Summary
The activation of inflammasomes containing NBD-LRR (NLRs) or non-NLRs is critical for effective host defense against microbial pathogens. Recent discoveries have uncovered a plethora of pathogenic strategies to inhibit inflammasome-mediated processing of IL-1β and IL-18. We review recent evidence for viral and bacterial manipulation of the inflammasome ranging from perturbation of caspase-1 activation to targeting of specific inflammasome components.
The role of the inflammasome in combating pathogenic infection
The innate immune response stands at the forefront of detecting “danger” signals whether foreign or intrinsic. Germline-encoded pattern-recognition receptors (PRRs) mediate the detection of viral and bacterial cell components. These PRRs include toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and nucleotide-binding domain and leucine-rich repeat proteins (NLRs). Pathogens activate the transcriptional and translational induction of a range of proinflammatory cytokines, but also elicit the activation of a multimeric protein complex known as the inflammasome that is critical in the proteolytic processing of pro-IL-1β and pro-IL18 into their mature active forms. The inflammasome is classically composed of an NLR, the adaptor molecule PYCARD/ASC, and pro-caspase-1, which when proteolyzed to caspase-1 provides the enzymatic activity of the inflammasome. Pro-caspase-1 forms the core of the inflammasome, however the constitution of NLRs within the inflammasome varies according to the pathogen involved. NLRP3 (cryopyrin, NALP3) represents the most widely used inflammasome NLR by bacterial and viral pathogens, while NLRP1 and NLRC4 inflammasomes respond to a subset of bacterial pathogens. Non-NLR inflammasomes also have been described containing the HIN2 protein AIM2 and the viral sensor RIG-I (Pedra 2009, Poeck 2010, Hornung 2010).
IL-1β is critical in the host defense against a number of bacteria and viruses, and pathogens have evolved mechanisms to prolong their survival by inhibiting IL-1β transcription and by producing IL-1β decoy receptors (Dinarello 2009). Additionally, recent studies have revealed an increasing number of pathogenic virulence factors that function to target the inflammasome and inhibit IL-1β processing. Examples of the mechanisms by which viruses and bacteria repress inflammasome activation to inhibit IL-1β secretion are outlined within the context of the two-step activation of IL-1β (Fig. 1). Additionally, specific information about the genes involved is provided in the sections below and summarized in Table I. Since host inhibition of the inflammasome has many parallels to pathogen-mediated inhibition of the inflammasome, the former will be briefly discussed.
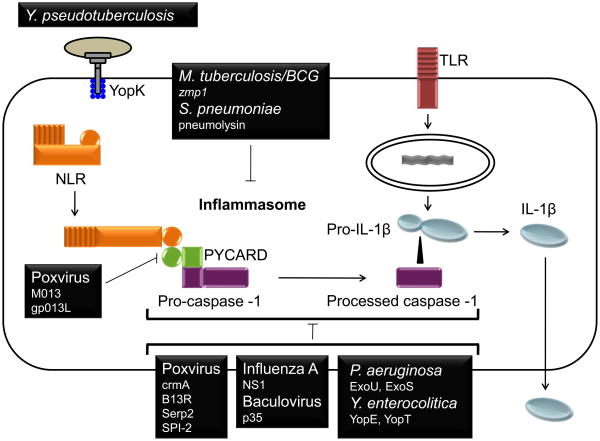
Figure 1. Suppression of the two step regulation of IL-1β production by virus and bacteria.
Host IL-1β release is prompted by a two step process. First, the activation of NF-κB and AP-1 leads the transcription of pro-IL-1β. A second signal sensed by an NLR triggers the assembly of the inflammasome and the subsequent activation of caspase-1. Active caspase-1 proteolytically processes pro-IL-1β into IL-1β for release. Viruses and bacteria have developed an array of strategies to interfere with inflammasome activation. Myxoma and Shope fibroma viruses utilize POP-like proteins M013 and gp013L to bind ASC/PYCARD and prevent the inflammasome assembly. Poxviruses also express the serpins crmA, Serp2, B13L, and SPI-2 which disrupt the proteolytic activity of caspase-1, potentially through direct binding to caspase-1. Influenza A virus and baculovirus express NS1 and p35, and Y. enterocolitica expresses Yop E and YopT that disrupt caspase-1 oligomerization.. The P. aeruginosa effector molecules ExoU and ExoS block the activation of the NLRC4 inflammasome, while the Mycobacterium tuberculosis gene zmp1 may target the NLRP3 inflammasome. Pneumolysin of Streptococcus pneumonia also blocks IL-1β release by diminishing caspase-1 activity. YopK of Yersinia pseudotuberculosis has a unique mechanisms in that it binds to T3SS and masks detection by the inflammasome Though each of these proteins inhibits caspase-1 activation, it is not known in most cases whether caspase-1 is directly targeted or whether effects are mediated indirectly by targeting of an upstream regulator.
