Abstract
OBJECTIVE
The (CTG)n polymorphism in the serum carnosinase (CN-1) gene affects CN-1 secretion. Since CN-1 is heavily glycosylated and glycosylation might influence protein secretion as well, we tested the role of N-glycosylation for CN-1 secretion and enzyme activity. We also tested whether CN-1 secretion is changed under hyperglycemic conditions.
RESULTS
N-glycosylation of CN-1 was either inhibited by tunicamycin in pCSII-CN-1–transfected Cos-7 cells or by stepwise deletion of its three putative N-glycosylation sites. CN-1 protein expression, N-glycosylation, and enzyme activity were assessed in cell extracts and supernatants. The influence of hyperglycemia on CN-1 enzyme activity in human serum was tested in homozygous (CTG)5 diabetic patients and healthy control subjects.
Tunicamycin completely inhibited CN-1 secretion. Deletion of all N-glycosylation sites was required to reduce CN-1 secretion efficiency. Enzyme activity was already diminished when two sites were deleted. In pCSII-CN-1–transfected Cos-7 cells cultured in medium containing 25 mmol/l d-glucose, the immature 61 kilodaltons (kDa) CN-1 immune reactive band was not detected. This was paralleled by an increased GlcNAc expression in cell lysates and CN-1 expression in the supernatants. Homozygous (CTG)5 diabetic patients had significantly higher serum CN-1 activity compared with genotype-matched, healthy control subjects.
CONCLUSIONS
We conclude that apart from the (CTG)n polymorphism in the signal peptide of CN-1, N-glycosylation is essential for appropriate secretion and enzyme activity. Since hyperglycemia enhances CN-1 secretion and enzyme activity, our data suggest that poor blood glucose control in diabetic patients might result in an increased CN-1 secretion even in the presence of the (CTG)5 allele.
Diabetic nephropathy (DN) is the foremost cause for dialysis in the Western world (1). Prime risk factors for developing DN are poor glycemic control and high blood pressure; yet, appropriate treatment of individual patients to minimize these risk factors can only delay the onset, but does not eliminate susceptibility to develop DN (2,3). We recently demonstrated that susceptibility to DN is strongly associated with a polymorphism in the CNDP1 gene (4). This gene encodes the serum carnosinase (CN-1) protein which degrades carnosine into β-alanine and histidine. We have also demonstrated that the CNDP1 polymorphism affects carnosinase secretion, that is, a low CN-1 secretion is observed when (CTG)5 is present in the signal peptide of CN-1 (5). This is in line with the finding that CN-1 activity is low in (CTG)5 homozygous individuals (4). Overexpression of CNDP1 in the db/db model results in early onset of diabetes, whereas in db/db mice that were fed with carnosine, the onset of diabetes was delayed and glucose metabolism was improved (6). This unambiguously shows the relevance of the carnosine carnosinase system for diabetic complications, and can also explain why low serum CN-1 activity is protective. Several mechanisms for the protective role of carnosine have been postulated (4,7–10), but definite proof for its beneficial effect on DN remains to be assessed.
Although CNDP1 has been confirmed as a susceptibility locus (11,12), more recently this association was questioned by Wanic et al. (13). The negative findings of Wanic et al. may be explained by the fact that baseline renal function, proteinuria, and mortality were not included in their Cox proportional hazards regression analyses (14). Alternatively, their data might indicate that apart from the (CTG)n polymorphism, CN-1 secretion is influenced by other factors.
N-glycosylation is initiated in the endoplasmatic reticulum (ER) by the transfer of preassembled glucose3-mannose9-N-acetylglucosamine2 (Glc)3(Man)9(GlcNAc)2 core oligosaccharides from the dolichylpyrophosphate carrier to asparagine residues in nascent proteins. Three different nucleotide-activated sugar donors are required as substrates for the assembly of dolichol-linked core oligosaccharides: UDP-N-acetylglucosamine (UDP-GlcNAc), GDP-mannose, and UDP-glucose. N-glycosidic bonds anchor the core oligosaccharides to side chains of asparagines located within a consensus sequence of Asn-X-Thr/Ser. Not all consensus sequences within a given protein are occupied. This results in generation of differentially N-glycosylated proteins. Moreover, both the supply of oligosaccharide substrates and the activity of the oligosaccharide-transferring enzyme determines N-glycosylation efficiency. N-glycosylation improves protein stability and solubility, and act as specific molecular recognition sites for the recruitment of chaperones (15). Many proteins need proper N-glycosylation to be correctly folded. Inaccurately N-glycosylated proteins may therefore not be secreted, and those that are still secreted may have altered substrate binding properties and modified enzyme activity.
The hexosamine biosynthesis pathway is responsible for both O- and N-glycosylation, using substrates (e.g., fructose-6P) that are key metabolites in carbon, nitrogen, and energy homeostasis (16). Hence, the extent of GlcNAc branching is dependent on enzyme kinetics and metabolic flux through the hexosamine pathway to generate UDP-GlcNAc. Lau et al. (17) showed that the-N-glycan branching pathway cooperates with the number of N-glycans on glycoproteins to differentially regulate their surface level in response to hexosamine flux. Similarly, Sasai et al. (18) demonstrated that the level of UDP-GlcNAc is a critical factor in the production of β1,6 branched N-glycans. If high glucose concentrations lead to a higher flux through the hexosamine (19) pathway, it is conceivable that this might have potential consequences for N-glycans such as CN-1. In fact, Bar-On et al. (20) showed in experimental diabetes that a dolichol-mediated increase in protein N-glycosylation occurs. They suggest that the dolichol-N-glycosylation pathway may represent another detrimental aspect of hyperglycemia and may operate by dolichol mass action rather than through glycosylating enzyme activity.
