Chloro-2-amino-α-chloroacetophenone (21).36
Boron trichloride-methyl sulfide complex (3.08 g, 17.2 mmol), 4-chloroaniline (19, 2.00 g, 15.6 mmol), chloroacetonitrile (1.47 g, 19.5 mmol), and aluminum chloride (2.50 g, 18.7 mmol), in dichloroethane, afforded a black gum. This residue was adsorbed onto SiO2 (4.17 g) and purified by flash column chromatography (SiO2, 56.8 g), eluting with 50% hexanes in CH2Cl2, to yield an iridescent green solid (0.230, 7.2%): mp 130-133 °C (lit36 mp 140-141 °C.) 1H NMR (300 MHz, CDCl3) δ 7.59 (d, J = 2.3 Hz, 1 H), 7.27-7.23 (m, 1 H), 6.67 (d, J = 8.9 Hz, 1 H), 6.30 (br s, 1 H), 4.64 (s, 2 H).
5.2.6. 4-Chloro-2-amino-α-chloroacetophenone (23), 6-Chloro-2-amino-αchloroacetophenone (24), and Their Respective N-Acetyl Derivates 25 and 26.36
Boron trichloride-methyl sulfide complex (4.63 g, 25.9 mmol), compound 22 (3.00 g, 23.5 mmol), chloroacetonitrile (2.21 g, 29.5 mmol), and aluminum chloride (3.76 g, 28.2 mmol), in dichloroethane, yielded a green solid. Both 23 and 24 were present by TLC. The residue was adsorbed onto SiO2 (11.8 g), and was purified by flash column chromatography (SiO2, 91.4 g), eluting with 25% hexanes in CH2Cl2, to remove unwanted by-products.
Synthesis and Separation of 25 and 26
The obtained residue containing 23 and 24 was diluted with acetic anhydride (10 mL), and the mixture was heated to 80 °C for 30 min, upon which it became a dark red. The mixture was concentrated to remove excess acetic anhydride, and was adsorbed onto SiO2 (8.52 g). The residue was purified by flash column chromatography (SiO2, 54.9 g), eluting with a gradient of 10% hexanes in CH2Cl2 to 1% MeOH in CH2Cl2 to yield, first, 25 (0.780 g, 13%), as white needles after recrystallization from 50% EtOH in CH2Cl2 and washing with hexanes: mp 131-132 (lit36 mp 139-140 °C). 1H NMR (300 MHz, CDCl3) δ 11.4 (br s, 1 H), 8.91 (d, J = 2.0 Hz, 1 H), 7.77 (d, J = 8.5 Hz, 1 H), 7.14 (dd, J = 6.9, 1.9 Hz, 1 H), 4.73 (s, 2 H), 2.26 (s, 3 H) and then 26 (0.270 g, 5%), as an orange solid: mp 115-117 °C (lit36 mp130-132 °C). 1H NMR (300 MHz, CDCl3) δ 8.19 (br s, 1 H), 8.02 (d, J = 8.2 Hz, 1 H), 7.44 (t, J = 8.2 Hz, 1 H), 7.27 (t, J = 9.5 Hz, 1 H), 4.71 (s, 2 H), 2.19 (s, 3 H).
Hydrolysis of the Acetanilides: 4-Chloro-2-amino-α-chloroacetophenone (23).36
Compound 25 (0.780 g, 3.17 mmol) was dissolved in EtOH (40 mL), and concd HCl (5 mL) was added. The mixture was heated to reflux for 1 h, and the resultant dark orange solution was cooled and poured into ice water (150 mL). 2 M NaOH was added until the pH was approximately 9. The mixture was extracted with CH2Cl2 (3 × 150 mL), and the organic phase was washed with H2O (200 mL), dried over anhydrous sodium sulfate, and concentrated to yield 23 as an orange iridescent solid (0.600 g, 93% from 25, 12% from 22): mp 126-128 °C (lit36 mp 134-135 °C). 1H NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.7 Hz, 1 H), 6.71 (d, J = 1.9 Hz, 1 H), 6.65 (dd, J = 7.5, 2.0 Hz, 1 H). 6.39 (br s, 2 H), 4.63 (s, 2 H).
5.2.7. 6-Chloro-2-amino-α-chloroacetophenone (24).36
Compound 26 (0.270 g, 1.10 mmol) was diluted in EtOH (12 mL), and concd HCl (3 mL) was added. The mixture was heated at reflux for 1 h 15 min, cooled, and poured into ice water (50 mL). 2 M NaOH was added until the pH was approximately 9, and the mixture was extracted with CH2Cl2 (2 × 40 mL). The organic layers were washed with H2O (1 × 100 mL), dried over anhydrous sodium sulfate, and concentrated. The residue was adsorbed onto SiO2 (5.0 g), and was purified by flash column chromatography (SiO2, 28.5 g), eluting with CH2Cl2 to yield a yellow-green solid (0.158 g, 71% from 26, 3.3% from 22): mp 50.5-52 °C (lit36 mp 60-61 °C). 1H NMR (300 MHz, CDCl3) δ 7.16 (t, J = 8.1 Hz, 1 H), 6.76 (d, J = 7.9 Hz, 1 H), 6.63 (d, J = 8.3 Hz, 1 H), 4.97 (br s, 1 H), 4.74 (s, 2 H).
5.3. Synthesis of Aromathecin Cores (27a-34a)
5.3.1. 14-Chloromethyl-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (27a)
Compound 8 (0.100 g, 0.502 mmol) and compound 10 (0.114 g, 0.502 mmol) were diluted with benzene (30 mL). p-TsOH monohydrate (0.005 g, 0.026 mmol) was added and the solution was heated at reflux for 16 h using a Dean-Stark trap to collect azeotroped water. The solution was concentrated, diluted with CHCl3 (175 mL) and washed with sat NaHCO3 (3 × 50 mL) and sat NaCl (50 mL). The organic layer was dried over sodium sulfate, concentrated, and purified by flash column chromatography (SiO2), eluting with a gradient of CHCl3 to 3% MeOH in CHCl3 to provide a yellow solid (0.107 g, 55%): mp 275 °C (dec). IR (KBr) 1655, 1628, 1605, 1288, 1243, 1226, and 1068 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 8.1 Hz, 1 H), 7.79-7.57 (m, 5 H), 7.55 (s, 1 H), 5.40 (s, 2 H), 4.95 (s, 2 H), 4.45 (s, 4 H); ESIMS m/z (rel intensity) 391/393 (MH+, 96/33). Anal. Calcd for C22H15ClN2O3·0.5 H2O: C, 66.09; H, 4.03; N, 7.01. Found: C, 66.02; H, 3.97; N, 7.14.
5.3.2. 14-(3′-Chloropropyl)-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28a)
Compound 11 (0.481 g, 1.88 mmol) was suspended in CHCl3 (5 mL). Compound 8 (0.300 g, 1.50 mmol) was added, followed by p-TsOH (0.285 g, 1.50 mmol), glacial acetic acid (2 mL), and toluene (60 mL). The mixture was heated at reflux for 17.5 h, using a Dean-Stark trap. The mixture was cooled to room temperature, concentrated, and the residue was suspended in a mixture of MeOH (30 mL) and CHCl3 (100 mL). The suspension was washed with sat NaHCO3 (200 mL) and H2O (200 mL). The aqueous layer was extracted with CHCl3 (50 mL). The organic layers were washed with H2O (200 mL), sat. NaCl (125 mL), dried over anhydrous sodium sulfate, and treated with decolorizing carbon (0.100 g). The mixture was filtered, concentrated, adsorbed onto SiO2 (3.80 g), and purified by flash column chromatography (SiO2, 62.8 g), eluting with CHCl3, to yield a yellow amorphous solid (0.275 g, 44%) after washing with MeOH (10 mL): mp 255-258 °C. IR (film) 3401, 2930, 1659, 1627, 1603, 1506, 1440, 1287, 1244, 1068, 915, 870, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 8.2 Hz, 1 H), 7.77-7.52 (m, 5 H), 7.48 (s, 1 H), 5.31 (s, 2 H), 4.43 (s, 4 H), 3.69 (t, J = 6.0 Hz, 2 H), 3.27 (t, J = 7.5 Hz, 2 H), 2.27-2.17 (m, 2 H). Anal. Calcd for C24H19ClN2O3 0.75 H2O: C, 66.67; H, 4.78; N, 6.48. Found: C, 66.28; H, 4.46; N, 6.40.
5.3.3. 14-Chloromethyl-2,3-methylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (29a)
Compound 8 (0.200 g, 1.00 mmol) and compound 15 (0.236 g, 1.10 mmol) were diluted with toluene (30 mL) and p-TsOH (0.190 g, 1.00 mmol) was added. The mixture was heated at reflux, using a Dean-Stark trap, for 23.5 h. The bright orange suspension was cooled and concentrated, and the residue was suspended in CHCl3 (100 mL) and washed with sat. NaHCO3 (100 mL). The aqueous layer was extracted with CHCl3 (5 × 50 mL). The combined organic layers were dried over anhydrous sodium sulfate and concentrated to yield a yellow amorphous solid (0.310 g, 82%) after washing with THF (40 mL) and drying: mp 325-327 °C (dec). IR (film) 2916, 1657, 1619, 1597, 1499, 1462, 1339, 1252, 1034, 866, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.1 Hz, 1 H), 7.79-7.70 (m, 2 H) 7.60-7.53 (m, 3 H), 7.43 (s, 1 H), 6.21 (s, 2 H), 5.40 (s, 2 H), 4.95 (s, 2 H); ESIMS m/z (rel intensity) 377/379 (MH+, 100/35).
5.3.4. 14-Chloromethyl-2,3-dimethoxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (30a)
Compound 8 (0.600 g, 3.01 mmol), compound 17 (0.760 g, 3.31 mmol) and p-TsOH (0.572 g, 3.01 mmol) were diluted with benzene (80 mL). The mixture was heated at reflux for 17 h using a Dean-Stark trap to collect azeotroped water. The mixture was cooled and the precipitate was filtered. The tan solid collected was washed with MeOH (10 mL), diluted with CHCl3, and the organic phase was washed with sat. NaHCO3 (200 mL), H2O (2 × 200 mL), and dried over anhydrous sodium sulfate. The solution was concentrated and adsorbed onto SiO2 (7.12 g) and the residue was purified by flash column chromatography (SiO2), eluting with CHCl3 to 0.5% MeOH in CHCl3, to yield a yellow amorphous solid (0.267 g, 23%) after washing with cold CHCl3 (5 mL), MeOH (10 mL), and ether (20 mL): mp 308-311 °C (dec). IR (KBr) 3427, 1654, 1626, 1606, 1590, 1576, 1507, 1482, 1434, 1358, 1255, 1222, 1012, 844, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 7.9 Hz, 1 H), 7.75-7.72 (m, 2 H), 7.59-7.54 (m, 3 H), 7.32 (s, 1 H), 5.42 (s, 2 H), 5.01 (s, 2 H), 4.10 (s, 6 H); ESIMS m/z (rel intensity) 393/335 (MH+, 100/33). Anal. Calcd for C22H17ClN2O3·1.5 H2O: C, 62.94; H, 4.80; N, 6.67. Found: C, 63.04; H, 4.57; N, 6.67.
