Abstract
OBJECTIVE
To determine the relative risk associated with DPA1 and DPB1 alleles and haplotypes in type 1 diabetes.
RESEARCH DESIGN AND METHODS
The frequency of DPA1 and DPB1 alleles and haplotypes in type 1 diabetic patients was compared to the family based control frequency in 1,771 families directly and conditional on HLA (B)-DRB1-DQA1-DQB1 linkage disequilibrium. A relative predispositional analysis (RPA) was performed in the presence or absence of the primary HLA DR-DQ associations and the contribution of DP haplotype to individual DR-DQ haplotype risks examined.
RESULTS
Eight DPA1 and thirty-eight DPB1 alleles forming seventy-four DPA1-DPB1 haplotypes were observed; nineteen DPB1 alleles were associated with multiple DPA1 alleles. Following both analyses, type 1 diabetes susceptibility was significantly associated with DPB1*0301 (DPA1*0103-DPB1*0301) and protection with DPB1*0402 (DPA1*0103-DPB1*0402) and DPA1*0103-DPB1*0101 but not DPA1*0201-DPB1*0101. In addition, DPB1*0202 (DPA1*0103-DPB1*0202) and DPB1*0201 (DPA1*0103-DPB1*0201) were significantly associated with susceptibility in the presence of the high risk and protective DR-DQ haplotypes. Three associations (DPB1*0301, *0402, and *0202) remained statistically significant when only the extended HLA-A1-B8-DR3 haplotype was considered, suggesting that DPB1 alone may delineate the risk associated with this otherwise conserved haplotype.
CONCLUSIONS
HLA DP allelic and haplotypic diversity contributes significantly to the risk for type 1 diabetes; DPB1*0301 (DPA1*0103-DPB1*0301) is associated with susceptibility and DPB1*0402 (DPA1*0103-DPB1*0402) and DPA1*0103-DPB1*0101 with protection. Additional evidence is presented for the susceptibility association of DPB1*0202 (DPA1*0103-DPB1*0202) and for a contributory role of individual amino acids and DPA1 or a gene in linkage disequilibrium in DR3-DPB1*0101 positive haplotypes.
Insulin-dependent autoimmune or type 1 diabetes is a common autoimmune disorder of unknown etiology which results in the destruction of the insulin-secreting pancreatic β-cells. The concordance rate in monozygotic twins is estimated to be 30 to 50% with an average risk to sibs of 6% (1) and an overall genetic risk ratio (λ-s) of about 15 (2). The increasing incidence of type 1 diabetes in a genetically homogeneous population, however, clearly indicates that environmental factors also play a key role (3,4).
Multiple association studies and genome linkage and association scans have confirmed that the greatest genetic risk is associated with variation within the HLA region located on chromosome six with evidence for modest associations at other regions in addition to HLA (5,6). In particular allelic, haplotypic or genotypic differences at the HLA class II DRB1, DQA1 or DQB1 loci have been shown in many studies, including a subset of this dataset (7), to have the greatest association.
The most plausible mechanism explaining the association with genes of the HLA class II region is their role in presentation of peptides derived from exogenous protein to CD4+ T-helper cells which, in the case of type 1 diabetes, may result in an inappropriate T cell immune response against self-antigens on the pancreatic β-cells. Allelic differences at the DRB1, DQA1 and DQB1 loci have been shown to influence the peptide binding and T cell stimulatory capacities of the individual HLA molecules (8), suggesting that such differences contribute to the association of individual or groups of alleles with autoimmune diseases. Genetic polymorphisms at other loci, both within and outside the HLA region may, in addition, contribute to and influence the magnitude of the immune response.
The HLA DPA1 and DPB1 genes are the third set of classical HLA class II loci which code for the DP antigen and have been associated with a lower immunostimulatory capacity and level of expression (9,10) although differences at individual DPB amino acids have been associated with an increased proliferative response in the mixed lymphocyte reaction (11,12). Similarly, a single DPB amino acid, glutamic acid at position 69, has been shown to contribute to graft versus host disease in otherwise HLA identical sibling bone marrow transplantation (13) and susceptibility to Beryllium disease (14).
Association studies of HLA-DPB1 and type 1 diabetes have shown multiple associations with conflicting results. The following have been reported as susceptibility alleles in populations of different ethnic backgrounds: DPB1*0201, *0202, *0301, *0401, *0402, *1701 and the following as protective alleles; DPB1*0101, *0202, *0401, *0402, *1701 (15–23). Other studies have reported weak or no association with HLA DPB1 alleles (24,25). The conflicting nature of these association studies may be a reflection of population specific differences, inconsistent typing approaches, differences in study design or inadequately powered studies.
The HLA DPA1 and DPB1 loci are highly polymorphic with 28 DPA1 and 136 DPB1 alleles defined as of October, 2009 (http://www.ebi.ac.uk/imgt/hla/). Association analyses in the HLA region are complicated by the occurrence of extensive linkage disequilibrium between loci such that the classical HLA loci, A, B, C, DR, DQ and DP, as well as other genes in this region, are often inherited as a “block”. The DR/DQ recombination frequency per meiosis between DR-DQ and DP has been estimated to range between 1–3% (13,26). Estimates of the relative contribution of HLA DP to susceptibility or protection against type 1 diabetes must therefore consider the potential influence of co-inherited loci, some of which are strongly associated with type 1 diabetes.
The Type 1 Diabetes Genetic Consortium (T1DGC) is a large worldwide collaborative study of type 1 diabetes families that have been collected in a highly standardized fashion from various populations (27). High resolution HLA typing has been performed at eight loci at four genotyping centers using standardized typing protocols and reagents (28). The large sample size and addition of DPA1 typing permits, for the first time, an association analysis of the DPA1 locus in addition to DPB1 and DPA1-DPB1 haplotypes, which encode the antigen presenting alpha and beta chain heterodimer with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The dataset used comprised 1,149 new Caucasian type 1 diabetes multiplex families combined with a number of existing type 1 diabetes DNA collections that are largely Caucasian, some of which have previously been HLA typed and analyzed (20,23), including the Danish (n = 95), Human Biological Data Interchange (HBDI) repository (Philadelphia, PA; n = 418), Sardinian (n = 52), and Joslin Diabetes Collections (n = 57). Where the proband was not indicated an affected sib was chosen at random.
Genotyping.
The methods of HLA genotyping have previously been described (7). Briefly, four digit HLA typing was reported using oligonucleotide probes that were immobilized to a nylon membrane and hybridized with biotin-labeled locus specific PCR products. Genotyping was performed using the Stripscan and Score proprietary software prior to upload to the central coordinating committee. Regular quality assurance typing performed on blind samples and between laboratories indicate typing agreement to be greater than 98% for DPA1 and DPB1 (28).
