Abstract
OBJECTIVE
Based on its role as an energy storage compartment and endocrine organ, white adipose tissue (WAT) fulfills a critical function in the maintenance of whole-body energy homeostasis. Indeed, WAT dysfunction is connected to obesity-related type 2 diabetes triggered at least partly by an inflammatory response in adipocytes. The pseudokinase tribbles (TRB) 3 has been identified by us and others as a critical regulator of hepatic glucose homeostasis in type 2 diabetes and WAT lipid homeostasis. Therefore, this study aimed to test the hypothesis that the TRB gene family fulfills broader functions in the integration of metabolic and inflammatory pathways in various tissues.
RESEARCH DESIGN AND METHODS
To determine the role of TRB family members for WAT function, we profiled the expression patterns of TRB13 under healthy and metabolic stress conditions. The differentially expressed TRB1 was functionally characterized in loss-of-function animal and primary adipocyte models.
RESULTS
Here, we show that the expression of TRB1 was specifically upregulated during acute and chronic inflammation in WAT of mice. Deficiency of TRB1 was found to impair cytokine gene expression in white adipocytes and to protect against high-fat diet–induced obesity. In adipocytes, TRB1 served as a nuclear transcriptional coactivator for the nuclear factor κB subunit RelA, thereby promoting the induction of proinflammatory cytokines in these cells.
CONCLUSIONS
As inflammation is typically seen in sepsis, insulin resistance, and obesity-related type 2 diabetes, the dual role of TRB1 as both a target and a (co) activator of inflammatory signaling might provide a molecular rationale for the amplification of proinflammatory responses in WAT in these subjects.
Adipose tissue can be subdivided into two distinct categories, white and brown. Whereas brown adipose tissue (BAT) dissipates energy as heat, white adipose tissue (WAT) is specialized in the storage of chemical energy such as triglycerides (1), thereby providing a reserve storage compartment for excess nutrients as a protective measure against starvation (2). In addition, white adipocytes also serve as critical endocrine cells, secreting a variety of adipokines to control energy intake and expenditure at the systemic level (3). Importantly, under both acute and chronic metabolic stress, as exemplified by sepsis and obesity-related type 2 diabetes, respectively, impaired WAT function is characterized by WAT tissue inflammation and an increased release of proinflammatory cytokines from this tissue as well as dysregulation of the energy balance (4). Indeed, sepsis induces the metabolic rate by 30–60% in humans (5), and obesity has been linked to enhanced macrophage recruitment and T-cell receptor rearrangements in WAT (6,7). Additionally, proinflammatory cytokine expression is activated in adipocytes, per se, in response to inflammatory cues, including bacterial lipopolysaccharide (LPS) (8). Interestingly, recent evidence suggests that chronically elevated levels of LPS resulting from alterations in the gut microbiota during obesity contribute to obesity-related insulin resistance (9), promoting the idea that common molecular pathways may underlie the proinflammatory responses in adipocytes and WAT under both acute and chronic metabolic disturbances (10).
The tribbles (TRB) protein family consists of three related serine/threonine kinase-like proteins, TRB1, -2, and -3, which have been highly conserved throughout evolution and serve as critical regulators of cell-cycle progression during development in Drosophila and Xenopus (11). In mammals, TRB3 was originally identified as a critical checkpoint in hepatic glucose homeostasis during fasting and type 2 diabetes, mediated through its regulatory function on Akt/protein kinase B in the insulin signaling pathway (12). Consistently, subsequent studies have proposed TRB3 as a determinant of insulin sensitivity in liver, skeletal muscle, and WAT (13–15) and of lipolysis in WAT by triggering the degradation of acetyl-CoA carboxylase via association with an E3 ubiquitin ligase (16). Interestingly, both TRB2 and TRB3 were found to suppress white adipocyte differentiation in an insulin-/peroxisome proliferator–activated receptor (PPAR) γ–dependent manner (17,18). Apart from hormonal pathway control, all three tribbles family members have been implicated in the activation of macrophages and, particularly, TRB3 in the cellular endoplasmatic reticulum stress response (19–22), overall supporting the hypothesis that the TRB family may fulfill broader but largely unexplored functions in the integration of metabolic and inflammatory pathways in various tissues.
RESEARCH DESIGN AND METHODS
Recombinant adenoviruses.
Adenoviruses expressing a TRB1-specific or -nonspecific short-hairpin RNA (shRNA) under the control of the U6 promoter were cloned as described previously (23,24). Viruses were purified by the caesium chloride method and dialyzed against PBS containing 10% glycerol prior to use. TRB1 shRNA forward 5′-CACCGGGCTATGTTGACTCAGAAATC GAAATTTCTGAGTCAACATAGCCC-3′; TRB1 shRNA reverse 5′-AAAAGGGC TATGTTGACTCAGAAATTTCGATTTCTGAGTCAACATAGCCC-3′.
Animal experiments.
