Abstract
OBJECTIVE
To investigate the associations of plasma levels of soluble receptor for advanced glycation end products (sRAGE) with incident cardiovascular disease (CVD) and all-cause mortality in type 1 diabetes and the extent to which any such associations could be explained by endothelial and renal dysfunction, low-grade inflammation, arterial stiffness, and advanced glycation end products (AGEs).
RESEARCH DESIGN AND METHODS
We prospectively followed 169 individuals with diabetic nephropathy and 170 individuals with persistent normoalbuminuria who were free of CVD at study entry and in whom levels of sRAGE and other biomarkers were measured at baseline. The median follow-up duration was 12.3 years (7.6–12.5).
RESULTS
The incidence of fatal and nonfatal CVD and all-cause mortality increased with higher baseline levels of log-transformed sRAGE (Ln-sRAGE) independently of other CVD risk factors: hazard ratio (HR) 1.90 (95% CI 1.13–3.21) and 2.12 (1.26–3.57) per 1-unit increase in Ln-sRAGE, respectively. Adjustments for estimated glomerular filtration rate (eGFRMDRD), but not or to a smaller extent for markers of endothelial dysfunction, low-grade inflammation, arterial stiffness, and AGEs, attenuated these associations to HR 1.59 (95% CI 0.91–2.77) for fatal and nonfatal CVD events and to 1.90 (1.09–3.31) for all-cause mortality. In addition, in patients with nephropathy, the rate of decline of GFR was 1.38 ml/min/1.73 m2 per year greater per 1-unit increase of Ln-sRAGE at baseline (P = 0.036).
CONCLUSIONS
Higher levels of sRAGE are associated with incident fatal and nonfatal CVD and all-cause mortality in individuals with type 1 diabetes. sRAGE-associated renal dysfunction may partially explain this association.
Recent studies have suggested a potential role of the receptor for advanced glycation end products (RAGE) in the development of vascular disease in individuals with diabetes (1). At the molecular level, RAGE is upregulated in atherosclerotic lesions in diabetes (2). RAGE-induced production of adhesion molecules (3–5) and inflammatory cytokines (4) could contribute to endothelial (4,5) and renal dysfunction (6), low-grade inflammation (4,5), and arterial stiffening, all of which may partially explain the increased cardiovascular disease (CVD) in diabetes.
We have recently shown, in a large cross-sectional study of type 1 diabetes (EURODIAB), that plasma levels of sRAGE were positively associated with macro- and microvascular complications, and also with endothelial and renal dysfunction, and low-grade inflammation as pathophysiological mechanisms that explained in part the associations of sRAGE with vascular complications (7). Whether sRAGE is associated with incident fatal and nonfatal CVD as well as all-cause mortality in individuals with type 1 diabetes has never been investigated. In addition, the extent to which any such associations could be explained by markers of endothelial and renal dysfunction, low-grade inflammation, arterial stiffness, and AGEs is also not known. We hereby address these questions in a 12-year prospective follow-up study.
RESEARCH DESIGN AND METHODS
Study population and design.
In 1993, 199 of all 242 albuminuric patients >18 years of age attending the outpatient clinic at the Steno Diabetes Center agreed to participate and were enrolled in a prospective observational study. Diabetic nephropathy was diagnosed according to the following criteria: persistent macroalbuminuria (>300 mg/24 h) in at least two of three previous consecutive 24-h urine collections, presence of retinopathy, and absence of other kidney or urinary tract disease. In addition, 192 patients with persistent normoalbuminuria (i.e., urinary albumin excretion [UAE] rate <30 mg/24 h) and matched for age, sex, and duration of diabetes were also enrolled as control subjects (8). The present study refers to 339 of the original 391 patients included in the cohort; details on inclusion/exclusion criteria and study main outcomes are depicted in a flow chart (see the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1509/DC1). The study was approved by the local ethics committee, in accordance with the Helsinki Declaration, and all patients gave their informed written consent.
Baseline investigations.