5.3.5. 14-Chloromethyl-3-fluoro-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31a)
Compound 8 (0.400 g, 2.01 mmol), compound 20 (0.506 g, 2.51 mmol) and p-TsOH (0.382 g, 2.01 mmol) were diluted with benzene (70 mL) and the mixture was heated at reflux using a Dean-Stark trap. After 16.5 h, another 0.070 g (0.35 mmol) of 20 was added, and reflux was continued for 1 h 20 min. The mixture was cooled, concentrated, and the residue was suspended in CHCl3 (100 mL) and washed with sat. NaHCO3 (100 mL). The aqueous layers were extracted with CHCl3 (100 mL). The combined organic layers were washed with H2O (200 mL), following which the aqueous layer was again extracted with CHCl3 (50 mL). The combined organic layers were washed with sat NaCl (200 mL). The aqueous phase was again extracted with CHCl3 (50 mL). The combined organic layers were dried over anhydrous sodium sulfate, concentrated, and adsorbed onto SiO2 (8.30 g). Purification by flash column chromatography (SiO2), eluting with a gradient of CHCl3 to 4% MeOH in CHCl3, resulted in precipitation of the compound on the column. Elution was continued with CHCl3. The obtained orange powder was boiled in tetrahydrofuran (80 mL), filtered, and washed with CHCl3 and ether to yield the title compound as a pale orange powder (0.464 g, 63%): mp 300-303 °C (dec). IR (KBr pellet) 3028, 3923, 2363, 2342, 1657, 1631, 1602, 1480, 1435, 1341, 1232, 1222, 1133, 899, 768, 726, 690 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.57 (d, J = 7.8 Hz, 1 H), 7.99 (d, J = 7.8 Hz, 1 H), 7.86-7.57 (m, 5 H), 5.44 (s, 2 H), 5.03 (s, 2 H), 2.58 (s, 3 H); ESIMS m/z (rel intensity) 365 (MH+, 100).
5.3.6. 2-Chloro-14-chloromethyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (32a)
Compound 8 (0.200 g, 1.00 mmol), compound 21 (0.230 g, 1.12 mmol) and p-TsOH (0.190 g, 1.00 mmol) were diluted with toluene (30 mL) and the mixture was heated at reflux for 13.5 h using a Dean-Stark trap. The mixture was cooled, concentrated, and the residue was diluted with CHCl3 (100 mL). The solution was washed with sat. NaHCO3 (100 mL). H2O (100 mL) was added, and the aqueous phase was exhaustively extracted with CHCl3 (1 × 50, 6 × 25 mL). The resultant cloudy suspension was dried over anhydrous sodium sulfate and concentrated to yield a yellow-green solid (0.339 g, 92%) after washing with MeOH (50 mL) and ether (10 mL): mp 276-277 °C (dec). IR (film) 2917, 1665, 1537, 1442, 1343, 1138, 822, 751, 686 cm−1; 1H NMR(300 MHz, CDCl3) δ 8.57 (d, J = 8.0 Hz, 1 H), 8.21 (d, J = 9.0 Hz, 1 H), 8.15 (d, J = 2.1 Hz, 1 H), 7.81-7.59 (m, 5 H), 5.46 (s, 2 H), 5.01 (s, 2 H); ESIMS m/z (rel intensity) 367 (MH+, 100).
5.3.7. 3-Chloro-14-chloromethyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (33a)
Compound 8 (0.200 g, 1.00 mmol) and compound 23 (0.230 g, 1.12 mmol) were diluted with toluene (40 mL) and p-TsOH (0.190 g, 1.00 mmol) was added. The mixture was heated at reflux for 17 h using a Dean-Stark trap. The mixture was cooled and concentrated, and the residue was diluted with CHCl3 (100 mL), and the suspension was washed with sat. NaHCO3 (100 mL). The aqueous layer was extracted with CHCl3 (4 x 50 mL), and the organic layers were dried over anhydrous sodium sulfate and concentrated to yield a yellow solid (0.314 g, 86%) after washing with MeOH (25 mL): mp 301.5-303 °C (dec). IR (film) 3584, 3369, 2922, 1656, 1622, 1604, 1449, 1342, 1183, 1075, 923, 751, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.58 (d, J = 7.8 Hz, 1 H), 8.28 (d, J = 2.0 Hz, 1 H), 8.15 (d, J = 9.0 Hz, 1 H), 7.80-7.61 (m, 5 H), 5.46 (s, 2 H), 5.04 (s, 2 H); ESIMS m/z (rel intensity) 368 (MH+, 65). 331 (MH-HCl, 100).
5.3.8. 1-Chloro-14-chloromethyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (34a)
Compound 8 (0.175 g, 0.88 mmol) and compound 24 (0.200 g, 0.98 mmol) were diluted with toluene (40 mL) and p-TsOH (0.167 g, 0.88 mmol) was added. The mixture was heated to reflux for 4.5 h using a Dean-Stark trap. The mixture was stirred at room temperature for 13 h. The mixture was concentrated, and the residue was diluted with CH2Cl2 (150 mL), and the suspension was washed with sat. NaHCO3 (150 mL). The aqueous layer was exhaustively extracted with CH2Cl2 (3 × 75, 3 × 50 mL). MeOH was added to the remaining aqueous suspension, which was extracted with CH2Cl2 (3 × 50 mL). This suspension was washed with H2O (2 × 100 mL). The suspension and combined organic layers were dried over anhydrous sodium sulfate and concentrated to yield a yellow amorphous solid (0.305 g, 95%) after washing with MeOH (50 mL) and ether (30 mL): mp 300-302 °C (dec). IR (film) 1657, 1628, 1444, 1380, 1206, 1124, 925, 883, 850, 814, 766, 754, 725, 688 cm−1; 1H NMR (300 MHz, CDCl3/CF3COOD) δ 8.61 (d, J = 8.2 Hz, 1 H), 8.47 (dd, J = 6.8, 3.0 Hz, 1 H), 8.33 (s, 1 H), 8.08-7.90 (m, 5 H), 5.78 (s, 2 H), 5.57 (s, 2 H); APCI-MS m/z (rel intensity) 367 (MH, base-peak).
5.4. General Procedure for Synthesis of 14-Substituted Aromathecins (Series 27 and 29-34).27-28
The chlorinated aromathecin core (between 0.050 and 0.115 g, or 0.140-0.300 mmol) was diluted in DMSO (20-30 mL, for room temperature reactions), or an equivalent amount of dry DMF (for some reactions performed at higher temperature). A nucleophile (between a three- and tenfold excess, typically between 5-6 equivalents) was then added. In the case of amine salts, approximately 10 equivalents of Et3N were added. The mixture was stirred overnight (between 12-24 h). The product was extracted by either diluting the reaction mixture with CHCl3 (between 50-100 mL) and washing with water (at least 90 mL) and sat. NaCl, or alternatively, by pouring the reaction mixture into H2O (~100 mL) and extracting with CHCl3 (at least 100 mL). The organic layers were dried over anhydrous sodium sulfate and concentrated, and the residue was purified by flash column chromatography (loading by either diluting the residue in an appropriate solvent or adsorbing onto between 3-5 g of SiO2) on 20-30 g SiO2. The obtained solids were then washed with ether or hexane (30-50 mL) and dried. The preparation of salts is described under the subheadings for individual compounds.
5.4.1. 14-N,N-Dimethylaminomethyl-2,3-ethylenedioxy-12H-5,11adiazadibenzo[b,h]fluoren-11-one (27b)
Compound 27a (0.090 g, 0.230 mmol), dimethylamine hydrochloride (0.094 g, 1.15 mmol) and Et3N (0.16 mL), 1.15 mmol), in DMSO yielded the title compound as a brown solid (0.057 mg, 57%) after flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 4% MeOH in CHCl3) and washing with ether (50 mL): mp 249-251 °C (dec). IR (KBr) 1659, 1632, 1607, 1505, 1287, 1245, 1064 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.34 (d, J = 7.7 Hz, 1 H), 7.95 (d, J = 7.8 Hz, 1 H), 7.81-7.75 (m, 2 H), 7.60 (m, 1 H), 7.58 (s, 1 H), 7.49 (s, 1 H), 5.29 (s, 2 H), 4.42 (s, 4 H), 3.86 (s, 2 H), 2.24 (s. 6 H); ESIMS m/z (rel intensity) 400 (MH+, 100). Anal. Calcd for C24H21N3O3: C, 72.16; H, 5.30; N,10.52. Found: C, 72.08; H, 5.19; N, 10.24.
5.4.2. 2,3-Ethylenedioxy-14-(1′-imidazolylmethyl)-2H-5,11a-diazadibenzo[b,h]fluoren-11-one (27c)
Compound 27a (0.116 g, 0.297 mmol) and imidazole (0.060 g, 0.891 mmol) in DMSO at 100 °C for 2 h, yielded the title compound as a yellow solid (0.056 mg, 45%) after flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 7% MeOH in CHCl3) and washing with diethyl ether (50 mL): mp 270 °C (dec). IR (KBr) 1656, 1627, 1605, 1505, 1291, 1246 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.33 (d, J = 7.7 Hz, 1 H), 7.96-7.92 (m, 2 H), 7.82-7.76 (m, 1 H), 7.65 (s, 1 H), 7.61-7.54 (m, 3 H), 7.25 (s, 1 H), 6.92 (s, 1 H), 5.80 (s, 2 H), 5.20 (s, 2 H), 4.42 (s, 4 H); ESIMS m/z (rel intensity) 423 (MH+, 100). Anal. Calcd for C25H18N4O30.75 H2O: C, 68.88; H, 4.51; N, 12.85. Found: C, 69.15; H, 4.30; N, 12.66.
5.4.3. 2,3-Ethylenedioxy-14-(N-methylpiperazinylmethyl)-12H-5,11adiazadibenzo[b,h]fluoren-11-one Trifluoroacetate (27d)
Compound 27a (0.090 g, 0.230 mmol) and N-methylpiperazine (0.069 g, 0.690 mmol) in DMSO yielded the desired compound as a brown solid after flash column chromatography (SiO2), eluting with a gradient of CHCl3 to 7% MeOH in CHCl3. The obtained compound was washed with ether and diluted with CHCl3 (40 mL) and trifluoroacetic acid (2 mL) was added. The reaction mixture was allowed to stir at room temperature for 30 min, concentrated, and the residue was triturated with diethyl ether. The obtained precipitate was filtered and washed with diethyl ether (50 mL) to provide a yellow solid (0.113 g, 86%): mp 238-242 °C. IR (KBr) 3430, 1661, 1505, 1287, 1243, 1181, 1067 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 9.39 (br s, 1 H) 8.36 (d, J = 7.4 Hz, 1 H), 7.98 (d, J = 7.6 Hz, 1 H), 7.82-7.78 (m, 2 H), 7.62-7.57 (m, 2 H), 7.54 (s, 1 H), 5.36 (s, 2 H), 4.50-4.00 (br m, 2 H), 4.44 (s, 4 H), 4.07 (s, 2 H), 3.37 (m, 2 H), 3.03 (m, 4 H), 2.80 (d, J = 4.2 Hz, 3 H); ESIMS m/z (rel intensity) 455 (MH+, 100). Anal. Calcd for C29H27F3N4O50.25 H2O: C, 60.78; H, 4.84; N, 9.78. Found: C, 60.68; H, 4.74; N, 9.56.
5.4.4. 2,3-Ethylenedioxy-14-(1′-morpholinomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (27e)
Compound 27a (0.090 g, 0.230 mmol) and morpholine (0.060 g, 0.690 mmol) in
DMSO yielded the desired compound as a brown solid after flash column chromatography (SiO2), eluting with a gradient of CHCl3 to 2% MeOH in CHCl3. The product was washed with ether (50 mL) and treated with TFA (as described for 27d), but only the free base was obtained upon drying, as a yellow-brown solid (0.088 g, 86%): mp 150-155 °C (dec). IR (KBr) 1661, 1630, 1507, 1290, 1246, 1200, 1176, 1129, 1068 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.34 (d, J = 8.2 Hz, 1 H), 7.96 (d, J = 7.9 Hz, 1 H), 7.81-7.76 (m, 2 H), 7.61-7.56 (m, 2 H), 7.50 (s, 1 H), 5.34 (s, 2 H), 4.43 (s, 4 H), 3.96 (s, 2 H), 3.56 (br s, 4 H), 2.50 (br s, 4 H); ESIMS m/z (rel intensity) 442 (MH+, 100). Anal. Calcd for C26H23N2O40.5 H2O: C, 69.32; H, 5.37; N, 9.33. Found: C, 69.46; H, 5.04; N, 9.08.
