Abstract
Traumatic brain injury is a devastating neurological injury associated with significant morbidity and mortality. Medical therapies to limit cerebral edema, a cause of increased intracranial hypertension and poor clinical outcome, are largely ineffective, emphasizing the need for novel therapeutic approaches. In the present study, pre-treatment with curcumin (75, 150 mg/kg) or 30 minute post-treatment with 300 mg/kg significantly reduced brain water content and improved neurological outcome following a moderate controlled cortical impact in mice. The protective effect of curcumin was associated with a significant attenuation in the acute pericontusional expression of interleukin-1β, a pro-inflammatory cytokine, after injury. Curcumin also reversed the induction of aquaporin-4, an astrocytic water channel implicated in the development of cellular edema following head trauma. Notably, curcumin blocked IL-1β-induced aquaporin-4 expression in cultured astrocytes, an effect mediated, at least in part, by reduced activation of the p50 and p65 subunits of NFκB. Consistent with this notion, curcumin preferentially attenuated phosphorylated p65 immunoreactivity in pericontusional astrocytes and decreased the expression of glial fibrillary acidic protein, a reactive astrocyte marker. As a whole, these data suggest clinically-achievable concentrations of curcumin reduce glial activation and cerebral edema following neurotrauma, a finding which warrants further investigation.
Keywords: intracranial pressure, neurotrauma, controlled cortical impact, neuroinflammation, cellular edema, glia
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of death and disability for over 1.5 million Americans annually (Nortje and Menon 2004). The development of cerebral edema and the ensuing rise in intracranial pressure (ICP) reduces cerebral blood flow, decreases cerebral perfusion pressure, and contributes toward a poor patient prognosis (Miller et al. 1977; Saul and Ducker 1982; Sarabia et al. 1988; Levin et al. 1991). Current medical therapies do not adequately control cerebral edema and the effectiveness of invasive neurosurgical approaches to alleviate brain swelling remains limited. These shortcomings emphasize the need for novel approaches to limit post-traumatic edema.
Aquaporins (AQP) are a family of water channels that permit selective, bidirectional water movement in response to the osmotic gradient (Borgnia et al. 1999; Yang et al. 2008). Aquaporin-4 (AQP4), the predominant isoform in the brain, regulates fast water transport within the perivascular end feet of astrocytes (Nielsen et al. 1997; Nicchia et al. 2004). AQP4-deficient mice exhibit improved survival, decreased brain edema, and reduced swelling of astrocytic foot processes following cerebral ischemia or acute water intoxication (Bloch and Manley 2007), although a functional role for AQP4 after TBI remains controversial. In support of a detrimental role, increased expression of AQP4 within the pericontusional cortex was associated with edema development and disruption of the blood-brain barrier (Vizuete et al. 1999; Guo et al. 2006; Aggarwal et al. 2007). In contrast, decreased expression of AQP4 aided in edema resolution and improved neurological outcome (Dietrich et al. 1999; Kiening et al. 2002). Thus, a better understanding of the biological actions and cellular regulation of AQP4 may yield novel strategies to limit cerebral edema following head trauma.
Inflammation contributes to post-traumatic neurological deterioration (Lenzlinger et al. 2001; Morganti-Kossmann et al. 2002; Hutchinson et al. 2007; Laird et al. 2008). Increased cerebrospinal fluid levels of the pro-inflammatory cytokine, interleukin-1β (IL-1β), clinically correlate with elevated ICP and neurological demise after TBI (Hayakata et al. 2004; Hutchinson et al. 2007). Consistent with a causative role for IL-1β in the development of cerebral edema, mice deficient in IL-1 type 1 receptor (IL-1R) exhibited a reduction in both vasogenic and cellular edema following mild hypoxia-ischemia (Lazovic et al. 2005). Although the downstream factors involved in mediating these actions remain largely unstudied, intracerebral administration of IL-1β increased AQP4 expression via activation of the pro-inflammatory transcription factor, NFκB (Ito et al. 2006). These data raise the unexplored possibility that IL-1β promotes cerebral edema via the regulation of AQP4 following TBI.
Curcumin, a low molecular weight, curry spice derived from the rhizome Curcuma longa, has been consumed by humans for centuries as an anti-inflammatory and antioxidant in Ayurvedic medicine (Singh and Aggarwal 1995; Garodia et al. 2007). Recent clinical trials showed curcumin was safe, even when orally administered at high doses (>8 grams/day) (Cheng et al. 2001). Curcumin is a potent inhibitor of NFκB (Singh and Aggarwal 1995) and recent work by our laboratory and others showed that curcumin limits neuroinflammation and neurological injury in experimental models of Alzheimer’s disease, ischemic stroke, and subarachnoid hemorrhage (Lim et al. 2001; Thiyagarajan and Sharma 2004; Cole et al. 2007; Wakade et al. 2008). Based on these observations, we hypothesized that curcumin may limit the development of cerebral edema, a major cause of mortality following TBI, via a reduction in AQP4.
MATERIALS AND METHODS
Supplies
Curcumin (>98% pure) and recombinant human IL-1β were purchased from MP Biomedical (Solon, OH) and Endogen (Rockford, IL), respectively. Helenalin and pyrrolidine dithiocarbamate (PDTC) were from Biomol (Plymouth Meeting, PA).
Controlled cortical impact (CCI)
Animal studies were approved by the Committee on Animal Use for Research and Education at the Medical College of Georgia, in compliance with NIH guidelines. Male CD-1 mice (8-10 weeks old; Charles River, Wilmington, MA) were anesthetized with xylazine (8mg/kg)/ketamine (60mg/kg) and treated with an intraperitoneal injection of curcumin (75, 150, 300 mg/kg) or vehicle (100 μL DMSO) either 15 minutes prior to TBI or post-treated (0.5 or 1h post-TBI). Mice were placed in a stereotaxic frame (Amscien Instruments, Richmond, VA) and a 3.5 mm craniotomy was made in the right parietal bone midway between bregma and lambda with the medial edge 1 mm lateral to the midline, leaving the dura intact. Mice were impacted at ~4.5 m/s with a 20 ms dwell time and 1 mm depression using a 3 mm diameter convex tip, mimicking a moderate TBI in humans. The incision was surgically stapled, and mice were allowed to recover. Sham-operated mice underwent the identical surgical procedures and received vehicle injections, but were not impacted. Throughout all procedures, body temperature was maintained at 37°C using a small animal temperature controller (Kopf Instruments, Tujunga, CA).
Assessment of cerebral edema
Brain water content, a sensitive measure of cerebral edema, was quantified using the wet-dry method (Dempsey et al. 2000; Hewett et al. 2006). At 24h post-injury, a timepoint associated with significant edema formation following experimental TBI (Kiening et al. 2002; Zweckberger et al. 2006; Griebenow et al. 2007), brain water content was estimated in a 3 mm coronal tissue section of the ipsilateral cortex (or corresponding contralateral cortex), centered on the impact site. Tissue was immediately weighed (wet weight), then dehydrated at 65°C. The sample was reweighed 48h later to obtain a dry weight. The percentage of water content in the tissue samples was calculated using the following formula: [(wet weight – dry weight)/wet weight]*100.
Assessment of neurological injury
Functional outcomes were determined using established tests of neurological function after TBI. The open field activity test provides a sensitive assessment of general locomotor activity, exploration habits, and anxiety. Briefly, mice were placed in a 14 × 14 inch black box that was divided into a 2 × 2 inch square grid for 2 days prior to injury to allow habituation and the establishment of a baseline reading, as detailed by our group (Wakade et al. 2009). Mice were then injured and open-field activity was re-assessed at time points indicated in the Figure Legend. The average number of crosses within a 3 minute trial (3 trials/mouse), was measured by independent investigators who were blinded to experimental conditions. Open-field activity was calculated as the percentage of the average number of crosses following injury/treatment divided by baseline value.