CN-1 is a heavily glycosylated protein with three putative N-glycosylation sites at asparagine N322, N382, and N402 (21). In the present study, therefore, we investigated whether N-glycosylation affects CN-1 protein secretion and enzyme activity of CN-1. Because an increased glucose flux through the hexosamine pathway increases GlcNAc concentrations (19), we hypothesized that N-glycosylation of CN-1 is affected by hyperglycemia, resulting in an increased CN-1 secretion and enzyme activity.
RESEARCH DESIGN AND METHODS
Construction of CNDP1 mutants.
A cDNA clone of the CNDP1 gene containing six CTG-repeats (RZPD Library 983, entry No BX094414), kindly provided by Dr. M. Moeller (Department of Nephrology, RWTH, Aachen, Germany), was used as a template to construct different CNDP1 variants by PCR. Glycosylation sites of CN-1 were deleted stepwise using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene Europe, Amsterdam, The Netherlands). In brief, single amino acid exchanges, that is, asparagine for glycine, were induced in the wild-type CNDP1 cDNA by mutagenic primers. The sequence of the primers that were used is depicted in Table 1. PCR reactions were performed according to standard procedures. For mutants lacking one glycosylation site, either one of the asparagines residues at N322, N382, or N402 were exchanged. For mutants lacking two glycosylation sites, combinations of residues N322 and N382, N322, and N402 or N382 and N402 were mutated. Finally, mutants that lack all three N-glycosylation sites at position N322, N382, and N402 were constructed. All CNDP1 constructs and mutants were cloned into pCSII + mt vector. For the N-glycosylation-lacking mutants, stopcodons were removed to generate a myc-tagged CN-1 protein.
TABLE 1.
Primers used for site-directed mutagenesis
| Forward primer | Reverse primer | |
|---|---|---|
| N322G | 5′gacctagaagaataccggggtagcagccgggttgagaa3′ | 5′ttctcaacccggctgctaccccggtattcttctaggtc3′ |
| N382G | 5′cgtctagtccctcacatgggtgtgtctgcggtggaaaa3′ | 5′ttttccaccgcagacacacccatgtgagggactagacg3′ |
| N402G | 5′cttgaagatgtgttctccaaaagaggtagttccaacaagatggttgtttc3′ | 5′gaaacaaccatcttgttggaactacctcttttggagaacacatcttcaag3′ |
N-glycosylation sites were step-wise deleted using mutagenic primers. Mismatched base pairs containing the mutations are underlined.
Cell culture and transfection.
Cos-7 cells (Invitrogen, Karlsruhe, Germany) were cultured in Dulbecco's modified Eagle's medium (DMEM, PAA Laboratories, Pasching, Austria) enriched with 10% FCS and 1% penicillin/streptomycin at 37°C and 5% CO2. The cells were cotransfected with the various CNDP1 constructs and pCruz-green fluorescent protein (Santa Cruz Biotechnology, Heidelberg, Germany) by Nanofectin according to the manufacturer's instructions (PAA Laboratories). Four hours later, medium was replaced by DMEM medium containing 1% penicillin/streptomycin. To inhibit N-glycosylation, 10 μg/ml of tunicamycin (Sigma-Aldrich Chemie, Steinheim, Germany) was added to the medium. Cells and supernatants were harvested 48 h later. For high glucose experiments, Cos-7 cells were transfected with the CNDP1 (CTG)5 variant and cultured for 72 h in DMEM containing 25 mmol/l d-(+)-glucose (Sigma-Aldrich) and 1% penicillin/streptomycin. To decrease GlcNAc production, hexosamine biosynthesis pathway was inhibited by supplementing 20 μmol/l azaserine (Santa Cruz Biotechnology) to the glucose-conditioned DMEM medium during the incubation period. No cell toxicity was observed using this concentration, as determined by trypan blue exclusion. Transfected cells cultured for the same time period in standard medium (DMEM, 5 mmol/l d-(+)-glucose, 1% penicillin/streptomycin) were used as control. Hereafter, supernatants were harvested and transfection efficiency was assessed on an aliquot of the cell suspension by fluorescence-activated cell sorter analysis using green fluorescent protein as read-out. In general, transfection efficiency was above 70%. In all experiments, supernatants were concentrated using a Centricon centrifugal filter device 30,000MW (Millipore, Schwalbach, Germany). Cells were lysed on ice by addition of 1% Triton X-100 containing lysis buffer, supplemented with 1 mmol/l 1,4-dithiol-dL-threitol (Fluka Chemie GmbH, Buchs, Germany), phosphatase inhibitor (Sigma-Aldrich) and protease inhibitor (Roche, Mannheim, Germany). Cell lysates were centrifuged for 10 min (14,000g at 4°C) to remove insoluble debris.
Western blot analysis.
