Abstract
OBJECTIVE
Disturbances in podocytes are typically associated with marked proteinuria, a hallmark of diabetic nephropathy. This study was conducted to investigate modulation of Notch-1 signaling in high glucose (HG)-stressed human podocytes and in a diabetic animal model.
RESEARCH DESIGN AND METHODS
Expression of the Notch signaling components was examined in HG-treated podocytes, human embryonic kidney cells (HEK293), and kidneys from diabetic animals by RT-qPCR, Western blot analysis, and immunohistochemical staining. The association between the Notch signaling, VEGF expression, and podocyte integrity was evaluated.
RESULTS
Notch-1 signaling was significantly activated in HG-cultured human podocytes and HEK293 cells and kidneys from diabetic animals. HG also augmented VEGF expression, decreasing nephrin expression and podocyte number—a critical event for the development of proteinuria in diabetic nephropathy. After use of pharmacological modulators or specific shRNA knockdown strategies, inhibition of Notch-1 signaling significantly abrogated VEGF activation and nephrin repression in HG-stressed cells and ameliorated proteinuria in the diabetic kidney.
CONCLUSIONS
Our findings suggest that upregulation of Notch-1 signaling in HG-treated renal podocytes induces VEGF expression and subsequent nephrin repression and apoptosis. Modulation of Notch-1 signaling may hold promise as a novel therapeutic strategy for the treatment of diabetic nephropathy.
Diabetic nephropathy is now the most common cause of end-stage renal disease worldwide (1). Like many renal diseases, diabetic nephropathy is characterized by the development of proteinuria followed by decreased glomerular filtration in association with glomerulosclerosis (2). Development of proteinuria is mainly due to injury of the glomerular filtration barrier, which consists of the glomerular endothelium, the glomerular basement membrane, and podocytes located outside of the capillary. Although each layer within the filtration barrier contributes to the prevention of proteinuria, emerging evidence suggests that podocytes function as the predominant component of this barrier (3).
The slit diaphragm (SD) represents the only cell-cell contact between mature podocytes. A major component of the SD complex is nephrin, which plays a critical role in maintaining the glomerular filtration barrier. Mutation or inactivation of the nephrin gene or reduction of nephrin expression may result in destabilization of the SD and consequent proteinuria (4). By contrast, overactive vascular endothelial growth factor (VEGF)/VEGF receptor system was observed in the diabetic kidney (2). VEGF is a proangiogenic factor that is expressed in podocytes during kidney morphogenesis (5). Evidence shows that increased VEGF activity in podocytes mediates the pathogenesis of focal segmental glomerulosclerosis (6) and is associated with proteinuria in diabetic nephropathy (7). Attenuation of the VEGF/VEGF receptor system by VEGF neutralization antibodies or VEGF receptor antagonists significantly ameliorates proteinuria in diabetic mice (6,8,9). Moreover, amelioration of proteinuria by inhibiting VEGF signaling in these kidney diseases is linked to restoration of SD density and nephrin quantity in podocytes (5,7,10), suggesting that downregulation of nephrin in diabetic nephropathy may be dependent on overactive VEGF signaling. Although modulation of VEGF signaling in diabetic nephropathy and other kidney diseases remains unclear, it must be subject to exquisite control in response to various environmental stimuli or stresses (11).
Notch signaling is known to play a critical role in mammalian kidney development (12). Notch proteins are single-pass transmembrane receptors with an extracellular epidermal growth factor and an intracellular domain. Notch receptors on the cell surface bind various ligands, including Jagged-1, resulting in a series of sequential proteolytic cleavage events of the Notch receptor by proteases, metalloproteases, and γ-secretase. The resulting Notch intracellular domain (NICD) translocates to the nucleus (13), where it associates with a DNA-binding protein, retinol-binding protein-Jκ, and the coactivator, Mastermind like-1 (MAML-1), to form a ternary complex, which activates the expression of downstream target genes (14–17). Vooijs et al. (18) have shown that Notch-1 is highly active in the developing kidney; however, in the mature kidney, very little active Notch-1 can be detected. Consistent with this observation, Cheng et al. (19,20) demonstrated that inhibition of Notch signaling during early development of the mouse kidney using a γ-secretase inhibitor resulted in a severe deficiency in the proximal tubules and glomerular podocytes, emphasizing the importance of Notch signaling during kidney development. However, sustained Notch activation in the mature kidney may be disastrous; Niranjan et al. (21) reported that Notch signaling functioned as a driving force behind podocyte damage and subsequent kidney failure. Inactivation of Notch signaling via genetic or pharmacologic intervention was sufficient to prevent and even reverse glomerular damage (21).
Although much evidence suggests that Notch-1 signaling is involved in glomerular disease, the relationship between the Notch-1 signaling pathway and diabetic proteinuria remains to be elucidated. In the present study, we investigated the modulation of the Notch-1 pathway in human podocytes and human embryonic kidney (HEK)293 cells cultured in HG conditions. We also evaluated the effects of Notch-1 signaling on VEGF and nephrin expression in podocytes and in the kidneys of diabetic animals to further elucidate the role of Notch-1 in diabetic nephropathy.
RESEARCH DESIGN AND METHODS
Human podocyte and HEK293 cell cultures.
Conditionally immortalized human podocytes (22) were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% insulin transferrin disodium selenite (Sigma, St. Louis, MO) at 33°C. To stimulate cell differentiation, the culture temperature was changed from 33 to 37°C for 14 days, and the FBS was replaced with human plasma (23).
HEK 293 cells were maintained in Dulbecco's Modified Eagle's Medium with 10% FBS. Additional incubations with 10 units/ml bovine erythrocyte SOD-PEG (Sigma), 1 μmol/l N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) (Calbiochem, Gibbstown, NJ), or specific monoclonal antibodies (R&D Systems, Minneapolis, MN) were also performed in cultured cells.
RNA extraction, RT-PCR, and quantitative RT-PCR (RT-qPCR).