Plasma levels of sRAGE were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Quantikine; R&D systems, Minneapolis, MN) according to the manufacturer's protocol. Briefly, a monoclonal antibody generated against the NH2-terminal extracellular domain of human RAGE was used to capture sRAGE from plasma. Captured sRAGE was detected with a polyclonal anti-human sRAGE antibody. After washing, plates were incubated with streptavidin–horseradish peroxidase, developed with appropriate substrate, and OD450 was determined using an ELISA plate reader. Measurements were performed in duplicate and the intra- and interassay coefficient of variation (CV) values were 2 and 17.5%, respectively.
Measurements of other biomarkers and risk factors have been described in detail elsewhere (8,9) and in the online appendix.
Follow-up and study end points.
All patients were followed up to the last visit at the Steno Diabetes Center, until 1 September 2006 or until death (n = 82) or emigration (n = 3). All patients were traced through the national register during autumn 2006. If a patient had died before 1 September 2006, the date of death was recorded and the primary cause of death was obtained from the death certificate, which was reviewed by two independent observers. Additional available information from necropsy reports was also included. All deaths were classified as cardiovascular unless an unequivocal noncardiovascular cause was established. In all patients alive at the end of follow-up, nonfatal CVD data were retrieved from their patient files at Steno Diabetes Center or other hospital records. The primary end point was a combination of fatal and nonfatal CVD (i.e., myocardial infarction, percutaneous coronary intervention, coronary bypass grafting, amputation due to ischemia, vascular surgery for peripheral atherosclerotic disease, and stroke), and the secondary end point was all-cause mortality.
Statistical analysis.
All analyses were performed with SPSS version 15.0 for Windows (SPSS, Chicago, IL).
Variables with a skewed distribution were loge-transformed before further analyses. Comparisons of baseline characteristics between groups were performed with Student's t or χ2 tests, as appropriate. The associations between Ln-sRAGE and study end points were investigated with Cox proportional hazards regression models adjusted first for sex, age, duration of diabetes, case-control status, and A1C; second for other traditional cardiovascular risk factors; and third for the use of renin-angiotensin-aldosterone system inhibitors and/or other antihypertensive treatment or whether subjects did or did not withhold their medication before baseline examinations. Further adjustments for markers of renal dysfunction (i.e., estimated glomerular filtration rate [eGFRMDRD] or Ln-UAE rate), low-grade inflammation (average of the z scores of Ln–interleukin-6, Ln–C-reactive protein, soluble intracellular adhesion molecule-1 [sICAM-1], and Ln-secreted phospholipase-A2), endothelial dysfunction (average of the z scores of soluble vascular cell adhesion molecule-1 and sICAM-1), and arterial stiffness (i.e., pulse pressure) were added into this model to ascertain the extent these could explain (i.e., attenuate the strength of) the association between Ln-sRAGE and study end points. The cross-sectional associations between Ln-sRAGE and markers of these pathophysiological mechanisms and AGEs [average of the z scores of Ln-pentosidine, Nε-(carboxymethyl)lysine, and Nε-(carboxyethyl)lysine] were examined with the use of linear regression analyses.
Finally, we investigated whether the associations listed above differed between patients with normoalbuminuria versus nephropathy by adding interaction terms between Ln-sRAGE and case-control status to our models; whenever the P value of such interactions were <0.1, results were presented for the two groups separately.
RESULTS
During the course of follow-up (median 12.3 years [interquartile range 7.6–12.5]), 82 individuals (24.2%) died; 85 (25.1%) suffered a fatal (n = 48) and/or nonfatal (n = 53) CVD event. Individuals with incident CVD events or who had died at follow-up had at baseline higher levels of Ln-sRAGE and a more adverse atherosclerotic risk (Table 1).
TABLE 1.