Male 8- to 12-week-old C57BL6, db/db, ob/ob, toll-like receptor (TLR) 4, tumor necrosis factor (TNF) receptor (TNFR)1/2, jun NH2-terminal kinase (JNK), p50, and TNFR1/interleukin (IL)-1β receptor knockout mice were obtained from Charles River Laboratories (Brussels, Belgium). Mice with TRB1 haploinsufficiency have been described previously (19). All animals were maintained on a 12-h light-dark cycle with regular unrestricted diet. For LPS experiments, animals were fasted for 18 h with free access to water and injected with 20 mg/kg body weight LPS. In each experiment, three to seven animals received identical treatments and were analyzed 2.5 h after LPS administration. For tumor induction in the cachexia model, 1.5 × 106 colon 26 (C26) cells (25) in PBS were injected subcutaneously into 10-week-old CD2F1 mice (Charles River Laboratories). Control mice were injected with heat-inactivated C26 cells. Subcutaneous implantation of C26 cells promoted severe reduction of body weight, as well as skeletal muscle and adipose tissue mass. In addition, C26 mice displayed reduced levels of serum triglycerides and substantial hepatic steatosis as described (26). In high-fat diet experiments, TRB1+/− mice and wild-type littermates were either fed a standard diet (D12450B, 10% energy from fat; Research Diets, New Brunswick, NJ) or a high-fat diet (D12492, 60% energy from fat; Research Diets) for a period of 13 weeks. Insulin and glucose tolerance tests were performed as described previously (27). Organs including liver, epididymal fat pads, and gastrocnemius muscles were collected after the corresponding time period, snap frozen, and used for further analysis. Total body fat content was determined by an Echo magnetic resonance imaging body composition analyzer (Echo Medical Systems, Houston, TX). Animal handling and experimentation was done in accordance with National Institutes of Health guidelines and approved by local authorities.
Blood metabolites.
Serum levels of glucose, insulin, triglycerides, cholesterol, and cytokines were determined using an automatic glucose monitor (One Touch; Lifescan, Neckargemünd, Germany) or commercial kits (MP Biomedicals, Orangeburg, NY; Mercodia, Uppsala, Sweden; Sigma, Munich, Germany; Randox, Wako, Neuss, Germany; Millipore, Schwalbach, Germany).
Cell culture and transient transfection assays.
3T3-L1 preadipocytes and HEK293 cells were transfected using lipofectamine (Invitrogen, Karlsruhe, Germany) reagent according to the manufacturer's instructions. Cell extracts were prepared 48 h after transfection, and luciferase assays were performed as described (23), normalizing to the activity from cotransfected β-galactosidase expression plasmid. Primary stromal-vascular fractions (SVFs) were isolated from mouse epididymal fat depots, cultured, and differentiated into mature primary adipocytes (essentially) as described (28). Cells were infected with recombinant adenoviruses at a multiplicity of infection of 1,000 and stimulated with 1.5 ng/ml TNF-α (Biomol, Hamburg, Germany). Cytokine-conditioned medium was obtained by stimulating RAW264.7 mouse macrophages with 100 ng/ml LPS for 3.5 h and collecting the medium. Medium harvested from RAW264.7 cells not exposed to LPS was used as the control μ in all experiments.
Analysis of the macrophage-derived conditioned medium and control was done by the Milliplex MAP Kit against mouse cytokines and chemokines (Millipore, Schwalbach, Germany). 3T3-L1 and primary adipocytes were treated with pharmacological inhibitors (50 μmol/l parthenolide, 50 μmol/l SP600125, 50 μmol/l PD98059, 10 μmol/l SB202190; Calbiochem, Darmstadt, Germany) 30 min prior to stimulation with conditioned medium or control. Cells were lysed in QIAzol (QIAgen, Hilden, Germany), and RNA was isolated according to standard procedures.
Cell separation studies of adipose tissue macrophages (ATMs) from epididymal fat pads of LPS- or PBS-injected mice were done by magnetic immunoaffinity isolation using anti-CD11b antibodies, conjugated to magnetic beads (MACS Cell Separation System; Miltenyi Biotec). Following isolation of ATMs from the SVFs using positive selection columns (MS columns; Miltenyi Biotec), the remaining cells were eluted as the SVFs. For the analysis of mRNA expression levels, eluted cells (CD11b-positive [+] and SVFs) and the floating adipocyte fraction were resuspended in QIAzol reagent.
Quantitative Taqman RT-PCR.
Total RNA was extracted from homogenized mouse WAT or cell lysates using QIAzol and the RNeasy (Qiagen, Hilden, Germany) kit. cDNA was prepared by reverse transcription using Superscript II (Invitrogen) and Oligo dT primer (Fermentas, St. Leon-Rot, Germany). cDNAs were amplified using assay-on-demand kits and an ABI Prism 7300 sequence detector (Applied Biosystems, Darmstadt, Germany). RNA expression data were quantified according to the ΔCt method as described (29) and normalized to levels of TATA-box binding protein RNA.
Protein analysis.