Total RNA from 1 × 106 cells or from rat kidneys was prepared with QIAzol reagent (Qiagen, Valencia, CA) or Tri reagent (Sigma) according to the manufacturers' instructions. RT-qPCR was performed according to the MIQE guidelines (24). Total RNA (1 μg) was reverse transcribed to cDNA with the Moloney murine leukemia virus reverse transcriptase (Fermentas, Glen Burnie, MD). The PCR mixture (25 μl) containing the cDNA template equivalent to 20 ng total RNA, 2.5 μmol/l forward and reverse primers, and 2X iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) was prepared and subjected to PCR amplification. The specific primer pairs targeting the Jagged-1, Notch-1, Notch-2, MAML-1, VEGF, hairy and enhancer of split (Hes)-1, nephrin, and β-actin genes were generated using the MIT Primer3 software (http://fokker.wi.mit.edu/primer3/). The individual PCR primer sequences and cycle number used in this study included the following: 5′-GACTCATCAGCCGTGTCTCA-3′ and 5′-TGGGGAA CACTCACACTCAA-3′ for amplifying Jagged-1, 25–28 cycles; 5′-CAATGTG GATGCCGCAGTTGTG-3′ and 5′-CAGCACCTTGGCGGTCTCGTA-3′ for Notch-1, 25–30 cycles; 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACT GTGTTGGCGTACAG-3′ for Notch-2, 25–30 cycles; 5′-CCCTGATGAGATC GAGTACATCTT-3′ and 5′-AGCAAGGCCCACAGGGATTT-3′ for VEGF, 25–28 cycles; 5′-CAGCATCAGTTGCTTTTGGA-3′ and 5′-CCCTGTGAACTGTC CAACCT-3′ for MAML-1, 25–30 cycles; 5′-CAACACGACACCGGATAAAC-3 and 5′-TTCAGCTGGCTCAGACTTTC-3′ for HES-1, 25–30 cycles; 5′-CACGGTCAG CACAACAGAGG-3′ and 5′- GAAACCTCGGGAATAAGACACCT-3′ for nephrin, 30 cycles; 5′-TCCTTCCTGGGCATGGAGTC-3′ and 5′-TTCTGCATCCTGTCG GCAATG-3′ for β-actin, 25 cycles; and 5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-TTGATTTTGGAGGGATCTCG-3′ for glyceraldehyde-3-phosphate dehydrogenase, 25 cycles. All real-time PCR experiments were performed in duplicate from at least three independent treatments. The relative gene expression was calculated as previously described (25) and as described in the MIQE guidelines (24).
Protein extraction and Western blot analysis.
Membrane, cytosolic, and nuclear extracts from cultured cells were prepared as previously described (26). Antibodies specific for Jagged-1 (Rockland, Gilbertsville, PA), full-length Notch-1 (Santa Cruz, CA), NICD (Abcam, Cambridge, U.K.), MAML-1, HES-1 (Abcam), hypoxia-inducible factor (HIF)-1α (Cell Signaling, Beverly, MA), VEGF (Santa Cruz), nephrin (kindly provided by Prof. Karl Tryggvason, Karolinska Institute, Stockholm, Sweden), poly(ADP-ribose) polymerase (PARP)-1 (Cell Signaling), cleaved and full-length caspase-3 (Cell Signaling), and β-actin (Santa Cruz) were used.
Analysis of γ-secretase activity.
γ-Secretase activity was quantified by fluorescent spectroscopy using a γ-secretase assay kit according to the manufacturer's instructions (R&D). Briefly, the γ-secretase activity was determined using the amyloid precursor protein peptide (GVVIATVIV) conjugated with 5-[(2-aminoethyl)amino] naphthalene-1-sulfonic acid (EDANS) and 4-(4-dimethylaminophenylazo) benzoic acid (DABCYL) reporter molecules as a substrate. The cleavage-dependent release was measured using a fluorescent microplate reader (Anthos Zenyth 3100; Anthos Labtec, Salzburg, Austria) at 495 nm.
RNA interference.
Plasmids expressing human Notch-1 shRNA (5′-CCAC CAGTTTGAATGGTCATT-3′), MAML-1 shRNA (5′-CGAGCAACTCCCTGTT TCT-3′), and a control shRNA were purchased from SuperArray (Frederick, MD). Transfection of podocytes or HEK293 cells (1 × 106 cells/well in a six-well plate) with the indicated plasmids was performed using lipofectamine reagent (Invitrogen).
Plasmid construction and transfection.
The NICD expression plasmid has been described previously (27); it was transfected into HEK293 cells using lipofectamine reagent (Invitrogen), and the transfected cells were cultured in medium with 300 μg/ml G418 (Invitrogen) for screening transient transfectants as previously described (27).
Cell viability and proliferation assay.
For determination of cell viability and proliferation, podocytes under different stimulations were cultured and were evaluated with tetrazolium-based colorimetric assay (XTT assay; Roche, Mannheim, Germany). Colorimetric changes were measured with a microplate reader at a wavelength of 450–500 nm (Molecular Devices, Sunnyvale, CA).
Capillary/tube-like forming assay.
Conditioned medium isolated from podocytes treated with various modulators was collected. Angiogenesis induced by the conditioned medium was determined by coculturing the conditioned medium with human umbilical vein endothelial cells (HUVECs) on a Matrigel-coated (Becton Dickinson, Bedford, MA) 48-well culture plate (1 × 104 cells/well). Formation of capillary/tube-like structures was visualized and counted 2 h after coculture (28).
Streptozotocin-induced diabetes model and DAPT treatment.
Male Wistar rats 4 months of age and weighing between 220 and 250 g (National Experimental Animals Production Center, Tapei, Taiwan) were caged and subjected to streptozotocin (STZ) injection as previously described (29); rats with blood glucose levels >350 mg/dl were defined as diabetic. Diabetic rats were treated with DAPT (n = 6; 5–10 mg/kg) or vehicle (n = 6; 200 μl corn oil) via subcutaneous injection once a week for 35 consecutive days. Then, urine samples were collected from all rats for measurement of total protein and creatinine concentrations using assay kits (Sigma). The animals were killed, and the tissues were collected as described previously (25). All in vivo experimental protocols were approved by the Institutional Animal Care and Use Committee of the Chang Gung Memorial Hospital and carried out according to the National Institutes of Health guidelines.
Immunohistochemistry.
Paraffin-embedded tissues were sliced longitudinally into 5-μm-thick sections and subjected to immunohistochemical staining. Antibodies specific for Jagged-1, NICD, HIF-1, VEGF, nephrin, podocin (Santa Cruz), and CD2AP (Santa Cruz) were used. Six regions within the renal glomeruli from three separate sections were obtained from each rat; images were randomly analyzed (25,29).
Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate-biotin nick-end labeling.
Paraffin-embedded rat tissues were evaluated for apoptosis using an in situ cell death detection kit according to the manufacturer's instructions (Roche, Mannheim, Germany) and as previously described (29).
Statistical analyses.