Baseline characteristics according to the occurrence of cardiovascular events and death during follow-up
| Fatal or nonfatal CVD event (n = 85) | No CVD event (n = 254) | P | Patients dead at follow-up (n = 82) | Patients alive at follow-up (n = 257) | P | |
|---|---|---|---|---|---|---|
| Sex (% M/F) | 61/39 | 60/40 | 0.901 | 68/32 | 58/42 | 0.156 |
| Age (years) | 44.7 ± 9.0 | 40.3 ± 9.6 | <0.001 | 45.5 ± 9.9 | 40.1 ± 9.2 | <0.001 |
| Duration of diabetes (years) | 30.7 ± 8.8 | 26.8 ± 7.5 | <0.001 | 30.3 ± 10.0 | 27.0 ± 7.1 | 0.006 |
| Nephropathy (%) | 75 | 41 | <0.001 | 78 | 41 | <0.001 |
| Retinopathy: no/simplex/proliferative (%) | 8/37/55 | 21/45/34 | <0.001 | 6/37/57 | 21/45/34 | <0.001 |
| A1C (%) | 9.5 ± 1.5 | 8.9 ± 1.4 | <0.001 | 9.6 ± 1.5 | 8.8 ± 1.4 | <0.001 |
| Total cholesterol (mmol/l) | 5.70 ± 1.10 | 4.99 ± 1.12 | <0.001 | 5.80 ± 1.13 | 4.97 ± 1.10 | <0.001 |
| HDL (mmol/l) | 1.43 ± 0.42 | 1.55 ± 0.55 | 0.033 | 1.52 ± 0.48 | 1.52 ± 0.54 | 0.961 |
| Triglycerides (mmol/l) | 1.24 (0.93–1.70) | 0.81 (0.63–1.19) | <0.001 | 1.28 (0.90–1.66) | 0.81 (0.64–1.16) | <0.001 |
| Creatinine (μmol/l) | 104 (77–150) | 80 (71–92) | <0.001 | 105 (78–147) | 79 (72–92) | <0.001 |
| Estimated GFRMDRD (ml/min/1.73 m2) | 65.5 ± 29.1 | 86.8 ± 21.1 | <0.001 | 65.5 ± 27.8 | 86.6 ± 21.9 | <0.001 |
| GFREDTA (ml/min/1.73 m2)* | 60.8 ± 31.3* | 84.7 ± 30.1* | <0.001 | 60.6 ± 30.6* | 84.6 ± 30.5* | <0.001 |
| UAE rate (mg/24 h) | 644 (33–1,940) | 17 (7–525) | <0.001 | 720 (82–2,012) | 16 (7–468) | <0.001 |
| Systolic blood pressure (mmHg) | 157 ± 24 | 136 ± 20 | <0.001 | 159 ± 24 | 136 ± 19 | <0.001 |
| Diastolic blood pressure (mmHg) | 85 ± 13 | 79 ± 12 | 0.001 | 86 ± 14 | 79 ± 11 | <0.001 |
| Mean arterial pressure (mmHg) | 109 ± 15 | 98 ± 13 | <0.001 | 111 ± 15 | 98 ± 13 | <0.001 |
| Pulse pressure (mmHg) | 73 ± 21 | 57 ± 15 | <0.001 | 73 ± 21 | 57 ± 15 | <0.001 |
| Renin-angiotensin-aldosterone system inhibitors (%) | 51 | 20 | <0.001 | 50 | 20 | <0.001 |
| Other antihypertensive agents (%) | 64 | 28 | <0.001 | 68 | 27 | <0.001 |
| Lipid-lowering agents (%) | 0 | 0 | — | 0 | 0 | — |
| Continuation of medication (%) | 32 | 10 | <0.001 | 30 | 11 | <0.001 |
| Smoking: never/former/current (%) | 28/19/53 | 37/17/46 | 0.513 | 26/17/57 | 38/18/44 | 0.085 |