For detection of carnosinase in cell lysates and supernatants, gel electrophoreses and subsequent Western blotting was performed. In some experiments, samples were deglycosylated by PNGase F (New England Biolabs, Frankfurt, Germany) treatment according to the manufacturer's recommendations. All samples were boiled for 10 min in Laemmli sample buffer (Bio-Rad, München, Germany) before loading on an 8% SDS-PAGE. Proteins were transferred electrophoretically to a polyvinylidene fluoride membrane (Roche, Mannheim, Germany) by semidry blotting. Hereafter the membranes were blocked for 1 h at room temperature in TBS-Tween 20 (0.3%, Sigma-Aldrich) containing 10% milk powder. Anti-myc antibody (Abcam, Cambridge, U.K.) was used for the detection of CN-1 produced by transfected cells. For detection of CN-1 in human serum samples, mouse monoclonal anti-CN-1 antibody (Clone RYSK-173, raised against human recombinant CN-1) was used. To assess the amount of GlcNAc modified proteins, the membranes were incubated overnight with anti-N-acetylglucosamine antibody (Abcam). After incubation with appropriate horseradish-peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology) antibody binding was visualized by enhanced chemiluminescence (PerkinElmer, Boston, MA). Intensity of the bands was measured by densitometry using ImageJ 1.36b software.
Measurement of carnosinase activity.
Serum carnosinase activity (CN1) was assayed according to a method described by Teufel et al. (21). Shortly, the reaction was initiated by addition of carnosine and/or homocarnosine and was stopped by adding 1% trichloracetic acid. Liberated histidine was derivatized by adding o-phtaldialdehyd, and fluorescence was read using a MicroTek plate reader (λExc: 360 nm; λEm: 460 nm). The recombinant human CN1 was obtained from R&D Systems (Minneapolis, MN). CN-1 activity in cell supernatants was normalized to transfection efficiency.
Patients.
Diabetic patients and healthy control subjects were recruited from our clinic. Blood samples were collected for measuring CN1 activity as described above and for CN-1 genotyping as described previously (4). Eleven (CTG)5 homozygous diabetic patients were identified (6 female, 5 male; mean age, 62 years; range 36–84 years). There were 3 type 1 and 8 type 2 diabetic patients included. Healthy control subjects consisted of 15 individuals (7 male, 8 female; mean age, 41 years; range 36–84 years). Demographic characteristics of patients and control subjects are shown in Table 2. All participants gave informed consent. Ethical approval was given by the Second Ethics Committee of Heidelberg University (amendment no. 2 and 3 to ethical approval no. 0193/2001).
TABLE 2.
Demographic characteristics of patients and control subjects
| n | Age (years) | Male/female | HbA1c (%) | Duration of DM (years) | RR (n) | DR (n) | |
|---|---|---|---|---|---|---|---|
| Diabetic patients | 11 | 60 (37–76) | 5/6 | 7.4 (6.0–8.4) | 17.9 (6–35) | 7 | 3 |
| Control subjects | 15 | 41 (36–84) | 7/8 | nd | 0 | No | No |
All data are expressed as mean. Values in parentheses represent the range; DM, diabetes mellitus; DR, diabetic retinopathy; n, number of patients; nd, not done; RR, hypertension (defined as repeatedly elevated blood pressure >140/90 mmHg).
Statistical analysis.
Quantitative data are given as mean ± SD. Student t test was calculated to compare groups. Values of P < 0.05 were considered statistically significant.
RESULTS
N-glycosylation modulates secretion and enzyme activity of CN-1.
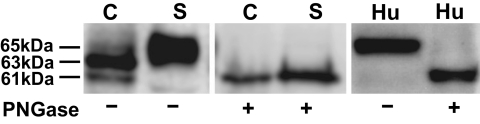
Three CN-1 immunoreactive bands, ranging from 61 to 65 kDa, were found by Western blotting in cell lysates of pCSII-CNDP1–transfected Cos-7 cells. Although the 65-kDa band was always weakly expressed in cell lysates, in supernatants it was the most prominent band. After PNGase F treatment, only the 61-kDa band was found, suggesting that the lower CN-1 band represents the immature CN-1 protein devoid of N-glycosylation (Fig. 1). In human serum, only one CN-1 reactive band was detected corresponding to a molecular weight of 65 kDa. PNGase F treatment of serum reduced its molecular weight to 61 kDa (Fig. 1).
FIG. 1.
CN-1 expression in CNDP1-transfected Cos-7 cells and human serum. After transfection, cell extracts (C) and supernatants (S) were harvested as described, and the samples were treated with PNGase (+) or were left untreated (−). Similarly, aliquots of human serum (Hu) were subjected to PNGase treatment. Note that in cell extracts, two major immunoreactive bands for CN-1 were detected by Western blot analysis with apparent molecular weight of 61 kDa and 63 kDa, respectively. The 65-kDa band was prominent in serum and supernatants of transfected cells, whereas the expression hereof was weak in cell extracts. After PNGase treatment, the molecular weight of CN-1 in serum, supernatants, and cell extracts shifted to 61 kDa. C, cell extracts; S, supernatants; Hu, human serum.