Statistical analysis.
Control haplotypes (n = 2,759) were determined based on the affected family-based control (AFBAC) method (29). The statistical significance of differences in allele frequencies between type 1 diabetes probands and AFBACs was assessed using a Pearson's χ2 test. P values were adjusted for multiple alleles or haplotypes by applying the Bonferroni correction of multiplying the nominal P value by the number of alleles or haplotypes compared (Padj). Only alleles or haplotypes for which f > 5% in either proband or AFBAC or where the nominal P < 0.05 and f > 1% were compared. We note that the Bonferroni correction is conservative for these data as the haplotypes are not independent.
Because only the proband from each family is used in the analysis and the AFBAC method uses at most two haplotypes from each pedigree, this approach does not introduce a bias due to the nonindependence between sibs.
Adjustment for linkage disequilibrium (LD) with DR-DQ (HLA-B) haplotypes.
The expected allele frequencies were computed, given LD and relative haplotype penetrance. In this case the null hypothesis (H0) is that DPA1, or DPB1 allele or DPA1-DPB1 haplotype frequencies will differ between patients and controls due to (1) linkage disequilibrium between DP and DRB1-DQB1 (and eventually also HLA-B) and (2) due to chance (sampling), thus implying that DP is neutral relative to disease predisposition.
Under H0 the expected allele frequencies at DPB1 or DPA1 alleles or DPA1-DPB1 haplotypes can be computed using the equation derived by Thomson (30):
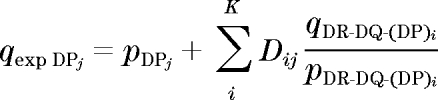 |
where Dij denotes pairwise linkage disequilibrium between the ith DR-DQ (HLA-B) haplotype and the jth DPB1 or DPA1 allele or DPA1-DPB1 haplotype in the control sample, q denotes the allele or haplotype frequency in probands, p denotes frequency in AFBACs, and qexp denotes the expected frequency in probands under the assumption of no involvement in disease. This method relies on sampling estimates of pairwise linkage disequilibrium between DP and DRB1-DQB1 and of the proband and control frequencies derived from the samples under study. Thus, there will be a sampling error associated with the computed value for expected DP allele or haplotype frequencies. The larger the control sample the smaller this error would be. This has been taken into account in the statistical tests carried out as previously described (31). Given the large number of DR-DQ-B haplotypes and the error that very rare haplotypes could introduce, only haplotypes at the susceptibility loci with an average frequency of 0.5% or higher in combined cases and controls were used. Moreover, only DP-DR-DQ (B) haplotypes where the expected frequency in controls in the absence of LD would be >0.05% were included in the analysis. Of the 75 observed DPA1-DPB1 haplotypes, a statistical test for association with type 1 diabetes conditional on DR-DQ or DR-DQ-HLA-B was carried out only on 22 haplotypes which had an expected frequency in type 1 diabetes of >0.5%.
In addition, the contribution of DP was considered on individual DRB1-DQA1-DQB1 and extended HLA haplotypes by a direct comparison between the allele and haplotype distribution between proband and AFBAC for individual haplotypes.
RESULTS
Frequency distribution of DPA1 and DPB1 alleles and haplotypes.
Eight DPA1 and thirty-eight DPB1 alleles were observed which formed seventy-four different DPA1-DPB1 haplotypes; nineteen DPB1 alleles were associated with multiple DPA1 alleles. The most frequent DPB1 allele, *0401, was observed in association with five different DPA1 alleles. While the DPA1-DPB1 haplotypic diversity is much greater than that previously observed, only fourteen DPA1-DPB1 haplotypes were observed at a frequency of greater than one percent in either the probands or AFBACs. The inclusion of DPA1 in HLA-DP associations with type 1 diabetes permits, for the first time, an analysis of the contribution of this locus and an analysis of DPA1-DPB1 haplotypes.
A direct comparison of the DPA1, DPB1, and DPA1-DPB1 frequency distribution, unadjusted for linkage disequilibrium, reveals a significant association with susceptibility to type 1 diabetes with DPB1*0101, 0202, 0301 and the DPA1-DPB1 haplotypes; 0201-0101, 0103-0202, 0103-0301. Protection against type 1 diabetes was significantly associated with DPA1*0202, DPB1*0402, 1001, 1101 and the DPA1-DPB1 haplotypes; 0103-0402, 0201-1101 (Table 1). DPB1*0101 was the only DPB1 allele observed with multiple DPA1 alleles that each had a frequency greater than 1% allowing analysis of the role of DPA1 in type 1 diabetes risk.
TABLE 1.
Proband and affected family-based control (AFBAC) HLA DPA1, DPB1 allele, and DPA1-DPB1 haplotype frequency distribution in 1,771 type 1 diabetes families
| AFBAC (%) (n = 2,759) | Proband (%) (n = 3,542) | Padj | Odds Ratio | 95% CI | AFBAC (%) (n = 2,759) | Proband (%) (n = 3,542) | Padj | Odds Ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPA1* | |||||||||||
| 0103 | 82.4 | 83.6 | 1.1 | ||||||||
| 0104 | 0.5 | 1.1 | 2.0 | 1.1–3.6 | |||||||
| 0201 | 13.7 | 13.5 | 1.0 | 0.8–1.2 | |||||||
| 0202 | 3.2 | 1.6 | 2E-4 | 0.5 | 0.4–0.7 | ||||||
| DPB1* | DPA1*-DPB1* | ||||||||||
| 0101 | 5.6 | 7.4 | 0.05 | 1.4 | 1.1–1.7 | 0103-0101 | 1.3 | 0.6 | 0.5 | 0.3–0.8 | |
| 0201-0101 | 3.4 | 6.4 | 2E-6 | 1.9 | 1.5–2.5 | ||||||
| 0202-0101 | 0.9 | 0.4 | 0.5 | 0.2–0.9 | |||||||
| 0201 | 13.5 | 15.2 | 1.1 | 1.0–1.4 | 0103-0201 | 12.6 | 14.9 | 1.2 | 1.0–1.4 | ||
| 0202 | 0.7 | 2.8 | 7E-9 | 4.4 | 2.6–7.3 | 0103-0202 | 0.5 | 2.8 | 5E-10 | 5.3 | 3.0–9.1 |
| 0301 | 10.4 | 15.9 | 5E-8 | 1.6 | 1.4–1.9 | 0103-0301 | 10.1 | 15.6 | 2E-8 | 1.7 | 1.4–2.0 |
| 0401 | 42.0 | 40.2 | 0.9 | 0103-0401 | 40.8 | 39.3 | 0.9 | ||||
| 0402 | 11.5 | 5.3 | 9E-17 | 0.4 | 0.4–0.5 | 0103-0402 | 11.2 | 5.1 | 1E-16 | 0.4 | 0.3–0.5 |
| 1001 | 1.8 | 1.0 | 0.04 | 0.5 | 0.3–0.8 | 0103-0601 | 1.5 | 2.2 | 1.5 | 1.0–2.2 | |
| 1101 | 2.1 | 0.9 | 9E-4 | 0.4 | 0.3–0.7 | 0201-1001 | 1.5 | 0.9 | 0.6 | 0.4–0.9 | |
| 1401 | 1.3 | 0.8 | 0.6 | 0.4–1.0 | 0201-1101 | 1.7 | 0.8 | 0.02 | 0.5 | 0.3–0.8 | |
| 1501 | 0.7 | 1.5 | 2.1 | 1.2–3.5 | 0104-1501 | 0.5 | 1.0 | 2.0 | 1.0–3.7 | ||
| 1701 | 1.7 | 1.0 | 0.6 | 0.4–0.9 | 0201-1701 | 1.6 | 1.0 | 0.6 | 0.4–1.0 |
Only alleles or haplotypes with a frequency >5% in an individual category or >1% where the nominal P value was <0.05 were compared. The nominal P value was adjusted (Padj) for multiple alleles or haplotypes by applying the Bonferroni correction of multiplying the nominal P value by the number of alleles or haplotypes compared.