| Soluble RAGE (ng/ml) | 1.02 (0.80–1.41) | 0.97 (0.72–1.37) | 0.057 | 1.12 (0.83–1.53) | 0.96 (0.72–1.33) | 0.001 |
| Nϵ-(carboxymethyl)lysine (μmol/l) | 3.60 ± 1.12 | 3.54 ± 0.84 | 0.634 | 3.56 ± 1.32 | 3.55 ± 0.75 | 0.967 |
| Nϵ-(carboxyethyl)lysine (μmol/l) | 1.02 ± 0.28 | 0.92 ± 0.19 | 0.004 | 1.03 ± 0.30 | 0.92 ± 0.18 | 0.003 |
| Pentosidine (pmol/mg) | 49.3 (35.7–71.8) | 40.8 (34.0–49.0) | 0.001 | 51.8 (34.2–73.1) | 41.0 (34.2–48.9) | 0.001 |
| C-reactive protein (mg/l) | 1.59 (0.64–3.22) | 0.96 (0.41–2.09) | 0.008 | 1.42 (0.59–3.26) | 1.02 (0.44–2.16) | 0.021 |
| Secreted phospholipase A2 (μg/ml) | 4.40 (2.80–7.00) | 4.00 (2.70–6.23) | 0.329 | 4.05 (2.80–6.55) | 4.00 (2.70–6.55) | 0.948 |
| Interleukin-6 (pg/ml) | 2.18 (1.52–3.45) | 1.49 (0.99–2.35) | <0.001 | 2.42 (1.75–3.89) | 1.44 (1.00–2.21) | <0.001 |
| Soluble vascular cell adhesion molecule-1 (sVCAM-1) (ng/ml) | 1,045 ± 269 | 984 ± 346 | 0.141 | 1,100 ± 346 | 967 ± 318 | 0.001 |
| Soluble intracellular adhesion molecule-1 (sICAM-1) (ng/ml) | 771 ± 258 | 726 ± 272 | 0.182 | 805 ± 286 | 715 ± 260 | 0.008 |
| Low-grade inflammation score | 0.21 ± 0.57 | −0.07 ± 0.68 | 0.001 | 0.23 ± 0.65 | −0.07 ± 0.65 | <0.001 |
| Endothelial dysfunction score | 0.13 ± 0.70 | −0.04 ± 0.81 | 0.074 | 0.28 ± 0.85 | −0.09 ± 0.75 | <0.001 |
| AGEs score | 0.26 ± 1.17 | −0.09 ± 0.79 | 0.012 | 0.27 ± 1.32 | −0.08 ± 0.72 | 0.024 |
Data are means ± SD, medians (interquartile range), or percent.
*Data of GFREDTA were only available in patients with diabetic nephropathy (n = 165).
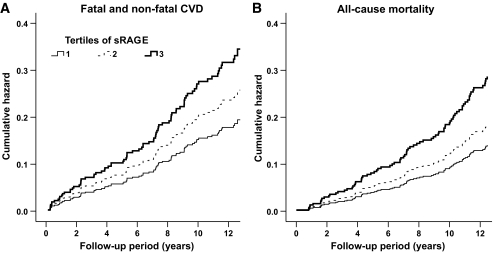
After adjustments for age, sex, case-control status, A1C, and duration of diabetes, the incidence of fatal and nonfatal CVD increased with a hazard ratio (HR) of 2.00 (95% CI 1.19–3.36), and the incidence of all-cause mortality increased with a HR of 2.44 (1.46–4.07), per unit increase in baseline levels of Ln-sRAGE (Table 2, model 1, Fig. 1A and B, respectively). The associations between Ln-sRAGE and study end points were attenuated, but remained significant after adjustments for other CVD risk factors and the use of medication: HR 1.90 (1.13–3.21) for fatal and nonfatal CVD events and 2.12 (1.26–3.57) for all-cause mortality (Table 2, models 2 and 3).
TABLE 2.