Neurological outcome was also assessed using the two-trial novel object recognition task, which assesses learning and memory processes. Briefly, mice were habituated one day prior to experiment initiation, then pre-tested with two identical objects for two days before the day of injury to establish a baseline. Mice generally spend equal time with each similar object. After injury, mice were returned to the enclosure and permitted to explore two identical objects located a set distance apart for 3 minutes. Mice were then removed from the enclosure for two hours, then retested in the same environment except one of familiar objects was replaced with a novel object that differed in shape, texture and appearance. The amount of time exploring all objects was recorded. Data were expressed as the percentage of time spent exploring the novel object, as compared to the familiar object, as reported previously (Biegon et al. 2004). For both tests, data are expressed as mean ± SEM from n=6-10 mice/group.
Tissue Culture
Primary astrocyte cultures were obtained from the cerebral cortices of 1-2 day old CD-1 mice, as described by our laboratory (Laird et al. 2008). Briefly, cortices were minced and placed in 0.025% trypsin diluted in Hank’s balanced salt solution supplemented with 15 mM glucose and 20 mM sucrose for 30 minutes at 37°C. Cells were centrifuged and resuspended in modified Eagle’s medium (MEM; Hyclone, Logan, UT) supplemented with 2 mM glutamine, 20 mM glucose, 5% fetal bovine serum (FBS), 5% bovine growth serum (BGS), 10% calf serum (CS), epidermal growth factor (10 ng/mL) and 50 IU/mL penicillin/50 μg/mL streptomycin (Hyclone, Logan, UT). Cells were plated at 2.5 hemispheres per 6- or 24-well plate, which routinely produces confluent monolayers of protoplasmic-like cells 10-12 days following plating. Upon reaching confluence, cells were exposed to 8 μM cytosine arabinoside (AraC) for 5-7 days to eliminate the growth of rapidly dividing cells, including microglia, macrophages, and oligodendrocytes. Astrocytes are contact inhibited and no longer dividing at this time, thus AraC does not affect astrocyte viability. Astrocyte cultures were maintained in MEM supplemented with 10% CS and antibiotics and used for experimentation between days 28-35 in vitro. Astrocyte cultures were routinely >97% pure, as assessed morphologically and immunocytochemically by the glial-specific marker, glial fibrillary acidic protein (GFAP) and were <1% microglia, as assessed by the microglial marker, CD11b.
NFκB activity assay
Nuclear extracts were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA) and stored at -80°C until the time of assay. Protein content was quantified by BCA protein assay (Pierce Biotechnology, Rockford, IL). 7.5 μg of nuclear protein was used to determine NFκB (p50 or p65) activation (TransAm, Active Motif, Carlsbad, CA), as described by our laboratory (Laird et al. 2008). This method rapidly detects activated NFκB complex binding and is 10x more sensitive than traditional electromobility shift assays, possessing a detection limit <0.5 μg nuclear extract or <0.4 ng recombinant p50 or p65 protein/well. Nuclear extracts from TPA-stimulated Jurkat cells (2.5 μg/well) were used as a positive control. To demonstrate binding specificity, a 20-fold excess of an NFκB wild-type oligonucleotide (20 pmol/well) was used as a competitor to block specific NFκB binding to the well. Conversely, a mutated consensus NFκB oligonucleotide had no effect on binding, further demonstrating the specificity of the reaction.
Western blotting
Following in vitro treatments, cultures were washed with phosphate-buffered saline (PBS) and whole cell lysates were collected in radioimmunoprecipitation (RIPA) buffer containing protease inhibitor cocktail, phosphatase-inhibitor cocktail and phenylmethylsulphonyl fluoride (PMSF) (Sigma, St. Louis, MO). For in vivo studies, 3 mm coronal sections were prepared with the aid of a brain matrix. A 1 mm micropunch was then used to collect tissue from the pericontusional cortex or from the corresponding contralateral hemisphere. Tissue was placed in complete RIPA buffer, sonicated, and then centrifuged for 5 minutes at 14,000xg at 4°C. Protein concentrations were quantified using a BCA protein assay kit (Pierce, Rockford, IL). 30 μg of protein were resolved on a 4-20% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. Blots were incubated overnight at 4°C in primary antibody (1:200 anti-AQP4 antibody, 1:200 anti-GFAP antibody, or 1:3000 anti-β actin) (Santa Cruz Biotechnology, Santa Cruz, CA) followed by a 2h incubation with an Alexa Fluor 750 secondary antibody at room temperature. Blots were visualized using a Li-Cor Odyssey near-infrared imaging system and densitometry analysis was performed using Quantity One software (Bio-Rad, Foster City, CA).
Immunohistochemistry
Deeply anesthetized mice were perfused with saline, followed by fixation with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were post-fixed overnight in paraformaldehyde followed by cryoprotection with 30% sucrose (pH 7.4) until brains permeated. Serial coronal sections (12 μM) were prepared from the pericontusional cortex, using a cryostat microtome (Leica, Wetzlar, Germany), and directly mounted onto glass slides. For comparisons, sections from sham-operated mice were prepared from tissue located directly below the craniectomy (e.g. same anatomical brain region). Sections were incubated at room temperature with 3% normal donkey serum in PBS containing 0.1% Triton X-100 for 1h, followed by incubation with the primary antibody (AQP4 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-p65 (1:100; Abcam, Cambridge, MA), or GFAP (1:100; Sigma, St. Louis, MO)) overnight at 4°C. Sections were then washed and incubated with an appropriate Alexa Fluor-tagged secondary antibody. Omission of primary antibody served as a negative control.
Confocal microscopy
Immunofluorescence was determined using a LSM510 Meta confocal laser microscope (Carl Zeiss), as described by our group (Khan et al. 2005; Laird et al. 2008). Cellular co-localization was determined in Z-stack mode using 63X oil immersion Neofluor objective (NA 1.3) with the image size set at 512 × 512 pixels. The following excitation lasers/emission filters settings were used for various chromophores: argon2 laser was used for Alexa Fluor 488, with excitation maxima at 490 nm and emission in the range 505-530 nm. HeNe1 laser was used for Alexa Fluor 594 with excitation maxima at 543 nm and emission in the range 568-615 nm. Z-stacks (20 optical slices) were collected at optimal pinhole diameter at 12-bit pixel depth and converted into three-dimensional projection images using LSM510 Meta imaging software.
Determination of lesion size
Serial coronal sections (12 μM) that included the contused cortex were incubated with 0.1% cresyl violet in 100% ethanol (pH 4.0) for 5 minutes. Sections were rinsed in distilled water, followed by successive ethanol washes (70%, 95%, 100%), and then briefly washed in xylene prior to cover-slipping. Digital images of ipsilateral cortices were captured on a Zeiss Axiophot microscope using a 2.5X objective and imported into the OsiriX v.2.7.5 32-bit program to quantify lesion size. A region of interest (ROI) was drawn along the perimeter of the injured cortex and the calculated area of this region was divided by the area of the total brain. Lesion areas were then compared between groups.
RNA Isolation and Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated (SV RNA Isolation kit, Promega, Madison, WI) and qRT-PCR performed on a Cepheid SmartCycler II (Cepheid, Sunnyvale, CA) using a Superscript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen, Carlsbad, CA), as detailed by our laboratory (Laird et al. 2008; Wakade et al. 2008). Primers were as follows: AQP4 : (FP 5’-CGGTTCATGGAAACCTCACT-3’; RP 5’-CATGCTGGCTCCGGTATAAT-3’), p50: (FP 5’-GAACAGCCTTGCATCTAGCC-3’; RP 5’-TCCGAGTCGCTATCAGAGGT-3’), p65: (FP 5’- GCGAGAGGAGCACAGATACC-3’; RP 5’-CTGATAGCCTGCTCCAGGTC-3’), IL-1β: (FP 5’-GCCCATCCTCTGTGACTCAT-3’; RP 5’-AGGCCACAGGTATTTTGTCG-3’), RPS3: (FP 5’-AATGAACCGAAGCACACCATA-3’ ; R P 5 ’-ATCAGAGAGTTGACCGCAGTT-3’). Product specificity was confirmed by melting curve analysis and visualization of a single, appropriately sized band on a 2% agarose gel. Gene expression levels were quantified using a cDNA standard curve and data were normalized to RPS3, a housekeeping gene that was unaffected by experimental manipulations (Rajeevan et al. 2001; Laird et al. 2008; Wakade et al. 2008). Data were expressed as fold change versus vehicle treatment. Experiments were performed in triplicate, with n=4/trial.