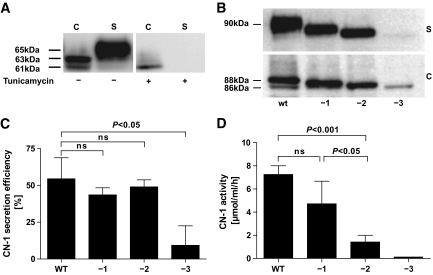
To study the role of N-glycosylation for CN-1 protein secretion, we first used tunicamycin to inhibit N-glycosylation. Tunicamycin is an antibiotic that blocks the reaction of UDP-GlcNAc and dolicholphosphate; as a consequence, the synthesis of all N-linked glycoproteins is inhibited. Tunicamycin completely prevented secretion of CN-1 in pCSII-CNDP1–transfected cells as demonstrated by Western blotting. CN-1 expression in cell extracts was quantitatively and qualitatively changed by tunicamycin. Only the immature 61-kDa CN-1 band was detected in significantly lower amounts (Fig. 2A).
FIG. 2.
Influence of N-glycosylation on CN-1 secretion. A: CNDP1-transfected cells were treated with tunicamycin (10 μg/ml) (+) for 48 h or left untreated (−). Hereafter, cell extracts (C) and supernatant (S) were subjected to Western blot analysis. Note that in the presence of tunicamycin, qualitative and quantitative differences for CN-1 expression in cell extracts were observed, whereas in supernatants of these cells, no CN-1 expression was found. B: N-glycosylation sites of wild-type CNDP1 were deleted by site-directed mutagenesis. Numbers below the blot represent the deletion of any one of three (−1), any two of three (−2), or all three (−3) glycosylation sites. The CNDP1 variants were transfected in Cos-7 cells and expressed as myc-tagged fusion protein as described. Cell extracts (C) and supernatants (S) were subjected to Western blot analysis using anti-myc antibody. In A and B, the results of a representative blot is depicted. Six different experiments were performed, all with essentially the same result. C: CN-1 secretion efficiency was measured by densitometry of the blots. Secretion efficiency was defined as the ratio of CN-1 in supernatant divided by the total amount of CN-1 (cell extracts + supernatant). The results are expressed as mean ratio ± SD of six individual experiments. D: CN-1 activity in supernatants of wild-type CNDP1-transfected cells and cells expressing CNDP1 variants lacking either one (−1), two (−2), or all three (−3) glycosylation sites. The results are expressed as mean CN-1 activity (μmol/ml/h) ± SD. Again, the supernatants of six individual experiments were included. C, cell extracts; S, supernatants; Hu, human serum; ns, not signifcant; WT, wild-type.
Because tunicamycin may also affect protein secretion independent of its inhibitory effect on N-glycosylation, we constructed myc-tagged CN-1 variants that lack one, two, or all three putative N-glycosylation sites. To this end, asparagine residues at N322, N382, and N402 were exchanged for glycine. Similar to tunicamycin treatment, the amount of total CN-1 produced by variants that were completely devoid of N-glycosylation sites was strongly decreased compared with wild-type CNDP1 (Fig. 2B). The low CN-1 expression in these variants was not caused by a low transfection efficiency, since the percentage of green fluorescent protein-expressing cells was not significantly different compared with wild-type CNDP1-transfected cells (wild-type, 68%; CNDP1 −3, 73%). In the supernatants of all CNDP1 variants that lack either one, two, or three N-glycosylation sites, CN-1 expression was lower compared with the wild-type CNDP1 variant (Fig. 2B).
CN-1 secretion efficiency, expressed as the proportion of CN-1 in supernatants to total CN-1 (cell extract + supernatant) was measured by densitometry. In comparison with wild-type CNDP1, CN-1 secretion efficiency was not affected for CN-1 variants lacking one or two N-glycosylation sites. In contrast, secretion efficiency was severely impaired for variants that lack all three N-glycosylation sites (Fig. 2C). In line with the Western blot, CN-1 enzyme activity in the supernatants decreased when the number of deleted N-glycosylation sites increased. Enzyme activity of CN-1 variants lacking only one N-glycosylation site was not significantly different to that of wild-type CN-1. However, enzyme activity of CN-1 variants lacking two N-glycosylation sites was significantly decreased (Fig. 2D). The combination of N-glycosylation sites that were deleted was not important in this regard (data not shown). As expected, CN-1 activity was not detected in supernatants of CN-1 variants that were devoid of N-glycosylation sites.
Hyperglycemia influences CN-1 secretion and enzyme activity.
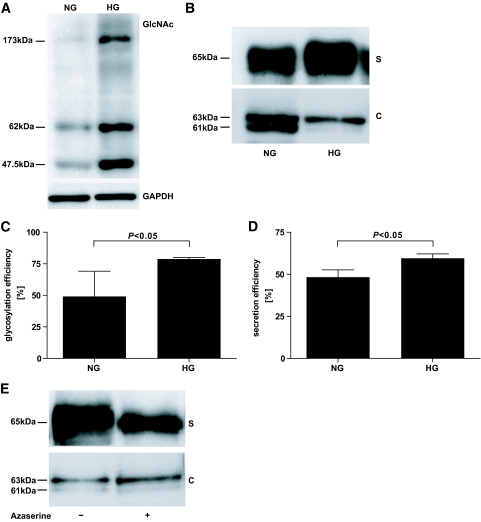
Because hyperglycemia results in an increased glucose flux through the hexosamine pathway and thereby increases GlcNAc concentrations, we speculated that hyperglycemia might increase N-glycosylation and subsequently affect CN-1 secretion and activity. Since among the different genetic CNDP1 (CTG)n variants, CN-1 secretion is the lowest for the (CTG)5, we chose this variant in the hyperglycemia experiments. CNDP1 (CTG)5-transfected cells were cultured for 3 days in high-glucose medium (25 mmol/l d-glucose) (HG) or kept in normal medium containing 5 mmol/l of d-glucose (NG). We first tested whether substrate supply for N-glycosylation, i.e., GlcNAc, was increased in cells that were cultured in HG medium. As demonstrated in Fig. 3A, the expression of GlcNAc-modified proteins was significantly increased under hyperglycemic conditions. We subsequently tested whether HG conditions would affect N-glycosylation of CN-1 and to what extent this influenced CN-1 secretion and enzyme activity.