Protein was extracted from frozen organ samples or cultured adipocytes in 2 × SDS-8 mol/l urea cell lysis buffer, and 20–30 μg of protein were loaded onto 10% SDS–polyacrylamide gels and blotted onto nitrocellulose membranes. Western blot assays were performed as described (23) using antibodies specific for TRB1 (30), RelA, P300, β-actin (Santa Cruz, Heidelberg, Germany), or valosin-containing protein (VCP) (Abcam, Cambridge, U.K.).
Chromatin immunoprecipitation assay.
3T3-L1 preadipocytes were transfected with a plasmid encoding Flag-tagged TRB1, stimulated with conditioned medium or control for 6 h and fixed with formaldehyde 48 h after transfection, and chromatin immunoprecipitation assays were performed as described (31) using Flag-specific antibodies (Upstate, Lake Placid, NY) or nonspecific anti-HA antibody (Santa Cruz). Precipitated DNA fragments were analyzed by PCR amplification, as described above, using primers directed against the IL-6, IL-1β, and TNF-α promoters. Primers against cytokine promoter regions lacking a RelA recognition site were used as negative controls as described (32). Primer sequences were mIL-6_forward TGTGT GTCGTCTGTCATGCG; mIL-6_reverse AGCTACAGACATCCCCAGTCTC; mIL-6_A forward (without nuclear factor [NF]-κB) CCTACTTTCAAGCCTG GAATC; mIL-6_A reverse (without NF-κB) TCAAGTCTTCTAGGCTGGGTC; mIL-1β_forward TGCCCATTTCCACCACG; mIL-1b_reverse TGCTACCCTGAA ATAATTTCTAATCCC; mIL-1b_forward (without NF-κB) CCCAAGGGAAAATT TCACAGC; mIL-1β _reverse (without NF-κB) ACCACTGCAGGGTTTGTTGTC; mTNF-α_forward CCCCCGCGATGGAGAAGAAACCGAGA; mTNF-α_reverse GCTAGTCCCTTGCTGTCCTCGCTGA.
Plasmids.
Wild-type or mutated NF-κB reporter plasmids have been described previously (32). Flag-TRB1 expression vector was generated by PCR-based standard procedures and cloned into pcDNA3.1 (Invitrogen) using standard protocols.
Glutathione-S-transferase pulldown assay.
Glutathione-S-transferase (GST) fusion proteins (pGEX5.1, pGEX5.1_GST_p65, pGEX5.1_GST_p65 RHD_1-305, and pGEX5.1_GST_p65_TA_441-551) (32) were produced in BL21 cells and affinity purified using glutathione Sepharose (Amersham Biosciences, Darmstadt, Germany). In vitro transcription/translation was performed using the TNT T7/T3 quick-coupled transcription/translation system (Promega, Mannheim, Germany), according to the manufacturer's instructions, and GST and in vitro translated proteins were incubated at 4°C overnight. After extensive washing, GST-precipitated proteins were separated by SDS-PAGE and detected by autoradiography.
Immunoprecipitation.
HEK293 cells were cotransfected with a RelA expression vector plus Flag-TRB1 or an empty Flag vector. Subsequently, cells were lysed, centrifuged, and the supernatant was incubated with anti-FLAG M2 agarose (Sigma) for 2 h. The immunoprecipitates were subsequently analyzed by Western blot as described.
Statistical analysis.
Statistical analyses were performed using a two-way ANOVA with Bonferroni-adjusted posttests or Student t test in one-factorial designs, respectively. The significance level was at P = 0.05.
RESULTS
TRB1 expression in WAT is elevated in acute and chronic inflammation.
To initially explore the expression profiles of TRB family members under conditions of metabolic and, particularly, WAT dysfunction, we extracted total RNA from various tissues of wild-type or db/db mice, the latter representing a standard model for obesity and the metabolic syndrome (33). Quantitative PCR analysis confirmed the previously reported upregulation of TRB3 expression in livers of obese mice (12), thereby verifying the experimental system (data not shown). In contrast, hepatic mRNA levels of TRB1 and TRB2 remained unchanged under these conditions (data not shown).
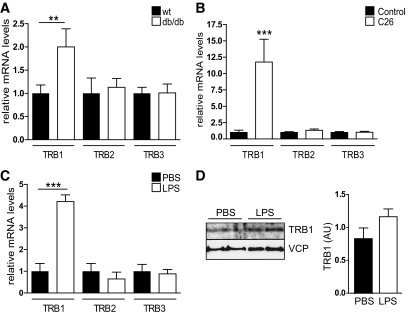
Intriguingly, TRB1 mRNA expression was found to be significantly elevated in WAT of db/db animals compared with wild-type controls (Fig. 1A). In contrast, TRB2 and TRB3 showed no difference in their WAT expression levels (Fig. 1A), suggesting a specific impact of obesity-related conditions on TRB1 adipose tissue expression. Indeed, correlating with increasing, age-dependent overweight condition a higher expression of WAT TRB1 levels was also observed in a second, early-onset obesity mouse model (supplemental Fig. 1A, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1537/DC1). Expression was, however, not affected by late-onset obesity as associated with high-fat diet feeding of adult wild-type mice (supplemental Fig. 1C). Furthermore, TRB1 mRNA levels were found to be elevated in WAT of tumor-bearing cachectic mice, while TRB2 and TRB3 again remained unchanged (Fig. 1B). Despite contrary states of energy availability, obesity actually shares many phenotypic features with severe wasting conditions such as cancer cachexia, including insulin resistance, hepatic steatosis, and particularly chronic inflammation (34). To test the hypothesis that proinflammatory conditions represent a common trigger for TRB1 expression in WAT, we provoked extensive proinflammatory cytokine production and signaling in mice by injecting sublethal doses of LPS. As shown in Fig. 1C and D, LPS treatment stimulated TRB1 mRNA and protein expression in WAT compared with controls (Fig. 1C and D), demonstrating that the expression of TRB1 in WAT is specifically induced by proinflammatory conditions, thereby representing a distinguishing feature from other TRB family members.