Physiological measurements
Physiological parameters were analyzed in mixed arterial-venous trunk blood using a CG8+ blood gas cartridge and iStat1 handheld blood gas analyzer (Abbott Inc., East Windsor, NJ), as described by our laboratory (Wakade et al. 2008). Data for each parameter were expressed as mean ± SEM (n=9-12 mice/group).
Statistical analysis
The effects of treatments were analyzed using a One-Way Analysis of Variance (ANOVA) followed by Student Newman Keul’s or Dunnett’s post-hoc test, as indicated in the Figure Legends. Results are expressed as mean ± SEM. A p<0.05 was considered to be statistically significant.
RESULTS
Curcumin attenuates cerebral edema following TBI
Brain water content, a measure of cerebral edema, was significantly increased in the ipsilateral cortex by 24h post-TBI in mice (83.1 ± 0.3% after TBI vs. 78.9 ± 0.3% in sham, p<0.001 vs. sham). The increase in brain water content following TBI was attenuated by a single injection of 150 mg/kg curcumin administered 15 minutes prior to injury (80.1 ± 0.4%; p<0.001 vs. TBI; not significantly different from sham-operated mice). Pre-treatment with 75 mg/kg curcumin similarly reduced edema after TBI (80.9 ± 0.7%; p<0.05 vs. sham and TBI). Administration of 150 mg/kg curcumin at 30 minutes post-TBI did not significantly reduce brain water content as compared to TBI alone (82.9 ± 0.5%). In contrast, 300 mg/kg curcumin significantly reduced edema when administered at 30 minutes post-TBI (81.5% ± 0.6%; p<0.05 vs. TBI), although this effect was lost when administered 1h post-injury (82.2 ± 0.6%; not significantly different from TBI alone). Brain water content did not significantly differ between any of the treatment groups within the contralateral cortices (FIGURE 1). Similarly, brain water content was unaffected by administration of curcumin (75-300 mg/kg) to uninjured (sham) (data not shown), suggesting an injury-specific reduction in edema. Although the ability of curcumin to reduce hippocampal neurodegeneration following lateral fluid percussion injury in rats was obscured by a protective effect of the vehicle (DMSO), administration of DMSO alone did not significantly reduce cerebral edema following TBI (Di Giorgio et al. 2008), as compared to untreated mice.
FIGURE 1. Curcumin attenuates cerebral edema following TBI.
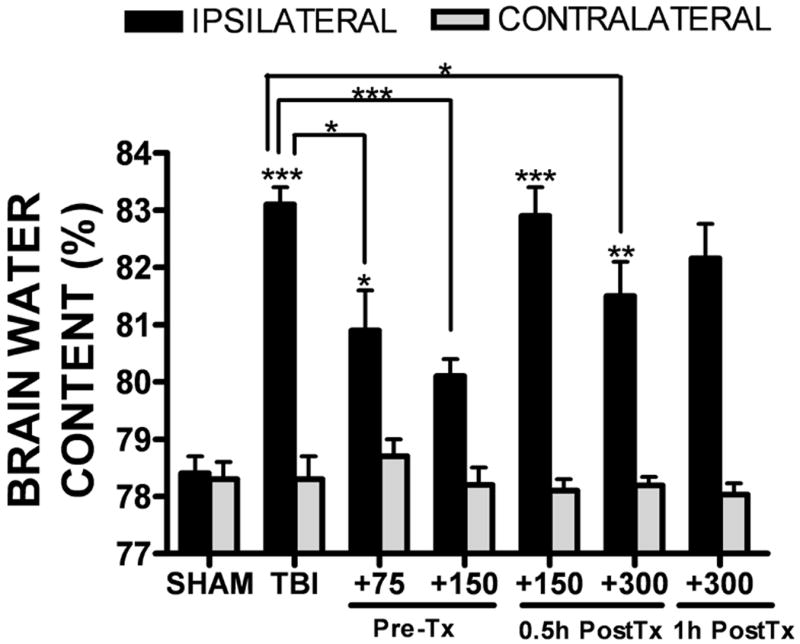
Mice (n=8-12/group) were administered curcumin (CCM; 75, 150, or 300 mg/kg) 15 minutes prior to injury (PreTx), 30 minutes post-injury (0.5h PostTx) or 1h post-injury (1h PostTx). Brain water content, a measure of cerebral edema, was assessed in the ipsilateral and contralateral hemisphere at 24h after TBI. Comparisons within each hemisphere between different treatments groups were done using a one-way ANOVA followed by Dunnett’s post-hoc test (* p<0.05, ** p<0.01, ***p<0.001 vs. the ipsilateral hemisphere in sham-operated mice). No significant differences in cerebral edema were observed between groups in the contralateral hemisphere. Data are represented as the mean ± SEM from 8-12 mice/group.
A significant lesion was observed in the ipsilateral cortex following TBI. Conversely, lesions were never observed in sham-operated mice, which underwent the same surgical procedures without trauma (FIGURE 2A). Despite the reduction in cerebral edema, pre-treatment with curcumin (150 mg/kg) did not significantly attenuate lesion size at any time point (1, 7, 14, or 21 days) post-injury, as compared to the TBI group (FIGURE 2A,B).
FIGURE 2. Effect of curcumin on cortical lesion size.
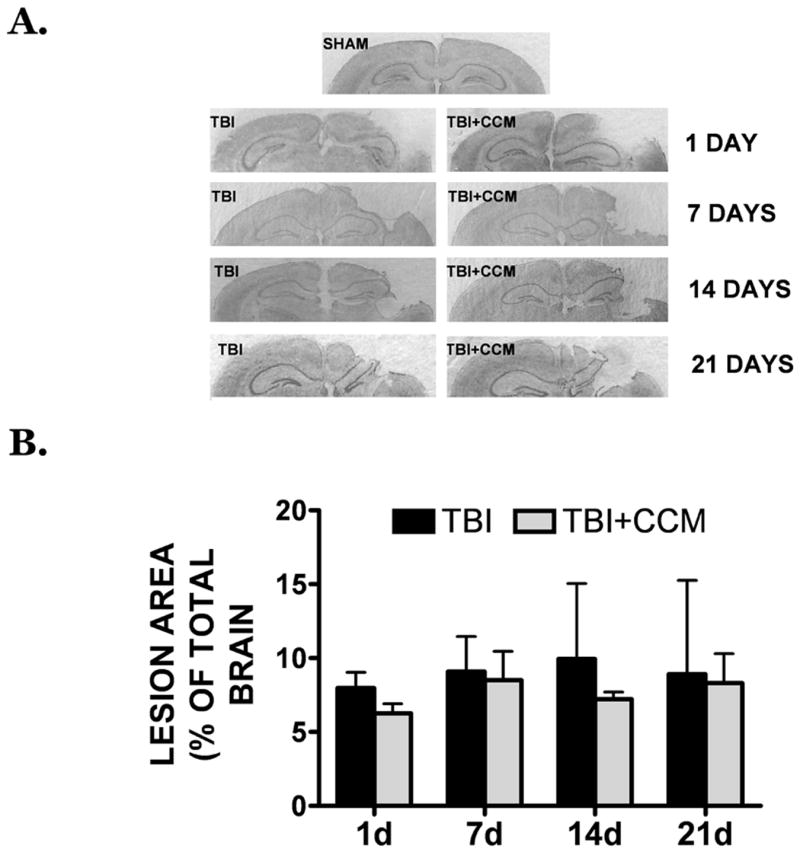
(A) Cortical lesion at 1, 7, 14, or 21 days following a moderate TBI. Representative brain sections are provided from sham, TBI, or TBI + pre-treatment with 150 mg/kg curcumin mice. A significant lesion is noted on the ipsilateral cortex (right side of images), as compared to the contralateral hemisphere (left side of images). (B) Quantification of lesion area at various time points following injury, as depicted in panel (A). Data are representative of n=4-6 mice/group. No significant differences in lesion size were observed between TBI and TBI + curcumin groups.