Table 1.
Examples of bacteria and viruses that inhibit IL-1β release as a pathogenic stealth mechanism.
| Gene/Protein | Pathogen | Classification | Function | References |
|---|---|---|---|---|
| M013 | Myxoma virus | cPOP homolog | Binds PYCARD and inhibits caspase-1 activation | Johnston 2005 |
| gp013L | Shope fibroma virus | cPOP homolog | Binds PYCARD and inhibits inflammasome activation | Dorfleutner 2007 |
| crmA | Cowpox virus | serpin | Inhibits caspase-1 activation | Ray 1992 |
| B13R | Vaccinia virus | serpin | Inhibits caspase-1 activation | Kettle 1997 |
| Serp2 | Myxoma virus | serpin | Binds caspase-1 and inhibits caspase-1 activation | Petit 1996 |
| SPI-2 | Ectromelia virus | serpin | Inhibits caspase-1 activity | Turner 2000 |
| NS1 | Influenza A virus | ds RNA-binding protein | Inhibits caspase-1 activation | Stasakova 2005 |
| p35 | Baculovirus | unknown | Inhibits caspase-1 activation | Bump 1995 |
| ExoU | Pseudomonas aeruginosa PA103 | exoenzyme | Inhibits IL-1β, caspase-1 activation within the NLRC4 inflammasome | Sutterwala 2007 |
| ExoS | Pseudomonas aeruginosa | GTPase-activating protein/ADP ribosytransferase | Inhibits IL-1β, caspase-1 activation | Galle 2008 |
| YopE | Yersinia enterocolitica | GTPase-activating protein | Inhibits IL-1β, caspase-1 activation/oligomerization | Schotte 2004 |
| YopT | Yersinia enterocolitica | cysteine protease | Inhibits IL-1β, caspase-1 activation/oligomerization | Schotte 2004 |
| YopK | Yersinia pseudotuberculosis | T3SS-binding protein | Prevents inflammasomal recognition of the T3SS | Brodsky 2010 |
| zmp1 | Mycobacterium tuberculosis/BCG | Zn2+ metalloproteinase | Inhibits IL-1β, caspase-1 activation by NLRP3 inducers | Master 2008 |
| pneumolysin | Streptococcus pneumoniae | pore-forming toxin | Inhibits IL-1β, caspase-1 activation | Littmann 2009 |
Host derived inhibition of the inflammasome
Host suppression of the inflammasome is essential since prolonged inflammatory response beyond eradication of a foreign or self-insult can lead to excessive tissue damage. Pathways of deactivation are important to understand since they provide potential targets or mimics for pathogens in their attempts to achieve immune stealth by minimizing the inflammatory response. Since PYCARD is an adaptor molecule shared by a variety of NLR and non-NLR inflammasome complexes, a PYCARD-binding molecule provides an attractive target for deactivation of the inflammasome. One well-characterized family of inhibitors of PYCARD is the cellular PYRIN domain (PYD)-only proteins (POP) family. Mammalian cPOP1 and cPOP2 disrupt inflammasome activation by binding PYCARD and blocking its interaction with NLRs (Stehlik 2003, Dorfleutner 2007, Bedoya 2007). The serpin proteinase inhibitor 9 (PI-9), on the other hand, directly inhibits caspase-1 activity through protein-protein interaction (Young 2000). PI-9 is expressed by vascular smooth muscle cells and a variety of normal tissues, and its expression correlates inversely with that of IL-1β. Caspase-12 also can directly associate with caspase-1 to inhibit its activity. Polymorphisms in the human caspase-12 gene confer increased sepsis (Saleh 2004), and its deficiency in mice impedes bacterial clearance (Saleh 2006). An additional class of host inflammasome inhibitors is represented by the Bcl family of cell survival proteins. Bcl-2 and Bcl-XL can directly bind to NLRP1 to disrupt the activation of its inflammasome and subsequent IL-1β processing (Bruey 2007). Another seminal study showed that T cells, in addition to profoundly regulating the adaptive immune response, are key regulators of innate immunity via the specific inhibition of inflammasomes. CD4+ T cells inhibit the activation of NLRP3 and NLRP1 inflammasomes but not the NLRC4 inflammasome (Guarda 2009). This suppression can be replicated by TNF family ligands such as CD40L. Collectively, these studies have expanded our understanding of host mechanisms for suppressing inflammasome activation and have provided rationales for pathways that pathogens have evolved to target.
