Abstract
OBJECTIVE
Our recent study demonstrated that Rac1 and NADPH oxidase activation contributes to cardiomyocyte apoptosis in short-term diabetes. This study was undertaken to investigate if disruption of Rac1 and inhibition of NADPH oxidase would prevent myocardial remodeling in chronic diabetes.
RESEARCH DESIGN AND METHODS
Diabetes was induced by injection of streptozotocin in mice with cardiomyocyte-specific Rac1 knockout and their wild-type littermates. In a separate experiment, wild-type diabetic mice were treated with vehicle or apocynin in drinking water. Myocardial hypertrophy, fibrosis, endoplasmic reticulum (ER) stress, inflammatory response, and myocardial function were investigated after 2 months of diabetes. Isolated adult rat cardiomyocytes were cultured and stimulated with high glucose.
RESULTS
In diabetic hearts, NADPH oxidase activation, its subunits' expression, and reactive oxygen species production were inhibited by Rac1 knockout or apocynin treatment. Myocardial collagen deposition and cardiomyocyte cross-sectional areas were significantly increased in diabetic mice, which were accompanied by elevated expression of pro-fibrotic genes and hypertrophic genes. Deficiency of Rac1 or apocynin administration reduced myocardial fibrosis and hypertrophy, resulting in improved myocardial function. These effects were associated with a normalization of ER stress markers' expression and inflammatory response in diabetic hearts. In cultured cardiomyocytes, high glucose–induced ER stress was inhibited by blocking Rac1 or NADPH oxidase.
CONCLUSIONS
Rac1 via NADPH oxidase activation induces myocardial remodeling and dysfunction in diabetic mice. The role of Rac1 signaling may be associated with ER stress and inflammation. Thus, targeting inhibition of Rac1 and NADPH oxidase may be a therapeutic approach for diabetic cardiomyopathy.
Diabetic cardiomyopathy has been defined as ventricular dysfunction that occurs in the absence of changes in blood pressure and coronary artery disease (1). Cardiac structural phenotypes of diabetic cardiomyopathy include cardiomyocyte apoptosis, cardiac hypertrophy, myocardial fibrosis, and interstitial inflammation (2,3), all of which significantly contribute to myocardial dysfunction. Three evident characteristic metabolic disturbances in diabetes, including hyperglycemia, hyperlipidemia, and hyperinsulinemia, are attributable to altered myocardial structure and function in diabetic cardiomyopathy (4). However, the signaling pathways associated with these metabolic triggers remain not fully understood in diabetic hearts.
Several mechanisms involved in diabetic myocardial dysfunction have been suggested, which include increased oxidative stress, impaired calcium homeostasis, upregulation of the renin-angiotensin system, altered substrate metabolism, and mitochondrial dysfunction (3). These changes are closely related to reactive oxygen species (ROS) production. ROS is mainly produced by mitochondria and NADPH oxidase in cardiomyocytes. A cross-talk between mitochondria and NADPH oxidase has been suggested to sustain cellular ROS production under stresses (5–9). Selective inhibition of mitochondrial ROS has been shown to prevent diabetic cardiac changes in type 1 diabetic mice, confirming an important role of mitochondrial ROS (10). Our recent study has revealed that Rac1 via NADPH oxidase activation induces mitochondrial ROS production and plays an essential role in cardiomyocyte apoptosis and myocardial dysfunction in streptozotocin (STZ)-induced diabetes (8). Cell death by apoptosis is the predominant damage in diabetic cardiomyopathy (2). Cardiomyocyte death causes a loss of contractile tissue, which initiates a cardiac remodeling (11). Furthermore, Rac1/NADPH oxidase signaling has also been demonstrated to directly induce cardiac hypertrophy (12,13) and skin fibrosis (14,15). However, direct evidence is lacking as for the contribution of Rac1/NADPH oxidase to myocardial remodeling in the development of diabetic cardiomyopathy.
In this study, we took advantage of the availability of mice with cardiomyocyte-specific Rac1 knockout to analyze the impact of Rac1 on NADPH oxidase activation, endoplasmic reticulum (ER) stress, hypertrophy, fibrosis, and inflammatory response in diabetic hearts. We further investigated the therapeutic effect of the NADPH oxidase inhibitor apocynin on diabetic cardiomyopathy in STZ-induced type 1 diabetic mice.
RESEARCH DESIGN AND METHODS
This investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH Publication No. 85-23). All experimental procedures were approved by the Animal Use Subcommittee at the University of Western Ontario, Canada. Breeding pairs of C57BL/6 mice and mice bearing the modified Rac1 gene containing loxP sites (floxed Rac1) were purchased from The Jackson Laboratory. Transgenic mice with cardiomyocyte-specific expression of Cre recombinase (Cre) under the control of α-myosin heavy chain (α-MHC) were provided by Dr. Dale Evan Abel (University of Utah). Mice with cardiomyocyte-specific Rac1 knockout (Rac1-ko) were generated by crossing the floxed Rac1 mice with mice overexpressing Cre under the control of α-MHC, as we recently described (8). Animals used for experiments were genotyped by PCR (PCR) to detect Rac1 and Cre as described previously (8). A breeding program for mice was implemented at our animal care facilities. (An expanded research design and methods section is available in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1800/DC1.)
Adult male rats (Sprague-Dawley, 200 g body weight) were purchased from Charles River Labs. Adult rat ventricle cardiomyocytes (ARVCs) were isolated and cultured as described previously (8,16,17).
Experimental protocol.
Diabetes was induced in adult male mice (2 months old) by consecutive peritoneal injection of STZ (50 mg/kg/day) for 5 days. Whole blood was obtained from the mouse tail vein 72 h after the last injection of STZ, and random glucose levels were measured using a OneTouch Ultra 2 blood glucose monitoring system (LifeScan, Milpitas, CA). The mice were considered diabetic and used for the study only if they had hyperglycemia (≥15 mmol/l) at 72 h after STZ injection, whereas citrate buffer–treated mice were used as a nondiabetic control (blood glucose <12 mmol/l). Two months after induction of diabetes, Rac1-ko mice and their wild-type (WT) littermates (n = 8–12 in each group) were killed for the following experiments.
