Abstract
Monocytes/macrophages are critical early innate immune responders during murine CMV (MCMV) infection. It has been established that inflammatory monocyte/macrophages are released from the bone marrow and into the peripheral blood before entry into infected tissue sites. We previously reported a role for IFN-α/β in promotion of CCR2-mediated recruitment of monocyte/macrophages into the liver in response to MCMV infection. However, the mechanisms that support the migration of monocyte/macrophages from the bone marrow and into the peripheral blood under conditions of MCMV infection have not been elucidated. Herein, we demonstrate an accumulation of monocyte/macrophages in the bone marrow of MCMV-infected CCR2-deficient mice, whereas circulating monocyte/macrophages are profoundly diminished. The CCR2 ligands MCP-1, MCP-3, and MCP-5 are detected in bone marrow and in serum from MCMV-infected mice. Furthermore, bone marrow leukocytes from naive mice produce high levels of MCP-1 and MCP-5, and moderate levels of MCP-3, when stimulated with recombinant IFN-α in culture. We identify bone marrow F4/80+ cells as major producers of MCP-1, MCP-3, and MCP-5. Moreover, induction of CCR2 ligands is dependent on IFN-α/β-mediated signals and MCMV infection. Taken together, the results reveal a critical role for inflammatory cytokines in stimulating production of CCR2-binding chemokines from F4/80+ cells in the bone marrow, and they suggest that local production of chemokines supports monocyte/macrophage egress from the bone marrow into the blood during a virus infection.
Cytomegaloviruses are species-specific betaherpesviruses. Human CMV causes asymptomatic infection in immunocompetent individuals, but it can cause debilitating disease in immunosuppressed patients such as those infected with HIV or transplant recipients (1, 2). Like human CMV, murine CMV (MCMV)3 is hepatotropic and serves as an animal model system for understanding human infection (3, 4). During acute MCMV infection, innate immune responses induced by type I IFNs (IFN-α/β) are critical to antiviral defense. Coordination of cytokine and chemokine networks is essential to early control of infection. Induction of IFN-α/β by plasmacytoid dendritic cells in the liver stimulates production of the chemokine MCP-1 (or CCL2) by resident liver macrophages (5). This is followed by an influx of trafficking macrophages that contribute to production of the chemokine MIP-1α (or CCL3) to recruit NK cells (6, 7). NK cells play a major role in liver antiviral defense by producing an early peak of IFN-γ and coordinating downstream adaptive T cell responses (8–11). Therefore, mobilization of inflammatory monocyte/macrophage populations to sites of MCMV infection is absolutely critical to antiviral defense.
Monocytes comprise a highly heterogeneous population of leukocytes that originate in the bone marrow and have the potential to develop into macrophages and dendritic cells (12). Murine monocytes express F4/80 and CD11b Ags and are subdivided into subsets according to their differential expression of CCR2, CX3CR1, and Ly6C (13–15). Homeostatic or resident monocytes are characterized as CCR2−CX3CR1+Ly6Clow and seed peripheral compartments to reconstitute resident macrophage or dendritic cell populations. Conversely, inflammatory monocytes are defined as CCR2+CX3CR1lowLy6Chigh and become mobilized in response to inflammatory cues. Expression of CCR2 on the surface of inflammatory monocytes directs their recruitment to inflamed tissue sites. The CC chemokines MCP-1 (CCL2), MCP-3 (CCL7), and MCP-5 (CCL12) can bind CCR2 and trigger activation (16–18). CCR2 and MCP-1 interactions are well documented as promoters of monocyte recruitment during infection and in various other models of inflammation (19–24). The in vivo role of MCP-3 and MCP-5 in monocyte chemotaxis and inflammation is less well characterized.
Recent studies using Listeria monocytogenes as a model of infection have defined a unique role for CCR2 in regulation of monocyte dissemination (25). Interestingly, in this model, CCR2 expression was required for inflammatory monocyte emigration from the bone marrow but not entry into the peripheral tissues from the circulation. Monocyte recruitment from bone marrow into circulation was also shown to be dependent on MCP-1 and MCP-3 during this bacterial infection and in a model of hypercholesterolemia (26, 27). These findings define a new role for chemokines, whose production by cells at peripheral sites of infection is conventionally thought to attract leukocytes that express the appropriate activating chemokine receptor from the circulation. The new model suggests that chemokine-chemokine receptor interactions may be required to attract leukocytes into the circulation from sites such as bone marrow, thus regulating the availability to peripheral tissue compartments.
During MCMV infection, CCR2 and MCP-1 have defined roles in recruiting inflammatory monocyte/macrophages to the liver (5, 28). CCR2- and MCP-1-deficient mice recruit fewer monocyte/macrophages into the liver by 48 h postinfection. This is associated with reduced downstream protective responses (28). It is not known whether CCR2-dependent effects influence the recruitment of monocyte/macrophages into the liver by indirectly promoting their release from the bone marrow into the blood during MCMV infection. Additionally, the in vivo cellular and molecular mechanisms promoting CCR2 ligand expression in the bone marrow have not yet been determined.
The studies presented herein were undertaken (1) to define CCR2-dependent roles in the regulation of monocyte/macrophage mobilization from the bone marrow into the blood, and (2) to identify a mechanism for induction of CCR2 ligand expression in the bone marrow during MCMV infection. The results indicate that inflammatory monocyte/macrophages accumulate in the bone marrow, but are diminished in the blood, of CCR2-deficient mice during MCMV infection. Furthermore, we demonstrate that MCMV infection induces MCP-1, MCP-3, and MCP-5 production in the bone marrow and in the blood. IFN-α/β-mediated effects are shown to elicit production of CCR2 ligands in the bone marrow. Furthermore, F4/80+ bone marrow leukocytes are identified as the primary responders to IFN-α/β stimulation for CCR2 ligand production. Collectively, these results clearly demonstrate that inflammatory cytokines promote chemokine production in the bone marrow, and they suggest that local production regulates monocyte/macrophage emigration from the bone marrow into the blood during a virus infection.
Materials and Methods
Mice
Pathogen-free C57BL/6 and B6-CCR2−/− mice were obtained from The Jackson Laboratory. B6-IFN-α/βR1−/− mice (provided by Dr. L. Brossay, Brown University, Providence, RI) were generated as described (29, 30). All mice, except C57BL/6, were bred and maintained in pathogen-free mouse facilities at Brown University. Age- and sex-matched mice were used in all experiments. Mouse handling and experimental procedures were conducted in accordance with institutional guidelines for animal care and use.
Virus infection
The Smith strain of MCMV was a salivary gland-passaged virus stock prepared from CD1 mice and titrated by plaque assay on NIH3T3 cells (American Type Culture Collection). Infections were initiated on day 0 by i.p. injection at a dose of 5 × 104 PFU per mouse. In vivo responses were examined either 40 or 48 h postinfection.