FIG. 3.
Influence of high glucose medium on GlcNAc expression, N-glycosylation of CN-1, and CN-1 secretion and impact of hexosamine synthesis pathway on CN-1 secretion. CNDP1-transfected Cos-7 cells were cultured for 72 h in the presence of 25 mmol/l (HG) ± 20 μmol/l azaserine or 5 mmol/l (NG) d-glucose. Hereafter, cell extracts were prepared and supernatants were harvested. A: Western blot analysis of GlcNAc modified proteins in cell extracts. B: Western blot analysis of CN-1 expression in supernatants (S) and cell extracts (C). Note that the immature 61-kDa band is not present in cells that were cultured under HG conditions. In A and B, the results of a representative experiment is depicted. Four different experiments were performed and all showed similar results. C: CN-1 glycosylation efficiency in cells extracts was measured by densitometry and defined as ratio of N-glycosylated CN-1 (upper band) to total CN-1 (upper band + lower band). D: CN-1 secretion efficiency was measured as described in Fig. 2. Four experiments were included. E: Result of a representative Western blot analysis of CN-1 expression in supernatants (S) and cell extracts (C) of cells cultured in the presence of HG with (+) or without (−) azaserine. Although the secretion of CN-1 is decreased by the limitation of GlcNAc, azaserine does not influence the relative increase in N-glycosylation in cell extracts under HG conditions. Four different experiments were performed, and all showed similar results. C, cell extracts; S, supernatants; HG, 25 mmol/l d-glucose; NG, 5 mmol/l d-glucose.
Similarly, as demonstrated in Fig. 1, in cell extracts obtained from cells that were cultured in NG medium, the immature non-N-glycosylated 61-kDa band was detected in Western blot analysis. However, in cells that were cultured in HG medium, the 61-kDa band was not detected, suggesting a relative increase in N-glycosylation under HG conditions. This was paralleled by a slight increase in CN-1 expression in the supernatants of these cells (Fig. 3B). Densitometric quantification of immunoblots revealed that N-glycosylation efficiency in cell extracts, expressed as the proportion of N-glycosylated CN-1 (upper band) to total CN-1 (upper band + lower band), was significantly increased in cells cultured in 25 mmol/l d-glucose containing medium (Fig. 3C, NG vs. HG, P < 0.05). In addition, this analysis revealed that CN-1 secretion efficiency, expressed as the proportion of CN-1 in cell extract to total CN-1 (cell extract + supernatant), was significantly increased under hyperglycemic conditions (Fig. 3D, NG vs. HG, P < 0.05). To analyze whether limitation of oligosaccharide substrate supply would influence N-glycosylation and secretion efficiency under HG, we used azaserine, an inhibitor of hexosamine biosynthesis pathway, to impede auxiliary UDP-GlcNAc formation. Azaserine, as a glutamine analog, inhibits, among others, the glutamine:fructose-6-phosphate amidotransferase enzyme. Restriction of UDP-GlcNAc caused a remarkable decrease in CN-1 expression in the supernatants. Nevertheless, increased N-glycosylation efficiency in cell extracts was not affected by azaserine conditioning (Fig. 3E).
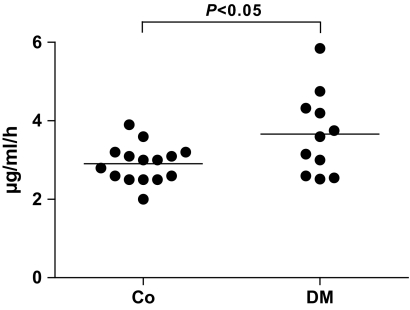
To test whether our in vitro observations also have an in vivo relevance, we next addressed whether serum CN-1 activity is different in homozygous (CTG)5 diabetic patients and genotype-matched healthy control subjects. Compatible with the in vitro findings, serum CN-1 activity was significantly higher in diabetic patients (control subjects vs. diabetic patients, P < 0.05) (Fig. 4).
FIG. 4.
CN-1 activity was measured in 5L homozygous healthy control subjects (Co, n = 15) as well as in 5L homozygous diabetic patients (DM, n = 11). Results are expressed for each individual patient and control. The line represents the mean of each group. Co, healthy control subjects; DM, 5L homozygous diabetic patients.