Association analyses of HLA-DP with type 1 diabetes are complicated by the presence of extensive linkage disequilibrium between HLA loci that include the established primary susceptibility and protective DRB1, DQA1, and DQB1 loci. To address the issue of linkage disequilibrium, association analyses were performed conditional on DRB1-DQA1-DQB1 linkage disequilibrium (Table 2) and HLA-B-DRB1-DQA1-DQB1 linkage disequilibrium (Fig. 1) to accommodate the more recent large association analyses which indicate an independent association of HLA-B (32,33). In addition, a relative predispositional analysis (RPA) (34) in which the primary susceptibility and protective DRB1-DQA1-DQB1 haplotypes are removed was performed to detect secondary associations not detected in the presence of the primary associations. Furthermore, an association analysis was performed comparing the frequency of DPA1-DPB1 haplotypes present on the more frequent individual DRB1-DQA1-DQB1 haplotypes.
TABLE 2.
Expected and observed proband DPA1, DPB1, and DPA1-DPB1 frequencies conditioned on HLA DRB1-DQA1-DQB1 linkage disequilibrium in 1,771 type 1 diabetes families
| Proband Fexp (%) | Proband Fobs (%) | Padj | Odds Ratio (Fobs/Fexp) | 95% CI | Proband Fexp (%) | Proband Fobs (%) | Padj | Odds Ratio (Fobs/Fexp) | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPA1* | DPA1-DPB1 | ||||||||||
| 0103 | 80.3 | 82.8 | 1.2 | ||||||||
| 0104 | 0.5 | 1.0 | 2.0 | 1.1–3.7 | |||||||
| 0201 | 17.2 | 13.4 | 5E-4 | 0.7 | 0.6–0.9 | ||||||
| 0202 | 2.0 | 2.8 | 1.4 | 1.0–2.0 | |||||||
| DPB1* | |||||||||||
| 0101 | 11.4 | 8.0 | 2E-4 | 0.7 | 0.6–0.8 | 0103-0101 | 2.8 | 0.7 | 2E-10 | 0.2 | 0.1–0.4 |
| 0201-0101 | 7.5 | 6.3 | 0.8 | 0.7–1.0 | |||||||
| 0201 | 13.0 | 15.2 | 1.2 | 1.0–1.4 | 0103-0201 | 12.1 | 14.7 | 1.3 | 1.1–1.5 | ||
| 0202 | 1.4 | 2.8 | 2E-3 | 2.0 | 1.4–3.0 | 0103-0202 | 1.4 | 2.8 | 2E-3 | 2.1 | 1.4–3.0 |
| 0301 | 10.1 | 15.6 | 4E-8 | 1.6 | 1.4–2.0 | 0103-0301 | 10.0 | 15.3 | 5E-8 | 1.6 | 1.4–1.9 |
| 0401 | 40.8 | 39.9 | 1.0 | 0103-0401 | 38.9 | 38.7 | 1.0 | 0.9–1.1 | |||
| 0201-0401 | 1.2 | 0.7 | 0.6 | 0.3–1.0 | |||||||
| 0402 | 10.9 | 5.3 | 2E-14 | 0.5 | 0.4–0.6 | 0103-0402 | 10.7 | 5.1 | 7E-15 | 0.5 | 0.4–0.5 |
| 0501 | 1.1 | 1.7 | 1.6 | 1.0–2.5 | |||||||
| 0601 | 1.5 | 2.2 | 1.5 | 1.0–2.3 | 0103-0601 | 1.4 | 2.2 | 1.6 | 1.1–2.3 | ||
| 1001 | 1.7 | 1.0 | 0.6 | 0.4–0.9 | 0201-1001 | 1.7 | 0.9 | 0.05 | 0.5 | 0.3–0.8 | |
| 1101 | 1.6 | 0.9 | 0.5 | 0.3–0.9 | 0201-1101 | 1.6 | 0.8 | 0.03 | 0.5 | 0.3–0.8 | |
| 1501 | 0.5 | 1.5 | 2E-3 | 3.0 | 1.6–5.4 | 0104-1501 | 0.5 | 1.0 | 2 | 1.1–3.7 |
Only alleles or haplotypes with a frequency >5% in an individual category or >1% where the nominal P value was <0.05 were compared. The nominal P value was adjusted (Padj) for multiple alleles or haplotypes by applying the Bonferroni correction of multiplying the nominal P value by the number of alleles or haplotypes compared.
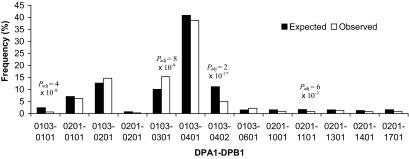
FIG. 1.
Expected and observed proband DPA1-DPB1 haplotype frequencies conditioned on HLA-B-DRB1-DQA1-DQB1 linkage disequilibrium. Alleles or haplotypes with a frequency of less than 5% for an individual category with a non significant comparison or less than 1% where the nominal P < 0.05 are not shown. Nominal P value was adjusted for the number of haplotypes compared (Padj).
Conditional analysis
DPA1.