Preparation of leukocytes, serum, and conditioned media
Whole blood was treated with NH4Cl2 to lyse erythrocytes and washed twice in PBS containing 1% heat-inactivated FCS (HyClone Laboratories). Bone marrow cells were obtained by flushing the femur with RPMI 1640 supplemented with 10% heat-inactivated FCS, followed by RBC lysis. The number of viable cells was determined by trypan blue exclusion. Serum was collected following centrifugation of collected whole blood. For generation of conditioned media, leukocytes were plated without additional stimulation in round-bottom microtiter plates at 106 cells per well in RPMI 1640 supplemented with 10% heat-inactivated FCS. After 24 h of incubation at 37°C, cell-free supernatants were collected and used for cytokine analyses.
Flow cytometric analyses and monocyte/macrophage enrichment
The following fluorochrome-labeled mAbs were used to distinguish inflammatory monocytes (12, 13, 25–28): PE-conjugated anti-F4/80 (Serotec), allophycocyanin-conjugated anti-CD11b (BD Biosciences or eBio-science), and FITC-conjugated Ly6C (BD Biosciences). Cells were incubated with anti-CD16/CD32 mAb (clone 2.4G2; BD Biosciences) to block nonspecific binding of Abs to the receptor of the Fc portion of the Ig followed by the indicated Abs. Isotype control Abs were used to correct for background fluorescence and set analysis gates. Cells were acquired using a FACSCalibur and analyzed with CellQuest software (BD Biosciences). Total bone marrow leukocytes labeled with PE-F4/80 were sorted using a high speed FACSAria (BD Biosciences) to enrich monocyte/macrophages. Cells negative for the selection markers were also retrieved. Purity and viability were analyzed immediately after enrichment. F4/80+ or F4/80−cells were typically enriched by 85–95%.
In vitro stimulation of cytokine production
Total bone marrow cells or sorted F4/80+ and F4/80− populations from uninfected C57BL/6 mice were cultured in 96-well microtiter plates with or without various amounts of recombinant IFN (rIFN)-αA/D (Pestka Biomedical Laboratories) as described before (28). After 24 h of incubation, cell supernatants were collected.
Cytokine analysis
Serum, bone marrow leukocyte-conditioned media, and culture supernatants after in vitro stimulation were tested for MCP-1, or MCP-5 using Quantikine kits or DuoSets (R&D Systems), and MCP-3 using Instant ELISA (Bender MedSystems). The limits of detection were 15–32 pg/106 cells.
Statistical analyses
Statistical significance of experimental results was analyzed by two-tailed Student’s t test where indicated (p ≤ 0.05).
Results
CCR2 deficiency affects monocyte/macrophage accumulation in bone marrow and blood following MCMV infection
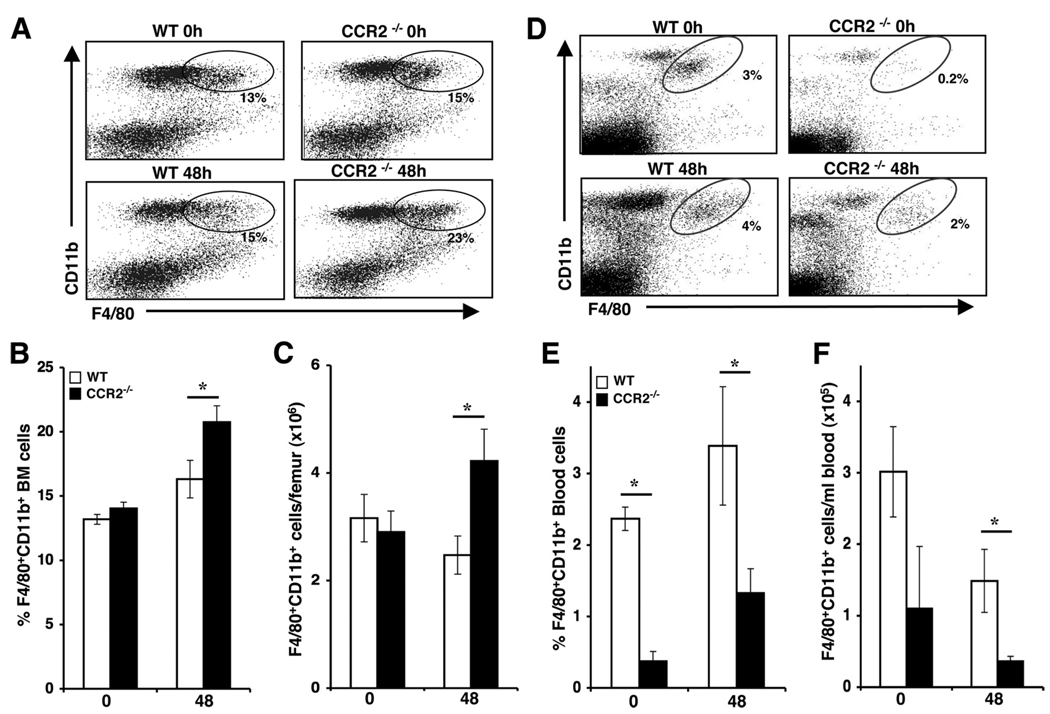
Myeloid lineage cells develop in the bone marrow and seed the periphery under homeostatic conditions or enter the circulation in response to inflammatory stimuli. Our previous studies identified a function for CCR2 in macrophage recruitment to the liver during MCMV infection (5, 28). In the absence of CCR2 signaling, fewer circulating monocyte/macrophages accumulate in the liver by 48 h postinfection. To examine whether this recruitment defect occurs at the level of monocyte/macrophage egress from bone marrow, we examined the expression of F4/80 and CD11b on cells in bone marrow leukocytes prepared from uninfected or 48 h MCMV-infected C57BL/6 mice that were immunocompetent (wild type, WT) or genetically deficient in CCR2 (CCR2−/−). The results demonstrate comparable monocyte frequencies and total numbers in uninfected WT and CCR2−/− mice using flow cytometric analysis (Fig. 1A–C). However, at 48 h following infection, the proportions and total numbers of F4/80+CD11b+ cells were significantly elevated in the bone marrow of CCR2-deficient mice as compared with WT controls. In contrast, the proportions (Fig. 1, D and E) and total numbers (Fig. 1F) of monocyte/macrophages in the blood were significantly reduced in uninfected and MCMV-infected CCR2−/− as compared with WT controls. These results indicate that CCR2-mediated effects contribute to monocyte release from bone marrow into blood under both homeostatic and viral infection conditions.
FIGURE 1.