FIG. 1.
TRB1 expression in WAT is elevated in acute and chronic inflammation. A–C: Quantitative PCR analysis of TRB1, TRB2, or TRB3 mRNA levels in abdominal WAT of wild-type (wt) and obese db/db mice (A), healthy control and tumor-bearing cachectic (C26) CD2F1 mice (B), and control (PBS)- and LPS-injected C57BL6 mice (means ± SE, n = 5). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. D: Western blot of WAT extracts from two representative control (PBS)- or LPS-injected animals as in C using antibodies against TRB1 or valosin-containing protein (VCP). Relative amounts of TRB1 protein levels normalized to VCP protein levels shown. AU, arbitrary units.
TRB1 expression is under the control of cytokine signaling in an adipocyte-autonomous manner.
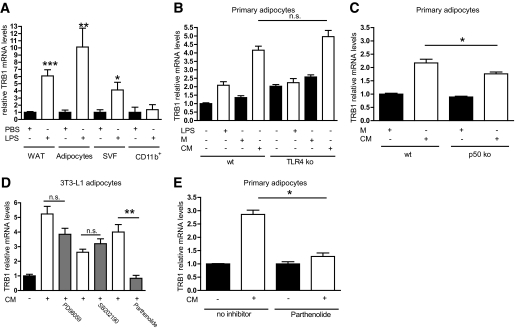
WAT is composed of a variety of different cell types, including mature adipocytes and the so-called SVF, comprising macrophages, endothelial cells, and corresponding progenitors (1). Cell separation studies using WAT explants from LPS-treated or nontreated wild-type mice demonstrated that TRB1 mRNA expression was specifically induced by LPS in whole WAT depots, mature adipocytes, and in the SVF, but not in CD11b+ macrophage-enriched cellular fractions (Fig. 2A), indicating that adipocytes indeed represent the major site of TRB1 regulation in response to proinflammatory signaling in WAT. To specifically explore potential signaling pathways involved in TRB1 induction under proinflammatory conditions in adipocytes, we isolated the SVFs from WAT depots of wild-type and TLR4 knockout mice, the latter deficient in the cellular LPS receptor (35). Isolated SVFs were differentiated into mature and primary adipocytes (data not shown) and treated either with cytokine-enriched conditioned medium from LPS-treated macrophages or LPS (supplementary Fig. 2A). Whereas conditioned medium efficiently stimulated TRB1 expression in both primary wild-type and TLR4 knockout adipocytes, heat-inactivated conditioned medium (supplementary Fig. 2B) and LPS treatment had no effect in either cell type (Fig. 2B), supporting the hypothesis that LPS/TLR4 signaling, per se, is not responsible for the induction of TRB1 under proinflammatory conditions but most likely LPS-triggered cytokines such as TNF-α and ILs. Interestingly, ablation of either TNFR1 and -2 or TNFR1 and IL-1β receptor in primary adipocytes had no effect on TRB1 mRNA induction in response to conditioned medium treatment (supplementary Fig. 2C), arguing that the induction of TRB1 expression in WAT is driven by multiple proinflammatory mediators in a combinatorial manner.
FIG. 2.
TRB1 expression is under the control of cytokine signaling in an adipocyte-autonomous manner. A: Quantitative PCR analysis of TRB1 mRNA levels in WAT depots, mature adipocytes after separation from SVFs, SVF- or CD11b+-enriched cellular fractions from WAT depots. B and C: Quantitative PCR analysis of TRB1 mRNA levels in primary adipocytes derived from the stromal-vascular WAT fractions of wild-type (wt) and TLR4 (B) or p50 (C) knockout (ko) mice. Cells were treated with LPS and LPS-conditioned (conditioned medium [CM]) or non–LPS-conditioned (control [M]) macrophage supernatant as indicated. D and E: Quantitative PCR analysis of TRB1 mRNA levels in mature adipocytes derived from 3T3–L1 preadipocytes (D) or the SVF of wild-type mice (E). Cells were pretreated with inhibitors against p38 (SB202190), the ERK (PD98059), and NF-κB (parthenolide) pathways and subsequently stimulated with LPS-conditioned (conditioned medium [CM]) or non–LPS-conditioned (control [M]) macrophage supernatant as indicated (means ± SE, n = 5). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n.s., non significant.