Viral inhibition of the inflammasome
Poxviruses produce cPOP and serpin homologs that bind and target PYCARD and caspase-1
In a 2005 study, a PYD containing protein analogous to the host-derived cPOP proteins was identified from myxoma virus, a member of the poxvirus family (Johnston 2005). This myxoma virus M013 protein is essential for productive viral infection, and like cPOP1 and cPOP2, binds PYCARD to prevent inflammasome activation. The poxvirus Shope fibroma virus encodes an additional cPOP homolog, gp013L, which like M013, co-localizes with PYCARD when transfected into cells, and can also reduce NLRP3-mediated IL-1β processing in a reconstituted cell system (Dorfleutner 2007).. Both M013 and gp013L were shown to possess an additional inhibitory activity on the transcription of IL-1β and other pro-inflammatory cytokines through repression of NF-κB (Dorfleutner 2007, Rahman 2009). These combined studies demonstrate that the poxvirus vPOPs simultaneously target inflammasome activation and NF-κB activation to provide dual regulation of IL-1β at the level of transcription and processing.
An additional strategy of poxviruses involves the production of viral serpins analogous to the mammalian PI-9 protein (Young 2000). In a landmark study, the cowpox gene crmA was identified as a potent host immune suppressor and a specific inhibitor of caspase-1 (Ray 1992). Extracts from cowpox infected cells can block the processing of IL-1β, and this property is lost when crmA is inactivated. Furthermore, purified CrmA protein abrogates the proteolytic activity of caspase-1. Vaccinia virus expresses a gene B13R that is highly homologous to crmA and is required for inhibition of IL-1β processing in vaccinia-infected THP1 monocytic cells (Kettle 1997). The myxoma virus counterpart of CrmA and B13R, Serp2, immunoprecipitates with caspase-1, suggesting that direct interaction may mediate the inhibitory function of the poxvirus serpins (Petit 1996). A similar caspase-1 inhibiting protein is encoded by ectromelia virus, a murine orthopoxvirus (Turner 2000). Thus the production of serpin PI-9 homologs appears to be an evolutionarily preserved and advantageous inhibitory mechanism adopted by multiple viruses to subvert host immunity.
Additional viral proteins that inhibit inflammasome activity
A dsRNA virus and a member of the Orthomyxoviridae, influenza A virus encodes approximately 10 proteins, among which the non-structural protein NS1 is critical for evasion of host immune defense (Stasakova 2005). NS1 is a 26KD protein that dimerizes to prevent nuclear export of host mRNA, activation of protein kinase (PKR) and the type I interferon response. Viruses expressing NS1 bearing mutations within the RNA binding and dimerization domains induce significantly elevated levels of caspase-1 activation and IL-1β and IL-18 secretion in primary human macrophages, suggesting that NS1 constitutes an additional viral protein that inhibits inflammasome activation.
Baculovirus expresses the antiapoptotic protein p35 which also can inhibit caspase-1 (Bump 1995). Co-expression of p35 with caspase-1 in a cell line that constitutively expresses pro-IL-1β decreases the release of IL-1β. Furthermore, purified recombinant p35 can inhibit the enzymatic activity of caspase-1. To date, this protein has no identifiable structural domains and no known human homolog, thus it could potentially comprise a novel class of inflammasome inhibitors.
Bacterial inhibition of the inflammasome
Pseudomonas aeruginosa and Yersinia species utilize the T3SS to prevent inflammasome activation
The Type III secretion system (T3SS) provides a mechanism for inserting bacterial virulence factors into the host cell, and it is not surprising that this system is exploited by several bacteria to minimize the inflammasome response. Pseudomonas aeruginosa induces NLRC4-dependent IL-1β release, however one strain of P. aeruginosa, PA103, fails to induce caspase-1 activation and IL-1β release (Sutterwala 2007). This effect was shown to be mediated by the T3SS effector molecule Exoenzyme U (ExoU). The ability of ExoU to suppress caspase-1 is attributed to its phospholipase activity, though it is not clear how this activity relates to its inhibitory function. ExoS also negatively regulates caspase-1-mediated IL-1β processing (Galle 2007). Interestingly, ExoS is a bifunctional protein with an N-terminal Rho GTPase domain and a C-terminal ADP-ribosyltransferase domain that could act to inhibit immune function.