All values were expressed as means ± SE from at least three independent experiments. Wilcoxon's test was used to evaluate differences between the sample of interest and its respective control. To analyze the time course studies, multiple-range ANOVA and post hoc tests were used. P values <0.05 were considered significant.
RESULTS
HG-induced Notch-1 signaling, VEGF expression, and nephrin downregulation in cultured human podocytes.
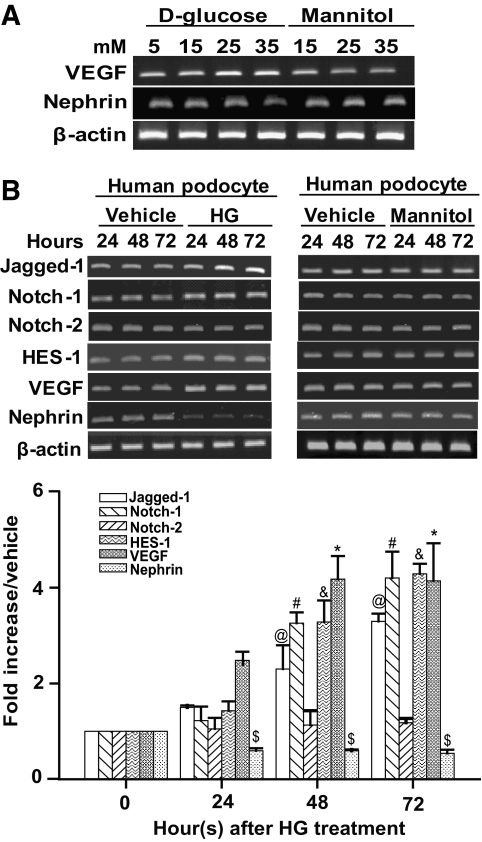
Dysregulation of VEGF and nephrin expression is commonly observed in diabetic nephropathy (7,10). To determine the effect of HG on human podocyte gene expression, cells were cultured with increasing amounts of d-glucose (15, 25, and 35 mmol/l) for 48 h. In normal control cells, 5 mmol/l d-glucose was maintained in the culture medium to provide the basal requirement for cell growth. Additionally, mannitol served as an osmotic control for HG. As illustrated in Fig. 1A, VEGF mRNA gradually increased with HG conditions in human podocytes; downregulation of nephrin mRNA was also observed. Neither VEGF nor nephrin expression was significantly altered in the mannitol-treated cells compared with normal control cells, indicating that HG modulated VEGF and nephrin expression in cultured podocytes.
FIG. 1.
HG exposure increased Jagged-1, Notch-1, HES-1, and VEGF expression, whereas nephrin expression was decreased in human podocytes. A: Human podocytes (1 × 105) were seeded and cultured in medium with increasing amounts of glucose (5, 15, 25, and 35 mmol/l) or mannitol for 48 h. The mRNA expression of VEGF, nephrin, and β-actin in these cultured cells was determined by RT-PCR. B: Human podocytes were cultured in medium with HG (35 mmol/l) or high mannitol (35 mmol/l) at different time points (24, 48, and 72 h). Total RNA was prepared from these cultured cells and subjected to analysis of Jagged-1, Notch-1, Notch-2, HES-1, VEGF, nephrin, and β-actin mRNA expression by RT-PCR. The RT-qPCR was also performed to determine the relative abundance of Jagged-1, Notch-1, Notch-2, HES-1, VEGF, and nephrin mRNAs in HG-treated cells (bottom panel). After normalization to β-actin expression, the fold activation of each gene was indicated. Experimental results are presented as means ± SE calculated from at least three experiments with duplicate samples. @#&$*Significant difference (P < 0.05) compared with the control group.
In addition to VEGF and nephrin, we also examined the effect of HG on the Notch signaling pathway in human podocytes. Cells cultured in medium with or without HG (35 mmol/l) were harvested at different time points (24, 48, and 72 h). mRNA expression of Notch signaling components, including Jagged-1, Notch-1, Notch-2, and HES-1, was determined by RT-PCR (Fig. 1B) or RT-qPCR (Fig. 1B). Because the expression of β-actin or glyceraldehyde-3-phosphate dehydrogenase genes in both untreated and glucose-treated podocytes at 24, 48, and 72 h remained invariant under the experimental conditions (supplementary Fig. 1 in the online appendix, which is available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0663), we subsequently used β-actin as an internal reference gene for expression studies. As shown in Fig. 1B, HG induced the Jagged-1, Notch-1, and HES-1 mRNA expression in a time-dependent manner; however, Notch-2 expression was unaltered. As expected, increased VEGF and decreased nephrin were also detected concomitantly in HG-treated cells. By contrast, no changes in gene expression were observed upon treatment with high mannitol (35 mmol/l) (Fig. 1B). These results raised the question whether signaling induced by HG may be linked to dysregulation of VEGF and nephrin expression in podocytes.
Inhibitors of Notch signaling abolished the effect of HG on VEGF and nephrin expression in human podocytes.
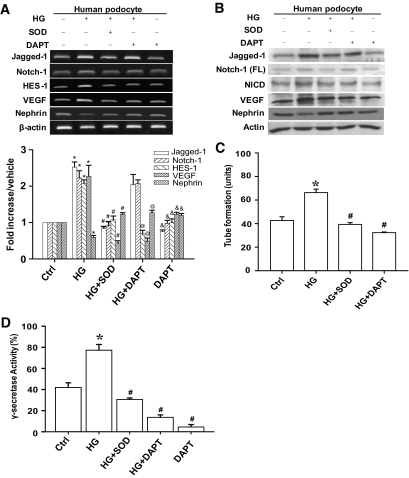
To examine the relationship between Notch-1 signaling and dysregulation of VEGF and nephrin expression in HG-stressed podocytes, a γ-secretase inhibitor, DAPT, was used to inhibit Notch signaling. Human podocytes were pretreated with DAPT for 2 h, and then the continual DAPT treatment proceeded in the presence or absence of HG. Treatment with DAPT markedly abolished the upregulation of HES-1 and VEGF and downregulation of nephrin in HG-treated human podocytes (P < 0.05) (Fig. 2A). Notably, DAPT did not alter upregulation of Jagged-1 or Notch-1 mRNAs in HG-treated podocytes (Fig. 2A). Similarly, Western blot analyses (Fig. 2B) revealed that DAPT significantly inhibited NICD expression alone; Jagged-1 and full-length Notch-1 expression induced by HG remained unchanged. Dysregulation of VEGF and nephrin induced by HG was also alleviated by DAPT. Superoxide dismutase (SOD), an enzyme that can scavenge superoxide, was also analyzed. Under normal culture conditions, treatment of human podocytes with exogenous SOD did not affect basal Notch-1 signaling (supplementary Fig. 2). However, exogenous SOD inhibited HG-induced Jagged-1, Notch-1, and HES-1 expression (Fig. 2A and B). Inhibition of Notch-1 signaling by SOD also relieved the dysregulation of VEGF and nephrin in HG-treated podocytes (Fig. 2A and B).