In a separate experiment, WT animals were divided into four groups (n = 8–12 in each group) that included control, control-treated, diabetes, and diabetes-treated groups. After induction of diabetes, apocynin, an inhibitor of NADPH oxidase, was administered to the control-treated and diabetes-treated groups in the drinking water (30 mg/kg/day) for 2 months.
Histological analysis.
Hearts were excised, washed with saline solution, and placed in 10% formalin. Hearts were then cut transversely close to the apex to visualize the left ventricle and right ventricle. Several sections of heart (5 μm thick) were prepared and stained with hematoxylin and eosin and a saturated solution of picric acid containing 1% Sirius red for collagen deposition (18). The sections were then visualized by light microscopy and photographed, and the collagen content of the sections was measured by using the computer-assisted morphometry (Image-Pro Plus Version 6.0). For each sample, all available fields (>30 fields) were measured, including the septum and the right and the left ventricle (all fields were analyzed with a ×40 objective lens).
For cardiomyocyte cross-sectional area, sections were stained for membranes with fluorescein isothiocyanate–conjugated wheat germ agglutinin (WGA; Invitrogen) and for nuclei with DAPI (19). A single cardiomyocyte was measured with an image quantitative digital analysis system (NIH Image version 1.6). The outline of 200 cardiomyocytes was traced in each section.
Tissue sections (5 μm) were stained with antibodies against tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-α1, and GRP78, respectively. Detection was carried out by using the EnVision+ system and diaminobenzidine (USCNLIFE, China) as described previously (20).
Statistical analysis.
All data were given as mean ± SD. ANOVA followed by Newman-Keuls test was performed for multigroup comparisons. A value of P < 0.05 was considered statistically significant.
RESULTS
Downregulation of NADPH oxidase activity, its expression, and ROS production in Rac1-ko and apocynin-treated hearts during diabetes.
Administration of STZ resulted in characteristic symptoms of diabetes including hyperglycemia and increased food and fluid intake when compared with age-matched controls (supplemental Table 2, available in the online appendix). Diabetic mice had higher plasma glucose levels (20 – 30 mmol/l) than nondiabetic control mice (<12 mmol/l) 72 h after STZ injection. All animals responded to STZ treatment, and no animal died or was excluded from the study. At termination, fluid intake, food consumption, and plasma glucose levels were higher in diabetic mice than in control mice, and neither Rac1 knockout nor treatment with apocynin for 2 months had significant effects on these changes (supplemental Table 2).
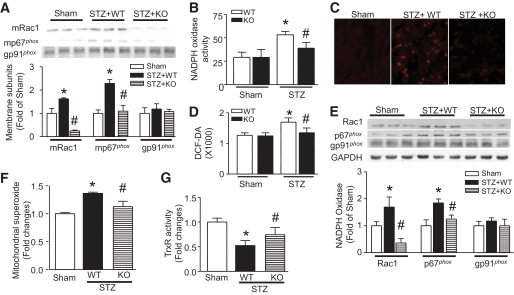
Consistent with our recent report (8), Rac1 protein levels were significantly reduced in Rac1-ko compared with WT hearts (Fig. 1A and C). In contrast, there was no change of Rac1 protein levels in cardiac fibroblasts from Rac1-ko mice compared with WT mice (supplemental Fig. 1). This result confirms cardiomyocyte-specific Rac1 deletion in Rac1-ko mice. Diabetes significantly increased membrane p67phox and Rac1 protein (Fig. 1A), NADPH oxidase activity (Fig. 1B), and ROS production (Fig. 1C and D), which were dramatically reduced in Rac1-ko mice. Diabetes also increased mRNA and/or protein expression of NADPH oxidase subunits (Rac1, gp91phox, p67phox, and p47phox) in the heart. However, their levels were significantly reduced in Rac1-ko diabetic hearts (Fig. 1E and supplemental Fig. 2A and B). Thus, deficiency of Rac1 not only blocks the translocation of p67pho to the membrane, NADPH oxidase activation, and ROS production, but also inhibits its expression in diabetic hearts.
FIG. 1.
Effects of Rac1 knockout on NADPH oxidase and ROS production. Rac1-ko mice (KO) and their WT littermates were injected with STZ. Two months later, NADPH oxidase activation and expression and ROS production in heart tissues were measured. A: Translocalization of Rac1 and p67phox to the membrane. The protein levels of Rac1 (mRac1) and p67phox (mp67phox) were decreased in the membrane fractions of Rac1 KO compared with WT diabetic hearts. The top panel is the representative Western blot for membrane mRac1, mp67phox, and gp91phox from three out of five to six different hearts in each group, and the lower panel is the quantification of mRac1, mp67phox, and gp91phox. NADPH oxidase activity (B), superoxide production (C), and H2O2 production (D) were decreased in diabetic Rac1 KO compared with WT hearts. C is the representative DHE staining (Red signal) for superoxide production from five to six different hearts in each group. E: Rac1, p67phox, and gp91pho protein expression. The protein levels of Rac1 and p67pho were decreased in Rac1 KO compared with WT diabetic hearts. The top panel is the representative Western blot for Rac1, p67phox, and gp91phox from three out of five to six different hearts in each group and the lower panel is the quantification of Rac1, p67phox, and gp91phox. F: Mitochondrial superoxide production was increased in WT diabetic hearts, which was significantly decreased in Rac1 KO hearts. G: Thioredoxin reductase activity was preserved in Rac1 knockout diabetic hearts. Magnification ×40. Data are means ± SD, n = 5–8. *P < 0.05 vs. sham; #P < 0.05 vs. STZ in WT. (A high-quality digital representation of this figure is available in the online issue.)