Associations between plasma Ln-sRAGE and incident CVD events and all-cause mortality in the whole study population (n = 339)
| Fatal and nonfatal CVD |
All-cause mortality |
|||||
|---|---|---|---|---|---|---|
| HR* | 95% CI | P | HR* | 95% CI | P | |
| Model: adjustments | ||||||
| 1: age, sex, A1C, case-control status, and duration of diabetes | 2.00 | 1.19–3.36 | 0.009 | 2.44 | 1.46–4.07 | 0.001 |
| 2: model 1 + mean arterial pressure, smoking status, and total cholesterol | 1.87 | 1.13–3.08 | 0.014 | 2.29 | 1.40–3.76 | 0.001 |
| 3a: model 2 + renin-angiotensin-aldosterone system inhibitors agents | 1.85 | 1.12–3.04 | 0.016 | 2.33 | 1.42–3.83 | 0.001 |
| 3b: model 2 + other antihypertensive agents | 1.68 | 1.01–2.81 | 0.048 | 1.97 | 1.18–3.28 | 0.010 |
| 3c: model 2 + continuation of medication use at baseline examination | 1.98 | 1.19–3.28 | 0.009 | 2.31 | 1.40–3.81 | 0.001 |
| 3: model 3a, 3b, and 3c | 1.90 | 1.13–3.21 | 0.016 | 2.12 | 1.26–3.57 | 0.005 |
| 4: model 3 + eGFRMDRD | 1.59 | 0.91–2.77 | 0.106 | 1.90 | 1.09–3.31 | 0.023 |
| 5: model 3 + Ln-UAE | 1.74 | 1.03–2.94 | 0.040 | 2.00 | 1.18–3.39 | 0.010 |
| 6: model 3 + inflammatory score | 1.89 | 1.12–3.19 | 0.018 | 2.12 | 1.26–3.57 | 0.005 |
| 7: model 3 + endothelial dysfunction score | 1.92 | 1.13–3.26 | 0.016 | 2.04 | 1.20–3.44 | 0.008 |
| 8: model 3 + pulse pressure | 1.67 | 0.98–2.87 | 0.059 | 1.91 | 1.12–3.25 | 0.017 |
| 9: model 3 + AGEs score | 1.74 | 1.02–2.98 | 0.042 | 1.98 | 1.16–3.38 | 0.012 |
*Hazard ratio for CVD morbidity and mortality or all-cause mortality per each unit increase in Ln-sRAGE levels at baseline.
FIG. 1.
Cumulative hazard for CVD morbidity and mortality (A) as well as all-cause mortality (B) across tertiles of plasma sRAGE. Data are adjusted for age, sex, case-control status, duration of diabetes, and A1C. Compared with patients in the lowest tertile of sRAGE, those in the middle and highest tertiles had increased risk for fatal and nonfatal CVD (HR 1.33 [95% CI 0.76–2.31] and 1.78 [1.03–3.06], respectively, P for trend 0.038) and all-cause mortality (1.31 [0.73–2.38] and 2.04 [1.17–3.55], respectively, P for trend = 0.010).
Further adjustment for eGFRMDRD attenuated the associations between Ln-sRAGE and incident fatal and nonfatal CVD as well as all-cause mortality by 28 and 14%, respectively (Table 2, model 4). Adjustments for Ln-UAE rate, pulse pressure, and AGEs attenuated these associations to a smaller extent, whereas adjustments for markers of endothelial dysfunction and low-grade inflammation did not (Table 2, models 5–9), despite the adverse associations between Ln-sRAGE and these variables (Table 3).
TABLE 3.
Associations between plasma Ln-sRAGE and potential mechanisms linking sRAGE to incident CVD and all-cause mortality
| Dependent variable | Model | All (n = 339) |
Normoalbuminuria (n = 170) |
Nephropathy (n = 169) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | ||
| Baseline eGFRMDRD* | 1 | −0.28 | −0.36 to −0.20 | <0.001 | −0.04 | −0.15 to 0.06 | 0.422 | −0.41 | −0.53 to −0.30 | <0.001 |
| 2 | −0.25 | −0.33 to −0.17 | <0.001 | −0.02 | −0.09 to 0.06 | 0.702 | −0.41 | −0.53 to −0.29 | <0.001 | |
| Ln-UAE | 1 | 0.07 | 0.03 to 0.11 | 0.001 | ||||||
| 2 | 0.06 | 0.02 to 0.10 | 0.002 | |||||||
| Low-grade inflammation score† | 1 | 0.03 | −0.04 to 0.10 | 0.384 | −0.05 | −0.16 to 0.05 | 0.316 | 0.10 | 0.01 to 0.19 | 0.039 |
| 2 | 0.03 | −0.04 to 0.10 | 0.400 | −0.08 | −0.17 to 0.02 | 0.130 | 0.09 | 0.00 to 0.18 | 0.056 | |
| Endothelial dysfunction score‡ | 1 | 0.16 | 0.08 to 0.24 | <0.001 | 0.07 | −0.03 to 0.16 | 0.168 | 0.23 | 0.10 to 0.36 | <0.001 |
| 2 | 0.16 | 0.08 to 0.24 | <0.001 | 0.07 | −0.03 to 0.17 | 0.159 | 0.23 | 0.10 to 0.36 | 0.001 | |
| Pulse pressure | 1 | 0.12 | 0.03 to 0.21 | 0.012 | ||||||