CCR2-mediated effects on monocyte/macrophage accumulation in bone marrow and blood during MCMV infection. Samples were prepared from C57BL/6 (WT) or mice genetically deficient in CCR2 (CCR2−/−) that were uninfected (day 0) or infected with MCMV for 48 h. To characterize the accumulation of monocyte/macrophages, bone marrow (A–C) and blood (D–F) leukocytes were labeled with F4/80 and CD11b and examined by flow cytometry. Representative dot plots for each group are shown after gating on live lymphocyte/monocyte populations (A and D). The percentages (B and E) and total numbers (C and F) of F4/80+CD11b+ cells are shown. Data shown represent the means ± SE of six to nine mice from at least two independent experiments. *, p ≤ 0.04 as compared with WT controls.
Monocyte/macrophages retained in the bone marrow in the absence of CCR2 have an inflammatory phenotype
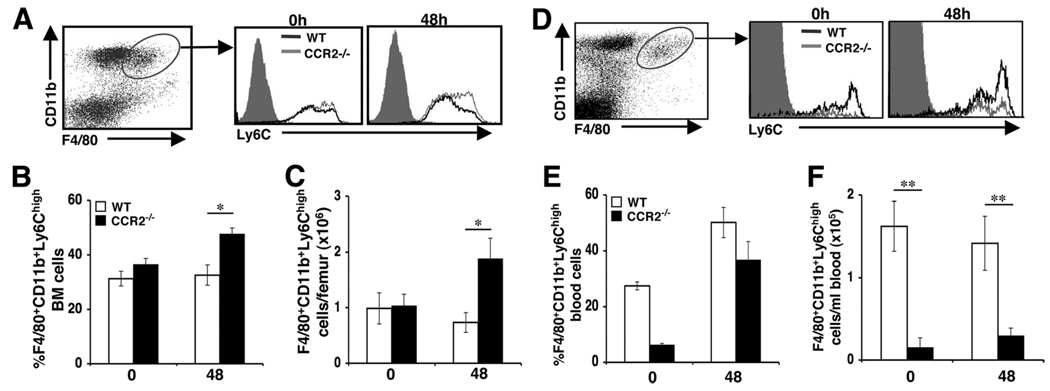
Ly6Chigh monocytes are correlated with CCR2 expression and are preferentially recruited to sites of inflammation. Conversely, Ly6Clow/int monocytes express little or no CCR2 and compose resident or homeostatic monocyte/macrophage populations (13–15). Recent studies have suggested that CCR2-mediated signals in bone marrow determine the release of Ly6Chigh monocytes into the circulation (25, 26, 31). To further investigate the role of CCR2 in regulating monocyte mobilization from the bone marrow during MCMV infection, we characterized inflammatory and homeostatic monocyte/macrophage populations on the basis of F4/80, CD11b, and Ly6C cell surface marker expression. Bone marrow leukocytes prepared from uninfected or 48 h MCMV-infected WT or CCR2−/− mice were analyzed for expression of Ly6C on gated F4/80+CD11b+ populations by flow cytometry. Two Ly6C populations were distinguished, one expressing high levels of Ly6C and one expressing low/intermediate levels. During infection, a significant increase was observed in the proportions of F4/80+CD11b+Ly6Chigh monocytes in CCR2−/− mice when compared with WT counterpart controls (Fig. 2, A and B). Likewise, CCR2−/− mice exhibited a 61% increase in the total numbers of F4/80+CD11b+Ly6C+ monocytes accumulating in the bone marrow when compared with control mice under conditions of MCMV infection (Fig. 2C). Significant differences in proportions or total numbers of inflammatory monocytes were not detected in bone marrow leukocyte samples from uninfected WT or CCR2−/− mice. In contrast, uninfected CCR2−/− mice had a profound reduction in the frequency and total number of inflammatory monocytes in blood when compared with WT mice (Fig. 2D–F). In response to MCMV infection, WT and CCR2−/− mice demonstrated 2- and 6-fold increases in frequencies of circulating inflammatory monocytes (Fig. 2E), respectively. Because total F4/80+CD11b+ blood leukocyte yields for WT and CCR2−/− mice were 1.5 × 105 ± 9 × 104/ml and 4 × 104 ± 2 × 104/ml, respectively, the CCR2−/− mice had statistically significant 4-fold lower numbers of inflammatory monocytes in blood (Fig. 2F). Examination of F4/80+CD11b+Ly6Clow/int cells in bone marrow or blood revealed no significant differences in the proportions or numbers between uninfected or MCMV-infected WT and CCR2−/− mice (data not shown). These results confirm that CCR2-mediated responses promote Ly6Chigh bone marrow monocyte release into the blood circulation. Additionally, infection in the presence or absence of CCR2 induces an increase in the frequency of circulating Ly6Chigh monocytes, but CCR2-mediated signals are needed to maintain significant numbers of the population in blood.
FIGURE 2.
Characterization of CCR2-mediated effects on inflammatory monocyte/macrophage accumulation in the bone marrow and blood. Samples were prepared from C57BL/6 (WT) or mice genetically deficient in CCR2 (CCR2−/−) that were uninfected (day 0) or infected with MCMV for 48 h. To characterize the accumulation of inflammatory monocyte/macrophages, bone marrow (A–C) and blood (D–F) leukocytes were labeled with F4/80, CD11b, and Ly6C and examined by flow cytometry. Inflammatory monocyte/macrophages were identified by analysis of Ly6C high expression after gating on the F4/80+CD11b+ cells. Representative dot plots and histograms for each group are shown (A and D). The percentages (B and E) and total numbers (C and F) of Ly6ChighF4/80+CD11b+ cells are shown. Data shown represent the means ± SE of six to nine mice from two independent experiments. *, p ≤ 0.04; **, p = 0.008 as compared with WT controls.