In this respect, the activator protein (AP) 1 and NF-κB transcriptional complexes represent common integration sites for divergent upstream proinflammatory signaling pathways in various cell types (10). Whereas the conditioned medium-dependent TRB1 induction in primary adipocytes was not affected by genetic deficiency for the AP1 upstream kinase JNK1 (supplemental Fig. 1D), knockout of the NF-κB subunit p50 significantly but incompletely impaired TRB1 gene expression upon cytokine stimulation in primary adipocytes (Fig. 2C), suggesting that the NF-κB transcriptional complex may represent a critical checkpoint for TRB1 gene regulation in these cells and that other NF-κB components apart from p50 are essential in this context. To test this hypothesis directly, we treated mature adipocytes derived from differentiated 3T3-L1 preadipocytes with various inhibitors for specific intracellular signaling pathways upon conditioned medium exposure. Inhibitors of p38 (SB202190), extracellular signal–regulated kinase (ERK) (PD98059), and protein kinase A pathways elicited no effect on conditioned medium–triggered TRB1 mRNA levels (Fig. 2D and data not shown). However, specific pharmacological inhibition of the NF-κB signaling axis by parthenolide as well as the non–isoform-specific JNK inhibitor SP600125 (36) completely eliminated the effect of conditioned medium on TRB1 mRNA levels in differentiated 3T3-L1 adipocytes as well as in SVF-derived primary adipocytes from wild-type mice (Fig. 2D and E; supplementary Fig. 2E), thereby demonstrating that the cell autonomous induction of TRB1 in white adipocytes under proinflammatory conditions is determined in a combinatorial fashion by distinct proinflammatory axes, including NF-κB and JNK signaling.
TRB1 controls cytokine gene expression in WAT.
The data thus far established TRB1 as a novel output gene of the proinflammatory pathway in WAT, prompting us to investigate the functional relevance of these findings in an in vivo setting. Due to the high perinatal mortality of homozygous TRB1 knockout mice on the C57BL6 background strain (unpublished data, obtained from T. Satoh, Japan), we studied mice with haploinsufficiency for TRB1 (19), displaying an ∼50% reduction in whole-body TRB1 mRNA levels (supplementary Fig. 3A). Notably, both TRB2 and TRB3 mRNA levels in WAT of these mice were substantially lower compared with TRB1 and not affected by TRB1 deficiency (supplmentary Fig. 3A). Under basal conditions, TRB1 haploinsufficiency slightly increased food consumption (supplementary Fig. 3B) but had no effect on body weight; total body fat content, as determined by magnetic resonance technology (Fig. 4A and B); blood glucose levels (supplementary Fig. 3C); serum insulin levels (supplementary Fig. 3D); or serum cholesterol (supplementary Fig. 3E), as well as nonesterified fatty acid (supplementary Fig. 3F) and triglyceride (supplementary Fig. 3G) levels as compared with wild-type littermates. In WAT, key genes in glucose and lipid regulatory pathways, including GLUT4, acetyl-carboxylase-2, fatty acid synthase, and fatty acid–binding protein-4, were not different when comparing wild-type and TRB1 haploinsufficient animals (supplementary Fig. 3H).
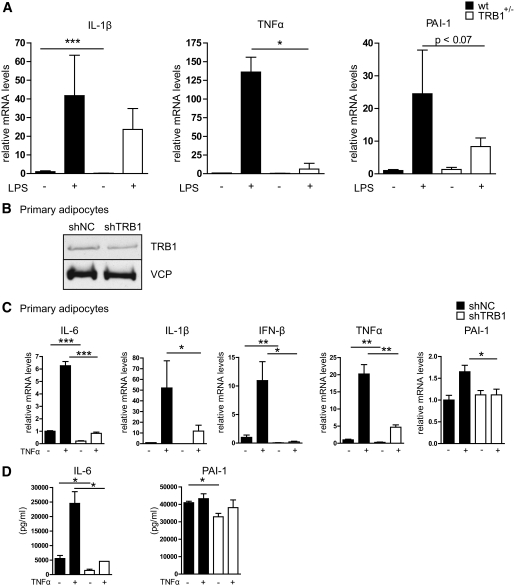
FIG. 3.