Deficiency of Rac1 also abrogated the increase of superoxide production in freshly isolated mitochondria from diabetic hearts on addition of pyruvate/malate (Fig. 1F). This result supports the fact that Rac1 activation induces mitochondrial superoxide generation in chronic diabetic hearts, which was also shown in STZ-induced acute diabetic hearts in our recent study (8).
It has been demonstrated that diabetes leads to a loss of antioxidant activity, in particular, thioredoxin system (21). Consistently, thioredoxin reductase activity was significantly reduced in diabetic hearts, which was preserved in Rac1-ko mice (Fig. 1G). This result suggests that Rac1 activation induces the reduction of thioredoxin reductase activity in diabetic hearts.
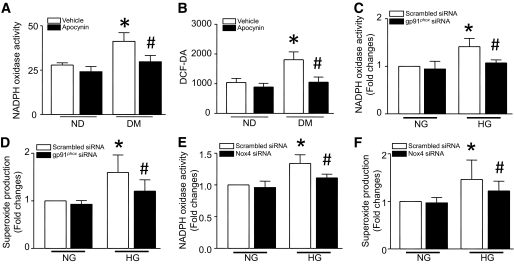
Similar to the effects of Rac1 knockout, administration of apocynin inhibited NADPH oxidase expression (supplemental Fig. 2C–E) and activation and ROS production in diabetic hearts (Fig. 2A and B). To determine the involvement of Nox isoforms, we focused on Nox2 and Nox4, since our previous study showed that cardiomyocytes express Nox2 and Nox4 (22). Cultured ARVCs were transfected with siRNA specific for Nox2 and Nox4, respectively, and then incubated with normal (5.5 mmol/l) or high glucose (33 mmol/l) for 24 h. A scrambled siRNA was used as a control. Knockdown of either Nox2 or Nox4 decreased NADPH oxidase activity and superoxide production in high glucose–stimulated ARVCs (Fig. 2C–F). Thus, both isoforms may be involved in diabetes-induced ROS production.
FIG. 2.
NADPH oxidase and ROS production. Wild-type mice were rendered diabetic by STZ injection, and apocynin was administrated in the drinking water for 2 months. Apocynin treatment significantly reduced NADPH oxidase activity (A) and H2O2 production (B) in diabetic heart tissues. Data are means ± SD, n = 6 – 8. *P < 0.05 vs. nondiabetes (ND) in vehicle; #P < 0.05 vs. diabetes (DM) in vehicle. Cultured adult rat cardiomyocytes were transfected with gp91phox siRNA, Nox4 siRNA, or a scrambled siRNA as a control and then incubated with normal glucose (NG, 5.5 mmol/l) or high glucose (HG, 33 mmol/l) for 24 h. NADPH oxidase activity (C and E) and superoxide production (D and F) were measured in cardiomyocytes. Data are means ± SD, n = 3–4. *P < 0.05 vs. scrambled siRNA in NG; #P < 0.05 vs. scrambled siRNA in HG.
Anti-hypertrophic effect of Rac1 knockout and apocynin in diabetic hearts.
Diabetes induces cardiac hypertrophy, which is one characteristic change of diabetic cardiomyopathy (23). Consistently, cardiomyocyte cross-sectional areas were significantly increased in diabetic compared with nondiabetic hearts, indicative of hypertrophy (Fig. 3A and D). In contrast, hearts from diabetic Rac1-ko mice showed a much smaller increase in cross-sectional areas (Fig. 3A). Similar to the finding of cardiomyocyte cross-sectional areas, induction of cardiac fetal gene expression (atrial natriuretic peptide [ANP] and β-MHC, markers of cardiac hypertrophy) was significantly reduced in Rac1-ko mice compared with their WT hearts in response to diabetes (Fig. 2B and C). These results indicate that Rac1 is critical for the development of cardiac hypertrophy in diabetic mice.
FIG. 3.
Role of Rac1/NADPH oxidase in cardiac hypertrophy. Diabetes was induced by injection of STZ in Rac1-ko and their WT littermates. In a separate experiment, WT diabetic mice were treated with vehicle or apocynin in drinking water for 2 months. A and D: The hearts were fixed, embedded, and sectioned. Sections were stained for membranes with fluorescein isothiocyanate (FITC)-WGA and for nuclei with DAPI. Cardiomyocyte cross-sectional area was measured with an image quantitative digital analysis system. The outline of 200 cardiomyocytes was traced in each section. The mRNA levels of β-MHC (B and E) and ANP (C and F) were determined by real-time RT-PCR in Rac1-ko and WT hearts. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes in WT or vehicle; #P < 0.05 vs. diabetes in WT or vehicle. O.D., optical density.
Having shown that deficiency of Rac1 blocked NADPH oxidase activity and ROS production, and attenuated cardiac hypertrophy in diabetic mice, we reasoned that administration of apocynin, a selective inhibitor of NADPH oxidase would prevent cardiac hypertrophy. In support of this hypothesis, inhibition of NADPH oxidase with apocynin resulted in significant attenuation of hypertrophy, as evidenced by a smaller increase in cardiomyocyte cross-sectional areas (Fig. 3D) and significant downregulation of ANP and β-MHC expressions in apocynin-treated hearts compared with vehicle-treated hearts in response to diabetes (Fig. 3E and F). However, this effect of apocynin was not observed in nondiabetic hearts. These findings support an important role of NADPH oxidase in the development of cardiac hypertrophy in diabetic mice, and thus, apocynin may provide a therapeutic effect on diabetic cardiac changes.
Contribution of Rac1 and NADPH oxidase to myocardial fibrosis in diabetic mice.