DISCUSSION
Secretion of the serum carnosinase is influenced by the length of the (CTG)n repeat, located in the signal peptide of this enzyme (10). Because CN-1 is N-glycosylated, and glycosylation might influence protein secretion, stability, and enzyme activity, it is conceivable that in hyperglycemic patients, CN-1 secretion or activity is not completely determined by the (CTG)n genotype. In the present study, we therefore assessed the relevance of N-glycosylation for CN-1 protein secretion and enzyme activity, and addressed whether hyperglycemia can influence serum CN-1 secretion and activity. The main findings of this study are the following. First, only deletion of all three N-glycosylation sites impairs CN-1 secretion efficiency, whereas CN-1 activity is already diminished when two sites are deleted. Second, hyperglycemia increases N-glycosylation efficiency and increases CN-1 secretion. This might be caused by an increased GlcNAc supply. Third, homozygous (CTG)5 diabetic patients have higher serum CN-1 activity compared with genotype-matched healthy control subjects.
The human CN-1 glycoprotein contains three potential N-glycosylation sites at asparagine numbers N322, N382, and N402 (21). In pCSII-CNDP1–transfected Cos-7 cells, three CN-1 immune reactive bands can be detected. The 61- and 63-kDa bands dominate in cell lysates whereas the 65-kDa band is almost nondetectable in cell extracts. In contrast, the 65-kDa band is strongly expressed in supernatants of transfected cells. The different CN-1 bands detected in Western blotting are likely due to differences in N-glycosylation, since after PNGase F treatment, only the 61-kDa band was detected. Hence, this band represents the immature non-N-glycosylated CN-1 protein. Macroheterogeneity occurs for a number of glycosylated proteins and describes the differential use of glycosylation sites within a given protein (22–24). The 61- or 63-kDa band was not found either in serum or in supernatants of transfected cells. Although this might suggest that CN-1 secretion occurs only after complete N-glycosylation, our findings that CNDP-1 variants lacking one or two glycosylation sites also secrete CN-1 argues against this assumption. It is therefore more likely that secretion of the completely N-glycosylated CN-1 protein is favored, but that complete N-glycosylation is not a prerequisite for CN-1 secretion. Nevertheless, when N-glycosylation is completely prevented, either by deletion of all three N-glycosylation sites or by tunicamycin treatment, CN-1 secretion is severely impaired. N-glycosylation occurs cotranslationally in the lumen of the ER and facilitates the protein-folding process by recruiting members of the calnexin chaperone system (25). Acquisition of the native structure of the protein may therefore fail or progress slowly when N-glycosylation is prevented. Folding-defective or terminally misfolded proteins are disposed from the ER through cytosolic transport and subsequent proteasomal degradation (26). This might explain why CN-1 is significantly less expressed in cells transfected with CNDP1 variants that are completely devoid of N-glycosylation sites as opposed to cells expressing wild-type CNDP1. The importance of N-glycosylation of CN-1 for protein secretion is not unique to CN-1 as it has also been reported for other proteins (24,27,28).
Our data also indicate that CN-1 enzyme activity was dependent on proper N-glycosylation since deletion of two N-glycosylation sites significantly decreased CN-1 activity. Enzyme activity as a function of N-glycosylation has also been reported by other groups (29,30). Why CN-1 activity severely drops after deletion of two glycosylation sites remains to be elucidated. Because CN-1 was poorly secreted by CNDP1 variants that were devoid of N-glycosylation sites, no CN-1 activity was detected in supernatants of these cells as expected.
In diabetic patients, the role of nonenzymatic advanced-glycation end products for cell activation and damage has been well studied (31,32). In contrast, the influence of enzymatic glycosylation and its possible pathogenic role in diabetic complications remains to be addressed. A few studies, however, have reported that hyperglycemia increases N-glycosylation in rats (33–35). Our own data are in line with this assumption, as we could show that the immature non-N-glycosylated CN-1 protein was not present in CNDP1-transfected cells that were grown under high-glucose conditions. Yet, a possible increase in glycosylation was not reflected by a difference in molecular weight of the mature secreted CN-1 protein. Although under hyperglycemic conditions, activation of the hexosamine pathway leads to synthesis of UDP-GlcNAc (19), an increase of substrates alone might not be sufficient to enhance N-glycosylation (36). These conclusions are in accordance with our observations, since in cells cultured under HG, N-glycosylation efficiency was not disturbed by inhibition of hexosamine synthesis pathway. Nevertheless, restriction of UDP-GlcNAc led to decreased CN-1 expression in the cell supernatants. The assembly of the N-glycosylation core oligosaccharide is dependent on two UDP-GlcNAc molecules. Therefore, it is conceivable that the N-glycosylation and secretion process is slowed down under azaserine treatment. As expected, azaserine did not completely block N-glycosylation of CN-1, because the oligosaccharide substrates can either be synthesized from glucose or be salvaged from glycoconjugates degraded within cells. The expression or activity of enzymes that attach sugars to growing proteins might equally have contributed to the increased N-glycosylation efficiency under hyperglycemic conditions; these enzymes were not measured in this study. Hence, it must be emphasized that the increased CN-1 secretion observed under hyperglycemic conditions may be related to changes in the N-glycosylation machinery and substrate increment.