TRB1 controls cytokine gene expression in WAT. A: Quantitative PCR analysis of IL-1β, TNF-α, and PAI-1 mRNA levels in WAT of wild-type (wt) or TRB1 heterozygous knockout mice (TRB1+/−) under basal or LPS-injected (20 mg/kg) conditions (means ± SE, n = 6–7). B: Western blot of extracts from primary mature adipocytes derived from the stromal-vascular WAT fraction of wild-type mice using antibodies against TRB1 or valosin-containing protein (VCP). Cells were infected with a nonspecific control (NC) or TRB1-specific shRNA adenovirus at a multiplicity of infection of 1,000. C: Quantitative PCR analysis of IL-6, IL-1β, interferon (IFN)-β, TNF-α, and PAI-1 mRNA levels in primary mature adipocytes derived from the stromal-vascular WAT fraction of wild-type mice. Cells were infected with a nonspecific control (NC) or TRB1-specific shRNA adenovirus at a multiplicity of infection of 1,000 and exposed to TNF-α for 6 h prior to harvesting. D: Release of IL-6 and PAI-1 from the same cells as in C was measured by enzyme-linked immunosorbent assay (means ± SEM, n = 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
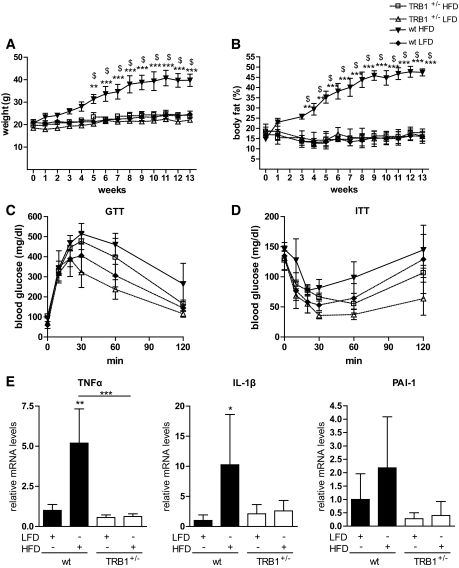
FIG. 4.
TRB1 haploinsufficiency protects against high-fat diet–induced obesity. A and B: Body weight (A) and total body fat (B) content as determined by magnetic resonance technology in wild-type (wt) or TRB1 heterozygous knockout mice (TRB1+/−) placed on a low-fat (LFD; 10% calories from fat) or high-fat (HFD; 60% calories from fat) diet for the indicated time points. C and D: Glucose (C) and insulin (D) tolerance tests in the same mice as in A and B. E: Quantitative PCR analysis of IL-1β, TNF-α, and PAI-1 mRNA levels in WAT of the same mice as in A and B after 13 weeks on a low- or high-fat diet (means ± SE, n = 3–4). *High-fat diet wild type versus low-fat diet wild type; $TRB1+/− high-fat diet vs. wild type high fat diet; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
In contrast, WAT mRNA expression of IL-1β, TNF-α, and plasminogen activator inhibitor (PAI)-1 was significantly reduced in TRB1 haploinsufficient mice under basal and/or LPS-stimulated conditions (Fig. 3A), supporting the hypothesis that TRB1 is specifically required for the execution of the proinflammatory program in WAT.
Given the variable impact of TRB1 haploinsufficiency on WAT cytokine gene expression (Fig. 3A), which can most likely be explained by differential effects of TRB1 on gene expression in different WAT cell types including macrophages (19), we next sought to determine the cell autonomous role of TRB1 in proinflammatory responses specifically in adipocytes. To this end, we infected 3T3-L1–derived mature adipocytes with an adenovirus carrying a TRB1-specific or a nonspecific control shRNA. TRB1-specific shRNA treatment substantially reduced TRB1 protein and mRNA expression in these cells (supplementary Fig. 4A and B) but had no influence on TRB2 and TRB3 expression levels (supplementary Fig. 4B), demonstrating the specificity of the shRNA knockdown strategy. Consistent with the results from TRB1 haploinsufficient mice (supplementary Fig. 3A), TRB1 deficiency impaired IL-6 and IL-1β gene expression (supplementary Fig. 4C) and release from these cells in response to proinflammatory stimulation (supplementary Fig. 4D) but exerted no discernable effect on metabolic gene expression (supplementary Fig. 4E).
To verify these findings in a primary cell system, we utilized differentiated SVF-derived mature adipocytes from wild-type mice infected with an adenovirus carrying a TRB1-specific or a nonspecific control shRNA as above. Adenoviral shRNA delivery substantially impaired TRB1 but not TRB2 and TRB3 expression in these primary cells (Fig. 3B and supplementary Fig. 4F). Recapitulating the results in TRB1 deficient mice and 3T3-L1–derived adipocytes, loss of TRB1 in fully differentiated mature adipocytes had no major influence on the expression of genes involved in either glucose metabolism, lipogenesis, or fatty acid oxidation, as demonstrated by quantitative PCR analysis (supplementary Fig. 4G). However, TRB1 deficiency substantially blunted the cytokine-triggered induction of proinflammatory genes, including IL-6, IL-1β, interferon-β, TNF-α, and PAI-1 (Fig. 3C), also reflected by a significantly impaired release of individual proinflammatory cytokines from these primary cells upon cytokine exposure as determined by enzyme-linked immunosorbent assay (Fig. 3D). Together with the in vivo data, these results demonstrated that TRB1 action is specifically required for the cell-autonomous cytokine production and release by white adipocytes during inflammation.
TRB1 haploinsufficiency protects against high-fat diet–induced obesity.