| 2 | 0.08 | 0.00 to 0.16 | 0.055 | |||||||
| AGEs score§ | 1 | 0.18 | 0.09 to 0.27 | <0.001 | 0.05 | −0.05 to 0.14 | 0.329 | 0.26 | 0.11 to 0.41 | 0.001 |
| 2 | 0.17 | 0.08 to 0.26 | <0.001 | 0.03 | −0.06 to 0.12 | 0.525 | 0.27 | 0.12 to 0.41 | 0.001 | |
β, standardized regression coefficient: indicates change in dependent variable (in SD) per 1-SD increase in Ln-sRAGE. Model 1, adjusted for age, sex, duration of diabetes, A1C, and case-control status when appropiate. Model 2, model 1 plus additional adjustments for smoking status, mean arterial pressure, total cholesterol, use of renin-angiotensin-aldosterone system inhibitors and other antihypertensive treatment, and continuation of medication at baseline examination. Symbols indicate significant interaction between sRAGE and case-control status, and therefore associations are also presented for each group separately (specifically,
*P < 0.001,
†P = 0.044,
‡P = 0.068, and
§P = 0.021).
However, the adverse associations between Ln-sRAGE on the one hand and baseline levels of eGFRMDRD, inflammation, endothelial dysfunction, and AGEs on the other were stronger in individuals with nephropathy than in those with normoalbuminuria (Table 3).
Additional analyses.
We investigated the role of eGFR in the associations between Ln-sRAGE and end points further and found 1) that in subjects with nephropathy, higher baseline Ln-sRAGE levels were associated with steeper declines in eGFRMDRD as well as GFR as estimated according to the 3.7-MBq 51Cr-EDTA method (GFREDTA) over the course of follow-up and 2) that the decline in (e)GFR attenuated the associations between Ln-sRAGE and study outcomes (see the online appendix).
DISCUSSION
The main findings of this study were twofold. First, in patients with type 1 diabetes, higher levels of plasma sRAGE are associated with incident fatal and nonfatal CVD as well as all-cause mortality, independently of other ‘traditional’ cardiovascular risk factors. Second, these associations could be partially explained by sRAGE-associated impairment in renal clearance, particularly in patients with nephropathy. Our findings are in agreement with studies that have examined the associations of sRAGE with CVD, but these were limited by cross-sectional designs (7,10–12). This is the first prospective study that has investigated the associations between plasma sRAGE and incident fatal and nonfatal CVD as well as all-cause mortality in a large sample of individuals with type 1 diabetes and has also addressed potential mechanisms that could explain the associations observed.
We hypothesized sRAGE to act as a putative marker of RAGE expression. The adverse associations found between sRAGE and CVD but also between sRAGE and markers of renal and endothelial dysfunction, low-grade inflammation, pulse pressure, and AGEs reported herein, which are in agreement with others (7,10–15), supported this hypothesis. However, sRAGE reflects the total pool of soluble RAGE in plasma and thus consists of different variants. These can result from alternative splicing (16) or from proteolytical cleavage of the membrane-bound RAGE (17). The exact functions of sRAGE in plasma are unknown, but these may differ across different variants. Indeed, several studies have reported inverse and thus “protective” associations between endogenous secretory RAGE (esRAGE) and (surrogate markers of) CVD (11,18–23). We have measured the total pool of plasma sRAGE only and therefore cannot discern whether the different variants of sRAGE have specific and potentially opposite associations with study outcomes. In addition, the extent to which levels of (e)sRAGE in plasma reflect the local concentrations and have the same effects as in tissues need to be further clarified.