If hyperglycemia influences CN-1 secretion, it is expected that diabetic patients would have more CN-1 activity compared with genotype-matched healthy control subjects. Because healthy individuals homozygous for CNDP1 (CTG)5 have low CN-1 activity (4), we stratified for this genotype and compared CN-1 activity of diabetic patients with that of healthy control subjects. Indeed, our data show that diabetic patients have significantly higher CN-1 activity. We are aware that this study included only a relative small group of diabetic patients (n = 11), and significant differences in the mean age between both groups were present. Because CN-1 activity increases with age until adulthood, but not thereafter (37), it is unlikely, but not excluded, that age differences are a confounding factor in our analysis. Therefore, these data should be confirmed in a larger cohort of patients matched for age, sex, and genotype before firm conclusions can be drawn.
Recently we showed that CNDP1 activity toward carnosine is inhibited by homocarnosine (38). If carnosine is considered to be protective in terms of diabetic complications, then clearly other factors than CNDP1 genotype that also affect carnosine metabolism (e.g., serum homocarnosine concentrations and blood glucose control) should be taken into account for risk assessment for development of diabetic complications in this group of patients. Diabetic patients who do not have the protective CNDP1 genotype might be protected because of good glycemic control or by having sufficient homocarnosine levels. Vice versa, diabetic patients with the protective genotype may still develop DN when glycemic control is poor or when a low serum concentration of homocarnosine is present.
Inasmuch as our study demonstrates that hyperglycemia increases CN-1 secretion, it was not our intention to investigate whether a low CN-1 activity is associated with a diminished oxidative stress or advanced glycation end product formation. CN-1 is only one of the parameters that influence the amount of serum carnosine. Activity of carnosine synthase and dietary carnosine intake are two additional factors. For future studies it would thus also be worthwhile to study whether CN-1 activity correlates with carnosine concentrations in diabetic patients and elucidate if this in turn correlates with parameters of oxidative stress and advanced glycosylation end product formation.
We conclude that apart from the (CTG)n polymorphism in the signal peptide of CN-1, N-glycosylation is essential for appropriate secretion and enzyme activity. Since hyperglycemia enhances CN-1 secretion and enzyme activity, our data suggest that poor blood glucose control in diabetic patients might result in an increased CN-1 secretion, even in the presence of the (CTG)5 allele.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
E.R. and H.K. researched data and prepared and edited the manuscript. F.P., V.P., S.S., and P.S. researched data. P.B., H.Z., and R.H.H. contributed to discussion. G.N., J.VDB., and B.J. reviewed the manuscript and contributed to discussion. S.J.L.B. reviewed the manuscript. F.J.vdW. contributed conception and design. B.A.Y. contributed conception and design and reviewed and edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ritz E, Rychlík I, Locatelli F, Halimi S: End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795–808 [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 3.Parving HH: Hypertension Optimal Treatment (HOT) Trial. Lancet 1998;352:574–575 [DOI] [PubMed] [Google Scholar]
- 4.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Koppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ: Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005;54:2320–2327 [DOI] [PubMed] [Google Scholar]
- 5.Riedl E, Koeppel H, Brinkkoetter P, Sternik P, Steinbeisser H, Sauerhoefer S, Janssen B, van der Woude F, Yard B: A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes 2007;56:2410–2413 [DOI] [PubMed] [Google Scholar]
- 6.Sauerhofer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N, Floege J, Kriz W, van der Woude F, Moeller MJ: L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007;56:2425–2432 [DOI] [PubMed] [Google Scholar]
- 7.Hipkiss A, Preston J, Himsworth D, Worthington V, Keown M, Michaelis J, Lawrence J, Mateen A, Allende L, Eagles P, Abbott N: Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann N Y Acad Sci 1998;854:37–53 [DOI] [PubMed] [Google Scholar]
- 8.Fouad A, El-Rehany M, Maghraby H: The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats. Eur J Pharmacol 2007;572:61–68 [DOI] [PubMed] [Google Scholar]
- 9.Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, Matsumura Y: Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther 2006;319:640–647 [DOI] [PubMed] [Google Scholar]
- 10.Rajanikant G, Zemke D, Senut M, Frenkel M, Chen A, Gupta R, Majid A: Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke 2007;38:3023–3031 [DOI] [PubMed] [Google Scholar]
- 11.Bowden DW, Colicigno CJ, Langefeld CD, Sale MM, Williams A, Anderson PJ, Rich SS, Freedman BI: A genome scan for diabetic nephropathy in African Americans. Kidney Int 2004;66:1517–1526 [DOI] [PubMed] [Google Scholar]
- 12.Freedman BI, Hicks PJ, Sale MM, Pierson ED, Langefeld CD, Rich SS, Xu J, McDonough C, Janssen B, Yard BA, van der Woude FJ, Bowden DW: A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant 2007;22:1131–1135 [DOI] [PubMed] [Google Scholar]