FIG. 2.
Effect of DAPT and exogenous SOD on Notch signaling in HG-stressed human podocytes. A: Human podocytes were pretreated with SOD or DAPT for 2 h, after which the cells were subjected to SOD or DAPT treatment and cultured in normal or HG medium for 48 h. Gel-based RT-PCR and RT-qPCR were carried out to analyze the mRNA expression of Notch signaling molecules, VEGF, and nephrin in these treated cells. For *control and @&#HG groups, P < 0.05. B: the protein expression of the indicated genes in the cell samples as described in A was determined by Western blot analysis with specific antibodies. Notch-1 (FL), full-length Notch-1 C: Tube-like formation of HUVECs induced by various conditioned medium. Conditioned medium from human podocytes treated with HG or a combination of HG and SOD or DAPT was used to stimulate tube formation in cultured HUVECs. At least six repeated experiments were performed, and the formation of a tube-like structure was calculated. For *control and #HG groups, P < 0.05. D: Repression of γ-secretase activity by SOD and DAPT in HG-stressed cells. For *control and #HG groups, P < 0.05.
To further determine the angiogenic activity of VEGF secretion from podocytes, a tube-like forming assay was performed using HUVECs cocultured with conditioned medium from cultured human podocytes treated with various reagents. Compared with the normal control conditions, larger and more abundant tube-like structures were observed upon coculture of HUVECs with conditioned medium isolated from HG-treated cells. However, reduced tube formation was observed using conditioned medium from the cells pretreated with SOD or DAPT and cultured in an HG environment compared with that from cells treated with HG alone (Fig. 2C). γ-Secretase activity was also examined in response to various stimuli. A significant increase (P < 0.05) in γ-secretase activity was detected in HG-stressed cells compared with cells cultured in normal conditions. Enhanced γ-secretase activity was inhibited by SOD and DAPT (Fig. 2D).
Effects of HG on Notch-1 signaling in HEK293 cells and human podocytes.
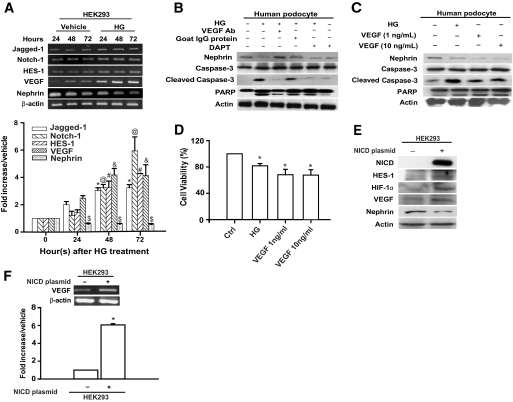
In addition to human podocytes, we also determined whether HG affected Notch-1 signaling and the expression of VEGF and nephrin in the human embryonic kidney cell line, HEK293. HEK293 cells are known to express nephrin (30,31), and the cell type-specific expression of nephrin protein in HEK293 cells was also confirmed by Western blot analysis (supplementary Fig. 3). Increased Jagged-1, Notch-1, HES-1, and VEGF expression and nephrin repression were detected in HG-treated HEK293 cells by RT-qPCR (Fig. 3A), indicating that similar regulatory signaling may exist in both podocytes and HEK293 cells in response to HG. Because of the pleiotropy of VEGF (7,10), we examined the causative relationship between VEGF and nephrin expression or cell apoptosis in both human podocytes and HEK293 cells cultured in HG conditions. In HG-stressed podocytes, nephrin repression and apoptotic cellular markers, cleaved caspase-3 and PARP-1, were consistently detected (Fig. 3B). Addition of a VEGF monoclonal antibody (10 ng/ml), but not a control antibody, efficiently blocked both nephrin repression and apoptosis induced by HG (Fig. 3B). Similar attenuation of nephrin repression and apoptosis by anti-VEGF antibodies was also observed in HG-stressed HEK293 cells (supplementary Fig. 4).
FIG. 3.
HG-induced Notch-1 signaling, nephrin repression, and apoptosis in HEK293 cells and human podocytes. A: HEK293 cells (1 × 106 cells/100-mm dish) were cultured in HG medium and harvested at 24, 48, and 72 h. The expression of Jagged-1, Notch-1, HES-1, VEGF, and nephrin in the HG-treated cells was measured by gel-based RT-PCR and RT-qPCR. The symbols @#$&*Significant difference (P < 0.05) in comparison with the control group. B: Neutralization of VEGF restored nephrin levels and prevented the apoptotic cascade in HG-treated podocytes. A VEGF neutralization antibody (Ab) (10 ng/ml) was incubated with HG medium for 48 h, and extracts of the treated podocytes were analyzed for the expression of nephrin, PARP-1, and caspase-3. C: Human podocytes (2 × 105 cells/60-mm dish) were treated with either HG or the exogenous VEGF protein (1 or 10 ng/ml, respectively) for 48 h. The cell lysates were subjected to Western blot analysis. D: the viability of cells treated with HG or VEGF was evaluated by MTT assay. E: HEK293 cells were transiently transfected with the NICD-expressing plasmid, and the cell extracts were analyzed for the indicated proteins. F: Relative abundance of VEGF mRNA in HEK293 and NICD-transfected HEK293 cells. Gel-based RT-PCR and RT-qPCR were used to determine the relative level of the VEGF mRNA. *P < 0.05 for HEK293 cells.
To further determine the effect of VEGF on nephrin expression and apoptosis, podocytes were treated with exogenous VEGF recombinant protein (1 and 10 ng/ml). As illustrated in Fig. 3C and D as well as in supplementary Fig. 5A, exogenous VEGF protein increased cleaved caspase-3 and PARP-1 levels but reduced nephrin expression and cell viability. However, reduced expression of nephrin was not due to reduced cell viability, as demonstrated in supplementary Fig. 5B. These results indicated that downregulation of nephrin and induction of the apoptotic cascade in HG conditions were mediated through Notch-1/VEGF signaling.