Curcumin improves neurological function following TBI
A significant reduction in open-field activity (total distance traveled), a measure of general health and anxiety, was observed over the first 28 days following TBI (FIGURE 3A). This reduction was associated with a concomitant increase in thigmotaxis (data not shown), suggesting an increase in post-traumatic anxiety. Curcumin (150 mg/kg) pre-treatment significantly increased overall locomotion and movement within squares in the center of the open-field chamber for the entire observation period after TBI (p<0.05 vs. TBI, not significantly different from sham-operated mice). Similarly, TBI worsened performance on the two-object novel recognition task, a sensitive test of learning and recognition memory (p<0.05 vs. sham) (FIGURE 3B). As was observed with open-field activity, curcumin increased the amount of time spent exploring a novel object (p<0.05 vs. TBI) such that curcumin-treated mice (with or without TBI) were not significantly different from sham-operated mice. In contrast, motor performance was unaffected in all treatment groups using this controlled cortical impact injury paradigm, as determined by the rotarod test (data not shown).
FIGURE 3. Curcumin improves long-term neurobehavioral outcomes following TBI.
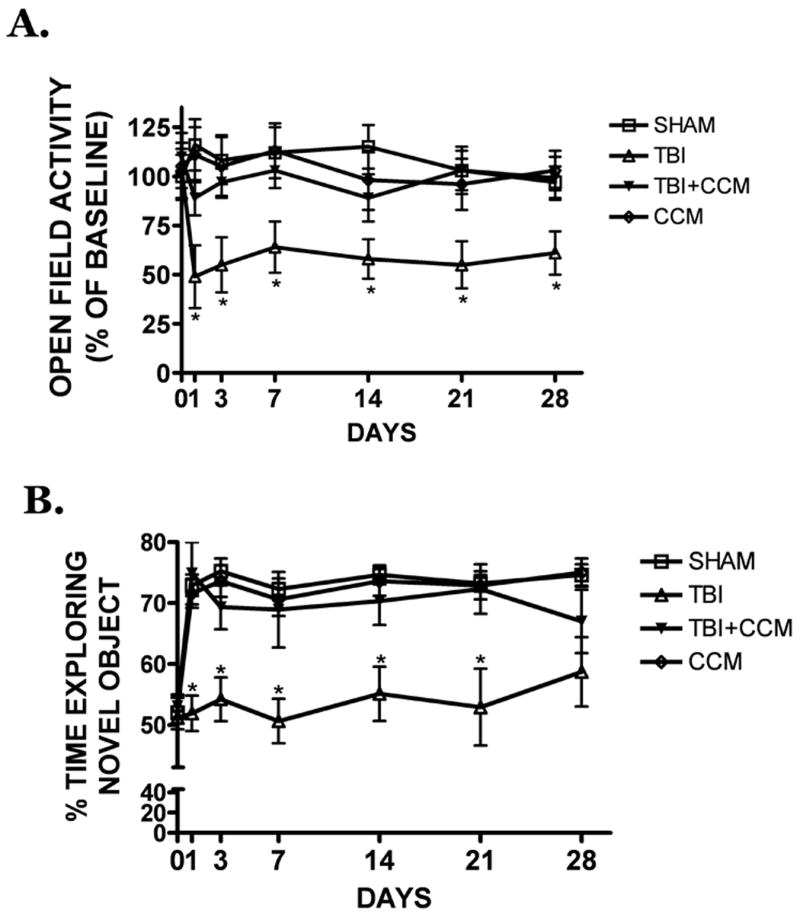
(A) Pre-treatment with curcumin (150 mg/kg) attenuated the reduction in open-field locomotion, a behavioral measure of anxiety/depression, in the 28 days after TBI. (B) Curcumin improved performance on the two-object novel recognition task, a sensitive measure of learning and memory, as compared to placebo-treated mice following TBI. Data are the mean ± SEM from 6-10 mice/group. * p<0.05 vs. sham.
Curcumin reduces AQP4 expression within the pericontusional cortex
AQP4 protein expression was elevated within the pericontusional cortex at 12h (2.29 ± 0.52 fold increase; p<0.05 vs. sham) and 24h (2.10 ± 0.41 fold increase; p<0.05 vs. sham) following TBI. Curcumin administration (150 mg/kg) 15 minutes prior to TBI attenuated the post-injury induction of AQP4 (1.54 ± 0.27 and 0.98 ± 0.11 fold increase vs. sham at 12h and 24h, respectively; p<0.05 vs. TBI, not significantly different from sham) (FIGURE 4A). Consistent with the results obtained by Western blotting, the intensity and number of pericontusional cells expressing AQP4 were strongly increased after TBI, and this induction was reduced by a single, pre-treatment injection of 150 mg/kg curcumin, a dose which also reduced cerebral edema (FIGURE 4B). Curcumin (300 mg/kg, i.p.) administration at 0.5h (data not shown) and 1h post-injury partially attenuated the post-traumatic induction of AQP4, whereas post-treatment with the 150 mg/kg dose did not reduce AQP4 expression, as compared to TBI alone (FIGURE 4C). These data indicate curcumin exhibits a relatively narrow therapeutic window to limit AQP4 expression and edema formation.
FIGURE 4. Pericontusional AQP4 expression following TBI.
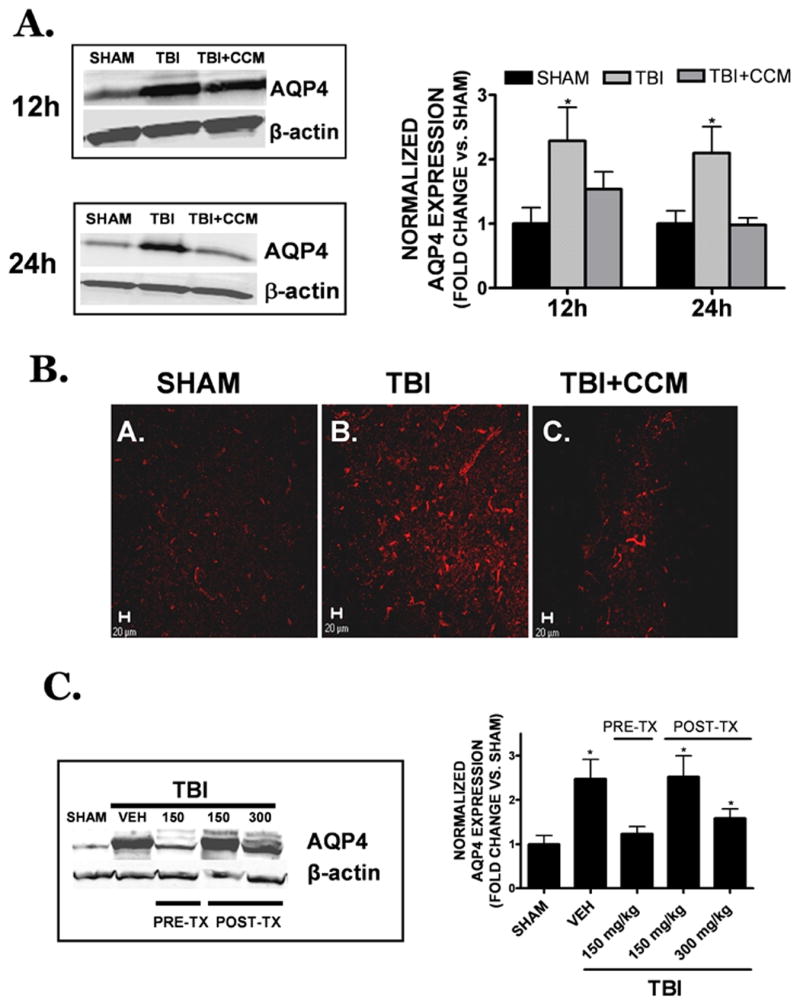
(A) Curcumin (150 mg/kg) was administered to mice at 15 minutes prior to injury and AQP4 expression was assessed 12h or 24h later by Western blotting. AQP4 expression was normalized to β-actin to control for equal protein loading. (B) Representative low power (20x objective) images of AQP4 immunoreactivity within the peri-contusional cortex following (A) sham, (B) TBI or (C) TBI + curcumin pre-treatment (150 mg/kg administered 0.5h prior to TBI). Data are representative of n=4 animals/group. Scale bar = 20 μm. (C) Representative Western blot of AQP4 expression (left) at 24h post-TBI following vehicle, 150 mg/kg curcumin pre-treatment (PRE-TX), or 1h post-treatment (150 or 300 mg/kg, POST-TX). For both panels, quantification of Western blotting (5-7 mice/group in each treatment condition) was performed by densitometry analysis. Data were represented as the mean ± SEM at each time point and expressed as fold change vs. sham. Data were analyzed using One-Way ANOVA followed by Student Newman Keul’s post-hoc test (*p<0.05 vs. sham).