- 13.Wanic K, Placha G, Dunn J, Smiles A, Warram JH, Krolewski AS: Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case-control and follow-up studies. Diabetes 2008;57:2547–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakker SJ, Alkhalaf A, Tarnow L, Navis G: Re: Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case-control and follow-up studies. Diabetes 2008;57:e16; author reply e17. [DOI] [PubMed] [Google Scholar]
- 15.Molinari M: N-glycan structure dictates extension of protein folding or onset of disposal. Nat Chem Biol 2007;3:313–320 [DOI] [PubMed] [Google Scholar]
- 16.Broschat KO, Gorka C, Page JD, Martin-Berger CL, Davies MS, Huang Hc HC, Gulve EA, Salsgiver WJ, Kasten TP: Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J Biol Chem 2002;277:14764–14770 [DOI] [PubMed] [Google Scholar]
- 17.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW: Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007;129:123–134 [DOI] [PubMed] [Google Scholar]
- 18.Sasai K, Ikeda Y, Fujii T, Tsuda T, Taniguchi N: UDP-GlcNAc concentration is an important factor in the biosynthesis of beta1,6-branched oligosaccharides: regulation based on the kinetic properties of N-acetylglucosaminyltransferase V. Glycobiology 2002;12:119–127 [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M: The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 20.Bar-On H, Nesher G, Teitelbaum A, Ziv E: Dolichol-mediated enhanced protein N-glycosylation in experimental diabetes—a possible additional deleterious effect of hyperglycemia. J Diabetes Complications 1997;11:236–242 [DOI] [PubMed] [Google Scholar]
- 21.Teufel M, Saudek V, Ledig J, Bernhardt A, Boularand S, Carreau A, Cairns N, Carter C, Cowley D, Duverger D, Ganzhorn A, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T: Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 2003;278:6521–6531 [DOI] [PubMed] [Google Scholar]
- 22.Antenos M, Stemler M, Boime I, Woodruff TK: N-linked oligosaccharides direct the differential assembly and secretion of inhibin alpha- and betaA-subunit dimers. Mol Endocrinol 2007;21:1670–1684 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Yang X, Nguyen AH, Brockhausen I: Requirement of N-glycosylation for the secretion of recombinant extracellular domain of human Fas in HeLa cells. Int J Biochem Cell Biol 2007;39:1625–1636 [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Tsai HM: N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity. Blood 2009;113:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivari S, Molinari M: Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett 2007;581:3658–3664 [DOI] [PubMed] [Google Scholar]
- 26.Ellgaard L, Molinari M, Helenius A: Setting the standards: quality control in the secretory pathway. Science 1999;286:1882–1888 [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Seri A, Kimura F, Matsudomi N: Site-specific glycosylation at Asn-292 in ovalbumin is essential to efficient secretion in yeast. J Biochem 2007;141:193–199 [DOI] [PubMed] [Google Scholar]
- 28.Pradere JP, Tarnus E, Gres S, Valet P, Saulnier-Blache JS: Secretion and lysophospholipase D activity of autotaxin by adipocytes are controlled by N-glycosylation and signal peptidase. Biochim Biophys Acta 2007;1771:93–102 [DOI] [PubMed] [Google Scholar]
- 29.Gladysheva IP, King SM, Houng AK: N-glycosylation modulates the cell-surface expression and catalytic activity of corin. Biochem Biophys Res Commun 2008;373:130–135 [DOI] [PubMed] [Google Scholar]
- 30.Pacheco B, Maccarana M, Goodlett DR, Malmstrom A, Malmstrom L: Identification of the active site of DS-epimerase 1 and requirement of N-glycosylation for enzyme function. J Biol Chem 2009;284:1741–1747 [DOI] [PubMed] [Google Scholar]
- 31.Wendt T, Tanji N, Guo J, Hudson B, Bierhaus A, Ramasamy R, Arnold B, Nawroth P, Yan S, D'Agati V, Schmidt A: Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol 2003;14:1383–1395 [DOI] [PubMed] [Google Scholar]
- 32.Bierhaus A, Hofmann M, Ziegler R, Nawroth P: AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovasc Res 1998;37:586–600 [DOI] [PubMed] [Google Scholar]
- 33.Bar-On H, Nesher G, Teitelbaum A, Ziv E: Dolichol-mediated enhanced protein N-glycosylation in experimental diabetes–a possible additional deleterious effect of hyperglycemia. J Diabetes Complications 1997;11:236–242 [DOI] [PubMed] [Google Scholar]
- 34.Berry E, Ziv E, Bar-On H: Protein and glycoprotein synthesis and secretion by the diabetic liver. Diabetologia 1980;19:535–540 [DOI] [PubMed] [Google Scholar]
- 35.Berry E, Ziv E, Bar-On H: Lipoprotein secretion by isolated perfused livers from streptozotocin-diabetic rats. Diabetologia 1981;21:402–408 [DOI] [PubMed] [Google Scholar]
- 36.Jones J, Krag SS, Betenbaugh MJ: Controlling N-linked glycan site occupancy. Biochim Biophys Acta 2005;1726:121–137 [DOI] [PubMed] [Google Scholar]
- 37.Lenney JF, George RP, Weiss AM, Kucera CM, Chan PW, Rinzler GS: Human serum carnosinase: characterization, distinction from cellular carnosinase, and activation by cadmium. Clin Chim Acta 1982;123:221–231 [DOI] [PubMed] [Google Scholar]
- 38.Peters V, Kebbewar M, Jansen EW, Jakobs C, Riedl E, Koeppel H, Frey D, Adelmann K, Klingbeil K, Mack M, Hoffmann GF, Janssen B, Zschocke J, Yard BA: Relevance of allosteric conformations and homocarnosine concentration on carnosinase activity. Amino Acids 2010;38:1607–1615 [DOI] [PubMed] [Google Scholar]