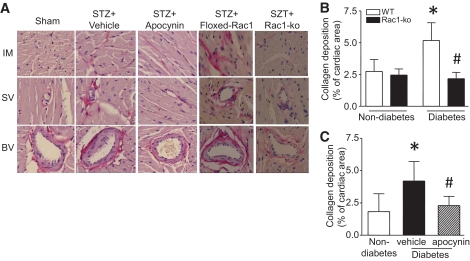
To investigate the role of Rac1 signaling in myocardial fibrosis, we first analyzed total collagen contents in diabetic hearts. In agreement with previous studies (24,25), diabetes significantly increased collagen deposition in mouse hearts (Fig. 4). The collagen deposition was present in both intra-myocardial and peri-vascular areas (Fig. 4A). Deficiency of Rac1 or apocynin treatment reduced diabetes-induced collagen deposition (Fig. 4B and C). Because changes in the collagen (Col) composition, and particularly in Col I and III, compromise cardiac performance (26), we then measured Col I and III expression in the heart. Consistently, the mRNA levels of Col I and III in hearts from diabetic Rac1-ko and apocynin-treated mice were much lower than those in diabetic WT and vehicle-treated mice, respectively (Figs. 5A and B and 6A and B).
FIG. 4.
Role of Rac1/NADPH oxidase in myocardial fibrosis. Diabetes was induced by injection of STZ in Rac1-ko mice and their WT littermates. In a separate experiment, WT diabetic mice were treated with vehicle or apocynin in drinking water for 2 months. The hearts were fixed, embedded, and sectioned. Sections of heart were stained with hematoxylin and eosin and a saturated solution of picric acid containing 1% Sirius red for collagen deposition (see research design and methods). A: Representative staining for collagen deposition is presented for intra-myocardium (IM), small vessel (SV), and big vessel (BV) from each group. Collagen deposition is stained as red color. B and C: Collagen deposition was quantified as percent of cardiac area. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes in WT or vehicle; #P < 0.05 vs. diabetes in WT or vehicle. (A high-quality digital representation of this figure is available in the online issue.)
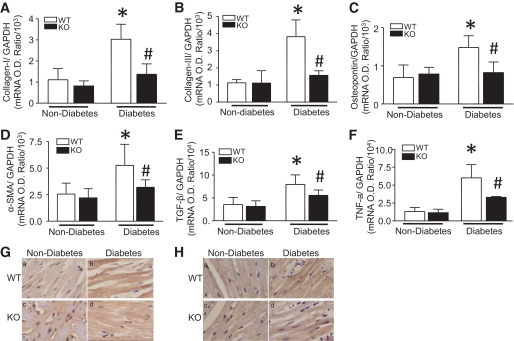
FIG. 5.
Effect of Rac1 knockout on pro-fibrotic genes expression. Diabetes was induced by injection of STZ in Rac1-ko (KO) and their WT littermates. Two months after STZ injection, the mRNA levels of Col I (A), Col III (B), osteopontin (C), α-SMA (D), TGF-β1 (E), and TNF-α (F) were quantified in heart tissues by real-time RT-PCR. G and H: Representative immunohistological stainings for TGF-β1 (G) and TNF-α (H) from four to six different hearts in each group (yellow-brown signal). Magnification ×40. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes in WT; #P < 0.05 vs. diabetes in WT. O.D., optical density. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 6.
Effect of apocynin on pro-fibrotic gene expression. Wild-type mice were rendered diabetic by STZ injection, and apocynin was administrated in the drinking water for 2 months. The mRNA levels of Col I (A), Col III (B), osteopontin (C), α-SMA (D), TGF-β1 (E), and TNF-α (F) were quantified in heart tissues by real-time RT-PCR. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes in vehicle; #P < 0.05 vs. diabetes in vehicle. O.D., optical density.
To further demonstrate the role of Rac1 and NADPH oxidase in diabetes-induced fibrosis, we also analyzed the expression of pro-fibrotic genes in diabetic hearts. In nondiabetic mice, there were no changes in mRNA levels of TGF-β1, α-SMA, and osteopontin between Rac1-ko and WT hearts and between vehicle and apocynin-treated hearts. In response to STZ, the mRNA levels of TGF-β1, α-SMA, and osteopontin were significantly upregulated in WT or vehicle-treated hearts. In parallel with changes in collagen deposition and Col I/III expression, deficiency of Rac1 or apocynin treatment reduced TGF-β1, α-SMA, and osteopontin mRNA expression by 54, 84, and 68% in diabetic Rac1-ko hearts (Fig. 5C–E) and 67, 80, and 62% in apocynin-treated diabetic hearts, respectively (Fig. 6C–E). Similarly, deficiency of Rac1 also inhibited diabetes-induced TGF-β1 protein in the myocardium (Fig. 5G).
Reduced inflammatory response in diabetic Rac1-ko and apocynin-treated hearts.
Rac1 has previously been suggested to be mediators of inflammation (27). Inflammation has been suggested to play an important role in the development of myocardial remodeling (28); we therefore analyzed inflammatory cytokine TNF-α expression in diabetic hearts. In agreement with another report (25), diabetes induced upregulation of TNF-α mRNA and protein expression in the heart. Similar to the hypertrophic and fibrotic effects, the increase in TNF-α mRNA and protein was significantly attenuated by Rac1 knockout or apocynin treatment in diabetic hearts (Figs. 5F, 5H, and 6F). These results suggest that inhibition of Rac1 and NADPH oxidase prevents inflammatory response in diabetic hearts.
Role of Rac1 in ER stress induction.