MCMV infection induces expression of CCR2 ligands in the bone marrow and blood
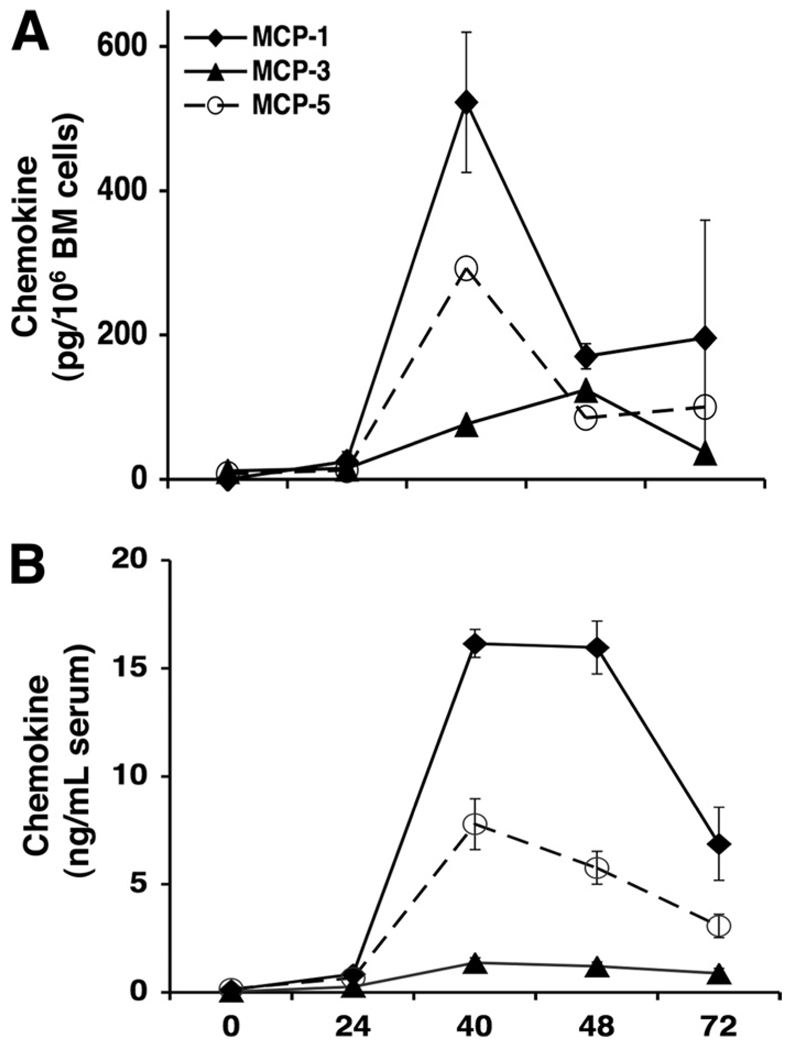
It has been established that the major chemokines controlling monocyte chemotaxis are the monocyte chemoattractant proteins, MCP-1, MCP-3, and MCP-5 (17, 26, 27, 32, 33), which bind and trigger signaling of CCR2. Considering the importance of CCR2 in bone marrow responses, we examined expression of these chemokines in bone marrow leukocyte conditioned media (CM) during MCMV infection. As shown in Fig. 3A, MCP-1 and MCP-5 followed a comparable pattern of production, with maximal chemokine levels evident at 40 h after infection and rapidly declining thereafter. However, the levels of MCP-1 were 2-fold greater than MCP-5. In contrast, MCP-3 was induced to lower concentrations and exhibited a delay in production, with maximal levels reached at 48 h, before subsiding by 72 h, following infection.
FIGURE 3.
Kinetics of MCP-1, MCP-3, and MCP-5 in the bone marrow and serum during MCMV infection. Serum samples and bone marrow leukocytes were prepared from C57BL/6 mice that were uninfected or infected with MCMV after the indicated hours. For generation of leukocyte conditioned media, bone marrow cells were cultured for 24 h in medium without additional stimulation. Spontaneous release of MCP-1, MCP-3, or MCP-5 in cell-free supernatants (A) or in serum (B) was measured by standard sandwich ELISA. Data represent the means ± SE (n = 2–3 mice tested individually). Results are representative of three independent experiments.
To further characterize kinetics and peak responses of CCR2 ligands, serum production of MCP-1, MCP-3, and MCP-5 was evaluated. The results demonstrate that MCP-1 protein reached maximal and sustained serum levels between 40 and 48 h after MCMV infection, with production declining by 72 h following infection (Fig. 3B). MCP-5 followed a similar pattern of production as with MCP-1, but levels were 2-fold less than MCP-1. MCP-3 was marginally induced and remained relatively low throughout infection when compared with MCP-1 and MCP-5. These results demonstrate that MCMV induces significant levels of MCP-1 and MCP-5, and to a lesser degree MCP-3, in bone marrow and blood, and also establish the kinetics of production.
IFN-α/β requirements for MCP-1, MCP-3, and MCP-5 production in bone marrow
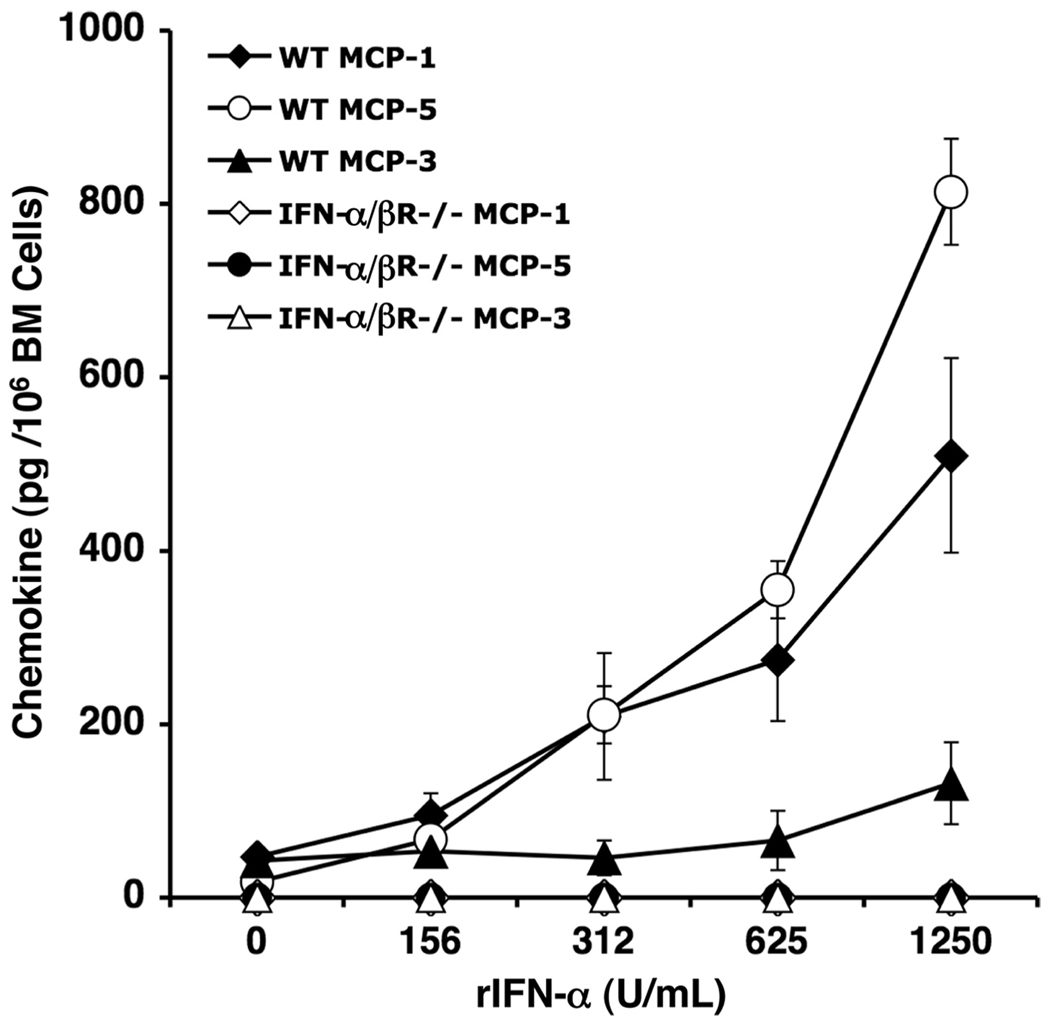
It has been established that maximal levels of IFN-α/β protein are induced in the serum and peripheral tissues within 40 h after infection with MCMV (7, 34–36). Previous studies have also identified IFN-α/β as a prominent factor eliciting production of MCP-1 in the liver during MCMV infection (5). We therefore assessed the contribution of IFN-α/β-dependent functions on chemokine production in the bone marrow. For these studies, bone marrow leukocytes were prepared from uninfected C57BL/6 mice that were immunocompetent (WT) or deficient in IFN-α/β receptor signaling (IFN-α/βR−/−) and incubated with increasing physiological doses of rIFN-α. ELISA was used to measure induction of MCP-1, MCP-3, and MCP-5 proteins in cell-free supernatants. The results show a dose-dependent induction of the three chemokines in samples from WT mice (Fig. 4). The most profound effects were on MCP-1 and MCP-5 production, whereas MCP-3 was only marginally induced. This effect was type 1 IFN-specific, as bone marrow cells from IFN-α/βR−/− mice failed to induce MCP-1, MCP-3, or MCP-5 in response to rIFN-α. Hence, rIFN-α treatment promotes MCP-1, MCP-5, and, to a lesser extent, MCP-3 production in the bone marrow.