5.4.5. 14-(N-Ethanolaminomethyl)-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trifluoroacetate (27f)
Compound 27a (0.090 g, 0.230 mmol) and ethanolamine (0.042 g, 0.691 mmol) in DMSO afforded the desired compound as a dark yellow solid after flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 7% MeOH in CHCl3) and washing with ether (50 mL). The obtained precipitate was treated with TFA as described for 27d, and was filtered and washed with diethyl ether (50 mL) to provide a brown-orange solid (0.101 g, 83%): mp 180-183 °C. IR (KBr) 3424, 1658, 1622, 1505, 1290, 1245, 1200 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.92 (br s, 2 H), 8.37 (d, J = 8.2 Hz, 1 H), 8.00 (d, J = 7.9 Hz, 1 H), 7.88 (s, 1 H), 7.85-7.79 (m, 1 H), 7.64-7.59 (m, 3 H), 5.53 (s, 2 H), 4.78 (br s, 2 H), 4.48 (s, 4 H), 3.79 (t, J = 5.51 Hz, 2 H), 3.37 (m, 2 H); the hydroxyl group is absent due to line-broadening; ESIMS m/z (rel intensity) 416 (MH+, 100). Anal. Calcd for C26H22F3N3O6: C, 58.98; H, 4.19; N, 7.94. Found: C, 59.22; H, 4.21; N, 7.89.
5.4.6. 2,3-Ethylenedioxy-14-[N-(S)-3′-hydroxypyrrolidinomethyl]-12H-5,11a diazadibenzo[b,h]fluoren-11-one (27g)
Compound 27a (0.065 g, 0.166 mmol), (S)-pyrrolidin-3-ol hydrogen maleate38 (0.102 g, 0.499 mmol), and Et3N (0.168 g, 1.66 mmol) in DMSO afforded the desired product as a tan solid (0.048 g, 64%) after flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 2% MeOH in CHCl3) and washing with hexanes: mp 235-237 °C (dec). IR (film) 3369, 2930, 1659, 1623, 1603, 1505, 1441, 1287, 1243, 1068, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 7.9 Hz, 1 H), 7.77-7.52 (m, 6 H), 5.38 (s, 2 H), 4.42 (s, 4 H), 4.36 (br s, 1 H), 4.08 (s, 2 H), 2.94-2.90 (m, 1 H), 2.70-2.62 (m, 2 H), 2.46-2.42 (m, 1 H), 2.26-2.20 (m, 1 H), 1.95 (bd, J = 7.0 Hz, 1 H), 1.79-1.75 (m, 1 H); ESIMS m/z (rel intensity) 442 (MH+, 100). Anal. Calcd for C26H23N3O4·H2O: C, 67.96; H, 5.48; N, 9.14. Found: C, 68.28; H, 5.22; N, 9.02.
5.4.7. 2,3-Ethylenedioxy-14-[(1′-imidazolyl)propylaminomethyl]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (27h)
Compound 27a (0.065 g, 0.166 mmol) and 1-(3-aminopropyl)imidazole (0.062 g, 0.499 mmol) in DMSO afforded the desired compound as a bright yellow amorphous solid (0.044 g, 56%) after flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 3% MeOH, with a few drops of Et3N) and washing with ether: mp 203-207 °C (dec.) IR (film) 3368, 2929, 1658, 1622, 1602, 1506, 1287, 1245, 1067 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.0 Hz, 1 H), 7.76-7.56 (m, 6 H), 7.47 (s, 1 H), 7.26 (s, 1 H), 6.87 (s, 1 H), 5.37 (s, 2 H), 4.44 (s, 4 H), 4.23 (s, 2 H), 4.08 (t, J = 6.9 Hz, 2 H), 2.76 (t, J = 6.6 Hz, 2 H), 2.01-1.90 (m, 2 H); the amine exchanges with residual water; ESIMS m/z (rel intensity) 480 (MH+, 100). Anal. Calcd for C28H25N5O3·1.5 H2O: C, 66.39; H, 5.57; N, 13.83. Found: C, 66.42; H, 5.57; N, 13.64.
5.4.8. 2,3-Ethylenedioxy-14-(3′-morpholinopropylaminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (27i)
Compound 27a (0.065 g, 0.166 mmol) and 3-morpholinopropylamine (0.072 g, 0.499 mmol) in DMSO afforded the title compound as a flocculent yellow amorphous solid (0.052 g, 63%) after flash column chromatography (SiO2, eluting with a gradient of 0.5% Et3N in CHCl3 to 1.5% MeOH-1% Et3N in CHCl3) and washing with diethyl ether: mp 202-205 °C. IR (film) 3401, 2921, 1658, 1629, 1603, 1506, 1446, 1289, 1244, 1115, 1068, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.0 Hz, 1 H), 7.77-7.53 (m, 6 H), 5.39 (s, 2 H), 4.42 (s, 4 H), 4.23 (s, 2 H), 3.66 (t, J = 4.5 Hz, 4 H), 2.81 (t, J = 6.5 Hz, 2 H), 2.42-2.38 (m, 6 H), 1.77-1.70 (m, 2 H), 1.60-1.50 (br m, obscured by residual water, 1 H); ESIMS m/z (rel intensity) 499 (MH+, 100). Anal. Calcd for C29H30N4O4·1.5 H2O: C, 66.27; H, 6.33; N, 10.66. Found: C, 66.29; H, 6.14; N, 10.63.
5.4.9. 2,3-Methylenedioxy-14-[1′-(N-methylpiperazinylmethyl)]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (29b)
Compound 29a (0.070 g, 0.185 mmol) and N-methylpiperazine (0.074 g, 0.743 mmol) in DMSO afforded the title compound as a pale-yellow amorphous solid (0.063 g, 77%) after flash column chromatography (SiO2, 24.2 g, eluting with 0.5% MeOH in CHCl3 to 5% MeOH in CHCl3) and washing with ether (60 mL): mp 288-291 °C (dec). IR (film) 3369, 2926, 2807, 1662, 1622, 1587, 1501, 1464, 1247, 1034, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 7.9 Hz, 1 H), 7.77-7.69 (m, 2 H), 7.66 (s, 1 H), 7.58-7.54 (m, 2 H), 7.48 (s, 1 H), 6.18 (s, 2 H), 5.39 (s, 2 H), 3.93 (s, 2 H), 2.70-2.30 (br m, 8 H), 2.29 (s, 3 H); ESIMS m/z (rel intensity) 441 (MH+, 100). Anal. Calcd for C26H24N4O3·0.25 H2O: C, 70.18; H, 5.55; N, 12.59. Found: C, 70.07; H, 5.45; N, 12.59.
5.4.10 14-(N-Ethanolaminomethyl)-2,3-methylenedioxy-12H-5,11a diazadibenzo[b,h]fluoren-11-one (29c)
Compound 29a (0.065 g, 0.172 mmol) and ethanolamine (0.042 g, 0.690 mmol) in DMSO afforded the title compound as a flocculent yellow solid (0.042 g, 61%) after flash column chromatography (SiO2, 24.8 g, eluting with a gradient of 0.5% MeOH in CHCl3 to 4% MeOH in CHCl3), washing with ether (25 mL) and drying in vacuo: mp 225-228 °C (dec). IR (film) 3401, 2917, 2342, 1655, 1618, 1493, 1462, 1337, 1250, 1038, 847, 757, 687 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.33 (d, J = 7.9 Hz, 1 H), 7.94 (d, J = 7.9 Hz, 1 H), 7.80 (t, J = 7.0 Hz, 1 H), 7.73 (s, 1 H), 7.59 (t, J = 7.3 Hz, 1 H), 7.52 (s, 1 H), 7.45 (s, 1 H), 6.26 (s, 2 H), 5.37 (s, 2 H), 4.55 (t, J = 5.2 Hz, 1 H), 4.21 (s, 2 H), 3.52 (q, J = 5.4 Hz, 1 H), 2.71 (t, J = 5.8 Hz, 2 H); the amine proton is not visible due to exchange with residual water; ESIMS m/z (rel intensity) 402 (MH+, 100). Anal. Calcd for C23H19N3O4·1.25 H2O: C, 65.16; H, 5.11; N, 9.91. Found: C, 65.35; H, 4.78; N, 9.87.
5.4.11. 14-(N,N-Dimethylaminomethyl)-2,3-methylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (29d)
Compound 29a (0.070 g, 0.186 mmol) and N,N-dimethylamine (0.56 mL, 2 M in THF) in DMSO afforded the title compound as a yellow powder (0.046 g, 64%) after flash column chromatography (SiO2, 29.4 g, eluting with 0.25% Et3N in CHCl3) and washing with ether (40 mL): mp 272-277 °C (dec). IR (film) 3585, 2916, 1661, 1623, 1605, 1463, 1337, 1247, 1034 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.13 Hz, 1 H), 7.75-7.66 (m, 3 H), 7.60-7.50 (m, 2 H), 7.48 (s, 1 H), 6.16 (s, 2 H), 5.38 (s, 2 H), 3.84 (s, 2 H), 2.33 (s, 6 H); ESIMS m/z (rel intensity) 386 (MH+, 100). Anal. Calcd for C23H19N3O3·0.5 H2O: C, 70.04; H, 5.11; N, 10.65. Found: C, 70.17; H, 5.02; N, 10.27.
5.4.12. 14-[N-(S)-3′-Hydroxypyrrolidinomethyl]-2,3-methylenedioxy-12H-5,11a diazadibenzo[b,h]fluoren-11-one (29e)
Compound 29a (0.065 g, 0.172 mmol), (S)-pyrrolidin-3-ol hydrogen maleate (0.141 g, 0.690 mmol) and Et3N (0.347 g, 3.44 mmol) in DMSO afforded the title compound as a yellowish-tan powder (0.051 g, 69%) after flash column chromatography (SiO2, 27.6 g, eluting with a gradient of 0.6% MeOH in CHCl3 to 1.75% MeOH in CHCl3) and washing with ether (25 mL): mp 260-263 °C (dec). IR (film) 3436, 2916, 2343, 1656, 1619, 1601, 1465, 1338, 1248, 1035, 944, 758, 687 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.33 (d, J = 8.0 Hz, 1 H), 7.94 (d, J = 8.0 Hz, 1 H). 7.80 (t, J = 7.2 Hz, 1 H), 7.76 (s, 1 H), 7.59 (t, J = 7.2 Hz, 1 H), 7.51 (s, 1 H), 7.44 (s, 1 H), 6.26 (s, 2 H), 5.32 (s, 2 H), 4.72 (d, J = 4.3 Hz, 1 H), 4.20-4.10 (m, 1 H) 4.05 (d, J = 2.9 Hz, 2 H), 2.80-2.66 (m, 2 H), 2.50-2.38 (m, 2 H), 2.02-1.96 (m, 1 H), 1.60-1.50 (m, 1 H); ESIMS m/z (rel intensity) 428 (MH+, 100). Anal. Calcd for C25H21N3O4·0.8 H2O: C, 67.96; H, 5.16; N, 9.51. Found: C, 67.68; H, 4.78; N, 9.49.
5.4.13. 14-[1′-(Imidazolyl)propylamino]methyl)-2,3-methylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (29f)
Compound 29a (0.055 g, 0.146 mmol) and 1-(3-aminopropyl)imidazole (0.091 g, 0.730 mmol) in DMSO afforded the title compound as a yellow amorphous solid (0.052 g, 77%) after flash column chromatography (SiO2, 28.1 g, eluting with 0.2% MeOH/0.2% Et3N in CHCl3 to 4% MeOH-0.2% Et3N in CHCl3) and washing with ether (25 mL): mp 195-199 °C (dec). IR (film) 3436, 2916, 2310 1655, 1622, 1602, 1488, 1465, 1238, 1037, 687 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.34 (d, J = 7.8 Hz, 1 H), 7.95 (d, J = 7.8 Hz, 1 H), 7.80 (t, J = 6.9 Hz, 1 H), 7.72 (s, 1 H), 7.59-7.53 (m, 3 H), 7.46 (s, 1 H), 7.13 (s, 1 H), 6.86 (s, 1 H), 6.27 (s, 2 H), 5.40 (s, 2 H), 4.21 (s, 2 H), 4.04 (t, J = 6.9 Hz, 2 H), 2.64 (br m, 2 H), 1.92 (m, 2 H); the amine proton is not visible due to residual water in the solvent; ESIMS m/z (rel intensity) 466 (MH+, 100). Anal. Calcd for C27H23N5O3·0.75 H2O: C, 67.60; H, 5.16; N, 14.62. Found: C, 67.90; H, 4.85; N, 14.47.