To further delineate the role of the Notch-1 signaling on dysregulation of VEGF and nephrin in HEK293, a NICD expression plasmid was transfected into HEK293 cells. NICD overexpression significantly induced HES-1, HIF-1α, and VEGF protein expression while reducing nephrin expression in HEK293 cells (Fig. 3E and F). Taken together, these results suggest that Notch-1 signaling stimulated by HG induces VEGF expression, which subsequently mediates nephrin repression and the apoptotic cascade in human podocytes and HEK293 cells.
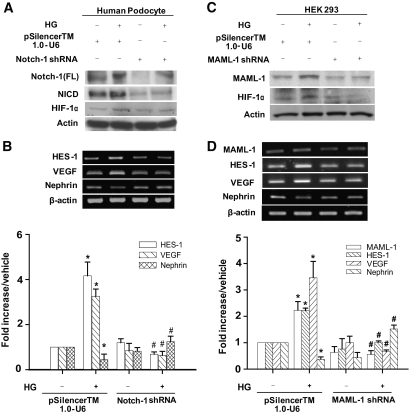
Knockdown of Notch-1 signaling molecules in human podocytes and HEK293 cells.
Cells expressing Notch-1 shRNA were created, and the expression of VEGF and nephrin were evaluated in these cells. To efficiently knock down the Notch-1 pathway, we generated a pool of transfected podocytes that expressed Notch-1 or control shRNAs. Endogenous full-length Notch-1, NICD, and HIF-1α were substantially reduced in cells expressing Notch-1 shRNA but not in those expressing control shRNA (Fig. 4A). Knockdown of Notch-1 in cells maintained in HG conditions resulted in reduction of HES-1 and VEGF expression and restoration of nephrin expression (Fig. 4B). Intriguingly, enhanced levels of MAML-1, a key coactivator of Notch signaling, were repeatedly detected in HG-treated cells (Fig. 4C and D and data not shown). Transfection of MAML-1 shRNA in HEK293 cells not only attenuated HG-induced MAML-1 but also abrogated HG-induced activation of HIF-1α, HES-1, and VEGF expression. Nephrin downregulation induced by HG was also restored in MAML-1 knockdown cells (Fig. 4C and D).
FIG. 4.
Genetic knockdown of Notch signaling components in human podocytes and HEK293 cells. A: Knockdown of Notch-1 in human podocytes was carried out using shRNA. Expression of the full-length Notch-1, NICD, and HIF-1α in response to different stimuli was determined by Western blot analysis in human podocytes. B: The relative expression of HES-1, VEGF, and nephrin mRNAs was assayed by RT-PCR. P < 0.05 for control shRNA in *normal and #HG medium. C: Knockdown of MAML-1 by shRNA in HEK293 cells. Protein expression of the MAML-1 and HIF-1α proteins in HEK293 cells in response to different treatments was examined by Western blot analysis. D: The relative mRNA expression of MAML-1, HES-1, VEGF, and nephrin was analyzed in the MAML-1 knockdown cells under normal or HG conditions. P < 0.05 for control shRNA in *normal and #HG medium.
Blockade of the Notch signaling pathway attenuated proteinuria in diabetic rats.
The effects of inhibition of Notch signaling by DAPT on diabetic nephropathy were also assessed. Compared with the normal group, diabetic rats had increased blood glucose and urinary protein excretion (Table 1). DAPT treatment significantly reduced urinary protein secretion in diabetic rats; blood glucose and A1C levels remained unchanged (Table 1). No differences in overall body weight, organ/body weight ratios as well as BUN, creatinine, AST, ALT, and CPK levels were observed between the untreated and DAPT-treated diabetic rats (Supplementary Table 1).
TABLE 1.
Biochemical properties and immunohistologic analyses in renal glomeruli of normal and diabetic rats and in diabetic rats subjected to DAPT treatment
| Normal rats | Diabetic rats |
|||
|---|---|---|---|---|
| Vehicle | 5 mg/kg DAPT | 10 mg/kg DAPT | ||
| Biochemical properties | ||||
| Blood glucose | 82 ± 1.6 | 407.2 ± 10.6* | 400.7 ± 7.1* | 397.4 ± 10* |
| A1C | 4.2 ± 0.1 | 9.6 ± 0.8* | 10.3 ± 0.3* | 9.5 ± 0.5* |
| Urinary protein secretion | 0.57 ± 0.13 | 4.73 ± 2.63* | 1.07 ± 0.35† | 1.08 ± 0.2† |
| Immunohistomorphormetry | ||||
| Jagged-1 | 10.4 ± 0.4 | 26.3 ± 0.1* | 22.9 ± 0.9* | 22.4 ± 0.9* |
| NICD | 4.9 ± 0.2 | 17.5 ± 0.7* | 7.4 ± 0.3*† | 3.7 ± 0.2†‡ |
| HIF-1 | 3.2 ± 0.1 | 32.17 ± 1.3* | 9.8 ± 0.4*† | 9.4 ± 0.4*† |
| VEGF | 13.8 ± 0.7 | 30.9 ± 1.3* | 23.5 ± 1.1*† | 17.3 ± 1*†‡ |
| Nephrin | 20.6 ± 1.2 | 8.2 ± 0.8* | 13.7 ± 0.8*† | 18.1 ± 0.6†‡ |
| CD2AP | 12.1 ± 0.5 | 16.5 ± 0.7 | 15.9 ± 0.7 | 14.6 ± 0.6 |
| Podocin | 16.1 ± 0.7 | 16.9 ± 0.7 | 18.2 ± 0.7 | 19.8 ± 0.8 |
| TUNEL apoptosis | 12.3 ± 1.0 | 35.2 ± 1.8* | 25.9 ± 1.2*† | 16.8 ± 0.9†‡ |
For the biochemical properties, the data represent means ± SE calculated from six rats. Glucose (milligrams per deciliter) and A1C (milligrams per deciliter) were measured from tail vein blood. Total urinary protein secretion (milligrams per milligram creatinine) was assayed using urinary protein kits and normalized to total creatinine concentrations in urine. For the immunohistologic analyses, the data are means ± SE calculated from the percentage of positively stained cells and total cells in each image. Six random images from three sections obtained from each rat were randomly selected and analyzed. P < 0.05 for
*normal mice,
†diabetic/vehicle mice, and
‡diabetic mice treated with DAPT (5 milligrams per kilogram).