FIGURE 4.
IFN-α/β effects on CCR2 ligand production. Total bone marrow leukocytes from naive C57BL/6 (WT) or IFN-α/βR−/− mice were pooled (n = 3–4 mice/group) and stimulated with rIFN-α at the doses indicated. Leukocytes were cultured overnight, and collected cell-free su-pernatants were evaluated for production of MCP-1, MCP-3, and MCP-5 by sandwich ELISA. Data represent the means ± SE of two independent experiments.
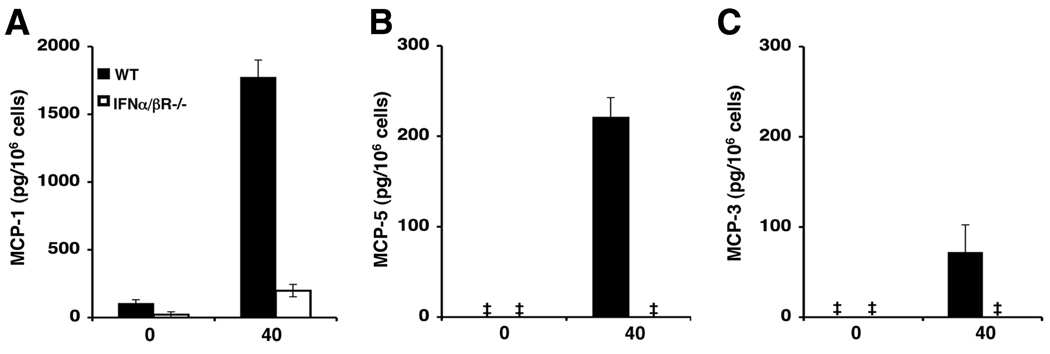
Additionally, to assess whether IFN-α/β signaling affects CCR2 ligand production in vivo, CM was generated from total bone marrow leukocytes prepared from uninfected or 40 h MCMV-infected WT or IFN-α/βR−/− mice. Cell-free supernatants were examined for production of MCP-1, MCP-3, and MCP-5 by ELISA. The results show a modest release of MCP-1 protein in uninfected WT mice (Fig. 5A), whereas the levels of MCP-5 and MCP-3 proteins were below the level of detection (Fig. 5, B and C). IFN-α/βR−/−mice lacked detectable levels of all three chemokines. Under conditions of MCMV infection, elevated levels of MCP-1, MCP-5, and MCP-3 proteins were revealed in bone marrow leukocytes prepared from WT mice. In contrast, moderate levels of MCP-1 protein, but undetectable levels of MCP-5 and MCP-3, were observed in bone marrow leukocytes prepared from infected IFN-α/βR−/− mice. Collectively, the results demonstrate a significant role for IFN-α/β signaling in inducing production of CCR2 ligands in the bone marrow during MCMV infection.
FIGURE 5.
IFN-α/β effects on CCR2 ligand production during MCMV infection. Total bone marrow leukocytes were collected from uninfected or 40 h MCMV-infected WT or IFN-α/βR−/− mice. Conditioned media was generated from leukocyte cultures as described in Materials and Methods and evaluated for (A) MCP-1, (B) MCP-5, and (C) MCP-3 production by ELISA. Shown are the means ± SE (n = 2–3 mice/group). Data are representative of three experiments. ‡, Below the level of detection.