Following the correction for multiple allele testing, only DPA1*0201 (Padj = 5 × 10−4, OR = 0.7) was significantly associated with protection to type 1 diabetes. DPA1*0201 was also protective when in haplotypic association with four DPB1 alleles; 0101, 0401, 1001 and 1101. The DPA1 allele in association with DPB1*0101 appears to significantly contribute to the risk associated with DPB1*0101 (described below).
DPB1.
DPB1*0301 was associated with susceptibility following the DRB1-DQA1-DQB1 conditional analysis (Padj = 4 × 10−8, OR = 1.6) and in haplotypic association with DPA1*0103 (Padj = 5 × 10−8, OR = 1.6). DPB1*0402 was associated with protection following the DRB1-DQA1-DQB1 conditional analysis (Padj = 2 × 10−14, OR = 0.5) and in haplotypic association with DPA1*0103 (Padj = 7 × 10−15, RR = 0.5). DPB1*0101 was associated with protection following the DRB1-DQA1-DQB1 conditional analysis (Padj = 2 × 10−4, OR = 0.7) and in haplotypic association with DPA1*0103 (Padj = 2 × 10−10, OR = 0.2). However, no association was observed with the more common DPA1*0201-DPB1*0101 haplotype; the nonoverlapping 95% confidence intervals for the Odds Ratios between the two DPB1*0101 haplotypes suggests a contributory role of DPA1 (Table 2). These observations were confirmed in the HLA-B-DRB1-DQA1-DQB1 conditional analysis (Fig. 1) and are the most widely reported and now replicated DP associations with type 1 diabetes.
The low frequency DPB1*0202 allele was associated with susceptibility following the DRB1-DQA1-DQB1 conditional analysis (Padj = 4 × 10−3, OR = 2.0) and in haplotypic association with DPA1*0103 (Padj = 7 × 10−3, OR = 2.1). This association was confirmed following the HLA-B-DRB1-DQA1-DQB1 conditional analysis where the expected frequency of DPA1*0103-DPB1*0202 was zero whereas the observed frequency in type 1 diabetic patients was 2.8% (data not shown).
Relative predispositional analysis.
The distinct hierarchy of risk and protection established for individual DRB1-DQA1-DQB1 haplotypes among the T1DGC families has been reported (7). The role of HLA DP was therefore considered both in the presence or absence of the DRB1*0301-DQA1*0501-DQB1*0201, DRB1*0401-DQA1*0301-DQB1*0302, DRB1*0402-DQA1*0301-DQB1*0302, DRB1*0404-DQA1*0301-DQB1*0302, DRB1*0405-DQA1*0301-DQB1*0302 high risk DR-DQ haplotypes and the DRB1*0701-DQA1*0201-DQB1*0303, DRB1*1401-DQA1*0101-DQB1*0503 and DRB1*1501-DQA1*0102-DQB1*0602 protective haplotypes (Table 3).
TABLE 3.
Relative predispositional analysis; DPA1, DPB1 allele, and DPA1-DPB1 haplotype frequency distribution in 1,771 type 1 diabetes families in the presence or absence of the high-risk and protective DRB1-DQA1-DQB1 haplotypes
| Presence of high-risk and protective DR-DQ haplotypes |
Absence of high-risk and protective DR-DQ haplotypes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % AFBACs (n = 1,102) | % Probands (n = 2,626) | Padj | Odds Ratio | 95% CI | % AFBACs (n = 1,656) | % Probands (n = 916) | Padj | Odds Ratio | 95% CI | |
| DPA1* | ||||||||||
| 0103 | 83.7 | 83.7 | 1.0 | 81.5 | 83.4 | 1.1 | 1.0–1.2 | |||
| 0104 | 0.7 | 2.2 | 0.002 | 3.3 | 1.6–7.0 | |||||
| 0201 | 14.2 | 14.4 | 1.0 | 0.8–1.3 | 13.3 | 10.9 | 0.8 | 0.6–1.0 | ||
| DPB1* | ||||||||||
| 0101 | 9.5 | 9.3 | 1.0 | 0.8–1.3 | ||||||
| 0201 | 9.6 | 15.3 | 1E-04 | 1.7 | 1.3–2.2 | 16.1 | 15.0 | 0.9 | 0.7–1.2 | |
| 0202 | 1.3 | 3.7 | 8E-04 | 2.9 | 1.7–5.2 | |||||
| 0301 | 8.7 | 14.9 | 1E-05 | 1.8 | 1.4–2.4 | 11.5 | 18.6 | 3E-05 | 1.8 | 1.4–2.2 |
| 0401 | 50.5 | 40.6 | 2E-04 | 0.7 | 0.5–0.8 | |||||
| 0402 | 6.4 | 4.8 | 0.7 | 0.5–1.0 | 14.9 | 6.8 | 7E-08 | 0.4 | 0.3–0.6 | |
| 1501 | 0.8 | 2.8 | 3E-04 | 3.7 | 1.9–7.2 | |||||
| 2301 | 1.3 | 0.2 | 5E-04 | 0.1 | 0.04–0.4 | |||||
| DPA1-DPB1 | ||||||||||
| 0103-0101 | 2.5 | 0.8 | 7E-04 | 0.3 | 0.2–0.6 | |||||
| 0201-0101 | 7.0 | 8.2 | 1.2 | 0.9–1.6 | ||||||
| 0103-0201 | 9.3 | 14.9 | 1E-04 | 1.7 | 1.4–2.2 | 14.9 | 14.7 | 1.0 | 0.8–1.3 | |
| 0103-0202 | 1.1 | 3.7 | 2E-04 | 3.4 | 1.9–6.3 | |||||
| 0103-0301 | 8.4 | 14.6 | 1–05 | 1.9 | 1.5–2.4 | 11.2 | 18.6 | 7E-06 | 1.8 | 1.4–2.3 |
| 0103-0401 | 49.5 | 39.7 | 2E-04 | 0.7 | 0.5–0.8 | 35.1 | 38.1 | 1.1 | 1.0–1.4 | |
| 0103-0402 | 6.4 | 4.6 | 0.7 | 0.5–1.0 | 14.4 | 6.4 | 6E-08 | 0.4 | 0.3–0.6 | |
| 0103-2301 | 1.3 | 0.2 | 5E-05 | 0.1 | 0.04–0.4 | |||||
| 0104-1501 | 0.3 | 0.2–0.6 | 0.6 | 2.2 | 2E-03 | 3.7 | 1.7–7.9 | |||
DPA1, DPB1, and DPA1-DPB1 alleles and haplotypes with a frequency >5% in an individual category or >1% where the nominal P value was <0.05 were compared in the presence or absence of the following high-risk and protective DRB1-DQA1-DQB1 haplotype: 0301-0501-0201, 0401-0301-0302, 0402-0301-0302, 0404-0301-0302, 0405-0301-0302, 0701-0201-0303, 1401-0101-0503 and 1501-0102-0602. The nominal P value was adjusted (Padj) for multiple alleles or haplotypes by applying the Bonferroni correction of multiplying the nominal P value by the number of comparisons.