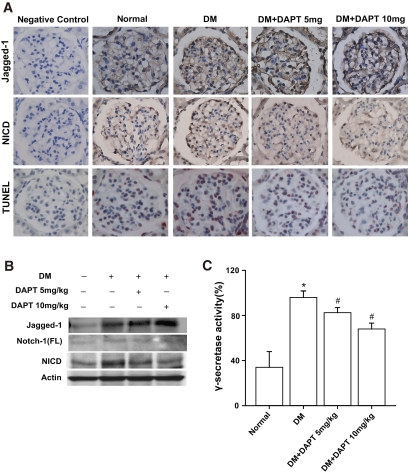
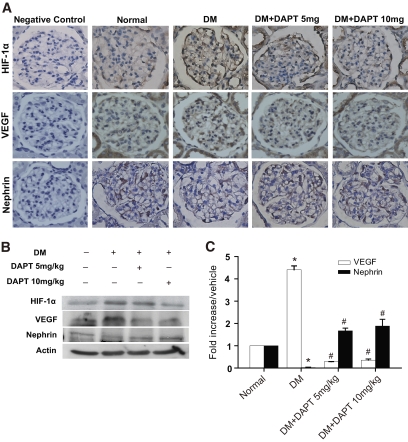
Involvement of Notch signaling in diabetic rats was examined by immunostaining and immunoblot analyses. Increased Jagged-1, NICD, HIF-1α, and VEGF expression were observed in podocytes of glomeruli in the diabetic group compared with the normal group (Figs. 5A and 6A and Table 1). In contrast, fewer nephrin-positive cells were observed in diabetic rats compared with normal rats (Fig. 6A; Table 1). In addition, elevated Jagged-1, full-length Notch-1, NICD, HIF-1α, and VEGF expression, as well as reduced nephrin, was detected in tissue lysates from the diabetic kidneys upon Western blot (Figs. 5B and 6B) and RT-qPCR (supplementary Fig. 6A) analyses. No significant differences in podocin or CD2AP expression levels were observed between the diabetic and normal rats (Table 1 and supplementary Fig. 6B). Using terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) analysis, elevated podocyte and mesangial cell apoptosis was detected in diabetic rats compared with normal rats (Fig. 5A and Table 1).
FIG. 5.
DAPT blocked the overactive Notch-1 signaling and apoptosis in renal tissue of diabetic rats. A: Immunostaining of the glomeruli of normal and diabetic kidneys. Podocytes within the glomeruli of diabetic kidneys expressed intensive Jagged-1 and NICD staining compared with normal kidneys. More apoptotic cells were also detected in diabetic kidneys by TUNEL assay. DAPT treatment attenuated NICD and TUNEL staining but not Jagged-1 staining in diabetic rats. Specimens were observed under 400× magnification. B: renal lysates from the same treatment as described in A were subjected to Western blot analysis. C: the corresponding γ-secretase activity in A was assayed. P < 0.05 for *normal and #diabetic (DM)/vehicle groups. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 6.
Representative photographs of HIF-1α, VEGF, and nephrin immunostaining in the glomeruli of normal and diabetic kidneys and diabetic kidneys with DAPT treatment. A: Intensive HIF-1α and VEGF expression and weak nephrin expression were observed in diabetic glomerular tissue compared with normal tissue. DAPT treatment attenuated HIF-1α and VEGF expression while restoring nephrin immunoreactivity in cells within the diabetic glomeruli—especially the podocytes. Immunostained cells are brown in color. Specimens were observed at 400× magnification. B: DAPT treatment abolished the increased HIF-1α and VEGF expression detected in the diabetic kidney while restoring nephrin expression as determine by Western blot analysis. C: Detection of VEGF and nephrin mRNA expression by RT-qPCR in renal tissue. The experimental results represent the relative abundance of the various genes normalized to the reference gene, β-actin. P < 0.05 for *normal and #diabetic (DM)/vehicle groups. (A high-quality digital representation of this figure is available in the online issue.)
Inhibition of Notch-1 signaling in diabetic rats by DAPT resulted in amelioration of enhanced NICD, HIF-1α, and VEGF (Figs. 5 and 6). DAPT treatment also restored nephrin downregulation in diabetic rats to the normal level observed in control rats (Fig. 6). Furthermore, DAPT treatment resulted in suppression of apoptosis in diabetic rats (Fig. 5A and Table 1). Although expression of several Notch-1 signaling molecules was blocked by DAPT, enhanced Jagged-1 in diabetic rats was not affected by DAPT treatment. The ability of DAPT to inhibit γ-secretase activity was confirmed by examining γ-secretase activity in DAPT-treated rats (Fig. 5C). As expected, DAPT treatment significantly repressed γ-secretase activity in diabetic rats. As shown above, SOD inhibited Notch-1 signaling and VEGF expression in HG-treated human podocytes (Fig. 2). The effects of SOD on Notch-1 signaling and expression of VEGF and nephrin in diabetic kidney were analyzed. Exogenous SOD significantly suppressed Jagged-1, Notch-1, HES-1, and VEGF expression and restored nephrin expression in the kidney of diabetic rats (supplementary Fig. 7A). γ-Secretase activity in diabetic rats was also inhibited by exogenous SOD (supplementary Fig. 7B). These results indicate the involvement of oxidative stress, at least in part, in regulating the Notch signaling pathway in diabetic rats.
DISCUSSION
Podocyte dysfunction is closely associated with marked proteinuria/albuminuria in diabetic nephropathy (12). In this study, we show that the Notch-1 signaling is activated in HG-treated human podocytes and HEK293 cells and within the glomeruli of diabetic rats (Figs. 1, 2, 3, and 5). Activation of Notch-1 signaling coincides with increased VEGF expression, nephrin downregulation, and increased apoptosis (Figs. 1, 3, 5, and 6). Importantly, overexpression of NICD in HEK293 cells and treatment of podocytes with recombinant VEGF are sufficient to induce nephrin repression and apoptosis (Fig. 3 and supplementary Fig. 4). Furthermore, repression of Notch-1 signaling by pharmacologic reagents, including DAPT and SOD, and through genetic knockdown of Notch-1 signaling components alleviated VEGF and nephrin dysregulation in HG-stressed cells and ameliorated urinary protein excretion in diabetic animal model (Figs. 2, 4, 5 and 6). These findings underscore the importance of the Notch/VEGF signaling pathway in diabetic proteinuria (Fig. 7).