F4/80-expressing cells are major cellular sources of CCR2 ligands in bone marrow during MCMV infection
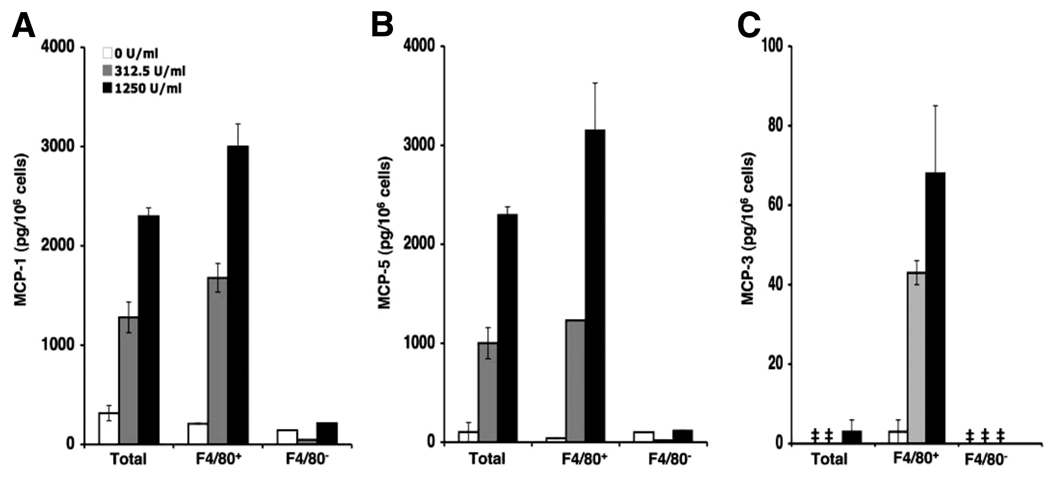
It has recently been demonstrated that the F4/80+ population of liver leukocytes are potent producers of MCP-1 during MCMV infection (5). We therefore determined whether F4/80-expressing cells were critical cellular sources of MCP-1, MCP-3, and MCP-5 in the bone marrow in response to MCMV infection. We first assessed the ability of these cells to respond to rIFN-α treatment for chemokine production. Total bone marrow cells were prepared from uninfected C57BL/6 mice and enriched or depleted of F4/80+ cells. Total, F4/80-enriched or F4/80-depleted cells were untreated or treated with rIFN-α at a dose of 312.5 or 1250 U/ml. CM was generated and ELISA was used to measure the levels of MCP-1, MCP-3, and MCP-5 proteins in cell-free supernatants. Untreated cells released low levels of MCP-1 (Fig. 6A) and marginal to below detectable levels of MCP-5 (Fig. 6B) and MCP-3 (Fig. 6C). Total and enriched F4/80+ bone marrow leukocytes released significant amounts of MCP-1 and MCP-5 in response to rIFN-α in a dose-dependent manner. The levels of chemokines were higher from enriched F4/80+ cell populations than from total bone marrow leukocytes. MCP-3 was modestly induced in total cells stimulated with a high dose of rIFN-α. However, production was substantially elevated in conditioned media from enriched F4/80+ cells in response to rIFN-α treatment. F4/80-depeleted cells from the same mice failed to respond to rIFN-α stimulation, and chemokine production was negligible. Thus, rIFN-α stimulates MCP-1, MCP-3, and MCP-5 production from F4/80+ bone marrow cells.
FIGURE 6.
Characterization of IFN-α/β-responding cells in bone marrow for CCR2 ligand production. Bone marrow leukocytes were prepared from uninfected C57BL/6 mice and enriched for F4/80 as described in Materials and Methods. Leukocyte-conditioned media were generated from pooled total, F4/80-enriched (F4/80+), and F4/80-depleted (F4/80−) cellular fractions after 24 h of incubation with or without rIFN-α at the doses indicated. Collected cell-free supernatants were evaluated in duplicate or triplicate for production of MCP-1 (A), MCP-3 (C), and MCP-5 (B) using ELISA. Data shown represent the means ± SE. Results are representative of two independent experiments. ‡, Below the level of detection.
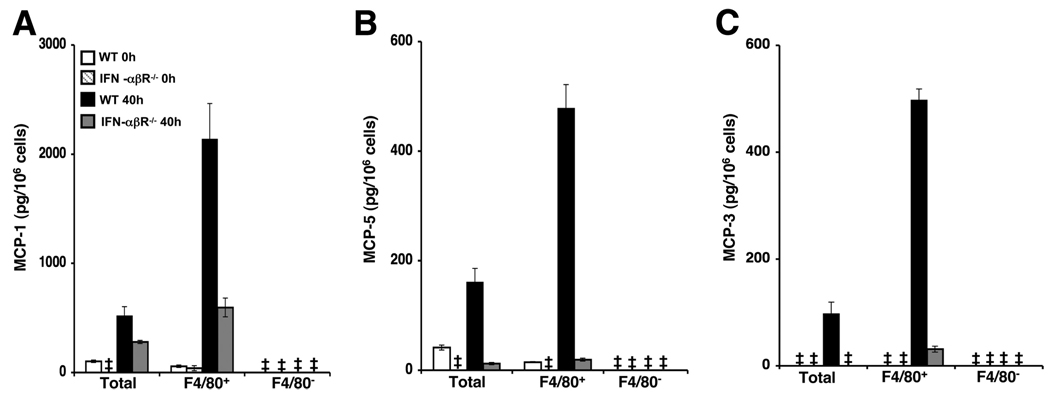
To establish and specifically evaluate the contribution of IFN-α/β responses in induction of CCR2 ligand production from bone marrow F4/80+ cells during MCMV infection, CM was prepared from total bone marrow leukocytes, enriched F4/80+ or F4/80− cells isolated from WT or IFN-α/βR−/− mice that were uninfected or infected with MCMV for 40 h. Enriched samples contained the same number of viable cells. ELISA was used to assess production of MCP-1, MCP-3, and MCP-5 in cell-free supernatants. As shown in Fig. 7, chemokine production was modest or undetectable in total and F4/80-enriched cells from uninfected WT and IFN-α/βR−/− mice. In response to MCMV infection, total cell populations from WT mice were induced to produce MCP-1, MCP-3, and MCP-5. However, the levels of chemokines produced by enriched F4/80+ cells were markedly elevated over the levels produced by the total leukocytes. The results also show induction of MCP-1 in total and F4/80+ enriched cells of IFN-α/βR−/− mice during infection (Fig. 7A). Nevertheless, the induced responses observed in IFN-α/βR−/− mice were reduced by 2-fold when compared with those in infected WT mice. Additionally, significant increases in MCP-1 levels were not observed in the F4/80-enriched populations from IFN-α/βR−/− mice when compared with total cells. MCP-5 and MCP-3 production was marginal or undetectable in total and F4/80+ cells from infected IFN-α/βR−/− mice (Fig. 7, B and C). CCR2 ligand production was below the level of detection in F4/80-depleted cell populations from MCMV-infected WT and IFN-α/βR−/− mice. Collectively, these results identify F4/80+ cells in the bone marrow as a major source of MCP-1, MCP-3, and MCP-5 production, and they clearly establish IFN-α/β as a critical signal promoting induction of these chemokines during MCMV infection.
FIGURE 7.
Effects of IFN-α/β signaling on CCR2 ligand production by F4/80+ bone marrow cells. Bone marrow leukocytes were prepared from C57BL/6 (WT) or IFN-α/β receptor-deficient (IFN-α/βR−/−) mice that were uninfected or infected for 40 h with MCMV. Bone marrow leukocytes were pooled from two to three mice per group and enriched or depleted of F4/80+ cells and plated to generate leukocyte conditioned media. Collected cell-free supernatants were evaluated in duplicate or triplicate for production of (A) MCP-1, (B) MCP-5, and (C) MCP-3 using ELISA. Data shown represent the means ± SE. Results are representative of at least two independent experiments. ‡, Below the level of detection.