DPB1*0301 was significantly associated with susceptibility both in the presence or absence of the high risk and protective DR-DQ haplotypes either individually or in association with DPA1*0103 with an odds ratio of between 1.8 and 1.9. DPB1*0402, either individually or in association with DPA1*0103, was observed to be significantly associated with protection only in the absence of the high risk and protective DR-DQ haplotypes with an odds ratio of 0.4.
The protective association of DPA1*0103-DPB1*0101 and susceptibility association of DPB1*0202 (DPA1*0103-DPB1*0202) only observed in the high risk and protective DR-DQ haplotypes is consistent with the presence of DR3 positive haplotypes in this group. The low frequency susceptibility association with DPB1*1501 (or DPA1*0104-DPB1*1501) observed in the absence of high risk and protective DR-DQ haplotypes and in the conditional analysis (Table 2) may represent an additional secondary association but requires confirmation.
DPA1-DPB1 haplotype distribution in individual DRB1-DQA1-DQB1 haplotypes.
A comparison of the DPA1-DPB1 odds ratios in the most frequent DRB1-DQA1-DQB1 haplotypes suggests that DPA1*0103-DPB1*0301 and DPA1*0103-DPB1*0201 are associated with susceptibility and DPA1*0103-DPB1*0402 with protection across most DR-DQ haplotypes (Table 4). Only a single DPA1-DPB1 association with either the combined or individual DR4-DQB1*0302 positive haplotypes reached statistical significance; susceptibility associated with DRB1*0401-DQA1*0301-DQB1*0302 was significantly reduced by the presence of DPA1*0103-DPB1*0101 (Padj = 0.04, OR = 0.2). In contrast, the risk associated with DR3 (DRB1*0301-DQA1*0501-DQB1*0201) positive haplotypes was significantly reduced by the presence of DPA1*0103-DPB1*0402 (Padj = 5 × 10−3, OR = 0.4) and the infrequent DPA1*0103-DPB1*0101 (Padj = 5 × 10−6, OR = 0.2) and increased by the presence of DPA1*0103-DPB1*0202 (Padj = 0.04, OR = 2.5).
TABLE 4.
DPA1-DPB1 haplotype frequency odds ratios for individual DRB1-DQA1-DQB1 haplotypes
| DRB1-DQA1-DQB1 | Proband (n) | AFBAC (n) | DPA1-DPB1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0103-0101 |
0201-0101 |
0103-0201 |
0103-0202 |
0103-0301 |
0103-0402 |
|||||||
| OR | Padj | OR | OR | OR | Padj | OR | P | OR | Padj | |||
| 04XX-0301-0302 | 1,393 | 239 | 1.4 | 1.6 | 0.7 | |||||||
| 0401-0301-0302 | 909 | 114 | 0.2 | 0.04 | 1.6 | 2.0 | 0.7 | |||||
| 0402-0301-0302 | 135 | 36 | 2.2 | 1.9 | 0.5 | |||||||
| 0404-0301-0302 | 223 | 90 | 1.2 | 1.6 | 0.9 | |||||||
| 0301-0501-0201 | 1,224 | 342 | 0.2 | 5.E-06 | 0.8 | 1.5 | 2.5 | 0.04 | 1.7 | 0.4 | 5.E–03 | |
| A1-B8-DR3 | 436 | 146 | 0.3 | 0.8 | 1.9 | 3.5 | 0.03 | 1.2 | 0.03 | 0.4 | 0.04 | |
| A30-B18-DR3 | 99 | 26 | 0.4 | 3.0 | 0.8 | 0.9 | ||||||
| DR3 other | 691 | 170 | 0.1 | 5.E-06 | 0.8 | 1.5 | 2.2 | 1.3 | 0.5 | |||
| 0101-0101-0501 | 195 | 236 | 0.8 | 2.2 | 0.3 | 1.E–03 | ||||||
| 1302-0103-0604 | 93 | 80 | 0.7 | 1.9 | 0.1 | 0.02 | ||||||
| 0701-0201-0201 | 94 | 270 | 1.0 | 1.1 | 0.3 | |||||||
| 1101-0501-0301 | 32 | 162 | 2.1 | 1.8 | 0.2 | |||||||
| 0401-0301-0301 | 63 | 111 | 2.2 | 0.4 | ||||||||
The odds ratio (OR) was calculated for DPA–DPB1 haplotypes with a frequency >5% in an individual category or >1% where the nominal P value was <0.05. The nominal P value was adjusted (Padj) for multiple alleles or haplotypes by applying the Bonferroni correction of multiplying the nominal P value by the number of comparisons. DR3 (DRB1*0301-DQA1*0501-DQB1*0201) positive haplotypes were subdivided into the presence or absence of the extended A*0101-B*0801-C*0701-DRB1*0301-DQA1*0501-DQB1*0201 and A*3002-B*1801-C*0501-DRB1*0301-DQA1*0501-DQB1*0201 positive haplotypes.
The effect of DP haplotypes on DR3 haplotypes was further divided into three categories based on the presence of the extended haplotypes A1-B8-DR3 (A*0101-B*0801-C*0701-DRB1*0301-DQA1*0501-DQB1*0201) and A30-B18-DR3 (A*3002-B*1801-C*0501-DRB1*0301-DQA1*0501-DQB1*0201) and “other” DR3 haplotypes. The susceptibility associated with the A1-B8-DR3 haplotype was significantly reduced by the presence of DPA1*0103-DPB1*0402 (Padj = 0.04, OR = 0.4) and increased by the presence of DPA1*0103-DPB1*0301 (Padj = 0.03, OR = 1.2) and the low frequency DPA1*0103-DPB1*0202 (Padj = 0.03, OR = 3.5). DPA1*0103-DPB1*0202 was associated with susceptibility on the A30-B18-DR3 extended haplotype as well as on “other” DR3 haplotypes but did not reach statistical significance in these haplotype comparisons.
The presence of DPA1*0103-DPB1*0101 (Padj = 5 × 10−6, OR = 0.1) but not DPA1*0201-DPB1*0101 significantly reduced the risk associated with “other” DR3 positive haplotypes with a nonoverlapping 95% CI (data not shown) and nonsignificantly reduced the risk associated with the A1-B8-DR3 haplotype. On the extended A1-B8-DR3 haplotype the only difference between those carrying DPA1*0101-DPB1*0101 and DPA1*0201-DPB1*0101 is likely to be the DPA1 allele or a gene in linkage disequilibrium.