5.4.14. 14-[N-(S)-3′-Hydroxypyrrolidinomethyl]-2,3-dimethoxy-12H-5,11a diazadibenzo[b,h]fluoren-11-one (30b)
Compound 30a (0.060 g, 0.153 mmol), (S)-pyrrolidin-3-ol hydrogen maleate (0.094 g, 0.458 mmol) and Et3N (0.154 g, 1.50 mmol) in DMSO afforded the title compound as a yellow amorphous solid (0.051 g, 75%) after flash column chromatography (SiO2, eluting with 2% MeOH in CHCl3), and washing with ether (20 mL) and hexanes (20 mL): mp 223-226 °C. IR (KBr) 3430, 2933, 2342, 1660, 1627, 1603, 1505, 1478, 1431, 1250, 1167, 846, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.1 Hz, 1 H), 7.76-7.68 (m, 2 H), 7.64 (s, 1 H), 7.57-7.52 (m, 3 H), 5.39 (s, 2 H), 4.40 (br m, 1 H), 4.20 (q, J = 13.4 Hz, 2 H), 4.09 (s, 3 H), 4.05 (s, 3 H), 2.94, (q, J = 5.5 Hz, 1 H), 2.80-2.70 (m, 2 H), 2.48 (q, J = 6.2 Hz, 1 H), 2.30-2.20 (m, 1 H), 1.90-1.70 (m, 2 H); ESIMS m/z (rel intensity) 444 (MH+, 100). Anal. Calcd for C26H25N5O4·1 H2O: C, 67.67; H, 5.90; N, 9.10. Found: C, 67.38; H, 5.76; N, 9.13.
5.4.15. 14-(1′-Imidazolylmethyl)-2,3-dimethoxy-2H-5,11a-diazadibenzo[b,h]fluoren-11-one (30c)
Compound 30a (0.070 g, 0.178 mmol) and imidazole (0.049 g, 0.713 mmol) were diluted with DMSO (25 mL) under an argon atmosphere. The mixture was heated to 60 °C for 4 h. Additional imidazole (0.012 g, 0.178 mmol) was added, and the mixture was stirred at room temperature for 16 h. TLC indicated that the reaction was incomplete, so additional imidazole (0.012 g, 0.178 mmol) was added, and the mixture was heated to 100 °C for 1 h. Extraction (following the general procedure) and flash column chromatography (SiO2, eluting with a gradient of CHCl3 to 5% MeOH in CHCl3) yielded a yellow amorphous solid (0.056 g, 73%) after washing with ether (20 mL): mp 309-312 °C (dec). IR (KBr) 3468, 3027, 1654, 1627, 1571, 1509, 1482, 1360, 1256, 1170, 1086, 1033, 853, 758, 689 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.34 (d, J = 7.4 Hz, 1 H), 7.96 (d, J = 6.8 Hz, 1 H), 7.94 (s, 1 H), 7.82 (t, J = 7.9 Hz, 1 H), 7.61 (t, J = 7.3 Hz, 1 H), 7.53 (s, 2 H), 7.48 (s, 1 H), 7.29 (s, 1 H), 6.92 (s, 1 H), 5.98 (s, 2 H), 5.20 (s, 2 H), 3.98 (s, 3 H), 3.93 (s, 3 H); ESIMS m/z (rel intensity) 425 (MH+, 100). Anal. Calcd for C25H20N4O3·1.5 H2O: C, 66.51; H, 5.13; N, 12.41. Found: C, 66.64; H, 4.89; N, 12.53.
5.4.16. 2,3-Dimethoxy-14-[1′-(N-methylpiperazinylmethyl)]-2H-5,11a-diazadibenzo[b,h]fluoren-11-one (30d)
Compound 30a (0.055 g 0.140 mmol), and N-methylpiperazine (0.042 g, 0.420 mmol) in DMSO afforded the title compound as a yellow-green amorphous solid (0.046 g, 72%) after flash column chromatography (SiO2, eluting with a gradient of 1% MeOH in CHCl3 to 5% MeOH in CHCl3), and washing with ether (20 mL): mp 256-259 °C (dec). IR (KBr) 3435, 2926, 2791, 1665, 1636, 1601, 1504, 1480, 1431, 1249, 1167, 842, 816, 686 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.57 (d, J = 8.0 Hz, 1 H), 7.77-7.89 (m, 3 H), 7.58-7.56 (m, 2 H), 7.53 (s, 1 H), 5.40 (s, 2 H), 4.09 (s, 3 H), 4.07 (s, 3 H), 4.01 (s, 2 H), 2.70-2.30 (br m, 8 H), 2.30 (s, 3 H); ESIMS m/z (rel intensity) 457 (MH+, 100). Anal. Calcd for C27H28N4O3·0.5 H2O: C, 69.66; H, 6.28; N, 12.03. Found: C, 69.59; H, 6.21; N, 12.13.
5.4.17. 14-[(1′-Imidazolyl)propylaminomethyl]-2,3-dimethoxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (30e)
Compound 30a (0.050 g, 0.127 mmol) and 1-(3-aminopropyl)imidazole (0.048 g, 0.381 mmol) in DMSO afforded the title compound as a yellow powder (0.040 mg, 66%) after flash column chromatography (SiO2, eluting with a gradient of 0.5% MeOH/0.1% Et3N in CHCl3 to 2.5% MeOH/0.2% Et3N in CHCl3) and washing with ether (20 mL): mp 213-216 °C (dec). IR (film) 3306, 2918, 1657, 1626, 1602, 1507, 1478, 1356, 1253, 1111, 687 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.54 (d, J = 8.0 Hz, 1 H), 7.76 (t, J = 7.6 Hz, 1 H), 7.73 (t, J = 7.7 Hz, 1 H), 7.57-7.53 (m, 3 H), 7.49 (s, 1 H), 7.44 (s, 1 H), 7.03 (s, 1 H), 6.85 (s, 1 H), 5.39 (s, 2 H), 4.30 (s, 2 H), 4.09 (s, 3 H), 4.07 (s, 3 H), 4.05 (t, J = 6.9 Hz, 2 H), 2.77 (t, J = 6.7 Hz, 2 H), 2.01 (pent, J = 6.7 Hz, 2 H); the amine proton is not visible due to residual water in the solvent; ESIMS m/z (rel intensity) 482 (MH+, 100). Anal. Calcd for C28H27N5O3·1 H2O: C, 67.32; H, 5.85; N, 14.02. Found: C, 66.95; H, 5.73; N, 13.92.
5.4.18. 3-Fluoro-2-methyl-14-[1′-(N-methylpiperazinylmethyl)]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31b)
Compound 31a (0.070 g, 0.192 mmol) and N-methylpiperazine (0.058 g, 0.575 mmol) in DMSO afforded the desired compound as a pale yellow-green amorphous solid (0.0530 g, 64%) after flash column chromatography (SiO2, eluting with 5% MeOH in CHCl3) and washing with ether: mp 238-242 °C (dec). IR (KBr pellet) 3435, 2930, 2790, 2764, 2372, 2348, 1670, 1641, 1504, 1441, 1161, 1139, 821, 686 cm −1; 1H NMR (300 MHz, CDCl3) δ 8.57 (d, J = 8.0 Hz, 1 H), 8.17 (d, J = 7.9 Hz, 1 H), 7.80-7.56 (m, 5 H), 5.44 (s, 2 H), 4.03 (s, 2 H), 2.70-2.40 (br m, 8 H), 2.54 (s, 3 H), 2.30 (s, 3 H); ESIMS m/z (rel intensity) 429 (MH+, 100). Anal. Calcd for C26H25FN4O 0.5 H2O: C, 71.38; H, 5.99; N, 12.81. Found: C, 71.34; H, 5.67; N, 12.70.
5.4.19. 3-Fluoro-14-[N-(S)-3′-Hydroxypyrrolidinomethyl]-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31c)
Compound 31a (0.070 g, 0.192 mmol) was diluted with DMSO (25 mL) and (S)-pyrrolidin-3-ol hydrogen maleate (0.118 g, 0.576 mmol) was added, followed by Et3N (0.194 g, 1.92 mmol). The mixture was stirred at room temperature for 16.5 h. TLC indicated incomplete reaction; 0.040 g (0.192 mmol) of additional pyrrolidinol and Et3N (0.065 g, 0.642 mmol) were added, and the mixture was heated at 50 °C for 45 min. Extraction (by general procedure) and flash column chromatography (SiO2, eluting with 1% MeOH in CHCl3 to 1.5% MeOH in CHCl3) afforded a cream-colored powder (0.050 g, 62%) after washing with ether: mp 239-242 °C (dec). IR (KBr pellet) 3369, 2903, 2806, 1661, 1614, 1597, 1506, 1482, 1440, 1347, 1229, 1135, 1024, 862, 760, 689 cm −1; 1H NMR (300 MHz, DMSO-d6) δ 8.37 (dd, J = 4.6, 3.9 Hz, 2 H), 7.99 (d, J = 7.9 Hz, 1 H), 7.84-7.78 (m, 2 H), 7.63-7.58 (m, 2 H), 5.39 (s, 2 H), 4.75 (d, J = 4.3 Hz, 1 H), 4.23-4.18 (m, 3 H), 2.85-2.73 (m, 2 H), 2.57-2.42 (m, 2 H), 2.50 (s, 3 H, obscured by solvent peak), 2.02-2.98 (m, 1 H), 1.70-1.50 (m, 1 H); ESIMS m/z (rel intensity) 416 (MH+, 100). Anal. Calcd for C25H22FN3O20.25 H2O: C, 71.50; H, 5.40; N, 10.01. Found: C, 71.15; H, 5.04; N, 9.90.
5.4.20. 3-Fluoro-14-[(1′-imidazolyl)propylaminomethyl]-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31d)
Compound 31a (0.070 g, 0.19 mmol) was diluted with DMSO (25 mL), and 1-(3-aminopropyl)imidazole (0.072 g, 0.08 mmol) was added. The mixture was stirred at room temperature for 16.5 h. TLC indicated the reaction was incomplete, so an additional 0.024 g (0.192 mmol) of 1-(3-aminopropylimidazole) was added, and the mixture was heated at 50 °C for 1 h. Extraction (by general procedure) and flash column chromatography (SiO2, eluting with a gradient of 1% MeOH in CHCl3 to 6% MeOH and 0.2% Et3N in CHCl3) yielded a bright yellow powder (0.077 g, 89%) after washing with ether: mp 183-187 °C (dec). IR (KBr pellet) 3435, 3248, 3116, 3084, 1947, 2603, 1659, 1633, 1606, 1507, 1439, 1341, 1231, 1137, 912, 862, 759, 689 cm −1; 1H NMR (300 MHz, DMSO-d6) 8.37 (d, J = 8.0 Hz, 1 H), 8.31 (d, J = 8.4 Hz, 1 H), 7.99 (d, J = 7.8 Hz, 1 H), 7.84-7.77 (m, 2 H), 7.63-7.58 (m, 3 H), 7.14 (s, 1 H), 6.87 (s, 1 H), 5.44 (s, 2 H), 4.30 (s, 2 H), 4.05 (t, J = 7.0 Hz, 2 H), 2.66 (t, J = 6.3 Hz, 2 H), 2.50 (s, 3 H, obscured by solvent peak), 1.94-1.89 (m, 2 H); ESIMS m/z (rel intensity) 454 (MH+, 100). Anal. Calcd for C27H34FN5O·0.5 H2O: C, 70.11; H, 5.45; N, 15.14. Found: C, 69.73; H, 5.30; N, 14.97.