Yersinia also produces a set of T3SS effector molecules, the Yops, that can modulate inflammasome response. Y. enterocolitica deficient in YopE and YopT induces elevated caspase-1 maturation and IL-1β secretion in a murine macrophage cell line (Schotte 2004). Furthermore, YopE and YopT were found to modulate the oligomerization of caspase-1. Like ExoS of P. aeruginosa, YopE serves as a Rho GTPase activating protein, while YopT is a cysteine protease. Though the relevance of these activities to caspase-1 inhibition is unclear, this suggests a commonality in function of the T3SS effector molecules in blocking inflammasome activation.
A recent study shows that YopK of Y. pseudotuberculosis has an entirely unique mechanism for interfering with inflammasome activation (Brodsky 2010). The T3SS of Y. pseudotuberculosis activates NLRP3 and NLRC4-dependent caspase-1 processing, but YopK associates with the T3SS translocon to mask its detection by the inflammasome. This is significant because it represents the first study where an effector molecule binds to T3SS to avoid host detection. It is likely that analgous proteins may exist for other T3SS bacteria.
Mycobacterium tuberculosis produces a Zn2+ metalloprotease that inhibits inflammasome activation by NLRP3 inflammasome inducers
IL-1β is not highly activated during infection with Mycobacterium tuberculosis (Mtb) or related Mycobacterium species such as the vaccine strain Mycobacterium bovis BCG. The BCG gene zmp1 encodes a Zn2+ metalloprotease that is required for its suppression of IL-1β processing (Master 2008). BCG infection also prevents the activation of caspase-1 triggered by the simultaneous administration of NLRP3 inflammasome agonists nigericin and ATP. This is a significant finding because it suggests that BCG undergoes an active process to repress inflammasome activation induced by an independent second signal. Interestingly, caspase-1−/− mice infected with Mtb exhibit wt levels of both pro-IL-1β and processed IL-1β. Despite the persistance of IL-1β, these mice display elevated lung burdens and decreased survival compared to wt mice (Mayer-Barber 2010). This suggests that caspase-1 plays other protective roles in Mtb infection besides IL-1β processing, such as regulating cell death.
Streptococcus pneumoniae produces a pore-forming toxin that suppresses the inflammasome
Streptococcus pneumoniae utilizes a number of virulence factors for successful colonization. Among these, the cholesterol-dependent pore-forming exotoxin, pneumolysin, is implicated in S. pneumoniae-induced host tissue damage. Pneumolysin-deficient bacteria induce elevated caspase-1 activation and IL-1β secretion in human DCs (Littmann 2009). This is surprising since other pore-forming toxins, by contrast, are known to activate caspase-1 (Cordoba-Rodriguez 2004, Craven 2009). Pneumolysin-deficient S. pneumoniae also exhibit elevated levels of immature IL-1β, suggesting that pneumolysin modifies host cell signaling pathways such NF-κB or MAP kinase to simultaneously suppress the expression and processing of IL-1β.