Discussion
These studies evaluate the effects of CCR2-dependent functions in the bone marrow during MCMV infection, and they establish a role for IFN-α/β in promotion of CCR2 ligands at this site. The results demonstrate impaired monocyte/macrophage trafficking from the bone marrow and into circulating blood in the absence of CCR2 signaling. Specifically, CCR2-deficient mice show an accumulation of monocyte/macrophages with an inflammatory phenotype in the bone marrow that is associated with a profound reduction of these cells in the blood following infection. MCP-1, MCP-3, and MCP-5, which are known CCR2 ligands, were produced by leukocytes in the bone marrow and detected in blood under conditions of MCMV infection; however, variability in the induction kinetics and levels of production were observed. Importantly, we identify bone marrow F4/80-expressing cells as critical sources of MCP-1, MCP-3, and MCP-5, and demonstrate a dependence on IFN-α/β signaling and MCMV infection for induction.
It has been clearly established that monocyte/macrophages are generated in the bone marrow and enter the circulation, where they are made available to sites of inflammation (13, 14, 37, 38). During MCMV infection, macrophage trafficking to the liver is largely dependent on CCR2 signaling (28). Here, CCR2 activation within the bone marrow is shown to control the magnitude of monocyte/macrophage release from this compartment following MCMV infection. In support of a role for CCR2 in mediating this process, only inflammatory monocyte/macrophages expressing high levels of Ly6C, which correlates with CCR2 expression (12–15), were found to accumulate in the bone marrow during MCMV infection. Importantly, Ly6Chigh monocyte/macrophages were dramatically reduced in the blood of CCR2-deficient mice. Consistent with other studies (25, 26, 31, 39), this cell population was also reduced in the circulation of uninfected CCR2-deficient mice, indicating a homeostatic role for CCR2 in regulating monocyte/macrophage egress from the bone marrow. During MCMV infection, the proportions of inflammatory monocyte/macrophages in the blood of CCR2-deficient mice were comparable to those of WT mice. Thus, our results do not exclude other chemokine/chemokine receptor responses in promoting monocyte mobilization into the circulation in the absence of CCR2 signaling during infection. Nevertheless, total numbers of inflammatory monocyte/macrophages were profoundly abrogated in the circulation of CCR2-deficient mice, indicating that CCR2-mediated signals are predominant. Furthermore, no difference was detected between WT and CCR2−/− mice in the number of bone marrow or blood monocytes expressing low/intermediate levels of Ly6C (data not shown), and therefore low or no CCR2. Thus, it can be inferred that CCR2-expressing monocytes respond to inflammatory cues during MCMV infection to be mobilized to the peripheral circulation. These results are consistent with recent observations in models of bacterial infection (25, 31). Additionally, these aforementioned studies demonstrated that CCR2 was not required for monocyte entry into bacteria-infected spleen or bladder. Likewise, under conditions of MCMV infection CCR2 is not absolutely required for monocyte/macrophage trafficking into the liver from the blood (M. J. Crane and T. P. Salazar-Mather, unpublished observations). Taken together, these studies suggest that the availability of monocyte/macrophages to peripheral tissues is indirectly regulated by CCR2-mediated signals in the bone marrow.
It follows that one or more CCR2 ligands must be present within the bone marrow or expressed systemically to activate CCR2 on bone marrow monocyte/macrophages. Expression of three chemo-kines known to bind CCR2 and promote monocyte chemotaxis in vivo was examined. We demonstrate induced bone marrow and serum production levels of MCP-1, MCP-3, and MCP-5 after MCMV infection. MCP-1 and MCP-5 protein rose sharply in the bone marrow by 40 h following infection and rapidly declined thereafter. In contrast, MCP-3 was modestly induced with altered kinetics. In the blood, maximal levels of MCP-1 and MCP-5 proteins were sustained beyond the production kinetics observed in the bone marrow; MCP-3 remained low throughout the infection time points that were evaluated.
Previous studies have demonstrated that MCP-1-deficient mice recruit fewer monocyte/macrophages to the liver during MCMV infection (28). However, the monocyte/macrophage trafficking defect to the liver is moderate in MCP-1-deficient mice compared with CCR2-deficient mice. This suggests that other CCR2 ligands function together with MCP-1 in the regulation of monocyte/macrophage trafficking. Studies by others have shown that MCP-1 and MCP-3 have a combined role in signaling monocyte release from the bone marrow in other, nonviral models of infection and inflammation (26, 27). Herein, our results suggest that MCP-1 and MCP-5, because they were produced at high levels in bone marrow and blood, are dominant in directing monocyte/macrophage migration during MCMV infection. However, the extent to which the function of these chemokines overlap or act independently is not known.
An important finding in this study is the identification of F4/80-expressing bone marrow leukocytes as major contributors to MCP-1, MCP-3, and MCP-5 production. This indicates that production of these chemokines does occur within the bone marrow itself during MCMV infection. It is therefore plausible that the concentration of CCR2 ligands at this site contributes to a chemo-tactic gradient that promotes monocyte/macrophage trafficking either within the bone marrow or across the bone marrow sinusoidal endothelium into the blood, where concentrations of these chemokines are much higher. In this context, the production of CCR2 ligands would be comparable to the role of CXC chemokines in the rapid mobilization of neutrophils from the bone marrow in response to inflammatory stimuli (40–43). Further investigations will be required to understand the relative contribution of individual CCR2 ligands in the bone marrow or blood, and the mechanisms that facilitate their function in inflammatory monocyte/macrophage migration.
Expression of IFN-α/β can affect leukocyte trafficking (44–46). During MCMV infection, bone marrow-derived macrophages migrate to secondary sites in response to IFN-α/β-mediated effects (5, 7, 28, 46). We previously demonstrated that IFN-α/β-induced production of MCP-1 in liver contributed to the accumulation of macrophages at this site following MCMV infection (5, 28). Here, the immunoregulatory effects of IFN-α/β are extended to include induction of MCP-1, MCP-3, and MCP-5 in the bone marrow. Moreover, we demonstrate that F4/80+ cells in the bone marrow are induced to produce MCP-1, MCP-3, and MCP-5 in response to IFN-α/β signals and MCMV infection. MCP-3 and MCP-5 production required IFN-α/β for induction. However, because moderate levels of MCP-1 were evident in the absence of IFN-α/β signals, we conclude that other factors induced during infection may also contribute to production by bone marrow F4/80+ cells. To our knowledge this is the first characterization of CCR2 ligand producers in the bone marrow during a virus infection. Whether this response is unique to MCMV or common to infectious agents that rapidly induce type 1 IFNs remains to be determined and warrants further studies in other viral models. High circulating levels of IFN-α/β have been detected within 40 h of MCMV infection (34, 36); however, induction was not evident in bone marrow (Ref. 47 and M. J. Crane and T. P. Salazar-Mather, unpublished observations). These results collectively suggest that circulating levels of IFN-α/β induced in response to MCMV infection stimulate MCP-1, MCP-3, and MCP-5 expression in the bone marrow compartment. As a result, inflammatory monocyte/macrophage trafficking is regulated at the level of the bone marrow through cytokine signals induced during infection.