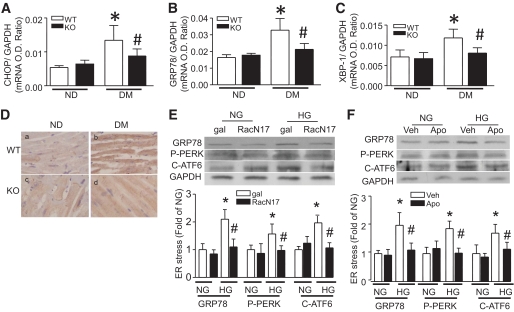
Oxidative stress is involved in ER stress induction, which contributes to myocardial dysfunction (29). A recent study has shown that ER stress is induced in diabetic hearts and plays a role in the development of diabetic cardiomyopathy (30). We therefore hypothesized that blocking Rac1 signaling could prevent ER stress in diabetic hearts. To test this hypothesis, we determined ER stress by analyzing ER stress markers' expression (CHOP, XBP1, and GRP78) (31). Consistent with a previous report (30), diabetes increased CHOP, XBP1, and GRP78 mRNA and/or protein expression in the heart, confirming the presence of ER stress. However, the increases in mRNA and/or protein levels of CHOP, XBP1, and GRP78 were abolished in Rac1-ko mice compared with their WT hearts in response to diabetes (Fig. 7A–D). Thus, these results suggest that disruption of Rac1 signaling prevents ER stress induction in diabetic hearts.
FIG. 7.
Effect of Rac1 knockout on ER stress induction. Diabetes was induced by injection of STZ in Rac1-ko (KO) and their WT littermates. Two months after STZ injection, the mRNA levels of CHOP (A), GRP78 (B), and XBP1 (C) were quantified in heart tissues by real-time RT-PCR. D: GRP78 protein was also determined by immunohistological staining (yellow-brown signal). Magnification ×40. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes (ND) in WT; #P < 0.05 vs. diabetes (DM) in WT. Cultured adult rat cardiomyocytes were infected with Ad-RacN17 (RacN17) or Ad-gal (gal) and then incubated with normal glucose (NG, 5.5 mmol/l) or high glucose (HG, 33 mmol/l) for 24 h. In a separate experiment, cardiomyocytes were incubated with normal or high glucose in the presence of apocynin (Apo) or vehicle (Veh) for 24 h. Western blot was performed for detection of phosphorylated PERK, cleaved ATF-6, GRP78, and GAPDH protein. Infection of Ad-RacN17 (E) and apocynin administration (F) reduced phosphorylated PERK, cleaved ATF-6, and GRP78 protein. The top panel is the representative blot from at least three different cell cultures and the lower panel is the quantification of phosphorylated PERK, cleaved ATF-6, and GRP78 protein. Data are means ± SD, n = 3–4. *P < 0.05 vs. gal or Veh in NG; #P < 0.05 vs. gal or Veh in HG. O.D., optical density. (A high-quality digital representation of this figure is available in the online issue.)
To further demonstrate the role of Rac1 signaling in ER stress and explore the involved pathways, we exposed cultured ARVCs to high glucose and analyzed phosphorylation of PERK, cleaved ATF-6, and GRP78 expression. ARVCs were infected with Ad-RacN17, an adenoviral vector expressing a dominant-negative mutant of Rac1, which specifically blocks Rac1 activation (32), or Ad-gal as an adenoviral control and were then incubated with normal (5.5 mmol/l) or high glucose (33 mmol/l) for 24 h. High glucose significantly increased GRP78 protein, phosphorylated PERK, and cleaved ATF-6 (50 kDa) in ARVCs (Fig. 7E), indicative of induction of ER stress and activation of PERK and ATF-6 pathways (31). In line with a reduction of superoxide production (supplemental Fig. 3), inhibition of Rac1 abrogated high glucose–induced increases in GRP78 protein and activation of PERK and ATF-6 in ARVCs. Similarly, incubation of apocynin inhibited ER stress in high glucose–stimulated ARVCs (Fig. 7F).
Attenuation of myocardial dysfunction in apocynin-treated diabetic mice.
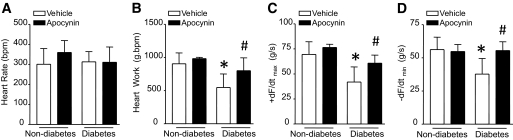
We recently showed that deficiency of Rac1 attenuates myocardial dysfunction in diabetic hearts (8). In the present study, we extended our experiments to examine the therapeutic potential of NADPH oxidase inhibition with apocynin for myocardial dysfunction in diabetic mice. Consistently, STZ-induced diabetic animals showed a significant reduction of +dF/dtmax and −dF/dtmin compared with nondiabetic ones. Importantly, myocardial function was significantly improved in apocynin-treated STZ mice compared with STZ controls (Fig. 8). Apocynin treatment in nondiabetic animals did not affect myocardial function, indicating no potential side effects of apocynin. Thus, administration of apocynin protects myocardial function in diabetic mice.
FIG. 8.
Myocardial function in diabetic mice. Wild-type mice were rendered diabetic by STZ injection, and apocynin was administrated in the drinking water for 2 months. Mouse hearts were isolated and perfused in Langendorff system. Contractile function of heart was determined. Changes in heart rate (A), heart work (B), rate of contraction (+ dF/dTmax, C), and relaxation (−dF/dTmin, D) are presented. Data are means ± SD, n = 6–8. *P < 0.05 vs. nondiabetes in WT; #P < 0.05 vs. diabetes in WT.
DISCUSSION
The present study used mice with cardiomyocyte-specific Rac1 knockout to investigate the role of Rac1 signaling in myocardial remodeling in chronic diabetes. We demonstrated that deficiency of Rac1 reduced cardiac hypertrophy and fibrosis in STZ-induced type 1 diabetic mice. Diabetes-induced NADPH oxidase activation and expression, ROS production, ER stress, and TNF-α expression were also attenuated by Rac1 knockout. Furthermore, pharmacological inhibition of NADPH oxidase prevented myocardial remodeling and alleviated myocardial dysfunction in diabetic mice. Thus, Rac1 and NADPH oxidase activation play a critical role in myocardial remodeling during the development of diabetic cardiomyopathy, and this action of Rac1/NADPH oxidase may be associated with ER stress and inflammatory response in diabetic hearts.
Rac1/NADPH oxidase and diabetic cardiac hypertrophy.