Concluding Remarks
Throughout evolution, pathogens have developed mechanisms to circumvent host immune detection via a wide range of strategies. Since IL-1β is critical in the effort to eradicate foreign microbial infection, inflammasome-mediated signaling pathways provide an obvious target for achieving pathogenic stealth by minimizing the host immune response. Though IL-1β appears to function as the primary target for pathogen-mediated inflammasome inhibition, studies with Mtb demonstrate that inflammatory processes other than IL-1β processing, such as pathogen-mediated cell death, may be targeted for the benefit of the pathogen. Viruses and bacteria inhibitt multiple stages of inflammasome activation. Poxviruses produce cPOP homologs that directly bind PYCARD and thereby prevent inflammasome assembly. Additionally, abundant evidence points to caspase-1 as a prime target for suppression of IL-1β production; however in many cases it not known whether caspase-1 is directly targeted or whether an unknown upstream mediator is involved. NLRP1 is a target for host Bcl family proteins (Bruey 2007), however as yet no pathogen-derived IL-1β inhibitors have been shown to function through direct interaction with an NLR component of the inflammasome. It is intriguing that several pathogen-encoded proteins show specificity for particular NLR inflammasome pathways, suggesting that such regulators may exist. The pathogen-derived inflammasome inhibitors identified thus far range from serpins to enzymes to pore forming toxins, suggesting that the mechanisms that have evolved to perturb the inflammasome are widespread. In each of the examples presented, inflammasome inhibition is beneficial to the pathogen, however it is possible that in cases of excessive inflammasome activation leading to tissue destruction inhibition might be beneficial to the host. Future challenges include the elucidation of mechanisms connecting pathogen-derived ligands to caspase-1 activation. For example, in most cases it has not been determined whether pathogenic proteins affect inflammasome assembly, the function of the mature caspase-1 protein, or an upstream mediator of the inflammasome. Elucidation of these mechanisms will lead to the development of novel approaches towards controlling infectious diseases and other inflammasome-dependent inflammatory processes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedoya F, Sandler LL, Harton JA. Pyrin-only protein 2 modulates NF-kappaB and disrupts ASC:CLR interactions. J Immunol. 2007 Mar 15;178(6):3837–45. doi: 10.4049/jimmunol.178.6.3837. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, Flavell RA, Bliska JB, Medzhitov R. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 2010 May 20;7(5):376–87. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, Matsuzawa S, Terskikh AV, Faustin B, Reed JC. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007 Apr 6;129(1):45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Bump NJ, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995 Sep 29;269(5232):1885–8. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 5.Cordoba-Rodriguez R, Fang H, Lankford CS, Frucht DM. Anthrax lethal toxin rapidly activates caspase-1/ICE and induces extracellular release of interleukin (IL)-1beta and IL-18. J Biol Chem. 2004 May 14;279(20):20563–6. doi: 10.1074/jbc.C300539200. [DOI] [PubMed] [Google Scholar]
- 6.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009 Oct 14;4(10):e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 8.Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect Immun. 2007 Mar;75(3):1484–92. doi: 10.1128/IAI.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galle M, Schotte P, Haegman M, Wullaert A, Yang HJ, Jin S, Beyaert R. The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of caspase-1 mediated IL-1beta maturation. J Cell Mol Med. 2008 Sep-Oct;12(5A):1767–76. doi: 10.1111/j.1582-4934.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009 Jul 9;460(7252):269–73. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 11.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010 Feb;10(2):123–30. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 12.Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, McFadden G. poxvirus-encoded pyrin domain protein interacts with PYCARD to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005 Dec;23(6):587–98. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Kettle S, Alcamí A, Khanna A, Ehret R, Jassoy C, Smith GL. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997 Mar;78(Pt 3):677–85. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 14.Littmann M, Albiger B, Frentzen A, Normark S, Henriques-Normark B, Plant L. Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression. EMBO Mol Med. 2009 Jul;1(4):211–22. doi: 10.1002/emmm.200900025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008 Apr 17;3(4):224–32. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010 Apr 1;184(7):3326–30. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009 Feb;21(1):10–6. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petit F, Bertagnoli S, Gelfi J, Fassy F, Boucraut-Baralon C, Milon A. Characterization of a myxoma virus-encoded serpin-like protein with activity against interleukin-1 beta-converting enzyme. J Virol. 1996 Sep;70(9):5860–6. doi: 10.1128/jvi.70.9.5860-5866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschläger N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010 Jan;11(1):63–9. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 20.Rahman MM, Mohamed MR, Kim M, Smallwood S, McFadden G. Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 2009 Oct;5(10):e1000635. doi: 10.1371/journal.ppat.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 22.Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 23.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006 Apr 20;440(7087):1064–8. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 24.Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis GR, Beyaert R. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J Biol Chem. 2004 Jun 11;279(24):25134–42. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- 25.Stasakova J, Ferko B, Kittel C, Sereinig S, Romanova J, Katinger H, Egorov A. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J Gen Virol. 2005 Jan;86(Pt 1):185–95. doi: 10.1099/vir.0.80422-0. [DOI] [PubMed] [Google Scholar]
- 26.Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J. 2003 Jul 1;373(Pt 1):101–13. doi: 10.1042/BJ20030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007 Dec 24;204(13):3235–45. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner SJ, Silke J, Kenshole B, Ruby J. Characterization of the ectromelia virus serpin, SPI-2. J Gen Virol. 2000 Oct;81(Pt 10):2425–30. doi: 10.1099/0022-1317-81-10-2425. [DOI] [PubMed] [Google Scholar]
- 29.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schönbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000 May 1;191(9):1535–44. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]