The critical role of TRB1 in the execution of WAT cytokine gene expression during acute inflammation prompted us to assess the potential impact of TRB1 on chronic, low-grade inflammatory conditions as those associated with adiposity and other components of the metabolic syndrome. To this end, heterozygous TRB1 knockout mice and wild-type littermates were placed on a high-fat diet or a control diet, with 60 or 10% of calories from fat, respectively, for 13 weeks. Wild-type mice on the high-fat diet showed a significant body weight as well as fat mass gain throughout the experimental period (Fig. 4A and B), associated with the increased expression of proinflammatory markers in WAT as reported (Fig. 4E) (37). Under the high-fat diet conditions, TRB1 heterozygosity had no effect on plasma glucose (supplementary Fig. 3C), serum insulin (supplementary Fig. 3D), or serum triglyceride (supplementary Fig. 3G) levels, as compared with wild-type controls. In contrast, weight gain and adiposity were almost completely prevented in TRB1+/− mice after 13 weeks on the high-fat diet, remaining at body weight and body fat mass levels of wild-type mice on the control diet (Fig. 4A and B). Consistent with the lean phenotype and improvements in body composition, high-fat diet–induced glucose intolerance tended to be improved in TRB1+/− mice (Fig. 4C), and TRB1+/− mice exhibited a trend toward increased blood glucose clearance after exogenous insulin administration, indicative of improved systemic insulin sensitivity (Fig. 4D). Moreover, TRB1 haploinsufficiency inhibited the HFD-mediated increase in proinflammatory gene expression in WAT of these animals (Fig. 4E), overall demonstrating the specific regulatory function of TRB1 for obesity-induced proinflammatory and metabolic programs under conditions of chronic energy excess in vivo.
TRB1 controls cytokine gene expression in adipocytes via direct promoter recruitment.
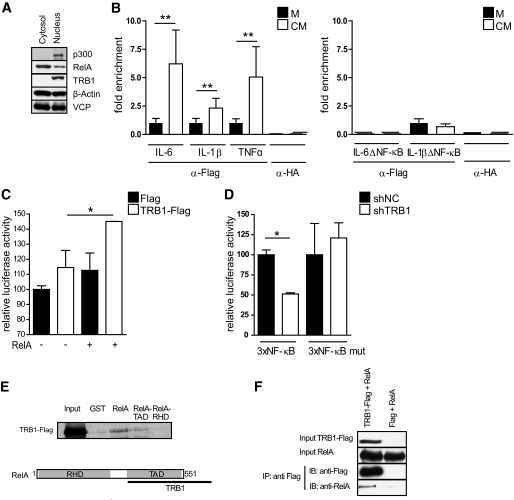
Finally, we sought to define the molecular mechanism of the regulatory function of TRB1 in adipocyte inflammation. To this end, we performed cellular fractionation studies to determine the principal localization of TRB1 within primary white adipocytes. In agreement with previous reports (38), TRB1 protein was exclusively detected in the adipocyte nuclear fraction (Fig. 5A), suggesting that TRB1 exerts its proinflammatory role on cytokine gene expression mainly through nuclear functions.
FIG. 5.
TRB1 controls cytokine gene expression in adipocytes via direct promoter recruitment. A: Representative Western blot of cytosolic or nuclear extracts from mature 3T3–L1–derived adipocytes using antibodies against p300, RelA, TRB1, β-actin, or valosin-containing protein (VCP) as indicated. B: ChIP assay of 3T3–L1 preadipoctes transfected with a Flag-TRB1 cDNA using antibodies specific for Flag (αFlag) or nonspecific IgG (αHA). Cells were treated with LPS-conditioned (conditioned medium [CM]) or non–LPS-conditioned (control [M]) macrophage media as indicated. Precipitated fragments were analyzed by real-time PCR using IL-6, IL-1β, and TNF-α promoter primers. Data show fold enrichment relative to control IgG. Left: Primer pairs including NF-κB sites; Right: Primer pairs without NF-κB sites (means ± SE, n = 4). C and D: Transient transfection assay of 3T3–L1 preadipocytes cotransfected with 3×NF-κB-Luc (containing wild-type NF-κB binding sites) (C and D) or 3×-NF-κB-mut-Luc (containing mutated NF-κB binding sites) (D), together with plasmids encoding RelA and TRB1 (C) or TRB1-specific or nonspecific control shRNA constructs as indicated (means ± SE, n = 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. E: Pull-down assays performed with full-length RelA, the transactivation domain (TA) or the Rel-homology domain (RHD) of RelA fused to GST and GST alone as a control. GST fusion proteins were incubated with in vitro translated full-length TRB1. Bound proteins were resolved by SDS-PAGE and visualized by autoradiography. Input lanes represent 20% of the input. Schematic representations of TRB1-RelA interactions are shown. F: Coimmunoprecipitation of TRB1 and RelA from HEK293 cells transfected with Flag-TRB1 or an empty vector using anti-Flag M2 antibody. Bound proteins were resolved by SDS-PAGE and subsequently detected by Western blot using RelA and Flag M2 antibodies.