Previous studies have provided direct evidence that demonstrates that NADPH oxidase activation is required for cardiac hypertrophy in different models (33). For example, cardiac hypertrophy induced by angiotensin II and myocardial infarction was prevented in gp91phox (34) and p47phox knockout mice (35), respectively. Rac1 activation is critical for the assembly of active NADPH oxidase to produce superoxide (36). In this regard, overexpression of active Rac1 induces cardiomyocyte hypertrophy (13), and cardiomyocyte specific Rac1 knockout prevents angiotensin-induced hypertrophy in mice (12). Thus, Rac1/NADPH oxidase signaling is important in cardiac hypertrophy. However, the role of NADPH oxidase may be both isoform specific and depending on stimuli (34,37). In diabetes, it remains unclear whether Rac1 and NADPH oxidase are involved in diabetic cardiac hypertrophy. Previous studies have shown that activation of a gp91phox-containing NADPH oxidase results in nuclear factor–κB activation and upregulation of atrial natriuretic factor mRNA in cardiomyocytes in response to early-glycated Amadori products (38), and cardiac hypertrophy is attenuated in association with downregulation of NADPH oxidase by N-acetylcysteine in STZ-induced diabetic rats (39). However, conclusive evidence is lacking to link Rac1/NADPH oxidase to the development of cardiac hypertrophy in diabetes. In this study, we have provided convincing evidence that demonstrates a critical role of Rac1 in diabetic cardiac hypertrophy. Cardiomyocyte-specific deletion of Rac1 reduced cardiomyocyte cross-sectional areas and prevented hypertrophic gene ANP and β-MHC expression in diabetic hearts. Furthermore, the role of Rac1 is mediated through NADPH oxidase, since deficiency of Rac1 blocks NADPH oxidase activation, its expression, and ROS production, and pharmacological inhibition of NADPH oxidase with apocynin reduces cardiac hypertrophy in diabetic mice. Although apocynin may have other antioxidant effects independent of NADPH oxidase inhibition (40), our data showed that administration of apocynin prevented NADPH oxidase activity and reduced ROS production in diabetic hearts, confirming the inhibitory effect of apocynin on NADPH oxidase activation. Thus, the present study extends the role of Rac1 via NADPH oxidase to the development of diabetic cardiac hypertrophy. However, future studies will be required to investigate whether NADPH oxidase–independent pathways are also involved in Rac1-induced cardiac hypertrophy in diabetes.
In addition to a direct pro-hypertrophic role of Rac1 in cardiomyocyte, the anti-hypertrophic effects of Rac1 knockout and NADPH oxidase inhibition may also partly result from the prevention of cardiomyocyte apoptosis, which otherwise will lead to compensative hypertrophy, since deficiency of Rac1 or inhibition of NADPH oxidase reduces cardiomyocyte apoptosis in diabetic hearts (8).
Rac1/NADPH oxidase and diabetic myocardial fibrosis.
Myocardial fibrosis is one of the most important mechanisms for the pathogenesis of diabetic cardiomyopathy (41). Consistent with previous studies (24,25), we found increased collagen deposition in the hearts of STZ-induced diabetic mice, correlating with myocardial dysfunction. Col I and III, which constitute 90% of cardiac collagen and are especially important for cardiac hemodynamics (26), were also upregulated in diabetic hearts. In contrast, cardiomyocyte-specific deletion of Rac1 blunted total cardiac collagen deposition and reduced the levels of Col I and III expression. Similarly, pharmacological inhibition of NADPH oxidase prevented myocardial fibrosis and Col I and III expression, in line with the improved myocardial function. Thus, Rac1/NADPH oxidase activation leads to the induction of fibrosis in diabetic hearts. Further evidence to support the role of Rac1/NADPH oxidase was from previous studies that demonstrated that atorvastatin inhibited Rac1 activity and reduced myocardial fibrosis in diabetic hearts (25), and inhibition of NADPH oxidase attenuated interstitial fibrosis of noninfarcted myocardium after myocardial infarction in type 2 diabetes (42).
It is known that fibroblasts play an important role in fibrosis. Activation of fibroblasts with limited proliferative capacity undergoes a conversion to myofibroblasts, leading to the formation of fibrosis (43). The change in fibroblast properties is initiated by TGF-β1, which stimulates the expression of genes that are characteristic of myofibroblasts, including α-SMA and osteopontin. In this regard, TGF-β1, α-SMA and osteopontin expression were significantly upregulated in diabetic hearts. However, their levels were decreased by deficiency of Rac1 or pharmacological inhibition of NADPH oxidase. This data provides mechanistic insight into the involvement of Rac1/NADPH oxidase in myocardial fibrosis.
Nevertheless, the mechanisms by which Rac1/NADPH oxidase induces fibrosis in diabetic hearts are not fully understood. Our data showed that diabetes induced myocardial TNF-α expression, which was also prevented by Rac1 knockout and inhibition of NADPH oxidase. Because myocardial fibrosis in diabetic cardiomyopathy is partly mediated by the upregulation of cytokines that have a pro-fibrotic action, including TNF-α (44), the reduction of myocardial TNF-α expression may be one of the mechanisms involved in the anti-fibrotic effects of Rac1 knockout and NADPH oxidase inhibition in STZ diabetic mice. In addition, we recently demonstrated that Rac1 via NADPH oxidase activation is required for cardiomyocyte apoptosis in diabetes (8). Loss of cardiomyocytes through both necrosis and apoptosis are replaced by fibrosis, since cardiomyocytes are not able to proliferate and the generation of new cardiomyocytes is largely limited. Thus, inhibition of cardiomyocyte apoptosis may be another possible mechanism for reduction of fibrosis by disruption of Rac1/NADPH oxidase signaling in diabetic hearts.