Curcumin attenuates IL-1β expression and signaling
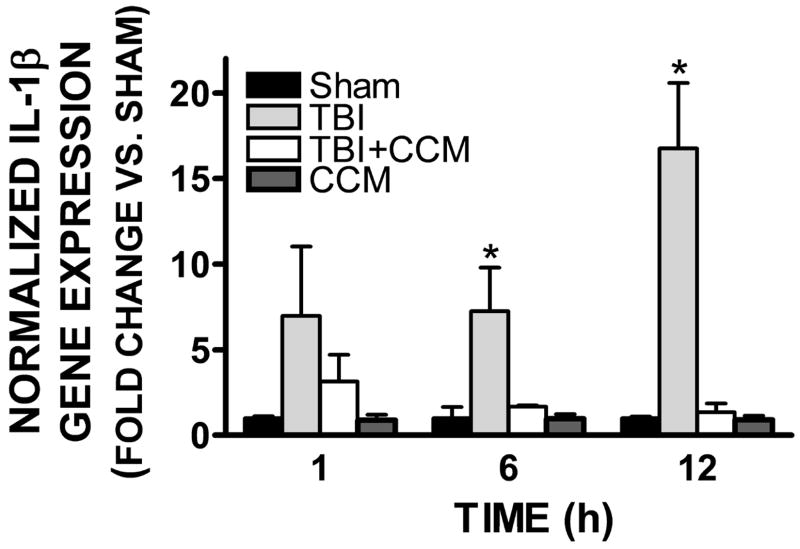
Expression of the pro-inflammatory cytokine, interleukin-1β (IL-1β), was elevated within the pericontusional cortex, a region exhibiting increased edema and AQP4 expression. Differences in IL-1β were not observed prior to 1h post-injury; however, expression was increased by 7.3 ± 2.5 fold and 6.8 ± 3.8 fold at 6h (p<0.05 vs. sham) and 12h (p<0.001 vs. sham) following TBI, respectively (FIGURE 5). Pretreatment with curcumin (150 mg/kg) reduced TBI-induced IL-1β expression, such that IL-1β mRNA levels were not significantly different from sham-operated mice at 6h and 12h post-injury (p<0.05 and p<0.001 vs. TBI, respectively).
FIGURE 5. Effect of curcumin on TBI-induced IL-1β expression.
Mice were pre-treated with curcumin (150 mg/kg, 15 minutes prior to injury) followed by a moderate TBI. IL-1β gene expression was assessed in the cerebral cortex at 1, 6, or 12h post-injury using qRT-PCR. Data were normalized to a housekeeping gene and expressed as fold change vs. sham-operated mice (mean ± SEM; n=6-8 mice/group) Data were analyzed using One-Way ANOVA and Student Newman-Keul’s post-hoc test (*p<0.05, ***p<0.001 vs. sham).
Curcumin reduced cerebral edema, an effect that correlated with a reduction in the pericontusional expression of IL-1β and AQP4. Consistent with a possible functional association between these factors, IL-1β (10 ng/mL) increased AQP4 gene (FIGURE 6A) and protein (FIGURE 6B) expression in cultured cortical astrocytes within 18h. Specifically, IL-1β increased AQP4 gene expression by 3.3 ± 0.3 fold (p<0.001 vs. vehicle), an effect that was significantly attenuated by co-treatment with 5 μM curcumin (1.3 ± 0.1 fold; p<0.001 vs. IL-1β, not significantly different from vehicle-treated cultures). AQP4 protein expression, as assessed by Western blotting, was similarly increased by 1.8 ± 0.1 fold after IL-1β treatment (p<0.01 vs. vehicle) and this induction was similarly reduced by 5 μM curcumin (0.6 ± 0.10 fold changes vs. vehicle; p<0.01 vs. IL-1β, p<0.05 vs. vehicle) (FIGURE 6B). In contrast, curcumin alone failed to significantly reduce basal AQP4 levels, as compared to vehicle, suggesting a specific inhibition following IL-1β treatment.
FIGURE 6. Curcumin reduces IL-1β induced AQP4 expression in cultured astrocytes.
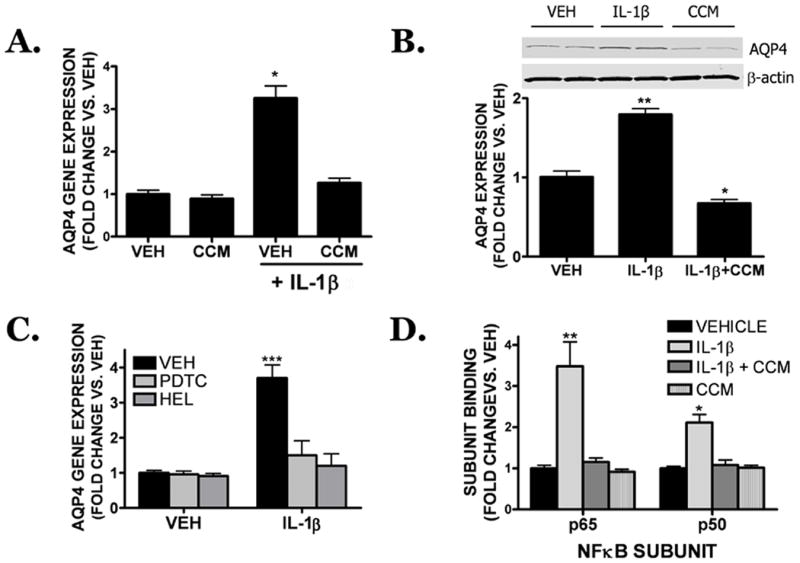
Mouse cortical astrocytes were treated with IL-1β (10 ng/mL) in the presence of curcumin (5 μM) or vehicle and AQP4 (A) gene and (B) protein expression were assessed 18h later. (C) The NFκB inhibitors, PDTC (100 μM), or the p65 translocation inhibitor, helenalin (10 μM; HEL) attenuated IL-1β-induced AQP4 gene expression following an 18h treatment. (D) Curcumin reduced IL-1β-induced NFκB (p50 and p65 subunits) binding in cortical astrocytes following a 6h treatment. For all panels, data are expressed as a fold change vs. vehicle and are the mean ± SEM from three independent experiments (n=4/experiment). Data were analyzed using One-Way ANOVA and Student Newman-Keul’s post-hoc test (*p<0.05, **p<0.01, ***p<0.001 vs. vehicle).
The NFκB transcription factor is a primary molecular target of curcumin and important effecter of IL-1β; thus, the contribution of NFκB toward IL-1β-induced AQP4 expression was determined in cultured cortical astrocytes. Co-treatment with either PDTC (100 μM), a general NFκB inhibitor, or helenalin (10 μM), a specific p65 translocation inhibitor, reduced IL-1β induced AQP4 gene expression to 1.5 ± 0.2 fold and 1.2 ± 0.1 fold over vehicle, respectively (p<0.001 vs. IL-1β alone; not significantly different from vehicle-treated cultures) (FIGURE 6C). In contrast, neither PDTC nor HEL alone significantly inhibited basal AQP4 expression. Treatment with IL-1β significantly increased the activation of the p50 (2.1 ± 0.2 fold increase; p<0.05 vs. vehicle) and p65 (3.5 ± 0.6 fold increase; p<0.01 vs. vehicle) subunits of NFκB following a 6h treatment. Treatment with curcumin (5 μM) reversed IL-1β-induced p50 (1.1 ± 0.1 fold increase; p<0.05 vs. IL-1β, not significantly different from vehicle) and p65 activation (1.2 ± 0.1 fold increase; p<0.01 vs. IL-1β, not significantly different from vehicle) (FIGURE 6D).