DISCUSSION
Previous association studies of HLA DP polymorphism with type 1 diabetes using a variety of typing approaches in different populations have indicated a susceptibility association with DPB1*0301 and *0202 and a protective association with DPB1*0402 as well as a number of conflicting associations. In this publication, the Type 1 Diabetes Genetic Consortium provides a large scale association analysis of DPA1, DPB1 allele and DPA1-DPB1 haplotypes in a new type 1 diabetes Caucasian family collection combined with existing collections that were re-typed for HLA using the same platform. Seventy four different DPA1-DPB1 haplotypes were observed; 19 DPB1 alleles were associated with multiple DPA1 alleles which permits, for the first time, a more precise type 1 diabetes association analysis for DP and an assessment of the role of DPA1.
A direct comparison of proband and AFBAC frequency distributions showed that the frequency of one DPA1, four DPB1 alleles and four DPA1-DPB1 haplotypes differed significantly when probands and AFBACs were compared; the most significant association was protection with DPA1*0103-DPB1*0402.
HLA-DP association analyses in type 1 diabetes are complicated by the extensive linkage disequilibrium across the HLA region, in particular with the HLA-DR-DQ region, which confers high risk for type 1 diabetes. In order to adjust for these factors, analyses were performed conditional on HLA-DRB1-DQA1-DQB1 and HLA-B-DRB1-DQA1-DQB1 linkage disequilibrium and in the presence or absence of the primary susceptibility and protective haplotypes (relative predispositional analysis).
The conditional analyses confirmed the previously described HLA-DP associations, now reported as DPA1-DPB1 haplotypes; susceptibility with DPB1*0301 (DPA1*0103-DPB1*0301), DPB1*0202 (DPA1*0103-DPB1*0202) and protection with DPB1*0402 (DPA1*0103-DPB1*0402). A novel haplotype association observed here, reflecting the potential role of DPA1, is the protective effect of DPB1*0101 in association with DPA1*0103 but not when linked to DPA1*0201. These alleles and haplotypes may either encode secondary class II HLA molecules involved in the presentation of a putative “diabetogenic peptide” or are in linkage disequilibrium with another putative causal gene.
The relative predispositional analysis, performed in the presence or absence of the high risk susceptibility and protective DR-DQ haplotypes, confirmed the association of susceptibility with DPA1*0103-DPB1*0301 across both groups, whereas protection with DPA1*0103-DPB1*0402 only reached statistical significance in the absence of the high risk susceptibility and protective DR-DQ haplotypes. The susceptibility association with DPA1*0103-DPB1*0202 and protective association with DPA1*0103-DPB1*0101 were observed only in the presence of the high risk susceptibility and protective DR-DQ haplotypes. This is consistent with the presence of DR3 positive haplotypes in this group.
The contribution of DP to DR3 positive haplotypes was further examined by comparing the risk associated with the presence or absence of the extended A1-B8-DR3 and A30-B18-DR3 haplotypes. The A1-B8-DR3 haplotype has previously been shown to be associated with a lower risk and the A30-B18-DR3 haplotype with a high risk to type 1 diabetes (35). The risk associated with the A1-B8-DR3 haplotype, which shows remarkable conservation of sequence between unrelated individuals (36), was significantly increased by the presence of DPA1*0103-DPB1*0301 and DPA1*0103-DPB1*0202 and reduced by the presence of DPA1*0103-DPB1*0402. DPB1 allele differences therefore appear to stratify the susceptibility risk on an otherwise conserved HLA haplotype. In contrast only a single DP haplotypic association with DR4-DQB1*0302 positive haplotypes was observed; the DRB1*0401-DQA1*0301-DQB1*0302 haplotype was significantly reduced by the presence of DPA1*0103-DPB1*0101. The DP haplotype *0103-*0202 is also increased among patient A30-B18-DR3 haplotypes, but the difference does not reach statistical significance, perhaps due to a power issue as DPA1*0103-DPB1*0202 has previously been associated with increased susceptibility when not in association with A30-B18-DR3 in Filipino type 1 diabetic patients (37). The risk associated with the remaining DR3 positive haplotypes was significantly decreased by the presence of DPA1*0103-DPB1*0101 but not by DPA1*0201-DPB1*0101 with nonoverlapping confidence intervals. It is plausible that HLA-DPA1 or another gene in linkage disequilibrium may contribute to the significant differences in associated risk. DPA1 is the closest expressed gene centromeric to HLA-DOA, which has recently been significantly associated with type 1 diabetes risk in a study that initially utilized the conservation of the A30-B18-DR3 haplotype to examine other DR3 positive haplotypes (38).
A comparison of the amino acid sequences of DPA1*0103 and DPA1*0201 shows they differ by two substitutions at positions 31 (M versus Q) and 50 (Q versus R); the latter substitution results in a charge difference with a limited effect on peptide binding (39). However, the same DPA1 allele difference in association with DPB1*0301 has been observed to significantly alter T cell recognition (40) and monoclonal antibody recognition (41), suggesting that these DPA1 differences do contribute to functional and antigenic differences. Evidence for the potential role of individual amino acids to type 1 diabetes susceptibility is also highlighted by the protective association of DPA1*0103-DPB1*0402 and the susceptibility association of DPA1*0103-DPB1*0201. A comparison between the two haplotype sequences reveals a single K to E difference at position 69 of DPB1. It is of note that a second susceptibility association, DPA1*0103-DPB1*0202, is also E positive at position 69, suggesting a critical role for this residue. This substitution has previously been implicated in influencing T cell recognition (12,42,43) and changing peptide binding properties, resulting in an increase in the affinity of pocket four for positively charged aromatic and polar residues (44). The corresponding position in the DR beta chain, amino acid residue 71, has been shown to strongly influence peptide binding in autoimmune related DRB1 alleles (45) and pocket four to be important in determining susceptibility of DRB1*04 alleles in type 1 diabetes (46).
The observations reported here confirm and further delineate the contributory role of DPA1 and DPB1 allelic and haplotypic diversity with the risk associated to type 1 diabetes. Susceptibility is associated with DPB1*0301 (DPA1*0103-DPB1*0301) and DPB1*0202 (DPA1*0103-DPB1*0202) and protection with DPB1*0402 (DPA1*0103-DPB1*0402) and DPA1*0103-DPB1*0101 but not DPA1*0201-DPB1*0101. The pattern of DP association on several different DR-DQ haplotypes is consistent with the notion that specific DPA1 and DPB1 alleles play a potential causal role in type 1 diabetes susceptibility rather than as haplotype markers. Additional evidence is presented to suggest that individual amino acid substitutions may influence type 1 diabetes risk.