The associations of sRAGE with CVD and all-cause mortality were attenuated when further adjusted for baseline eGFR, the former association being no longer statistically significant. However, this adjustment explained only 28% of the increased CVD risk associated with higher sRAGE. In addition, independent associations of both sRAGE and creatinine with CVD have been reported in individuals with diabetes (10). Furthermore, we found that sRAGE contributed to an accelerated decline in GFR, as estimated by eGFRMDRD or by GFREDTA in subjects with diabetic nephropathy. This is in line with a recent study in which sRAGE was inversely associated with changes in eGFR in the course of 1 year of follow-up in a large population of elderly women (24). These findings support the hypothesis of renal dysfunction being one of the intermediate factors linking sRAGE (as a reflection of RAGE expression) to vascular complications. However, we cannot discard the alternative (or concomitant) possibility, i.e., that baseline sRAGE levels may have been elevated as a consequence of impaired renal clearance. Indeed, our data also support this possibility; specifically, the association between higher baseline eGFR (per 10 ml/min per 1.73 m2) and incident CVD (HR 0.89 [95% CI 0.78–1.00]) was attenuated by ∼24% when further adjusted for Ln-sRAGE (to HR 0.92 [95% CI 0.81–1.04]). These observations reflect the interrelationships between sRAGE and eGFR at baseline, the exact directions of which we cannot fully unravel in the present study. Nevertheless, and altogether, these observations support the view that higher sRAGE levels, due to, but also to a great extent independent of, impaired GFR, are positively associated with CVD and all-cause mortality. Further studies with repeated data on both sRAGE and GFR are needed to disentangle the temporal order of these associations.
There are limitations to our study. Samples for analyses of sRAGE and other biomarkers were taken at baseline only, which impedes evaluation of the impact of changes in these variables on study outcomes. In addition, the relatively high interassay CV in the measurement of plasma sRAGE, and the potential misclassification of nonspecific mortality as CVD-related mortality may have introduced nondifferential biases, in which case the estimates reported herein may have been underestimated. However, we cannot discard the possibility that possible underreporting of nonfatal CVD introduced some differential bias affecting our results. Finally, although our findings did not suggest strong mediating effects of endothelial dysfunction, low-grade inflammation, arterial stiffness, and AGEs in the associations between sRAGE and study outcomes, we cannot fully exclude their potential mediating role due to the use of a selection of markers representing these processes.
In conclusion, higher plasma sRAGE levels, as a reflection of RAGE expression, are associated with incident fatal and nonfatal CVD as well as all-cause mortality in type 1 diabetes, and this may partially be explained by sRAGE-associated renal dysfunction in patients with nephropathy.
ACKNOWLEDGMENTS
I.F. was supported by a postdoctoral research grant from the Netherlands Heart Foundation (Grant 2006T050).
No potential conflicts of interest relevant to this article were reported.
J.W.M.N. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. A.J. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. I.F. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. C.G.S. researched data, contributed to discussion, wrote manuscript, and reviewed/edited manuscript. M.P. researched data, contributed to discussion, and reviewed/edited manuscript. H.-H.P. contributed to discussion and reviewed/edited manuscript. L.T. researched data and reviewed/edited manuscript. P.R. researched data, contributed to discussion, and reviewed/edited manuscript. C.D.A.S. researched data, contributed to discussion, and reviewed/edited manuscript.