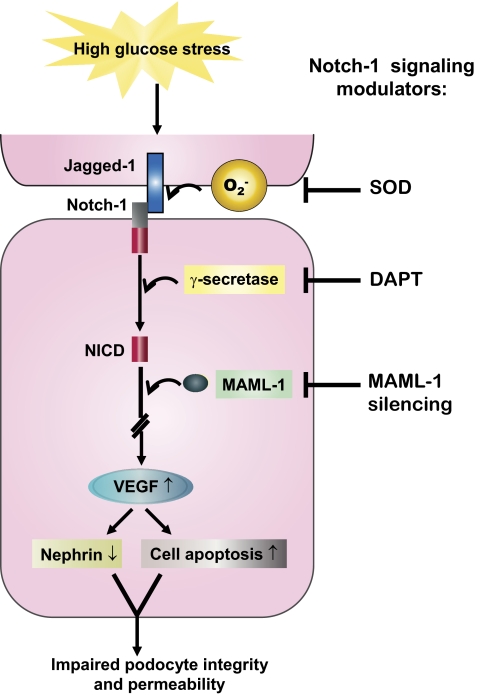
FIG. 7.
Involvement of superoxide, Notch-1 signaling, and VEGF in podocyte injury under HG stress. Human podocytes exposed to HG positively regulated several Notch-1 signaling components, including Jagged-1, Notch-1, γ-secretase, and MAML-1. Activation of Notch-1 signaling may contribute to the enhancement of VEGF expression, which in turn mediates downregulation of nephrin and promotes cell apoptosis cascade. Blockers that inhibit the Notch-1 signaling in podocytes markedly abolish the deleterious effect caused by HG. (A high-quality digital representation of this figure is available in the online issue).
Several studies have suggested that overactive Notch signaling in mature podocytes may result in loss of podocytes, glomerular failure, and the development of glomerulosclerosis and focal segmental glomerulosclerosis (21,32,33). Consistent with the importance of Notch signaling in the development of glomerular disease, Notch-1 signaling was detected in diabetic animal model (Figs. 5 and 6) along with marked proteinuria and reduction of podocyte SD. Administration of the γ-secretase inhibitor, DAPT, alleviated proteinuria found within the glomeruli (Table 1), suggesting that Notch-1 signaling may directly contribute to the pathogenesis of proteinuria. The strong correlation between overactive Notch-1 signaling and proteinuria indicates that components of the Notch signaling cascade could possibly be genetic biomarkers for prediction of the progression of diabetic nephropathy.
Elucidation of the molecular mechanism by which Notch-1 signaling is modulated in diabetic rats remains to be addressed. In cultured podocytes or HEK293 cells, an HG environment induced Notch-1 signaling through many distinct manners, including Jagged-1 (a ligand of Notch-1), Notch-1, and MAML-1 (a coactivator of Notch signaling) expression as well as enhancement of γ-secretase activity (Figs. 1, 2, and 4). Interestingly, exogenous SOD dramatically blocked Notch-1 signaling in HG-treated cells (Fig. 2). These results imply that superoxide overproduction under HG stress may act as a trigger in stimulating the Notch-1 pathway.
At the early stages of diabetic nephropathy, the expression of VEGF and VEGF receptor was upregulated within the glomerulus—specifically within podocytes (34). Several reports have shown that inhibition of VEGF in experimental models of diabetic nephropathy restored the slit pore density of podocytes and alleviated albuminuria (5,7,35). Based on our results, we observed elevated VEGF expression as well as reduced nephrin expression and podocyte number in diabetic rats compared with normal rats (Figs. 5 and 6 and Table 1), further supporting the relationship between VEGF signaling and proteinuria. Neutralization of VEGF with monoclonal antibodies reversed the repression of nephrin and attenuated the cleavage of PARP-1 and caspase-3 in HG-treated podocytes and HEK293 cells (Fig. 3 and supplementary Fig. 4). Although VEGF is generally regarded as a prosurvival factor (36), our results revealed that recombinant VEGF stimulates the apoptotic cascade in human podocytes (Fig. 3B–D). A growing number of studies have suggested that VEGF promotes cell death mediated by or in concert with other apoptotic signaling factors (37–39).
In addition to nephrin, other SD proteins that maintain the normal function of the glomerular filtration barrier may be controlled by Notch-1 and VEGF signaling in diabetic rats. However, no significant changes in CD2AP or podocin expression were observed in diabetic rats compared with normal controls (supplementary Fig. 6B and Table 1). Given these results, it can be concluded that overactive Notch/VEGF signaling specifically modulates the expression of nephrin.
Previous studies have shown that an HG environment activated oxidative stress through Ras/Rac/extracellular signal–regulated kinase–dependent pathways in renal mesangial cells and ameliorated the deleterious effects of proteinuria in diabetic nephropathy by SOD (29,40). Crosstalk between reactive oxygen radicals and Wnt/ß-catenin signaling in modulating the apoptosis of HG-stressed mesangial cells was also observed (40). In the present study, superoxide may also serve as a Notch1 signaling modulator, thereby regulating VEGF-mediated nephrin expression in diabetic nephropathy (Fig. 2 and supplementary Fig. 7). Therefore, at least two pathways, including Wnt/ß-catenin and Notch-1, that are controlled by redox reactions contribute to diabetic nephropathy.
Taken together, we provide evidence that HG augments Notch/VEGF signaling components. Modulation of the Notch-1 signaling pathway may provide a therapeutic strategy for controlling the deleterious effects of diabetic nephropathy.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Science Council of Taiwan (NMRPG180661 to C.-L.L.) and Chang Gung Memorial Hospital at Chiayi, Chiayi, Taiwan (CMRPG680351 to C.-L.L.).
We thank the Prevision Medical Corporation of Taiwan for technical assistance. No other potential conflicts of interest relevant to this article were reported.
C.-L.L. researched data and wrote, reviewed, and edited the manuscript. F.-S.W. researched data and contributed to discussion. Y.-C.H. researched data and contributed to discussion. C.-N.C. contributed to discussion. M.-J.T. researched data and contributed to discussion. M.S. researched data and contributed to discussion. P.-J.C. researched data and wrote, reviewed, and edited the manuscript. J.-Y.W. contributed to discussion.
We thank Professor Karl Tryggvason of the Karolinska Institute, Stockholm, Sweden, for kindly providing the nephrin monoclonal antibody.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1865.