5.4.21. 14-(N-Ethanolaminomethyl)-3-fluoro-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31e)
Compound 31a (0.070 g, 0.192 mmol) was diluted with DMSO (25 mL) and ethanolamine (0.059 g, 0.960 mmol) was added. The mixture was stirred overnight at room temperature for 17 h. TLC indicated incomplete reaction, so additional ethanolamine (0.029 g 0.480 mmol) was added, and the mixture was heated at 50 °C for 1 h. Extraction (by general procedure) and flash column chromatography (SiO2, eluting with a gradient of 1% MeOH in CHCl3 to 5% MeOH and 0.2% Et3N in CHCl3) afforded a residue that was recrystallized from boiling CHCl3 (20 mL) to yield a pale yellow amorphous solid (0.036 g, 48%) after washing with ether: mp 212-216 °C (dec) IR (KBr pellet) 3378, 3066, 2925, 1656, 1617, 1602, 1504, 1438, 1348, 1128, 1110, 1056, 878, 689 cm −1; 1H NMR (300 MHz, DMSO-d6) δ 8.35 (t, J = 7.3 Hz, 2 H), 7.98 (d, J = 8.0 Hz, 1 H), 7.83 (t, J = 10.2 Hz, 2 H), 7.62-7.58 (m, 2 H), 5.44 (s, 2 H), 4.59 (br s, 1 H), 4.34 (s, 2 H), 3.56 (q, J = 5.4 Hz, 2 H), 2.80-2.70 (s, 2 H), 2.50 (s, 3 H, obscured by solvent peak); ESIMS m/z (rel intensity) 390 (MH+, 100). Anal. Calcd for C23H20FN5O2·0.3 H2O: C, 69.97; H, 5.26; N, 10.64. Found: C, 69.90; H, 5.24; N, 10.57.
5.4.22. 14-Azidomethyl-3-fluoro-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (31f)
Compound 31a (0.070 g, 0.19 mmol) and sodium azide (0.037 g, 0.58 mmol) in DMSO afforded the title compound as a pale yellow amorphous solid (0.057 g, 79%) after flash column chromatography (SiO2, eluting with CHCl3) and washing with ether: mp 225-226 °C (dec). IR (KBr pellet) 3437, 2106, 1661, 1636, 1504, 1430, 1342, 1142, 877, 688 cm −1; 1H NMR (300 MHz, DMSO-d6) δ 8.35 (d, J = 7.9 Hz, 1 H), 8.26 (d, J = 8.2 Hz, 1 H), 7.99 (d, J = 7.9 Hz, 1 H), 7.88 (d, J = 10.9 Hz, 1 H), 7.83 (t, J = 7.5 Hz, 1 H), 5.43 (s, 2 H), 4.23 (s, 2 H), 2.49 (s, 3 H, obscured by solvent peak); ESIMS m/z (rel intensity) 372 (MH+, 100).
5.4.23. 14-Aminomethyl-3-fluoro-2-methyl-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Dihydrochloride (31g)
Compound 31f (0.047 g, 0.127 mmol) was diluted with benzene (30 mL). Triethyl phosphite (0.063 g, 0.380 mmol) was added, and the mixture was heated at reflux for 20 h. The mixture was cooled, methanolic HCl (3 M, 10 mL) was added, and the mixture was heated at reflux for 3 h. The solution was cooled and concentrated to yield an orange solid (0.053 g, 100%) after washing with ether and drying in vacuo: mp 278-282 °C (dec). IR (KBr pellet) 3428, 2047, 2915, 2866, 2632, 2346, 1902, 1656, 1624, 1603, 1528, 1346, 1254, 1128, 802, 689 cm −1; 1H NMR (300 MHz, D2O) δ 7.50-7.40 (m, 2 H), 7.36 (d, J = 7.3 Hz, 2 H), 7.13 (t, J = 6.6 Hz, 1 H), 6.87 (d, J = 10.4 Hz, 1 H), 6.59 (s, 1 H), 5.00 (s, 2 H), 4.47 (s, 2 H), 2.19 (s, 3 H); the amine is not visible due to exchange with the solvent; ESIMS m/z (rel intensity) 346 (MH+, 100). Anal. Calcd for C21H18Cl2FN3O·0.5 H2O: C, 59.03; H, 4.48; N, 9.83. Found: C, 58.89; H, 4.48; N, 9.63.
5.4.24. 2-Chloro-14-[(1′-imidazolyl)propylaminomethyl]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (32b)
Compound 32a (0.060 g, 0.163 mmol) and 1-(3-aminopropyl)imidazole (0.102 g, 0.817 mmol) in DMSO afforded the desired compound as a yellow amorphous solid (0.039 g, 53%) after flash column chromatography (SiO2, 25.8 g, eluting with a gradient of 1% MeOH-0.25% Et3N in CHCl3 to 2% MeOH-0.25% Et3N in CHCl3) and washing with ether (100 mL): mp 171-175 °C (dec). IR (film) 3293, 2930, 1659, 1617, 1602, 1497, 1481, 1341, 1089, 825, 752, 697 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 7.8 Hz, 1 H), 8.27 (s, 1 H), 8.17 (d, J = 9.1 Hz, 1 H), 7.79-7.58 (m, 5 H), 7.44 (s, 1 H), 7.03 (s, 1 H), 6.86 (s, 1 H), 5.43 (s, 2 H), 4.30 (s, 2 H), 4.08 (t, J = 6.7 Hz, 2 H), 2.78 (t, J = 6.7 Hz, 2 H), 2.03-1.98 (m, 2 H); the amine proton is not visible due to residual water in the solvent; ESIMS m/z (rel intensity) 456 (MH+, 100). Anal. Calcd for C26H22ClN5O2.2 H2O: C, 63.01; H, 5.37; N, 14.1. Found: C, 63.37; H, 4.99; N, 13.70.
5.4.25. 2-Chloro-14-[1′-(N-methylpiperazinylmethyl)]-12H-5,11a- diazadibenzo[b,h]fluoren-11-one (32c)
Compound 32a (0.060 g, 0.163 mmol) and N-methylpiperazine (0.066 g, 0.652 mmol) in DMSO afforded the title compound as a pale-yellow amorphous solid (0.055 g, 78%) after flash column chromatography (SiO2, 22.9 g, eluting with a gradient of 1% MeOH in CHCl3 to 4% MeOH in CHCl3) and washing with ether (30 mL): mp 238-241 °C (dec). IR (film) 2931, 2791, 1662, 1632, 1605, 1496, 1480, 1456, 1338, 1161, 1089, 1011, 826, 731, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.58 (d, J = 8.0 Hz, 1 H), 8.37 (d, J = 2.2 Hz, 1 H), 8.16 (d, J = 9.0 Hz, 1 H), 7.78-7.59 (m, 5 H), 5.46 (s, 2 H), 4.02 (s, 2 H), 2.70-2.30 (br m, 8 H), 2.30 (s, 3 H); ESIMS m/z (rel intensity) 431 (MH+, 100). Anal. Calcd for C25H23ClN4O0.25 H2O: C, 68.96; H, 5.44; N, 12.87. Found: C, 68.95; H, 5.66; N, 12.90.
5.4.26. 2-Chloro-14-[N-(S)-3′-hydroxypyrrolidinomethyl]-12H-5,11adiazadibenzo[b,h]fluoren-11-one (32d)
Compound 32a (0.060 g, 0.163 mmol), (S)-pyrrolidin-3-ol (0.133 g, 0.642 mmol) and Et3N (0.197 g, 1.96 mmol) in DMSO afforded the title compound as a yellow powder (0.040 g, 59%) after flash column chromatography (SiO2, 25.6 g, eluting with a gradient of 1% MeOH in CHCl3 to 1.5% MeOH in CHCl3) and washing with ether (30 mL): mp 235-238 °C (dec). IR (film) 3369, 2917, 2797, 1659, 1617, 1602, 1496, 1480, 1340, 1088, 827, 754, 730, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 8.1 Hz, 1 H), 8.35 (d, J = 2.2 Hz, 1 H), 8.15 (d, J = 9.0 Hz, 1 H), 7.77-7.58 (m, 5 H), 5.45 (d, J = 2.4 Hz, 2 H), 4.40 (br s, 1 H), 4.15 (s, 2 H), 2.95 (q, J = 5.8 Hz, 1 H), 2.78-2.72 (m, 2 H), 2.53-2.47 (m, 1 H), 2.30-2.23 (m, 1 H), 1.84-1.62 (m, 2 H); ESIMS m/z (rel intensity) 418 (MH+, 100). Anal. Calcd for C24H20ClN3O20.5 H2O: C, 67.52; H, 4.96; N, 9.84. Found: C, 67.80; H, 5.03; N, 9.80.
5.4.27. 2-Chloro-14-(N-ethanolaminomethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (32e)
Compound 32a (0.070 g, 0.191 mmol) and ethanolamine (0.047 g, 0.762 mmol) in DMSO afforded a yellow powder after flash column chromatography (SiO2, 27.9 g, eluting with a gradient of 1% MeOH-0.2% Et3N in CHCl3 to 1.5% MeOH-0.2% Et3N in CHCl3) and washing with ether (100 mL). This solid contained residual Et3NHCl, and was dissolved in CHCl3 (50 mL, with MeOH added for solubility) and washed with dilute NaOH (2 × 25 mL). The aqueous phase was extracted with CHCl3 (5 × 25 mL), and the organic phase was dried over anhydrous sodium sulfate and concentrated to yield a yellow powder (0.027 g, 36%) after washing with ether (30 mL): mp 218-220 °C (dec). IR (film) 3306, 2917, 2833, 1654, 1614, 1494, 1482, 1346, 845, 827, 757, 690 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.3 Hz, 1 H), 8.25 (d, J = 2.2 Hz, 1 H), 8.15 (d, J = 9.1 Hz, 1 H), 7.76-7.58 (m, 5 H), 5.46 (s, 2 H), 4.36 (s, 2 H), 3.79 (t, J = 4.6 Hz, 2 H), 2.96 (t, J = 5.2 Hz, 2 H), 2.08 (br s, 1 H); the hydroxyl proton is not visible due to residual water in the solvent; ESIMS m/z (rel intensity) 392 (MH+, 100). Anal. Calcd for C22H18ClN3O21 H2O: C, 64.47; H, 4.92; N, 10.25. Found: C, 64.35; H, 4.69; N, 9.93.
5.4.28. 2-Chloro-14-(1′-imidazolylmethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (32f)
Compound 32a (0.070 g, 0.191 mmol) and imidazole (0.065 g, 0.955 mmol) at 60 °C in DMF afforded the title compound as a yellow-green chalky solid (0.054 g, 70%) after flash column chromatography (SiO2, 26.7 g, eluting with a gradient of 1.5% MeOH in CHCl3 to 4% MeOH in CHCl3), washing with ether (30 mL) and drying: mp 296-298 °C (dec). IR (film) 2918, 1660, 1632, 1606, 1500, 1341, 1230, 1083, 828, 756, 689 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 8.34 (d, J = 2.1 Hz, 1 H), 8.31 (d, J = 8.0 Hz, 1 H), 8.17 (d, J = 9.0 Hz, 1 H), 7.98-7.83 (m, 4 H), 7.79 (s, 1 H), 7.61 (t, J = 7.7 Hz, 1 H), 7.28 (s, 1 H), 6.94 (s, 1 H), 5.92 (s, 2 H), 5.18 (s, 2 H); ESIMS m/z (rel intensity) 399 (MH+, 100). Anal. Calcd for C23H15ClN4O0.25 H2O: C, 68.49; H, 3.87; N, 13.89. Found: C, 68.33; H, 3.90; N, 13.92.
5.4.29. 3-Chloro-14-[(1′-imidazolyl)propylaminomethyl]-12H-5,11adiazadibenzo[b,h]fluoren-11-one (33b)
Compound 33a (0.060 g, 0.163 mmol) was diluted with DMSO (20 mL). 1-(3-Aminopropyl)imidazole (0.102 g, 0.817 mmol) was added, and the mixture was stirred at room temperature for 18 h. The reaction was incomplete by TLC, so the mixture was heated to 55 °C for 2 h. The product was extracted (following general procedures) and flash column chromatography (SiO2, 27.0 g, eluting with 1% MeOH-0.2% Et3N in CHCl3) yielded a yellow-orange amorphous solid (0.046 g, 61%) after washing with ether (20 mL): mp 163-176 °C (dec). IR (film) 3401, 2922, 1660, 1622, 1604, 1480, 1343, 1230, 1081, 923, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 7.8 Hz, 1 H), 8.21 (d, J = 1.5 Hz, 1 H), 8.20 (d, J = 8.7 Hz, 1 H), 7.78-7.57 (m, 5 H), 7.42 (s, 1 H), 7.03 (s, 1 H), 6.84 (s, 1 H), 5.42 (s, 2 H), 4.31 (s, 2 H), 4.06 (d, J = 6.9 Hz, 2 H), 2.76 (t, J = 6.7 Hz, 2 H), 2.01-1.97 (m, 2 H), 1.60-1.50 (br s, 1 H, obscured by solvent peak); ESIMS m/z (rel intensity) 456 (MH+, 100). Anal. Calcd for C26H22ClN5O2.25 H2O: C, 62.90; H, 5.38; N, 14.11. Found: C, 62.65; H, 4.99; N, 13.94.