We would like to thank all the participants who kindly participated in the study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kalea AZ, Schmidt AM, Hudson BI: RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (Lond) 2009;116:621–637 [DOI] [PubMed] [Google Scholar]
- 2.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A: The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation 2003;108:1070–1077 [DOI] [PubMed] [Google Scholar]
- 3.Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM: N(epsilon)-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem 1999;274:31740–31749 [DOI] [PubMed] [Google Scholar]
- 4.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM: Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest 2008;118:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R: Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 2002;105:816–822 [DOI] [PubMed] [Google Scholar]
- 6.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM: RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 2003;162:1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nin JW, Ferreira I, Schalkwijk CG, Prins MH, Chaturvedi N, Fuller JH, Stehouwer CD: EURODIAB Prospective Complications Study Group Levels of soluble receptor for AGE are cross-sectionally associated with cardiovascular disease in type 1 diabetes, and this association is partially mediated by endothelial and renal dysfunction and by low-grade inflammation: the EURODIAB Prospective Complications Study. Diabetologia 2009;52:705–714 [DOI] [PubMed] [Google Scholar]
- 8.Astrup AS, Tarnow L, Rossing P, Pietraszek L, Riis Hansen P, Parving HH: Improved prognosis in type 1 diabetic patients with nephropathy: a prospective follow-up study. Kidney Int 2005;68:1250–1257 [DOI] [PubMed] [Google Scholar]
- 9.Astrup AS, Tarnow L, Pietraszek L, Schalkwijk CG, Stehouwer CD, Parving HH, Rossing P: Markers of endothelial dysfunction and inflammation in type 1 diabetic patients with or without diabetic nephropathy followed for 10 years: association with mortality and decline of glomerular filtration rate. Diabetes Care 2008;31:1170–1176 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, Sato A, Imaizumi T: Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabete Metab Res Rev 2007;23:368–371 [DOI] [PubMed] [Google Scholar]
- 11.Yan XX, Lu L, Peng WH, Wang LJ, Zhang Q, Zhang RY, Chen QJ, Shen WF: Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis 2009;205:544–548 [DOI] [PubMed] [Google Scholar]
- 12.Humpert PM, Djuric Z, Kopf S, Rudofsky G, Morcos M, Nawroth PP, Bierhaus A: Soluble RAGE but not endogenous secretory RAGE is associated with albuminuria in patients with type 2 diabetes. Cardiovasc Diabetol 2007;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan KC, Shiu SW, Chow WS, Leng L, Bucala R, Betteridge DJ: Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia 2006;49:2756–2762 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita-Nakamura Y, Takeuchi M, Inoue H, Imaizumi T: Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc Res 2008;76:52–56 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Yamagishi S, Adachi H, Kurita-Nakamura Y, Matsui T, Yoshida T, Imaizumi T: Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol Med 2007;13:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H: Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 2003;370:1097–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME: A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 2008;22:3716–3727 [DOI] [PubMed] [Google Scholar]
- 18.Peng WH, Lu L, Hu J, Yan XX, Zhang Q, Zhang RY, Chen QJ, Shen WF: Decreased serum esRAGE level is associated with angiographically determined coronary plaque progression in diabetic patients. Clin Biochem 2009;42:1252–1259 [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Pu LJ, Zhang Q, Wang LJ, Kang S, Zhang RY, Chen QJ, Wang JG, De Caterina R, Shen WF: Increased glycated albumin and decreased esRAGE levels are related to angiographic severity and extent of coronary artery disease in patients with type 2 diabetes. Atherosclerosis 2009;206:540–545 [DOI] [PubMed] [Google Scholar]
- 20.Koyama H, Shoji T, Yokoyama H, Motoyama K, Mori K, Fukumoto S, Emoto M, Shoji T, Tamei H, Matsuki H, Sakurai S, Yamamoto Y, Yonekura H, Watanabe T, Yamamoto H, Nishizawa Y: Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 2005;25:2587–2593 [DOI] [PubMed] [Google Scholar]
- 21.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Yasuda T, Yamasaki Y: Endogenous secretory RAGE but not soluble RAGE is associated with carotid atherosclerosis in type 1 diabetes patients. Diab Vasc Dis Res 2008;5:190–197 [DOI] [PubMed] [Google Scholar]
- 22.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Yasuda T, Umayahara Y, Kosugi K, Yamasaki Y: Serum endogenous secretory RAGE level is an independent risk factor for the progression of carotid atherosclerosis in type 1 diabetes. Atherosclerosis 2009;204:288–292 [DOI] [PubMed] [Google Scholar]
- 23.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, Ohtoshi K, Hayaishi-Okano R, Kosugi K, Hori M, Yamasaki Y: Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care 2005;28:2716–2721 [DOI] [PubMed] [Google Scholar]
- 24.Semba RD, Ferrucci L, Fink JC, Sun K, Beck J, Dalal M, Guralnik JM, Fried LP: Advanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling women. Am J Kidney Dis 2009;53:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]