REFERENCES
- 1.Schieppati A, Remuzzi G: Chronic renal disease as a public health problem: epidemiology, social, and economic implications. Kidney Int 2005;98(Suppl.):S7–S10 [DOI] [PubMed] [Google Scholar]
- 2.Jefferson JA, Shankland SJ, Pichler RH: Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int 2008;74:22–36 [DOI] [PubMed] [Google Scholar]
- 3.Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW: Podocyte biology in diabetic nephropathy. Kidney Int 2007;106(Suppl.):S36–S42 [DOI] [PubMed] [Google Scholar]
- 4.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephritic syndrome. Mol Cell 1998;1:575–582 [DOI] [PubMed] [Google Scholar]
- 5.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 2006;291:F422–F428 [DOI] [PubMed] [Google Scholar]
- 6.Bennett MR, Czech KA, Arend LJ, Witte DP, Devarajan P, Potter SS: Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol 2007;107:e30–e40 [DOI] [PubMed] [Google Scholar]
- 7.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S: Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol 2006;17:3093–3104 [DOI] [PubMed] [Google Scholar]
- 8.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH: Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol 2001;12:993–1000 [DOI] [PubMed] [Google Scholar]
- 9.Ku CH, White KE, Dei Cas A, Hayward A, Webster Z, Bilous R, Marshall S, Viberti G, Gnudi L: Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes 2008;57:2824–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu E, Morimoto M, Kitajima S, Koike T, Yu Y, Shiiki H, Nagata M, Watanabe T, Fan J: Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol 200;18:2094–2104 [DOI] [PubMed] [Google Scholar]
- 11.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE: Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 2006;17:724–735 [DOI] [PubMed] [Google Scholar]
- 12.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F: Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 2008;14:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward P, Kalmar T, Arias AM: Wnt/Notch signaling and information processing during development. Development 2008;135:411–424 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Katzman RB, Delmolino LM, Bhat I, Zhang Y, Gurumurthy CB, Germaniuk-Kurowska A, Reddi HV, Solomon A, Zeng MS, Kung A, Ma H, Gao Q, Dimri G, Stanculescu A, Miele L, Wu L, Griffin JD, Wazer DE, Band H, Band V: The notch regulator MAML1 interacts with p53 and functions as a coactivator. J Biol Chem 2007;282:11969–11981 [DOI] [PubMed] [Google Scholar]
- 15.Del Bianco C, Aster JC, Blacklow SC: Mutational and energetic studies of Notch 1 transcription complexes. J Mol Biol 2008;376:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZJ, Xiao M, Balint K, Soma A, Pinnix CC, Capobianco AJ, Velazquez OC, Herlyn M: Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J 2006;20:1009–1011 [DOI] [PubMed] [Google Scholar]
- 17.McCright B: Notch signaling in kidney development. Curr Opin Nephrol Hypertens 2003;12:5–10 [DOI] [PubMed] [Google Scholar]
- 18.Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, Kopan R: Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 2007;134:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng HT, Kopan R: The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int 2005;68:1951–1952 [DOI] [PubMed] [Google Scholar]
- 20.Cheng HT, Miner JH, Lin M, Tansey MG, Roth K, Kopan R: γ-Secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 2003;130:5031–5042 [DOI] [PubMed] [Google Scholar]
- 21.Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K: The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 2008;14:290–298 [DOI] [PubMed] [Google Scholar]
- 22.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002;13:630–638 [DOI] [PubMed] [Google Scholar]
- 23.Coward RJ, Foster RR, Patton D, Ni L, Lennon R, Bates DO, Harper SJ, Mathieson PW, Saleem MA: Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, Podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol 2005;16:629–637 [DOI] [PubMed] [Google Scholar]
- 24.Bustin SA, Benes V, Garson JA, Hellemans J, Huggette J, Kubista M, Muller R, Nolan T, Pfaffl MW, Shipley GL, Vandersompele J, Wittwer CT: The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–622 [DOI] [PubMed] [Google Scholar]
- 25.Lin CL, Wang FS, Kuo YR, Huang YT, Huang HC, Sun YC: Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int 2006;69:1593–1600 [DOI] [PubMed] [Google Scholar]
- 26.Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD: Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem 2004;279:10331–10337 [DOI] [PubMed] [Google Scholar]
- 27.Yeh TS, Lin YM, Hsieh RH, Tseng MJ: Association of transcription factor YY1 with the high molecular weight Notch complex suppresses the transactivation activity of Notch. J Biol Chem 2003;278:41963–41969 [DOI] [PubMed] [Google Scholar]
- 28.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C: Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res 2003;93:664–673 [DOI] [PubMed] [Google Scholar]
- 29.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS: Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 2006;17:2812–2820 [DOI] [PubMed] [Google Scholar]
- 30.Wang SX, Menè P, Holthöfer H: Nephrin mRNA regulation by protein kinsase C. J nephrol 2001;14:98–103 [PubMed] [Google Scholar]
- 31.Huwiler A, Ren S, Holthöfer H, Pavenstädt, Pfeilschifter J: Inflammatory cytokines upregulate nephrin expression in human embryonic kidney epithelial cells and podocytes. Biochem Biophys Res Commun 2003;305:136–142 [DOI] [PubMed] [Google Scholar]
- 32.Ziyadeh FN, Wolf G: Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 2008;4:39–45 [DOI] [PubMed] [Google Scholar]
- 33.Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD: Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol 2008;19:1045–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME: Temporal renal expression of angiogenic growth factors and their receptors in experimental diabeties: role of the rennin-angiotensin system. J Hypertens 2005;23:153–164 [DOI] [PubMed] [Google Scholar]
- 35.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 2007;56:1127–1135 [DOI] [PubMed] [Google Scholar]
- 36.Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ: Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol 2005;288:F48–F57 [DOI] [PubMed] [Google Scholar]
- 37.Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P: VEGF, a prosurvival factor, acts in concert with TGF-β1 to induce endothelial cell apoptosis. Proc Natl Acad Sci U S A 2006;103:17260–17265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petreaca ML, Yao M, Ware C, Martins-Green M: Vascular endothelial growth factor promotes macrophage apoptosis stimulation of tumor necrosis factor superfamily member 13 (TNFSF14/LIGHT). Wound Rep Reg 2008;16:602–614 [DOI] [PubMed] [Google Scholar]
- 39.Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G: Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 2005;68:276–284 [DOI] [PubMed] [Google Scholar]
- 40.Lin CL, Wang JY, Ko JY, Surendran K, Huang YT, Kuo YH, Wang FS: Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology 2008;149:2934–2342 [DOI] [PubMed] [Google Scholar]