5.4.30. 3-Chloro-14-[1′-(N-methylpiperazinylmethyl)]-12H-5,11a- diazadibenzo[b,h]fluoren-11-one (33c)
Compound 33a (0.060 g, 0.163 mmol) and N-methylpiperazine (0.066 g, 0.652 mmol) in DMSO afforded the title compound as a pale-yellow amorphous solid (0.055 g, 78%) after flash column chromatography (SiO2, 24.4 g, eluting with a gradient of 1% MeOH in CHCl3 to 5% MeOH in CHCl3) and washing with ether (20 mL): mp 226-228 °C (dec). IR (film) 2931, 2798, 1662, 1631, 1605, 1460, 1341, 1291, 1162, 1077, 1011, 918, 751, 686 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.1 Hz, 1 H), 8.33 (d, J = 9.1 Hz, 1 H), 8.20 (d, J = 2.1 Hz, 1 H), 7.78-7.54 (m, 5 H), 5.43 (s, 2 H), 4.03 (s, 2 H), 2.70-2.30 (br m, 8 H), 2.30 (s, 3 H); ESIMS m/z (rel intensity) 431 (MH+, 100). Anal. Calcd for C25H23ClN4O0.50 H2O: C, 68.25; H, 5.50; N, 12.73. Found: C, 68.47; H, 5.34; N, 12.71.
5.4.31. 3-Chloro-14-[N-(S)-3′-hydroxypyrrolidinomethyl]-12H-5,11adiazadibenzo[b,h]fluoren-11-one (33d)
Compound 33a (0.060 g, 0.163 mmol) and (S)pyrrolidin-3-ol (0.133 g, 0.648 mmol) were diluted with DMSO (25 mL). Et3N (0.197 g, 1.96 mmol) was added, and the mixture was stirred at room temperature for 17 h. The reaction was incomplete by TLC, so the mixture was heated to 50 °C for 1 h and 30 min. Extraction followed general procedures, and flash column chromatography (SiO2, 24.9 g, eluting with a gradient of 1% MeOH in CHCl3 to 1.5% MeOH in CHCl3) yielded a pale-yellow amorphous solid (0.046 g, 68%) after washing with ether (30 mL): mp 247-249 °C (dec). IR (film) 3401, 2916, 2799, 1659, 1619, 1603, 1480, 1344, 1190, 922, 687 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.0 Hz, 1 H), 8.35 (d, J = 9.1 Hz, 1 H), 8.21 (d, J = 2.1 Hz, 1 H), 7.79-7.55 (m, 5 H), 5.45 (d, J = 1.6 Hz, 2 H), 4.39 (br s, 1 H), 4.17 (s, 2 H), 3.00-2.90 (m, 1 H), 2.73 (d, J = 3.7 Hz, 2 H), 2.50-2.44 (m, 1 H), 2.30-2.20 (m, 1 H), 1.84-1.76 (m, 2 H); ESIMS m/z (rel intensity) 418 (MH+, 100). Anal. Calcd for C24H20ClN3O20.75 H2O: C, 66.82; H, 5.02; N, 9.74. Found: C, 66.67; H, 4.77; N, 9.50.
5.4.32. 3-Chloro-14-(1′-imidazolylmethyl)-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (33e)
Compound 33a (0.070 g, 0.191 mmol) and imidazole (0.065 g, 0.955 mmol) in DMF at 80 °C afforded the title compound as a chalky yellow solid (0.054 g, 71%) after flash column chromatography (SiO2, 27.4 g, eluting with a gradient of 1.5% MeOH in CHCl3 to 3.5% MeOH in CHCl3), and washing with ether (30 mL): mp 279-281 °C (dec). IR (film) 3401, 2917, 1658, 1621, 1599, 1498, 1448, 1228, 1079, 687 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 8.30-8.29 (m, 2 H), 8.18 (s, 1 H), 7.97 (d, J = 7.0 Hz, 1 H), 7.89 (s, 1 H), 7.78-7.64 (m, 4 H), 7.24 (s, 1 H), 6.89 (s, 1 H), 5.90 (s, 2 H), 5.17 (s, 2 H); ESIMS m/z (rel intensity) 399 (MH+, 100). Anal. Calcd for C23H15ClN4O0.25 H2O: C, 66.99; H, 4.03; N, 13.59. Found: C, 66.81; H, 3.81; N, 13.39.
5.4.33. 1-Chloro-14-[1′-(N-methylpiperazinylmethyl)]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (34b)
Compound 34a (0.070 g, 0.191 mmol) and N-methylpiperazine (0.076 g, 0.762 mmol) in DMSO afforded the desired compound as a cream-colored solid after flash column chromatography (SiO2, 27.6 g, eluting with a gradient of 0.5% MeOH in CHCl3 to 3% MeOH in CHCl3). This solid was washed with ether (25 mL), dissolved in CHCl3 (35 mL) and methanolic HCl (3 M, 5 mL) was added slowly. The mixture was stirred at room temperature for 2 h and concentrated to yield an orange-yellow iridescent solid (0.047 g, 46%) after washing with ether (50 mL) and drying in vacuo: mp 203-206 °C (dec). IR (KBr) 3400, 2599, 2489, 1651, 1618, 1596, 1344, 1127, 821, 759, 692 cm−1; 1H NMR (300 MHz, DMSO-d6) δ 10.13 (br s, 1 H), 8.37 (d, J = 7.8 Hz, 1 H), 8.18 (dd, J = 7.7, 1.9 Hz, 1 H), 8.02 (d, J = 7.9 Hz, 1 H), 7.84-7.77 (m, 3 H), 7.70 (s, 1 H), 7.65 (t, J = 7.2 Hz, 1 H), 5.42 (s, 2 H), 4.40 (s, 2 H), 3.21-3.28 (m, 2 H), 3.00-2.80 (m, 4 H), 2.70-2.50 (m, 5 H); ESIMS m/z (rel intensity) 431 (MH+, 100). Anal. Calcd for C25H26Cl4N4O1.3 H2O: C, 53.26; H, 5.11; N, 9.94. Found: C, 53.63; H, 5.51; N, 9.87.
5.4.34. 1-Chloro-14-[N-(S)-3′-hydroxypyrrolidinomethyl]-12H-5,11a diazadibenzo[b,h]fluoren-11-one Hydrochloride (34c)
Compound 34a (0.070 g, 0.191 mmol) and (S)-pyrrolidin-3-ol hydrogen maleate (0.157 g, 0.764 mmol) were diluted with DMSO (25 mL) and Et3N (0.231 g, 0.764 mmol) was added. The mixture was stirred at room temperature for 16 h, and then heated to 55 °C for 1.5 h. Extraction followed general procedures, and flash column chromatography (SiO2, 25.7 g, eluting with a gradient of 0.25% MeOH in CHCl3 to 1.25% MeOH in CHCl3) afforded a yellow solid. This solid was treated (in CHCl3) with methanolic HCl as described for 34b to yield a dark-orange iridescent solid (0.031 g, 37%) after washing with ether (50 mL) and drying in vacuo: mp 208-210 °C (dec). IR (KBr) 3400, 2658, 1659, 1627, 1601, 1440, 1377, 1343, 1125, 1102, 926, 815, 758, 688 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 10.1-10.0 (br m, 1 H), 8.33 (d, J = 8.0 Hz, 1 H), 8.20 (dd, J = 7.8, 3.5 Hz, 1 H), 7.99 (d, J = 7.9 Hz, 1 H), 7.91 (d, J = 7.5 Hz, 1 H), 7.86 (td, J = 8.1, 2.7 Hz, 1 H), 7.81 (t, J = 7.1 Hz, 1 H), 7.70 (s, 1 H), 7.62 (t, J = 7.2 Hz, 1 H), 5.70-5.30 (m, 4 H), 4.10 (br s, 0.5 H), 4.52 (br s, 0.5 H), 4.39 (br s, 0.5 H), 3.68-2.54 (m, 3 H), 3.27-3.25 (br m, 0.5 H), 2.50-2.30 (br m, 0.5 H), 2.10-2.00 (br m, 0.5 H), 2.00-1.90 (br m, 0.5 H), 1.80-1.70 (br m, 0.5 H); ESIMS m/z (rel intensity) 418 (MH+, 100). Anal. Calcd for C24H21Cl2N3O22 H2O: C, 58.78; H, 5.14; N, 8.57. Found: C, 59.01; H, 4.84; N, 8.26.
5.4.35. 1-Chloro-14-[(1′-imidazolyl)propylaminomethyl]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one Trihydrochloride (34d)
Compound 34a (0.090 g, 0.245 mmol) was diluted with DMSO (25 mL) and 1-(3-aminopropyl)imidazole (0.153 g, 0.1.22 mmol) was added. The mixture was stirred at room temperature for 17 h, and then heated to 50 °C for 2 h. Extraction followed general procedures, and flash column chromatography (SiO2, 27.0 g, eluting with a gradient of 1% MeOH in CHCl3 to 4% MeOH in CHCl3) afforded a yellow solid. This solid was treated with methanolic HCl as described for 34b to yield the title compound as a flaky red iridescent solid (0.048 g, 35%) after washing with ether (50 mL) and drying in vacuo: mp 180-185 °C. IR (film) 3400, 2922, 2726, 1658, 1620, 1601, 1463, 1299, 1203, 1103, 818, 758, 683 cm−1; 1H NMR (500 MHz, DMSO-d6) δ 9.9 (br s, 2 H), 9.25 (s, 1 H), 8.33 (d, J = 7.9 Hz, 1 H), 8.22 (dd, J = 7.8, 1.6 Hz, 1 H), 7.99 (d, J = 8.0 Hz, 1 H), 7.89-7.77 (m, 4 H), 7.73 (s, 1 H), 7.71 (s, 1 H), 7.63 (t, J = 7.5 Hz, 1 H), 5.80 (s, 2 H), 4.99 (s, 2 H), 4.41 (t, J = 6.6 Hz, 2 H), 3.30-3.20 (br m, 2 H), 2.40-2.30 (m, 2 H); the protonated secondary amine is not visible due to exchange with residual water in the solvent; ESIMS m/z (rel intensity) 456 (MH+, 100). Anal. Calcd for C26H25Cl4N5O2.5 H2O: C, 51.16; H, 4.95; N, 11.47. Found: C, 50.90; H, 5.19; N, 11.11.
5.5. General Procedure for Synthesis of Extended Ethylenedioxyaromathecins 28b-g
Compound 28a (0.180-0.215 mmol) was diluted in DMSO or DMF (25-30 mL), and sodium iodide (six equivalents) and the nucleophile (between six and twelve equivalents) were added. The mixture was heated to 100 °C overnight (between 12 and 36 h or until TLC indicated completion of the reaction), and the mixture was poured into H2O (at least 100 mL) and extracted thoroughly with CHCl3 (at least 150 mL). The organic layers were washed with copious water (between 400 and 600 mL) and (when DMF was used), sat. NH4Cl (~200 mL), and dried over anhydrous sodium sulfate. The solution was concentrated, adsorbed onto SiO2 (between 3-5 g), and purified by flash column chromatography (SiO2, between 20-30 g), to afford the aromathecins as solids after washing with ether or suspending in methanol and precipitating with ether.