Curcumin reduces NFκB activation following TBI
IL-1β increased AQP4 expression via a NFκB-dependent pathway in cultured astrocytes; however, it remained unclear whether a similar increase in astrocytic NFκB activation occurs within the pericontusional cortex following TBI. To address this issue, immunohistochemistry was performed using antibodies against GFAP, an astrocyte-specific intermediate filament, and phospho-p65, which only detects the activated form of p65. Whereas GFAP and phospho-p65 positive cells were sparse in the brain of sham-operated mice, immunoreactivity for both markers was strongly increased after TBI, including a significant degree of cellular co-localization. Pre-treatment with curcumin (150 mg/kg) decreased immunoreactivity for GFAP and phospho-p65 following TBI resulting in few dually labeled (e.g. GFAP+/phospho-p65+) cells (FIGURE 7). Western blotting was next performed to quantify the reduction in the pericontusional expression of GFAP, a marker of reactive gliosis, following curcumin treatment. GFAP expression was increased by 2.4-fold following TBI, as compared to sham-operated mice (p<0.05 vs. sham). This increase was attenuated by pre-treatment with curcumin (150 mg/kg) (p<0.05 vs. TBI, not significantly different from sham), consistent with the pattern observed by immunohistochemistry (FIGURE 8).
FIGURE 7. Co-localization of activated p65 in astrocytes following TBI.
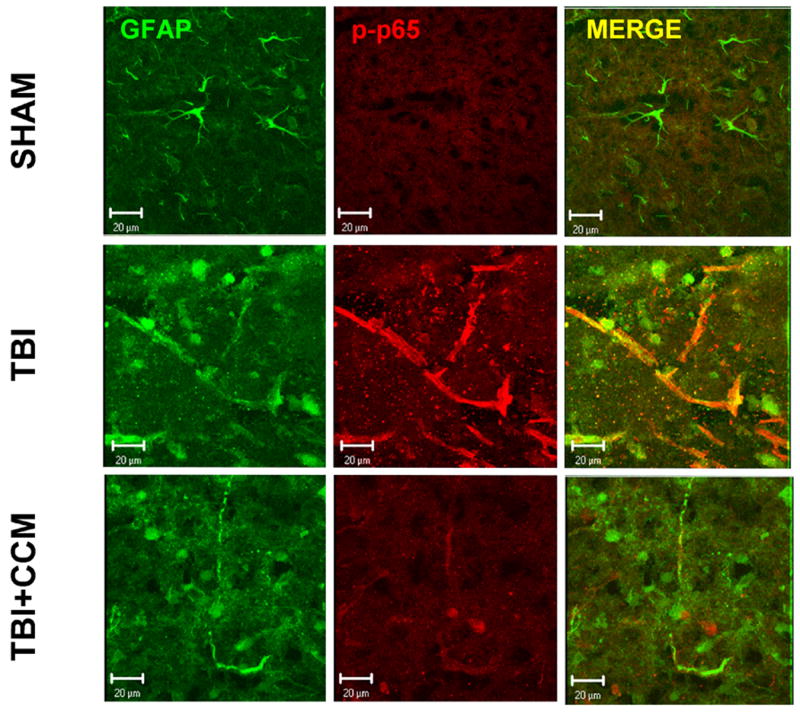
High-magnification projection z-stack images demonstrating the cellular localization of phosphorylated p65 (p-p65; red) and GFAP (green) in the pericontusional cortex at 24h post-TBI in sham, TBI or TBI + curcumin (150 mg/kg curcumin, 0.5h pre-treatment) treated mice. Images are representative of 5-7 mice/group. Scale bar = 20 μm.
FIGURE 8. Effect of curcumin on reactive gliosis following TBI.
Effect of curcumin (150 mg/kg; 0.5h pre-treatment) on GFAP expression with in the pericontusional cortex at 24h post-TBI. Data were quantified using densitometric analysis and are depicted as the mean ± SEM from 4 mice/group. Data were compared by one way ANOVA followed by Student Newman Keul’s post-hoc test (* p<0.05). A representative Western blot is shown in the inset.
Physiological parameters
TBI was well-tolerated (0% mortality) and body weight, grooming, and ambulation were unaffected by both injury and curcumin administration, allowing all mice to be included in data analyses. Analysis of physiological parameters in anesthetized mice (n=9-12/group) did not indicate any significant differences between sham, TBI, or TBI + curcumin, or curcumin-treated mice (TABLE 1). Blood pH was stable across groups and was consistent with pH values obtained in similar analyses of CD-1 mice (Koch et al. 2008; Wakade et al. 2009). No significant changes in saturated oxygen or total carbon dioxide were observed between any of the treatment groups. Similarly, bicarbonate, sodium, ionized calcium, hematocrit, and hemoglobin were unaffected by all treatments, as compared to sham-operated mice.
TABLE 1. Physiological parameters.
Physiological parameters were assessed in trunk blood collected from mice at 6h post-injury. Data are represented as the mean ± SEM from 9-12 mice/group. No significant differences were detected between any of the treatment groups.
| SHAM | TBI | TBI+CCM | CCM | |
|---|---|---|---|---|
| N | 9 | 12 | 10 | 9 |
| pH | 7.13±0.02 | 7.14±0.02 | 7.10±0.02 | 7.11±0.05 |
| sO2 | 75.3±1.3% | 75.0±3.6% | 76.4±4.5% | 76.8±4.2% |
| tCO2 | 25.0±1.5% | 25.7±1.0% | 24.3±0.5% | 24.8±1.1% |
| HCO3 | 23.7±1.3 | 23.7±1.0 | 23.6±0.5 | 23.9±0.5 |
| Na+ | 146.9±2.1 | 146.7±1.3 | 150.5±2.5 | 146.0±1.6 |
| iCa | 1.18±0.16 | 1.33±0.07 | 1.32±0.10 | 1.22±0.15 |
| Hct | 43.1±3.4% | 37.3±1.3% | 38.4±1.2% | 40.3±1.2% |
| Hb | 14.6±1.2 | 12.7±0.4 | 13.1±0.4 | 13.7±0.4 |
Abbreviations and units: Saturated oxygen (sO2; %), total carbon dioxide (tCO2; mM), bicarbonate (HCO3; mM), sodium (Na+; mM), ionized calcium (iCa; mM), hematocrit (Hct; % packed cell volume), hemoglobin (Hb; g/dL)
DISCUSSION
Cerebral edema and the subsequent rise in ICP remain serious complications which contribute to increased patient mortality and long-term disability (Katayama et al. 1990; Aldrich et al. 1992). The present study suggests, for the first time, that clinically-achievable doses of curcumin attenuate cerebral edema, decrease pericontusional expression of AQP4, a glial water channel which promotes brain swelling, and improve neurological outcome following TBI.
Elevated ICP, secondary to edema formation, is a key determinant of long-term prognosis. Along these lines, the degree of swelling on the first computed tomography scan directly correlates with patient outcome, demonstrating the importance of limiting edema following head trauma (Eisenberg et al. 1990). In the present study, curcumin reduced cerebral edema and improved performance on neurobehavioral tests following TBI. Notably, curcumin normalized exploratory locomotion and reduced thigmotaxis to sham levels over the four weeks after TBI. The reduction in locomotion following trauma was unlikely to be a result of motor deficits, as differences in the rotarod test were not observed between any of the groups, suggesting curcumin may limit post-traumatic stress and/or anxiety, an important long-term sequela of TBI (Vallee et al. 1997). Coupled with a report suggesting curcumin enhances synaptic plasticity and cognitive function after fluid percussion injury in rats (Wu et al. 2006), curcumin may represent a potent therapeutic agent which exerts multiple beneficial effects after TBI.