It is important to mention that the present study demonstrated that Rac1 in cardiomyocytes contributes to fibrosis, since the levels of Rac1 protein are not altered in cardiac fibroblasts from cardiomyocyte-specific Rac1 knockout mice compared with their WT littermates. It is likely that Rac1 in fibroblasts, albeit speculation, also plays a role in myocardial fibrosis in diabetes, since mice containing a fibroblast-specific deletion of Rac1 showed resistance to the bleomycin-induced model of skin fibrosis (15) and impaired myofibroblast formation in the dermal punch model of cutaneous wound healing (14).
ER stress and diabetic cardiomyopathy.
ER stress is induced by accumulation of unfolded proteins, resulting from oxidative stress, ischemia, disturbance of calcium homeostasis, and overexpression of normal and/or incorrectly folded proteins (31). The resulting ER stress triggers the unfolded protein response, which activates ER transmembrane sensors to initiate the adaptive responses. These ER transmembrane sensors include protein kinase–like ER kinase (PERK), inositol-requiring kinase 1 (IRE1), and activating transcription factor 6 (ATF6), and their activation results in phosphorylation of eukaryotic translation initiation factor-2α (eIF2α), transcription factor ATF4 translation, XBP1 splicing, and finally the induction of the unfolded protein response related genes, including chaperones GRP78 and GRP94, XBP1, and CHOP. If ER stress is prolonged or overwhelming, however, it can induce cell death through CHOP and/or other pathways. Studies have revealed that ER stress is implicated in the pathophysiology of heart failure and ischemic heart disease (45). In diabetes, more recent studies have shown that cardiac ER stress was induced and linked to cell death in STZ-induced type 1 diabetes, which may play a part in diabetic cardiomyopathy (30). Levels of ER stress makers (phosphorylated PERK, IRE-1, and eIF2α) were significantly elevated in cardiomyocyte from type 2 diabetic db/db mice, presumably contributing to cardiomyocyte dysfunction (46). ER stress was also observed in type 2 diabetic rats and compromised myocardial response to cytoprotective signaling (47). These studies suggest that ER stress may play a part in diabetic heart diseases. In agreement with these previous studies, our data also showed the induction of ER stress in STZ-induced diabetic hearts. More importantly, we demonstrated for the first time that deficiency of Rac1 inhibited the expression of ER stress markers, suggesting a critical role of Rac1 signaling in ER stress in diabetic hearts. To characterize whether the role of Rac1 signaling in ER stress could be reproduced by high glucose levels, we extended our analyses to cardiomyocytes. Direct exposure of cardiomyocytes to high glucose induced ER stress. Selective inhibition of Rac1 or NADPH oxidase prevented ER stress in high glucose–stimulated cardiomyocytes. We further demonstrated that high glucose–induced ER stress was associated with activation of PERK- and ATF-6–dependent pathways. This is consistent with a previous report in diabetic hearts (30). Furthermore, activation of PERK and ATF-6 was dependent on Rac1/NADPH oxidase signaling in high glucose–induced ER stress, since inhibition of Rac1 or NADPH oxidase prevented phosphorylation of PERK and reduced cleaved ATF-6 in cardiomyocytes. Given the association of ER stress with apoptosis (31), hypertrophy, and myocardial dysfunction (48), ER stress may be one of the mechanisms by which Rac1/NADPH oxidase induces diabetic cardiomyopathy.
In summary, whereas studies have implied the involvement of Rac1 and NADPH oxidase in diabetic cardiomyopathy (39,49), this study provided conclusive evidence that supports a critical role of Rac1/NADPH oxidase in the development of cardiac hypertrophy, fibrosis, and inflammatory response, leading to myocardial dysfunction in type 1 diabetic mice. The role of Rac1 signaling may be associated with ER stress. Thus, targeting inhibition of Rac1 and NADPH oxidase may be a therapeutic approach for diabetic cardiomyopathy.
ACKNOWLEDGMENTS
This study was supported by an operating grant awarded to T.P. from the Canadian Institutes of Heart Research (MOP93657). T.P. is a recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada.
No potential conflicts of interest relevant to this article were reported.
J.L. wrote manuscript, researched data. H.Z. researched data. E.S. researched data. L.W. researched data. M.A. contributed to discussion, reviewed/edited manuscript. T.P. researched data, wrote manuscript, reviewed/edited manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Zarich SW, Nesto RW: Diabetic cardiomyopathy. Am Heart J 1989;118:1000–1012 [DOI] [PubMed] [Google Scholar]
- 2.Cai L, Kang YJ: Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 2003;3:219–228 [DOI] [PubMed] [Google Scholar]
- 3.Boudina S, Abel ED: Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]
- 4.Poornima IG, Parikh P, Shannon RP: Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605 [DOI] [PubMed] [Google Scholar]
- 5.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX: Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doughan AK, Harrison DG, Dikalov SI: Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008;102:488–496 [DOI] [PubMed] [Google Scholar]
- 7.Lee SB, Bae IH, Bae YS, Um HD: Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 2006;281:36228–36235 [DOI] [PubMed] [Google Scholar]
- 8.Shen E, Li Y, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, Peng T: Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 2009;58:2386–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK: Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther 2005;4:1367–1373 [DOI] [PubMed] [Google Scholar]
- 10.Shen X, Zheng S, Metreveli NS, Epstein PN: Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 2006;55:798–805 [DOI] [PubMed] [Google Scholar]
- 11.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P: IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 2001;50:1414–1424 [DOI] [PubMed] [Google Scholar]