In summary, our study demonstrates a role for CCR2 in regulating the trafficking of inflammatory monocyte/macrophages from the bone marrow into the blood in a model of MCMV infection. It is also established that high levels of the CCR2 ligands MCP-1 and MCP-5, and moderate levels of MCP-3, are produced in the bone marrow and blood. Furthermore, we identify F4/80-expressing bone marrow leukocytes as critical producers of these chemokines and clearly establish IFN-α/β as a prominent inducer of these responses. Collectively, the results suggest that CCR2-mediated regulation of inflammatory monocyte/macrophage trafficking from the bone marrow into the blood is supported by local induction of CCR2 ligands in response to virus-induced cytokines.
Acknowledgments
We thank Pamela Gaddi and Tania Nevers for assistance with experiments.
Footnotes
This work was supported by National Institutes of Health Grant CA102708 (to T.S.-M.). Meredith Crane is supported by a Nicole Rosenthal Hartnett ’91 Graduate Fellowship.
Abbreviations used in this paper: MCMV, murine CMV; WT, wild type; rIFN-α, recombinant IFN-α CM, conditioned media.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Betts RF, Hanshaw JB. Cytomegalovirus (CMV) in the compromised host(s) Annu. Rev. Med. 1977;28:103–110. doi: 10.1146/annurev.me.28.020177.000535. [DOI] [PubMed] [Google Scholar]
- 2.Drew WL. Cytomegalovirus infection in patients with AIDS. J. Infect. Dis. 1988;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 3.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Scalzo AA, Corbett AJ, Rawlinson WD, Scott GM, Degli-Esposti MA. The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol. Cell Biol. 2007;85:46–54. doi: 10.1038/sj.icb.7100013. [DOI] [PubMed] [Google Scholar]
- 5.Hokeness-Antonelli KL, Crane MJ, Dragoi AM, Chu WM, Salazar-Mather TP. IFN-α/β-mediated inflammatory responses and antiviral defense in liver is TLR9-independent but MyD88-dependent during murine cytomegalovirus infection. J. Immunol. 2007;179:6176–6183. doi: 10.4049/jimmunol.179.9.6176. [DOI] [PubMed] [Google Scholar]
- 6.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces natural killer (NK) cell inflammation and protection through macrophage inflammatory protein-1α (MIP-1α)-depen-dent pathways. J. Exp. Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar-Mather TP, Lewis CA, Biron CA. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein-1α delivery to liver. J. Clin. Invest. 2002;110:321–330. doi: 10.1172/JCI15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pien GC, Satoskar AR, Takeda K, Akira S, Biron CA. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-y responses during viral infection. J. Immunol. 2000;165:4787–4791. doi: 10.4049/jimmunol.165.9.4787. [DOI] [PubMed] [Google Scholar]
- 10.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP. CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J. Virol. 2007;81:1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 14.Sunderkötter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 15.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Franci C, Wong LM, Van DJ, Proost P, Charo IF. Monocyte chemoattractant protein-3, but not monocyte chemoattractant protein-2, is a functional ligand of the human monocyte chemoattractant protein-1 receptor. J. Immunol. 1995;154:6511–6517. [PubMed] [Google Scholar]
- 17.Sarafi MN, Garcia-Zepeda EA, Maclean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J. Exp. Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja SK, Ahuja SS. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1, or CCR2-deficient mice. J. Immunol. 1999;163:5519–5525. [PubMed] [Google Scholar]
- 23.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 24.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 26.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J. Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-α/β-induced inflammatory responses and antiviral defense in liver. J. Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 29.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 30.Wesley JD, Tessmer MS, Paget C, Trottein F, Brossay L. A Y chromosome-linked factor impairs NK T development. J. Immunol. 2007;179:3480–3487. doi: 10.4049/jimmunol.179.6.3480. [DOI] [PubMed] [Google Scholar]
- 31.Engel DR, Maurer J, Tittel AP, Weisheit C, Cavlar T, Schumak B, Limmer A, van Rooijen N, Trautwein C, Tacke F, Kurts C. CCR2 mediates homeostatic and inflammatory release of Gr1high monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J. Immunol. 2008;181:5579–5586. doi: 10.4049/jimmunol.181.8.5579. [DOI] [PubMed] [Google Scholar]
- 32.Salazar-Mather TP, Hokeness KL. Cytokine and chemokine networks: pathways to antiviral defense. Curr. Top. Microbiol. Immunol. 2006;303:29–46. doi: 10.1007/978-3-540-33397-5_2. [DOI] [PubMed] [Google Scholar]
- 33.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 34.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis CA, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon-α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biron CA, Dalod M, Salazar-Mather TP. Innate immunity and viral infections. In: Kaufmann SHE, Sher A, Ahmed R, editors. Immunology of Infectious Diseases. Washington, DC: ASM Press; 2002. pp. 139–160. [Google Scholar]
- 36.Krug A, French AR, Barchet W, Fischer AJ, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol. Cell Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 39.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Combadiere C, Potteaux S, Rodero M, Simon T, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 40.De Bruyn PPH, Michelson S, Thomas TB. The migration of blood cells of the bone marrow through the sinusoidal wall. J. Morphol. 1971;133:417–437. doi: 10.1002/jmor.1051330406. [DOI] [PubMed] [Google Scholar]
- 41.Martin C, Burdon PCE, Gutierrez-Ramos JC, Bridger G, Williams TJ, Rankin SM. Chemokines acting via CXCR4 and CXCR2 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 42.Burdon PCE, Martin C, Rankin SM. The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d dependent manner. Blood. 2005;105:2543–2548. doi: 10.1182/blood-2004-08-3193. [DOI] [PubMed] [Google Scholar]
- 43.Burdon PCE, Martin C, Rankin SM. Migration across the sinusoidal endothelium regulates neutrophil mobilization in response to ELR + CXC chemokines. Br. J. Haematol. 2008;142:100–108. doi: 10.1111/j.1365-2141.2008.07018.x. [DOI] [PubMed] [Google Scholar]
- 44.Korngold R, Blank KJ, Murasko DM. Effect of interferon on thoracic duct lymphocyte output: induction with either polyI:C or vaccinia virus. J. Immunol. 1983;130:2236–2243. [PubMed] [Google Scholar]
- 45.Wiltrout RH, Pilaro AM, Gruys ME, Talmadge JE, Longo DL, Ortaldo JR, Reynolds CW. Augmentation of mouse liver-associated natural killer cell activity by biological response modifiers occurs largely via rapid recruitment of large granular lymphocytes from the bone marrow. J. Immunol. 1989;143:372–378. [PubMed] [Google Scholar]
- 46.Salazar-Mather TP, Biron CA. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J. Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- 47.Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int. Immunol. 2008;20:45–56. doi: 10.1093/intimm/dxm119. [DOI] [PMC free article] [PubMed] [Google Scholar]