ACKNOWLEDGMENTS
This research utilizes resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD), and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Todd JA: Genetic control of autoimmunity in type 1 diabetes. ImmunToday 1990;11:122–129 [DOI] [PubMed] [Google Scholar]
- 2.Risch N: Assessing the role of HLA-linked and unlinked determinants of disease. Am J Hum Genet 1987;40:1–14 [PMC free article] [PubMed] [Google Scholar]
- 3.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA: The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004;364:1699–1700 [DOI] [PubMed] [Google Scholar]
- 4.Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, Harrison LC: The rising incidence of type 1 diabetes is accounted for by cases with lower risk human leucocyte antigen genotypes. Diabetes Care 2008;31:1546–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concannon P, Erlich HA, Julier C, Morahan G, Nerup J, Pociot F, Todd JA, Rich SS:; Type 1 Diabetes Genetics Consortium Type 1 diabetes evidence for susceptibility loci from four genome-wide linkage scans in 1,435 multiplex families. Diabetes 2005;54:2995–3001 [DOI] [PubMed] [Google Scholar]
- 6.Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, Akolkar B, Erlich HA, Hilner JE, Julier C, Morahan G, Nerup J, Nierras CR, Chen WM, Rich SS:; Type 1 Diabetes Genetics Consortium: A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes 2008;57:2858–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A: Moonsamy P; Type 1 Diabetes Genetics Consortium HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bondinas GP, Moustakas AK, Papadopoulos GK: The spectrum of HLA-DQ and HLA-DR alleles, 2006: a listing correlating sequence and structure with function. Immunogenetics 2007;59:539–553 [DOI] [PubMed] [Google Scholar]
- 9.Shaw S, Johnson AH, Shearer GM: Evidence for a new segregant series of B cell antigens that are encoded in the HLA-D region and that stimulate secondary allogenic proliferative and cytotoxic responses. J Exp Med 1980;152:565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawelec G, Bühring HJ: Expression of MHC class II epitopes on human T lymphocyte clones. Cell Immunol 1990;127:520–526 [DOI] [PubMed] [Google Scholar]
- 11.Cesbron A, Moreau P, Milpied N, Harousseau JL, Muller JY, Bignon JD: Crucial role of the third and fourth hypervariable regions of HLA-DPB1 allelic sequences in the mixed lymphocyte reaction. Hum Immunol 1992;33:202–207 [DOI] [PubMed] [Google Scholar]
- 12.Nicholson I, Varney M, Kanaan C, Grigg A, Szer J, Tiedemann K, Tait BD: Alloresponses to HLA-DP detected in the primary MLR-correlation with a single amino acid difference. Hum Immunol 1997;55:163–169 [DOI] [PubMed] [Google Scholar]
- 13.Nomura N, Ota M, Kato S, Inoko M, Tsuji K: Severe acute graft versus host disease by HLA-DPB1 disparity in recombinant family of bone marrow transplantation between serologically HLA identical siblings: an application of the polymerase chain reaction–restriction fragment length polymorphism method. Hum Immunol 1991;32:261–268 [DOI] [PubMed] [Google Scholar]
- 14.Richeldi L, Sorrentino R, Saltini C: HLA-DPB1 Glutamate 69: a genetic marker of beryllium disease. Science 1993;262:242–244 [DOI] [PubMed] [Google Scholar]
- 15.Baisch JM, Capra JD: Analysis of HLA genotypes and susceptibility to insulin-dependent diabetes mellitus: association maps telomeric to HLA-DP. Scand J Immunol 1992;36:331–340 [DOI] [PubMed] [Google Scholar]
- 16.Tait BD, Harrison LC, Drummond BP, Stewart V, Varney MD, Honeyman MC: HLA antigens and age at diagnosis of insulin-dependent diabetes mellitus. Hum Immunol 1995;42:116–122 [DOI] [PubMed] [Google Scholar]
- 17.Balducci-Silano PL, Layrisse ZE: HLA-DP and susceptibility to insulin-dependent diabetes mellitus in an ethnically mixed population. Associations with other HLA-alleles. J Autoimmun 1995;8:425–437 [DOI] [PubMed] [Google Scholar]
- 18.Erlich HA, Rotter JI, Chang JD, Shaw SJ, Raffel LJ, Klitz W, Bugawan TL, Zeidler A: Association of HLA-DPB1*0301 with IDDM in Mexican-Americans. Diabetes 1996;45:610–614 [DOI] [PubMed] [Google Scholar]
- 19.Nishimaki K, Kawamura T, Inada H, Yagawa K, Nose Y, Nabeya N, Isshiki G, Tatsumi N, Niihira S: HLA DPB1*0201 gene confers disease susceptibility in Japanese with childhood onset type I diabetes, independent of HLA-DR and DQ genotypes. Diabetes Res Clin Pract 2000;47:49–55 [DOI] [PubMed] [Google Scholar]
- 20.Noble JA, Valdes AM, Thomson G, Erlich HA: The HLA class II locus DPB1 can influence susceptibility to type 1 diabetes. Diabetes 2000;49:121–125 [DOI] [PubMed] [Google Scholar]
- 21.Cucca F, Lampis R, Congia M, Angius E, Nutland S, Bain SC, Barnett AH, Todd JA: A correlation between the relative predisposition of MHC class II alleles to type 1 diabetes and the structure of their proteins. Hum Mol Genet 2001;10:2025–2037 [DOI] [PubMed] [Google Scholar]
- 22.Al-Hussein KA, Rama NR, Ahmad M, Rozemuller E, Tilanus MG: HLA-DPB1*0401 is associated with dominant protection against type 1 diabetes in the general Saudi population and in subjects with a high-risk DR/DQ haplotype. Eur J Immunogenet 2003;30:115–119 [DOI] [PubMed] [Google Scholar]
- 23.Cruz TD, Valdes AM, Santiago A, Frazer de Llado T, Raffel LJ, Zeidler A, Rotter JI, Erlich HA, Rewers M, Bugawan T, Noble JA: DPB1 alleles are associated with type 1 diabetes susceptibility in multiple ethnic groups. Diabetes 2004;53:2158–2163 [DOI] [PubMed] [Google Scholar]
- 24.Johansson S, Lie BA, Pociot F, Nerup J, Cambon-Thomsen A, Kockum I, Thorsby E, Undlien DE: HLA associations in type 1 diabetes: DPB1 alleles may act as markers of other HLA-complex susceptibility genes. Tissue Antigens 2003;61:344–351 [DOI] [PubMed] [Google Scholar]