Both cellular and vasogenic edema contribute to brain swelling, although cellular edema predominates after TBI (Ito et al. 1996; Unterberg et al. 2004; Kleindienst et al. 2006). Astrocytic swelling, a hallmark of cellular edema, commences within the first hours after injury and contributes to reactive astrocytosis (Kimelberg 1992; Nilsson et al. 1993; Kimelberg et al. 1995; Richardson et al. 2009). While astrocytes may exert diametrically opposing actions (Myer et al. 2006; Laird et al. 2008), glial activation temporally mirrors the neuroinflammatory response and development of edema after experimental and clinical TBI (Hinkle et al. 1997; Dietrich et al. 1999). Serum and CSF levels of the reactive astrocyte markers, S100β and GFAP, also retrospectively correlated with a negative patient outcome (Pelinka et al. 2004a; Pelinka et al. 2004b), suggesting a detrimental, yet poorly defined, role for astrocytes after TBI.
Increased pericontusional expression of AQP4 correlates with the development of cellular edema after TBI in humans and rodents (Mason et al. 2001; Hu et al. 2005; Guo et al. 2006; Aggarwal et al. 2007; Griffiths et al. 2007). Unfortunately, the molecular regulation of AQP4 remains poorly understood and clinically-useful inhibitors of AQP4 are lacking, complicating the exploitation of this potential therapeutic target. Herein, we show clinically-feasible doses of curcumin, which produce serum concentrations approximating oral administration of 8 grams/daily in humans (Cheng et al. 2001), attenuated pericontusional AQP4 expression in a manner which paralleled the reduction in cerebral edema following TBI. These novel findings suggest curcumin may represent a clinically-safe AQP4 inhibitor, although the mechanism(s) underlying this potentially beneficial effect remained unresolved.
IL-1β, which promotes reactive astrogliosis after TBI (Lin et al. 2006), and IL-1R undergo an acute and sustained upregulation after experimental TBI (Nieto-Sampedro and Berman 1987; Taupin et al. 1993; Fan et al. 1995; Lee et al. 1995). Although the role of IL-1β following brain injury remains unresolved, astrocyte-specific over expression of the IL-1R antagonist (IL-1Ra) reduced neurological injury and attenuated neuroinflammatory activation following TBI (Tehranian et al. 2002) whereas IL-1R deficient mice exhibited a reduction in cerebral edema after stroke (Lazovic et al. 2005). Consistent with these experimental findings, IL-1β levels within the CSF and brain of neurotrauma patients positively correlate with elevated ICP and an unfavorable outcome (Hayakata et al. 2004; Chiaretti et al. 2005). Interestingly, IL-1β induces the expression of AQP4, a water channel implicated in the development of post-traumatic edema, via a NFκB-dependent mechanism (Ito et al. 2006). In line with these observations, curcumin reduced acute, pericontusional IL-1β expression and preferentially attenuated the activation of NFκB, a transcriptional regulator of IL-1β and downstream mediator of IL-1β-induced signaling (Nadjar et al. 2003; Moynagh 2005), within pericontusional astrocytes. Additionally, curcumin attenuated IL-1β-induced AQP4 expression in cultured astrocytes, suggesting AQP4 inhibition may occur at multiple points, including up- and down-stream from IL-1β expression.
Cerebral edema was maximally reduced when curcumin was administered prior to TBI, with a diminished effect observed when given up to 1h post-injury. A mechanistic explanation for this limited therapeutic window remains poorly defined; however, curcumin exhibits a short serum half-life (<40 minutes), due in part to rapid metabolism and excretion (Pan et al. 1999; Anand et al. 2007). Coupled with the 1h lag between intraperitoneal administration and peak brain concentrations of curcumin (Pan et al. 1999), pre-treatment may provide maximal serum concentrations immediately after injury, when the molecular changes underlying edema formation occur. The pro-inflammatory transcription factor, NFκB, promotes GFAP expression in injured human astrocytes (Bae et al. 2006), induces astrocytic inflammation following S100β treatment (Lam et al. 2001), and increases astrocytic swelling (Sinke et al. 2008). Of particular relevance to the present study, NFκB was activated within reactive astrocytes within 1h of TBI (Nonaka et al. 1999; Sanz et al. 2002), supporting acute, NFκB-dependent gene expression (e.g. IL-1β, AQP4). Thus, curcumin post-treatment beyond the first 30 minutes after TBI may follow the molecular changes underlying the development of post-traumatic edema.
Daily oral ingestion of 8 grams of curcumin is non-toxic in humans, producing peak serum concentrations (~1.8 μM) within 1h of administration (Cheng et al. 2001). This same dose functionally inhibited NFκB and attenuated inflammatory expression in peripheral blood mononuclear cells from pancreatic cancer patients, suggesting anti-inflammatory activity despite poor oral bioavailability (Dhillon et al. 2008). In mice, peak plasma concentrations (~1.6 μM) were achieved 15 minutes after administration of 100 mg/kg (i.p.) curcumin, followed by brain accumulation within 1h (Pan et al. 1999). Thus, the doses and route of administration utilized in the present study (75-300 mg/kg, i.p.) and in other pre-clinical studies (Thiyagarajan and Sharma 2004; Zhao et al. 2008) may simulate steady state serum concentrations in humans. The need for relatively large doses of curcumin remains unclear; however, a significant percentage of curcumin precipitated within the abdominal cavity immediately after an intraperitoneal injection, suggesting a relatively small percentage of administered curcumin actually enters the bloodstream (MDL and KMD, unpublished observation). Thus, approaches incorporating liposomes or nanoparticles may improve drug delivery and overcome issues of low bioavailability (Anand et al. 2007). Alternatively, the development of curcumin analogues exhibiting an improved pharmacological profile (e.g. improved solubility, half life, brain distribution), may more effectively reduce brain injury.
In summary, clinically-achievable doses of curcumin attenuated the development of cerebral edema and improved long-term neurological outcome following TBI. These effects were directly associated with a reduction in neuroinflammatory activation and attenuated expression of the glial water channel, AQP4. Given the lack of clinically-useful AQP4 inhibitors, further exploration of curcumin, both as a novel inhibitor of AQP4 and as an adjunct in the acute management of neurotrauma patients, may be warranted.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (NS065172) and American Heart Association (BGIA2300135) to KMD and by a fellowship from the American Heart Association (PRE2250690) to MDL
Abbreviations
- AQP
aquaporin
- ICP
intracranial pressure
- IL-1R
interleukin-1β type 1 receptor
- IL-1β
interleukin-1β
- GFAP
glial fibrillary acidic protein
- NFκB
nuclear factor κB
- TBI
traumatic brain injury
Footnotes
The authors report no conflicts of interest.