- 12.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK: Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A 2006;103:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pracyk JB, Tanaka K, Hegland DD, Kim KS, Sethi R, Rovira II, Blazina DR, Lee L, Bruder JT, Kovesdi I, Goldshmidt-Clermont PJ, Irani K, Finkel T: A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J Clin Invest 1998;102:929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu SW, Liu S, Eastwood M, Sonnylal S, Denton CP, Abraham DJ, Leask A: Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 2009;4:e7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Kapoor M, Shi-wen X, Kennedy L, Denton CP, Glogauer M, Abraham DJ, Leask A: Role of Rac1 in a bleomycin-induced scleroderma model using fibroblast-specific Rac1-knockout mice. Arthritis Rheum 2008;58:2189–2195 [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T: Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 2009;46:51–61 [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Li Y, Feng Q, Arnold M, Peng T: Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res 2009;84:100–110 [DOI] [PubMed] [Google Scholar]
- 18.Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kühl U, Schultheiss HP: Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 1999;99:2750–2756 [DOI] [PubMed] [Google Scholar]
- 19.Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, Huang Y, He CW, Shi Y, Chen X, Nghiem MP, Liu Y, Chen M, Dawood F, Fukuoka M, Maekawa Y, Zhang L, Leask A, Ghosh AK, Kirshenbaum LA, Liu PP: Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest 2009;119:2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng T, Sadusky T, Li Y, Coulton GR, Zhang H, Archard LC: Altered expression of Bag-1 in Coxsackievirus B3 infected mouse heart. Cardiovasc Res 2001;50:46–55 [DOI] [PubMed] [Google Scholar]
- 21.Luan R, Liu S, Yin T, Lau WB, Wang Q, Guo W, Wang H, Tao L: High glucose sensitizes adult cardiomyocytes to ischaemia/reperfusion injury through nitrative thioredoxin inactivation. Cardiovasc Res 2009;83:294–302 [DOI] [PubMed] [Google Scholar]
- 22.Peng T, Lu X, Feng Q: Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression. Circulation 2005;111:1637–1644 [DOI] [PubMed] [Google Scholar]
- 23.Shehadeh A, Regan TJ: Cardiac consequences of diabetes mellitus. Clin Cardiol 1995;18:301–305 [DOI] [PubMed] [Google Scholar]
- 24.Tschöpe C, Walther T, Königer J, Spillmann F, Westermann D, Escher F, Pauschinger M, Pesquero JB, Bader M, Schultheiss HP, Noutsias M: Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J 2004;18:828–835 [DOI] [PubMed] [Google Scholar]
- 25.Van Linthout S, Riad A, Dhayat N, Spillmann F, Du J, Dhayat S, Westermann D, Hilfiker-Kleiner D, Noutsias M, Laufs U, Schultheiss HP, Tschöpe C: Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 2007;50:1977–1986 [DOI] [PubMed] [Google Scholar]
- 26.Spinale FG: Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 2002;90:520–530 [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Shan L, Peng T: Rac1 mediates sex difference in cardiac tumor necrosis factor-alpha expression via NADPH oxidase-ERK1/2/p38 MAPK pathway in endotoxemia. J Mol Cell Cardiol 2009;47:264–274 [DOI] [PubMed] [Google Scholar]
- 28.Frantz S, Bauersachs J, Ertl G: Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 2009;81:474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo R, Ma H, Gao F, Zhong L, Ren J: Metallothionein alleviates oxidative stress-induced endoplasmic reticulum stress and myocardial dysfunction. J Mol Cell Cardiol 2009;47:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L: Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med 2009;13:1499–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Bailly-Maitre B, Reed JC: Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 2005;115:2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggiò M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G: Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res 2006;98:218–225 [DOI] [PubMed] [Google Scholar]
- 33.Murdoch CE, Zhang M, Cave AC, Shah AM: NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 2006;71:208–215 [DOI] [PubMed] [Google Scholar]
- 34.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM: Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 2003;93:802–805 [DOI] [PubMed] [Google Scholar]
- 35.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, Schaefer A, Holland SM, Sorrentino S, Manes C, Schieffer B, Drexler H, Landmesser U: Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 2007;100:894–903 [DOI] [PubMed] [Google Scholar]
- 36.Hordijk PL: Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 2006;98:453–462 [DOI] [PubMed] [Google Scholar]
- 37.Maytin M, Siwik DA, Ito M, Xiao L, Sawyer DB, Liao R, Colucci WS: Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation 2004;109:1168–1171 [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC: Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation 2006;113:1235–1243 [DOI] [PubMed] [Google Scholar]
- 39.Guo Z, Xia Z, Jiang J, McNeill JH: Downregulation of NADPH oxidase, antioxidant enzymes, and inflammatory markers in the heart of streptozotocin-induced diabetic rats by N-acetyl-L-cysteine. Am J Physiol Heart Circ Physiol 2007;292:H1728–H1736 [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM: Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension 2008;51:172–174 [DOI] [PubMed] [Google Scholar]
- 41.Asbun J, Villarreal FJ: The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 2006;47:693–700 [DOI] [PubMed] [Google Scholar]
- 42.Matsushima S, Kinugawa S, Yokota T, Inoue N, Ohta Y, Hamaguchi S, Tsutsui H: Increased myocardial NAD(P)H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Am J Physiol Heart Circ Physiol 2009;297:H409–H416 [DOI] [PubMed] [Google Scholar]
- 43.Singh M, Foster CR, Dalal S, Singh K: Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 2010;48:538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschöpe C, Walther T, Escher F, Spillmann F, Du J, Altmann C, Schimke I, Bader M, Sanchez-Ferrer CF, Schultheiss HP, Noutsias M: Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J 2005;19:2057–2059 [DOI] [PubMed] [Google Scholar]
- 45.Minamino T, Kitakaze M: ER stress in cardiovascular disease. J Mol Cell Cardiol 2010;48:1105–1110 [DOI] [PubMed] [Google Scholar]
- 46.Dong F, Ren J: Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 2009;17:262–268 [DOI] [PubMed] [Google Scholar]
- 47.Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K: Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes 2009;58:2863–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H, Liao Y, Minamino T, Asano Y, Asakura M, Kim J, Asanuma H, Takashima S, Hori M, Kitakaze M: Inhibition of cardiac remodeling by pravastatin is associated with amelioration of endoplasmic reticulum stress. Hypertens Res 2008;31:1977–1987 [DOI] [PubMed] [Google Scholar]
- 49.Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, Privratsky JR, Ren J: Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med 2006;40:1419–1429 [DOI] [PubMed] [Google Scholar]