5.5.1. 14-[3′-(N-Ethanolaminopropyl)]-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28b)
Compound 28a (0.090 g, 0.215 mmol), sodium iodide (0.193 g, 1.29 mmol) and ethanolamine (0.079 g, 1.29 mmol) in DMSO afforded the title compound as a yellow solid (0.044 g, 46%) after flash column chromatography (SiO2, 29.0 g, eluting with a gradient of 1.5% MeOH-0.5% Et3N in CHCl3 to 6% MeOH-0.5% Et3N in CHCl3) and precipitation by general procedure: mp 200-205 °C. IR (film) 3584, 2930, 1656, 1621, 1602, 1507, 1441, 1400, 1288, 1246, 1068, 914 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.54 (d, J = 8.0 Hz, 1 H), 7.78-7.51 (m, 6 H), 5.31 (s, 2 H), 4.42 (s, 4 H), 3.74 (t, J = 4.9 Hz, 2 H), 3.19 (t, J = 7.5 Hz, 2 H), 2.85-2.78 (m, 4 H), 2.00-1.95 (m, 2 H); the hydroxyl group and amine are not visible due to residual water in the solvent; ESIMS m/z (rel intensity) 444 (MH+, 100). Anal. Calcd for C26H25ClN3O41.25 H2O: C, 67.01; H, 5.95; N, 9.02. Found: C, 66.76; H, 5.67; N, 8.88.
5.5.2. 2,3-Ethylenedioxy-14-[3′-(1′-imidazolylpropyl)]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28c)
Compound 28a (0.075 g, 0.179 mmol), sodium iodide (0.160 g, 1.08 mmol), and imidazole (0.073 g, 1.08 mmol) were diluted with anhydrous DMF (30 mL) under an argon atmosphere. The mixture was heated to 100 °C for 21 h. As the reaction was incomplete by TLC, additional imidazole (0.073 g, 1.08 mmol) was added, and the mixture was kept at 100 °C for an additional 3 h, upon which a white precipitate formed (presumably NaCl). The mixture was held at 40 °C for 16.5 h, cooled, and diluted with H2O (100 mL). The mixture was extracted following general procedures, and the residue was purified by flash column chromatography (SiO2, 30.0 g), eluting with 1% MeOH-0.5% Et3N in CHCl3, to yield a yellow amorphous solid (0.040 g, 50%) after washing with ether (25 mL): mp 275-278 °C. IR (film) 3584, 2930, 1657, 1621, 1602, 1506, 1447, 1288, 1246, 1067, 917, cm−1; 1H NMR (300 MHz, CDCl3) δ 8.53 (d, J = 8.0 Hz, 1 H), 7.77-7.53 (m, 6 H), 7.20 (s, 1 H), 7.17 (s, 1 H), 7.01 (s, 1 H), 5.23 (s, 2 H), 4.42 (s, 4 H), 4.17 (t, J = 7.1 Hz, 2 H), 3.05 (t, J = 8.0 Hz, 2 H), 2.27-2.17 (m, 2 H); ESIMS m/z (rel intensity) 451 (MH+, 100). Anal. Calcd for C27H22N4O3·1 H2O: C, 69.22; H, 5.16; N, 11.96. Found: C, 69.31; H, 5.24; N, 11.77.
5.5.3. 2,3-Ethylenedioxy-14-[3′-(N-morpholinopropyl)]-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28d)
Compound 28a (0.075 g, 0.179 mmol), sodium iodide (0.160 g, 1.08 mmol) and morpholine (0.188 g, 2.15 mmol) in DMF afforded the title compound as a white amorphous solid (0.060 g, 72%) after flash column chromatography (SiO2, 30.1 g, eluting with 0.5% MeOH-0.25% Et3N in CHCl3) and precipitation by the general procedure: mp 259-260 °C. IR (film) 3584, 2929, 2382, 1659, 1630, 1603, 1506, 1448, 1398, 1287, 1244, 1116, 1068, 914 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 7.9 Hz, 1 H), 7.78-7.54 (m, 6 H), 5.32 (s, 2 H), 4.42 (s, 4 H), 3.76-3.73 (m, 4 H), 3.16 (t, J = 7.3 Hz, 2 H), 2.45-2.41 (m, 6 H), 1.97-1.93 (m, 2 H); ESIMS m/z (rel intensity) 470 (MH+, 100). Anal. Calcd for C28H27N3O4: C, 71.62; H, 5.80; N, 8.95. Found: C, 71.35; H, 5.75; N, 8.92.
5.5.4. 14-[3′-(N,N-Dimethylaminopropyl)]-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28e)
Compound 28a (0.075 g, 0.179 mmol), sodium iodide (0.160 g, 1.08 mmol), and N,N-dimethylamine (1.07 mL of a 2 M solution in THF) in DMF afforded the title compound as a yellow powder (0.051 g, 67%) after flash column chromatography (SiO2, 30.9 g, eluting with a gradient of 0.5% MeOH-0.25% Et3N in CHCl3 to 1% MeOH-0.5% Et3N in CHCl3) and precipitation by the general procedure: mp 221-225 °C. IR (film) 3400, 2924, 1658, 1622, 1603, 1507, 1448, 1289, 1247, 1067, 918, 751, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.55 (d, J = 8.1 Hz, 1 H), 7.78-7.51 (m, 6 H), 5.30 (s, 2 H), 4.43 (s, 4 H), 3.13 (t, J = 7.5 Hz, 2 H), 2.54 (t, J = 7.1 Hz, 2 H), 2.26 (s, 6 H), 1.95-1.90 (m, 2 H); ESIMS m/z (rel intensity) 428 (MH+, 100). Anal. Calcd for C26H25N3O3·2 H2O: C, 67.37; H, 6.31; N, 9.07. Found: C, 67.30; H, 6.05; N, 9.00.
5.5.5. 14-(3′-Azidopropyl)-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one (28f)
Compound 28a (0.075 g, 0.179 mmol) and sodium azide (0.058 g, 0.895 mmol) were diluted with anhydrous DMF (25 mL) under an argon atmosphere. The mixture was heated to 100 °C, upon which it became a bright pink solution. After 17 h, the color had discharged to pale orange, and the solution was cooled, sonicated briefly, and poured into H2O (100 mL). The mixture was extracted with CHCl3 (3 × 50 mL) and the organic layers were washed with H2O (3 × 200 mL), sat NH4Cl (200 mL), and dried over anhydrous sodium sulfate. The residue was adsorbed onto SiO2 (3.07 g) and purified by flash column chromatography (SiO2, 28.6 g), eluting with CHCl3 to yield a yellow-green microcrystalline solid (0.059 g, 78%) after washing with MeOH (10 mL) and ether (50 mL) and drying in vacuo for 72 h: mp 228-230 °C (dec). IR (film) 3584, 2930, 2097, 1660, 1628, 1604, 1507, 1448, 1289, 1248, 1068, 688 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.56 (d, J = 8.2 Hz, 1 H), 7.78-7.54 (s, 5 H), 7.47 (s, 1 H), 5.31 (s, 2 H), 4.44 (s, 4 H), 3.48 (t, J = 6.4 Hz, 2 H), 3.19 (t, J = 7.6 Hz, 2 H), 2.08 (m, 2 H); ESIMS m/z (rel intensity) 426 (MH+, 100). Anal. Calcd for C24H19N5O3: C, 67.76; H, 4.50; N, 16.46. Found: C, 67.47; H, 4.47; N, 16.29.
5.5.6. 14-(3′-Aminopropyl)-2,3-ethylenedioxy-12H-5,11a-diazadibenzo[b,h]fluoren-11-one dihydrochloride (28g)
Compound 28f (0.050 g, 0.118 mmol) was diluted with benzene (25 mL) and triethyl phosphite (0.059 g, 0.354 mmol) was added. The mixture was heated at reflux for 19 h. TLC indicated complete consumption of the azide and the mixture was cooled to room temperature. Methanolic HCl (10 mL, 5 M) was added, and the yellow-green mixture was heated at reflux for 3 h, cooled, and concentrated to afford a bright yellow amorphous solid (0.055 g, 99%) after washing with ether (30 mL) and drying in vacuo: mp 290-300 °C (dec). IR (KBr) 3445, 3040, 2708, 1665, 1622, 1603, 1501, 1480, 1401, 1299, 1257, 1156, 1062, 920, 761, 686 cm−1; 1H NMR (300 MHz, D2O) δ 7.20-7.10 (m, 1 H), 7.01-6.78 (m, 3 H), 6.37 (s, 1 H), 6.28 (s, 1 H), 6.05 (s, 1 H), 4.32 (s, 2 H), 4.14-4.10 (m, 4 H), 3.10-3.00 (br m, 2 H), 2.50-2.40 (br m, 2 H), 1.80-1.70 (br m, 2 H); the amino group exchanges with the solvent; ESIMS m/z (rel intensity) 400 (MH+, 100). Anal. Calcd for C24H23Cl2N3O3·0.5 H2O: C, 59.88; H, 5.03; N, 8.73. Found: C, 60.06; H, 5.29; N, 8.66.
5.7. Topoisomerase I-Mediated DNA Cleavage Reactions
Human recombinant Top1 was purified from Baculovirus as previously described.63 DNA cleavage reactions were prepared as previously reported27 (for review see64) with the exception of the DNA substrate. Briefly, a 117-bp DNA oligonucleotide (Integrated DNA Technologies) encompassing the previous identified Top1 cleavage sites identified in the 161-bp fragment from pBluescript SK(−) phagemid DNA was employed. This 117-bp oligonucleotide contains a single 5′-cytosine overhang, which was 3′-end labeled by fill-in reaction with [•-32P]-dGTP in React 2 buffer (50 mM Tris-HCl, pH 8.0, 100 mM MgCl2, 50 mM NaCl) with 0.5 units of DNA polymerase I (Klenow fragment, New England BioLabs). Unincorporated 32P-dGTP was removed using mini Quick Spin DNA columns (Roche, Indianapolis, IN), and the eluate containing the 3′-end-labeled DNA substrate was collected. Approximately 2 nM of radiolabeled DNA substrate was incubated with recombinant Top1 in 20 μL of reaction buffer [10 mM Tris-HCl (pH 7.5), 50 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 15 μg/mL BSA] at 25 °C for 20 min in the presence of various concentrations of compounds. The reactions were terminated by adding SDS (0.5% final concentration) followed by the addition of two volumes of loading dye (80% formamide, 10 mM sodium hydroxide, 1 mM sodium EDTA, 0.1% xylene cyanol, and 0.1% bromophenol blue). Aliquots of each reaction were subjected to 20% denaturing PAGE. Gels were dried and visualized by using a Phosphoimager and ImageQuant software (Molecular Dynamics). For simplicity, cleavage sites were numbered as previously described in the 161-bp fragment.63
5.8. Modeling Studies
The crystal structure of camptothecin or topotecan in complex with Top1 and a short DNA fragment were downloaded from the Protein Data Bank (PDB codes 1T8I and 1K4T).10,15 For the topotecan structure, an atom of Hg and one molecule of PEG were deleted. Hydrogens were added and minimized by the Powell method, using the MMFF94s force field and MMFF94 charges, in Sybyl 8.1 (Tripos, Inc). Analogues of aromathecins were constructed, fixed positive charges were assigned to amines using Sybyl atom types, and the structures were energy minimized using a conjugate gradient method, the MMFF94s force field, and MMFF94 charges. The structure of the ligand was aligned onto topotecan or camptothecin using the “fit atoms” function. The aligned ligand was overlapped in the crystal structure, and the structure of camptothecin or topotecan was deleted. This new ternary complex was re-subjected to energy minimization using a standard Powell method, the MMFF94s force field, and MMFF94 charges, converging to termination at 0.05 kcal/mol*Å, with a distance-dependent dielectric function. The structure of the ligand and a sphere with a radius of 5-8 Å were allowed to move during the minimization, and the surrounding structures were frozen in an aggregate. Ligand overlays were performed by aligning the protein backbones in Sybyl.