References
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Aldrich EF, Eisenberg HM, Saydjari C, Luerssen TG, Foulkes MA, Jane JA, Marshall LF, Marmarou A, Young HF. Diffuse brain swelling in severely head-injured children. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1992;76:450–454. doi: 10.3171/jns.1992.76.3.0450. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Bae MK, Kim SR, Lee HJ, Wee HJ, Yoo MA, Ock Oh S, Baek SY, Kim BS, Kim JB, Sik Y, Bae SK. Aspirin-induced blockade of NF-kappaB activity restrains up-regulation of glial fibrillary acidic protein in human astroglial cells. Biochim Biophys Acta. 2006;1763:282–289. doi: 10.1016/j.bbamcr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci U S A. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch O, Manley GT. The role of aquaporin-4 in cerebral water transport and edema. Neurosurg Focus. 2007;22:E3. doi: 10.3171/foc.2007.22.5.4. [DOI] [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Chiaretti A, Genovese O, Aloe L, Antonelli A, Piastra M, Polidori G, Di Rocco C. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005;21:185–193. doi: 10.1007/s00381-004-1032-1. discussion 194. [DOI] [PubMed] [Google Scholar]
- Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey RJ, Baskaya MK, Dogan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47:399–404. doi: 10.1097/00006123-200008000-00024. discussion 404-396. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Di Giorgio AM, Hou Y, Zhao X, Zhang B, Lyeth BG, Russell MJ. Dimethyl sulfoxide provides neuroprotection in a traumatic brain injury model. Restor Neurol Neurosci. 2008;26:501–507. [PubMed] [Google Scholar]
- Dietrich WD, Truettner J, Zhao W, Alonso OF, Busto R, Ginsberg MD. Sequential changes in glial fibrillary acidic protein and gene expression following parasagittal fluid-percussion brain injury in rats. J Neurotrauma. 1999;16:567–581. doi: 10.1089/neu.1999.16.567. [DOI] [PubMed] [Google Scholar]
- Eisenberg HM, Gary HE, Jr, Aldrich EF, Saydjari C, Turner B, Foulkes MA, Jane JA, Marmarou A, Marshall LF, Young HF. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73:688–698. doi: 10.3171/jns.1990.73.5.0688. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces expression of interleukin-1 beta mRNA in the rat brain. Brain Res Mol Brain Res. 1995;30:125–130. doi: 10.1016/0169-328x(94)00287-o. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- Griebenow M, Casalis P, Woiciechowsky C, Majetschak M, Thomale UW. Ubiquitin reduces contusion volume after controlled cortical impact injury in rats. J Neurotrauma. 2007;24:1529–1535. doi: 10.1089/neu.2007.0306. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int Rev Neurobiol. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, Shimazu T, Sugimoto H. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Silakova JM, Hewett JA. Oral treatment with rofecoxib reduces hippocampal excitotoxic neurodegeneration. J Pharmacol Exp Ther. 2006;319:1219–1224. doi: 10.1124/jpet.106.109876. [DOI] [PubMed] [Google Scholar]
- Hinkle DA, Baldwin SA, Scheff SW, Wise PM. GFAP and S100beta expression in the cortex and hippocampus in response to mild cortical contusion. J Neurotrauma. 1997;14:729–738. doi: 10.1089/neu.1997.14.729. [DOI] [PubMed] [Google Scholar]
- Hu H, Yao HT, Zhang WP, Zhang L, Ding W, Zhang SH, Chen Z, Wei EQ. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J Zhejiang Univ Sci B. 2005;6:33–37. doi: 10.1631/jzus.2005.B0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- Ito H, Yamamoto N, Arima H, Hirate H, Morishima T, Umenishi F, Tada T, Asai K, Katsuya H, Sobue K. Interleukin-1beta induces the expression of aquaporin-4 through a nuclear factor-kappaB pathway in rat astrocytes. J Neurochem. 2006;99:107–118. doi: 10.1111/j.1471-4159.2006.04036.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996;84:97–103. doi: 10.3171/jns.1996.84.1.0097. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Tsubokawa T, Miyazaki S, Kawamata T, Yoshino A. Oedema fluid formation within contused brain tissue as a cause of medically uncontrollable elevation of intracranial pressure: the role of surgical therapy. Acta Neurochir Suppl (Wien) 1990;51:308–310. doi: 10.1007/978-3-7091-9115-6_104. [DOI] [PubMed] [Google Scholar]
- Khan MM, Hadman M, Wakade C, De Sevilla LM, Dhandapani KM, Mahesh VB, Vadlamudi RK, Brann DW. Cloning, expression, and localization of MNAR/PELP1 in rodent brain: colocalization in estrogen receptor-alpha- but not in gonadotropin-releasing hormone-positive neurons. Endocrinology. 2005;146:5215–5227. doi: 10.1210/en.2005-0276. [DOI] [PubMed] [Google Scholar]
- Kiening KL, van Landeghem FK, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF. Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett. 2002;324:105–108. doi: 10.1016/s0304-3940(02)00180-5. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic edema in CNS trauma. J Neurotrauma. 1992;9(Suppl 1):S71–81. [PubMed] [Google Scholar]
- Kimelberg HK, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab. 1995;15:409–416. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, Fazzina G, Amorini AM, Dunbar JG, Glisson R, Marmarou A. Modulation of AQP4 expression by the protein kinase C activator, phorbol myristate acetate, decreases ischemia-induced brain edema. Acta Neurochir Suppl. 2006;96:393–397. doi: 10.1007/3-211-30714-1_81. [DOI] [PubMed] [Google Scholar]
- Koch JD, Miles DK, Gilley JA, Yang CP, Kernie SG. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- Lam AG, Koppal T, Akama KT, Guo L, Craft JM, Samy B, Schavocky JP, Watterson DM, Van Eldik LJ. Mechanism of glial activation by S100B: involvement of the transcription factor NFkappaB. Neurobiol Aging. 2001;22:765–772. doi: 10.1016/s0197-4580(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Lazovic J, Basu A, Lin HW, Rothstein RP, Krady JK, Smith MB, Levison SW. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke. 2005;36:2226–2231. doi: 10.1161/01.STR.0000182255.08162.6a. [DOI] [PubMed] [Google Scholar]
- Lee SC, Dickson DW, Brosnan CF. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav Immun. 1995;9:345–354. doi: 10.1006/brbi.1995.1032. [DOI] [PubMed] [Google Scholar]
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- Levin HS, Eisenberg HM, Gary HE, Marmarou A, Foulkes MA, Jane JA, Marshall LF, Portman SM. Intracranial hypertension in relation to memory functioning during the first year after severe head injury. Neurosurgery. 1991;28:196–199. doi: 10.1097/00006123-199102000-00004. discussion 200. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Basu A, Druckman C, Cicchese M, Krady JK, Levison SW. Astrogliosis is delayed in type 1 interleukin-1 receptor-null mice following a penetrating brain injury. J Neuroinflammation. 2006;3:15. doi: 10.1186/1742-2094-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–516. doi: 10.3171/jns.1977.47.4.0503. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Moynagh PN. The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain. J Anat. 2005;207:265–269. doi: 10.1111/j.1469-7580.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Combe C, Laye S, Tridon V, Dantzer R, Amedee T, Parnet P. Nuclear factor kappaB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. J Neurochem. 2003;87:1024–1036. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M, Frigeri A. The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129:935–945. doi: 10.1016/j.neuroscience.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sampedro M, Berman MA. Interleukin-1-like activity in rat brain: sources, targets, and effect of injury. J Neurosci Res. 1987;17:214–219. doi: 10.1002/jnr.490170303. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Olsson Y, Sheardown MJ, Hansen AJ. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Chen XH, Pierce JE, Leoni MJ, McIntosh TK, Wolf JA, Smith DH. Prolonged activation of NF-kappaB following traumatic brain injury in rats. J Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- Nortje J, Menon DK. Traumatic brain injury: physiology, mechanisms, and outcome. Curr Opin Neurol. 2004;17:711–718. doi: 10.1097/00019052-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004a;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- Pelinka LE, Kroepfl A, Schmidhammer R, Krenn M, Buchinger W, Redl H, Raabe A. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004b;57:1006–1012. doi: 10.1097/01.ta.0000108998.48026.c3. [DOI] [PubMed] [Google Scholar]
- Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Singh A, Sun D, Fillmore HL, Dietrich DW, Bullock MR. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J Neurosurg. 2009 doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- Sanz O, Acarin L, Gonzalez B, Castellano B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J Neurosci Res. 2002;67:772–780. doi: 10.1002/jnr.10140. [DOI] [PubMed] [Google Scholar]
- Sarabia R, Lobato RD, Rivas JJ, Cordobes F, Rubio J, Cabrera A, Gomez P, Munoz MJ, Madera A. Cerebral hemisphere swelling in severe head injury patients. Acta Neurochir Suppl (Wien) 1988;42:40–46. doi: 10.1007/978-3-7091-8975-7_9. [DOI] [PubMed] [Google Scholar]
- Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498–503. doi: 10.3171/jns.1982.56.4.0498. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Sinke AP, Jayakumar AR, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. NFkappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J Neurochem. 2008;106:2302–2311. doi: 10.1111/j.1471-4159.2008.05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–951. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, Cano J. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin Attenuates Vascular Inflammation and Cerebral Vasospasm After Subarachnoid Hemorrhage in Mice. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Yang B, Zador Z, Verkman AS. Glial cell aquaporin-4 overexpression in transgenic mice accelerates cytotoxic brain swelling. J Biol Chem. 2008;283:15280–15286. doi: 10.1074/jbc.M801425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Eros C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23:1083–1093. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]