- 25.Mbanya JC, Sobngwi E, Mbanya DN: HLA-DRB1, -DQA1, -DQB1 and DPB1 susceptibility alleles in Cameroonian type 1 diabetes patients and controls. Eur J Immunogenet 2001;28:459–462 [DOI] [PubMed] [Google Scholar]
- 26.Begovich AB, McClure GR, Suraj VC, Helmuth RC, Fildes N, Bugawan TL, Erlich HA, Klitz W: Polymorphism, recombination, and linkage disequilibrium within the HLA class II region. J Immunol 1992;148:249–258 [PubMed] [Google Scholar]
- 27.Rich SS, Concannon P, Erlich H, Julier C, Morahan G, Nerup J, Pociot F, Todd JA:; The Type 1 Diabetes Genetics Consortium. Ann N Y Acad Sci 2006;1079:1–8 [DOI] [PubMed] [Google Scholar]
- 28.Mychaleckyj JC, Noble JA, Moonsamy PV, Carlson J, Varney MD, Post J, Helmberg W, Pierce J, Bonella P, Fear AL, Lavant E, Louey A, Boyle S, Lane J, Sali P, Kim S, Rappner R, Williams DT, Perdue LH, Reboussin D, Tait BD, Akolkar B, Hilner JE, Steffes MW, Erlich HA: HLA genotyping in The International Type 1 Diabetes Genetics Consortium (T1DGC). Clinical Trials (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson G: Mapping disease genes: family-based association studies. Am J Hum Genet 1995;57:487–498 [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson G: HLA DR antigens and susceptibility to insulin-dependent diabetes mellitus. Am J Hum Genet 1984November;36(6):1309–1317 [PMC free article] [PubMed] [Google Scholar]
- 31.Noble JA, Valdes AM, Bugawan TL, Apple RJ, Thomson G, Erlich HA: The HLA class I A locus affects susceptibility to type 1 diabetes. Hum Immunol 2002;63(8):657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA:; Wellcome Trust Case Control Consortium Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007;450:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howson JM, Walker NM, Clayton D, Todd JA:; Type 1 Diabetes Genetics Consortium Confirmation of HLA class II independent type 1 diabetes associations in the major histocompatibility complex including HLA-B and HLA-A. Diabetes Obes Metab 2009;11Suppl. 1:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payami H, Joe S, Farid NR, Stenszky V, Chan SH, Yeo PP, Cheah JS, Thomson G: Relative predispositional effects (RPEs) of marker alleles with disease: HLA-DR alleles and Graves disease. Am J Hum Genet 1989;45:541–546 [PMC free article] [PubMed] [Google Scholar]
- 35.Bilbao JR, Calvo B, Aransay AM, Martin-Pagola A, Perez de Nanclares G, Aly TA, Rica I, Vitoria JC, Gaztambide S, Noble J, Fain PR, Awdeh ZL, Alper CA, Castaño L: Conserved extended haplotypes discriminate HLA-DR3-homozygous Basque patients with type 1 diabetes mellitus and celiac disease. Genes Immun 2006;7:550–554 [DOI] [PubMed] [Google Scholar]
- 36.Aly TA, Eller E, Ide A, Gowan K, Babu SR, Erlich HA, Rewers MJ, Eisenbarth GS, Fain PR: Multi-SNP analysis of MHC region: remarkable conservation of HLA-A1–B8-DR3 haplotype. Diabetes 2006;55:1265–1269 [DOI] [PubMed] [Google Scholar]
- 37.Bugawan TL, Klitz W, Alejandrino M, Ching J, Panelo A, Solfelix CM, Petrone A, Buzzetti R, Pozzilli P, Erlich HA: The association of specific HLA class I and II alleles with type 1 diabetes among Filipinos. Tissue Antigens 2002;59:452–469 [DOI] [PubMed] [Google Scholar]
- 38.Santin I, Castellanos-Rubio A, Aransay AM, Gutierrez G, Gaztambide S, Rica I, Vicario JL, Noble JA, Castaño L, Bilbao JR: Exploring the diabetogenicity of the HLA-B18-DR3 CEH: independent association with T1D genetic risk close to HLA-DO. Genes Immun 2009;10:596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder JA, Weston A, Tinkle SS, Demchuk E: Electrostatic potential on human leukocyte antigen: implications for putative mechanism of chronic beryllium disease. Environ Health Perspect 2003;111:1827–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaston JS, Goodall JC, Young JL, Young SP: Effect of polymorphism of the HLA-DPA1 chain on presentation of antigenic peptides. Hum Immunol 1997;54:40–47 [DOI] [PubMed] [Google Scholar]
- 41.Kishi H, Okumura A, Tong JJ, Sugiyama E, Matsuno H, Minowada J, Kanai T, Nishimura Y, Muraguchi A: A murine monoclonal antibody (928) recognizing a new epitope formed with a combination of HLA-DPA1*0201 and DPB1*0301 gene products. Hum Immunol 1997;56:114–124 [DOI] [PubMed] [Google Scholar]
- 42.Díaz G, Catálfamo M, Coiras MT, Alvarez AM, Jaraquemada D, Nombela C, Sánchez-Pérez M, Arroyo J: HLA-DPbeta residue 69 plays a crucial role in allorecognition. Tissue Antigens 1998;52:27–36 [DOI] [PubMed] [Google Scholar]
- 43.Lombardi G, Germain C, Uren J, Fiorillo MT, du Bois RM, Jones-Williams W, Saltini C, Sorrentino R, Lechler R: HLA-DP allele-specific T cell responses to beryllium account for DP-associated susceptibility to chronic beryllium disease. J Immunol 2001;166:3549–3555 [DOI] [PubMed] [Google Scholar]
- 44.Berretta F, Butler RH, Diaz G, Sanarico N, Arroyo J, Fraziano M, Aichinger G, Wucherpfennig KW, Colizzi V, Saltini C, Amicosante M: Detailed analysis of the effects of Glu/Lys beta69 human leukocyte antigen-DP polymorphism on peptide-binding specificity. Tissue Antigens 2003;62:459–471 [DOI] [PubMed] [Google Scholar]
- 45.Hammer J, Gallazzi F, Bono E, Karr RW, Guenot J, Valsasnini P, Nagy ZA, Sinigaglia F: Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med 1995;181:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cucca F, Lampis R, Congia M, Angius E, Nutland S, Bain SC, Barnett AH, Todd JA: A correlation between the relative predisposition of MHC class II alleles to type 1 diabetes and the structure of their proteins. Hum Mol Genet 2001;10:2025–2037 [DOI] [PubMed] [Google Scholar]