Acknowledgments
This work was facilitated by the National Institutes of Health (NIH) through support with Research Grant UO1 CA89566, Training Grant ST32 CA09634-12, and by the Lynn and McKeehan Fellowships (Purdue University, M.A.C.), and a Purdue Research Foundation Grant (#203202, M.C. and M.A.C.) This work was also supported in part by an by an ACS Medicinal Chemistry Pre-doctoral Fellowship sponsored by Pfizer Global Research and Development (A.M.), and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. M.A.C. thanks Drs. Karl Wood, Huaping Mo, and H. Daniel Lee for consultation and valuable discussion and Joe Fornefeld of Midwest Microlab for additional assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pommier Y. Nature Reviews Cancer. 2006;6:789. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. Nat. Rev. Mol. Cell Biol. 2002;3:430. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Stewart L, Redinbo MR, Qiu XY, Hol WGJ, Champoux JJ. Science. 1998;279:1534. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 4.Husain I, Mohler JL, Seigler HF, Besterman JM. Cancer. Res. 1994;54(2):539. [PubMed] [Google Scholar]
- 5.Pfister TD, Reinhold RC, Agama K, Gupta S, Khin SA, Kinders RJ, Parchment RE, Tomaszewski JE, Doroshow JH, Pommier Y. Mol. Cancer Ther. 2009;8:1878. doi: 10.1158/1535-7163.MCT-09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhold WC, Mergny J-L, Liu H, Ryan M, Pfister TD, Kinders R, Parchment R, Doroshow J, Weinstein JN, Pommier Y. Cancer Res. 2010;70(6):2191. doi: 10.1158/0008-5472.CAN-09-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J. Cancer. 2003;98(1):18. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- 8.Thomas CJ, Rahier NJ, Hecht SM. Bioorg. Med. Chem. 2004;12:1585. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Wall ME, Wani MC, Cook CE, Palmer KH, A.T. M, Sim GA. J. Am. Chem. Soc. 1966;88:3888. [Google Scholar]
- 10.Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, Burgin A. J. Med. Chem. 2005;48:2336. doi: 10.1021/jm049146p. [DOI] [PubMed] [Google Scholar]
- 11.Ioanaviciu A, Antony S, Pommier Y, Staker BL, Stewart L, Cushman M. J. Med. Chem. 2005;48:4803. doi: 10.1021/jm050076b. [DOI] [PubMed] [Google Scholar]
- 12.Marchand C, Antony S, Kohn KW, Cushman M, Ioanaviciu A, Staker BL, Stewart L, Pommier Y. Mol. Cancer Ther. 2006;5(2):287. doi: 10.1158/1535-7163.MCT-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X, Cushman M. J. Am. Chem. Soc. 2005;127:9960. doi: 10.1021/ja042485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiang YH, Hertzberg R, Hecht S, Liu LF. J. Biol. Chem. 1985;260:14873. [PubMed] [Google Scholar]
- 15.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr., Stewart L. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15387. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrencik JE, Staker BL, Burgin AB, Jr., Pourquier P, Pommier Y, Stewart L, Redinbo MR. J. Mol. Biol. 2004;339:773. doi: 10.1016/j.jmb.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 17.Teicher BA. Biochem. Pharmacol. 2008;75:1262. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Schaeppi U, Fleischman R, Cooney DW. Cancer Chemother. Rep. 1974;58:25. [PubMed] [Google Scholar]
- 19.Luzzio MJ, Besterman JM, Emerson DL, Evans MG, Lackey K, Leitner PL, McIntyre G, Morton B, Myers PL, Peel M, Sisco JM, Sternbach DD, Tong W, Truesdale A, Uehling DE, Vuong A, Yates J. J. Med. Chem. 1995;38:395. doi: 10.1021/jm00003a001. [DOI] [PubMed] [Google Scholar]
- 20.Mi Z, Burke TG. Biochemistry. 1994;33:10325. doi: 10.1021/bi00200a013. [DOI] [PubMed] [Google Scholar]
- 21.Nagarajan M, Morrell A, Fort BC, Meckley MR, Antony S, Kohlhagen G, Pommier Y, Cushman M. J. Med. Chem. 2004;47(23):5651. doi: 10.1021/jm040025z. [DOI] [PubMed] [Google Scholar]
- 22.Morrell A, Placzek M, Parmley S, Grella B, Antony S, Pommier Y, Cushman M. J. Med. Chem. 2007;50:4388. doi: 10.1021/jm070307+. [DOI] [PubMed] [Google Scholar]
- 23.Morrell A, Antony S, Kohlhagen G, Pommier Y, Cushman M. Bioorg. Med. Chem. Lett. 2004;14:3659. doi: 10.1016/j.bmcl.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan M, Morrell A, Ioanoviciu A, Antony S, Kohlhagen G, Hollingshead M, Pommier Y, Cushman M. J. Med. Chem. 2006;49:6283. doi: 10.1021/jm060564z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pommier Y, Cushman M. Mol. Cancer Ther. 2009;8(5):1008. doi: 10.1158/1535-7163.MCT-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antony S, Agama KK, Miao Z-H, Takagi K, Wright MH, Robles AI, Varticovski L, Nagarajan M, Morrell A, Cushman M, Pommier Y. Cancer Res. 2007;67(21):10397. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 27.Cinelli MA, Morrell A, Dexheimer T, Scher E, Pommier Y, Cushman M. J. Med. Chem. 2008;51(15):4609. doi: 10.1021/jm800259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cinelli MA, Cordero B, Dexheimer TS, Pommier Y, Cushman M. Bioorg. Med. Chem. 2009;17:7145. doi: 10.1016/j.bmc.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao X, Antony S, Pommier Y, Cushman M. J. Med. Chem. 2006;49:1408–1412. doi: 10.1021/jm051116e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox BM, Xiao X, Antony S, Kohlhagen G, Pommier Y, Staker BL, Stewart L, Cushman M. J. Med Chem. 2003;46:3275. doi: 10.1021/jm0300476. [DOI] [PubMed] [Google Scholar]
- 31.Cheng K, Rahier NJ, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM. J. Am. Chem. Soc. 2005;127(3):838. doi: 10.1021/ja0442769. [DOI] [PubMed] [Google Scholar]
- 32.Babjak M, Kanazawa A, Anderson RJ, Greene AE. Org. Biomol. Chem. 2006;4:407. doi: 10.1039/b516154a. [DOI] [PubMed] [Google Scholar]
- 33.Pin F, Comesse S, Sanselme M, Daïch A. J. Org. Chem. 2008;73(5):1975. doi: 10.1021/jo702387q. [DOI] [PubMed] [Google Scholar]
- 34.Shamma M, Novak L. Tetrahedron. 1969;25:2275. [Google Scholar]
- 35.Sugasawa T, Toyoda T, Adachi M, Sasakura K. J. Am. Chem. Soc. 1978;100:4842. [Google Scholar]
- 36.Sugasawa T, Adachi M, Sakasura K, Kitagawa A. J. Org. Chem. 1979;44(4):578. [Google Scholar]
- 37.Cevasco AA. US Patent No. 5,405,998 1995
- 38.Houghton PG, Humphrey GR, Kennedy DJ, Roberts DC, Wright SHB. J. Chem. Soc. Perkin Trans. 1993;1:1421. [Google Scholar]
- 39.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J. J. Natl. Cancer Inst. 1990;82(13):1107. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 40.Paull KD, Hamel E, Malspeis L. In: Cancer Chemotherapeutic Agents. Foye WO, editor. American Chemical Society; Washington, DC: 1995. pp. 9–45. [Google Scholar]
- 41.Antony S, Jayaraman M, Laco G, Kohlhagen G, Kohn KW, Cushman M, Pommier Y. Cancer Res. 2003;63:7428. [PubMed] [Google Scholar]
- 42.Morrell A, Placzek MS, Steffen JD, Antony S, Agama K, Pommier Y, Cushman M. J. Med Chem. 2007;50:2040. doi: 10.1021/jm0613119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emerson DL, Besterman JM, Brown HR, Evans MG, Leitner PP, Luzzio MJ, Shaffer JE, Sternbach DD, Uehling D, Vuong A. Cancer. Res. 1995;55:603. [PubMed] [Google Scholar]
- 44.Sessa C, Wanders J, Roelvink M, Dombernowsky P, Nielsen D, Morant R, Drings P, Wissel P, Hanauske A-R. Ann. Oncol. 2000;11:207. doi: 10.1023/a:1008372404504. [DOI] [PubMed] [Google Scholar]
- 45.Wall ME, Wani MC, Nicholas AW, Manikumar G, Tele C, Moore L, Truesdale A, Leitner P, Besterman JM. J. Med Chem. 1993;36:2689. doi: 10.1021/jm00070a013. [DOI] [PubMed] [Google Scholar]
- 46.Jew S-s., Kim MG, Kim H-J, Rho E-Y, Park H-g., Kim J-K, Han H-J, Lee H. Bioorg. Med. Chem. Lett. 1998;8:1797. doi: 10.1016/s0960-894x(98)00317-5. [DOI] [PubMed] [Google Scholar]
- 47.Werbovetz KA, Bhattacharjee AK, Brendle JJ, Scovill JP. Bioorg. Med. Chem. 2000;8:1741. doi: 10.1016/s0968-0896(00)00111-5. [DOI] [PubMed] [Google Scholar]
- 48.Xie Z, Ootsu K, Akimoto H. Bioorg. Med. Chem. Lett. 1995;5(19):2189. [Google Scholar]
- 49.O'Connor PM, Kerrigan D, Bertrand R, Kohn KW, Pommier Y. Cancer Commun. 1990;2:395. doi: 10.3727/095535490820873912. [DOI] [PubMed] [Google Scholar]
- 50.Wani MC, Nicholas AW, Wall ME. J. Med Chem. 1986;29:2358. doi: 10.1021/jm00161a035. [DOI] [PubMed] [Google Scholar]
- 51.Vladu B, Woynarowski JM, Manikumar G, Wani MC, Wall ME, Von Hoff DD, Wadkings RM. Mol. Pharm. 2000;57:243. [PubMed] [Google Scholar]
- 52.Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y. Cancer Res. 1989;49:1465. [PubMed] [Google Scholar]
- 53.Fan Y, Weinstein JN, Kohn KW, Shi LM, Pommier Y. J. Med. Chem. 1998;41:2216. doi: 10.1021/jm9605445. [DOI] [PubMed] [Google Scholar]
- 54.Versace RW. Expert. Opin. Ther. Patents. 2003;13(6):751. [Google Scholar]
- 55.Wani MC, Nicholas AW, Manikumar G, Wall ME. J. Med. Chem. 1987;30:1774. doi: 10.1021/jm00393a016. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Wang L-K, Kingsbury WD, Johnson RK, Hecht SM. Biochemistry. 1998;37:9399. doi: 10.1021/bi980451k. [DOI] [PubMed] [Google Scholar]
- 57.Morrell A, Antony S, Kohlhagen G, Pommier Y, Cushman M. J. Med. Chem. 2006;49:7740. doi: 10.1021/jm060974n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Souza PL, Cooper MR, Imondi AR, Myers CE. Clin. Cancer Res. 1997;3:287. [PubMed] [Google Scholar]
- 59.Dallavalle S, Rocchetta DG, Musso L, Merlini L, Morini G, Penco S, Tinelli S, Beretta GL, Zunino F. Bioorg. Med. Chem. Lett. 2008;18:2781. doi: 10.1016/j.bmcl.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Huang M, Gao H, Chen Y, Zhu H, Cai Y, Zhang X, Miao Z, Jiang H, Zhang J, Shen H, Lin L, Lu W, Ding J. Clin. Cancer. Res. 2007;13:1298. doi: 10.1158/1078-0432.CCR-06-1277. [DOI] [PubMed] [Google Scholar]
- 61.Quintas-Cardama A, Kantarjian H, O' Brien S, Jabbour E, Giles F, Ravanti F, Faderi S, Pierce S, Shan J, Verstovsek S, Cortes J. Cancer. 2006;107(7):1525. doi: 10.1002/cncr.22186. [DOI] [PubMed] [Google Scholar]
- 62.Phillips AP. U.S. Patent 4374146 1983
- 63.Pourquier P, Ueng L-M, Fertala J, Wang D, Park H-K, Essigmann JM, Bjornsti M-A, Pommier Y. J. Biol. Chem. 1999;274:8516. doi: 10.1074/jbc.274.13.8516. [DOI] [PubMed] [Google Scholar]
- 64.Dexheimer TS, Pommier Y. Nature Protocols. 2008;3(11):1736. doi: 10.1038/nprot.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]