Consistent with this notion, in chromatin immunoprecipitation assays of 3T3-L1 preadipocytes TRB1, but not negative-control precipitates, efficiently recovered endogenous IL-6, IL-1β, and TNF-α promoter fragments, carrying critical inflammation-responsive regulatory DNA elements (Fig. 5B, left). Interestingly, TRB1 was found to be further enriched on these promoter sites upon conditioned medium stimulation as compared with the basal state (Fig. 5B, left). On the contrary, no or reduced TRB1 promoter association was observed with IL-6 and IL-1β promoter regions, respectively, which lacked recognition elements for proinflammatory activator complexes, including the NF-κB subunit RelA (Fig. 5B, right), demonstrating the specificity of the observed effects. Taken together, these results showed that TRB1 is directly recruited to cytokine gene promoters in the nucleus and particularly directed to the NF-κB/RelA recognition site–containing promoter regions.
To test whether TRB1 can indeed functionally modulate RelA-dependent gene transcription, we utilized promoter reporter constructs harboring isolated wild-type or mutated RelA binding sites in transient transfection assays of 3T3-L1 preadipocytes. Cotransfection of a TRB1 cDNA expression plasmid coactivated RelA-driven wild-type promoter activity by 1.5-fold as compared with controls (Fig. 5C). Furthermore, shRNA-mediated knockdown of TRB1 in these preadipocytes significantly impaired RelA-dependent promoter activity but had no effect on mutated promoter function (Fig. 5D), demonstrating the requirement of endogenous TRB1 for full RelA transcriptional activity in this context.
Overall, these data supported the hypothesis that TRB1 acts as a direct transcriptional coactivator for NF-κB/RelA in the control of cytokine gene expression in white (pre-) adipocytes. Indeed, GST-tagged RelA but not GST alone recovered in vitro translated TRB1 in GST pulldown assays via its transactivator domain (Fig. 5E), and TRB1 was found to bind to RelA in cellular coimmunoprecipitation assays (Fig. 5F). Taken together, these results underline the notion that TRB1 can affect RelA transcriptional activity via direct physical interaction upon promoter recruitment.
DISCUSSION
Our results suggest a novel nuclear coactivator function of TRB1 on proinflammatory cytokine promoters in white adipocytes, mediated by its recruitment to NF-κB DNA recognition sites. Also, given the discovery that TRB1 gene expression is induced via proinflammatory pathways in these cells, our data are consistent with a model in which TRB1 acts as both a target and effector of proinflammatory signaling in adipocytes via its direct physical interaction with the RelA subunit of NF-κB.
A biological function for TRB1 has been reported only in a very limited number of studies. Thus far, TRB1 has been suggested to represent a biomarker for antibody-mediated allograft failure, expressed mainly in antigen-presenting cells and activated endothelial cells (39). In addition, TRB1 controls vascular smooth muscle cell proliferation and chemotaxis via regulation of mitogen-activated protein kinase activity (40) and has been shown to control NF–IL-6–mediated gene expression in macrophages in an NF-κB–independent manner (19). By acting as a positive mediator of inflammatory cues in adipocytes, the coactivator function of TRB1 for cytokine gene expression in these cells may thereby reflect a specific “adipose” aspect of a broader involvement of TRB1 in (pro-) inflammatory programs and pathologies at a systemic level. Interestingly, TRB1 haploinsufficiency resulted in no obvious metabolic phenotype under basal conditions (supplementary Fig. 3A–G), and TRB1 haplodeficiency did not seem to influence adipocyte differentiation, per se, as the body fat content was not different from wild-type animals under standard diet–fed conditions (Fig. 4B), thereby contrasting the essential requirements of both TRB2 and TRB3 for the adipogenic program (17,18). However, TRB1 expression has been found to be elevated in human atherosclerotic arteries (40), and recent genome-wide association studies have pinpointed variants of the TRB1 locus as risk factors for hypertriglyceridemia and coronary artery disease (41–43), conditions tightly linked to a chronic inflammatory status (44). Indeed, during low-grade inflammatory conditions TRB1 heterozygosity protected against high-fat diet–induced obesity, glucose intolerance, as well as insulin resistance and inhibited cytokine gene expression in WAT (Fig. 4). Rather than controlling metabolic pathways directly, it is tempting to speculate that the proinflammatory action of TRB1 in adipocytes contributes to the above-mentioned (metabolic) pathologies by enhancing the release of circulating cytokines from WAT in response to a primary, proinflammatory hit (e.g., macrophage-derived cytokines). This notion is consistent with the induction of TRB1 expression in synovial fibroblasts upon IL-1β exposure (45) as well as with our data showing that the transcriptional activation of TRB1 in adipocytes does not rely on direct TLR4/LPS signaling (Fig. 2A) but rather on a complex cytokine cocktail as produced by tissue macrophages or stressed adipocytes, per se (Fig. 2A). Given the whole-body heterozygosity, the observed resistance against diet-induced obesity could be further enhanced by nonadipose tissue functions of TRB1, including increased uncoupled respiration in BAT, increased physical activity, and/or differences in intestinal absorption, which will be addressed in future studies.
Overall, TRB1 may serve as a functional receptor in the communication between metabolic (adipocytes) and immune cells (e.g., macrophages) in WAT, thereby amplifying WAT inflammation in response to activated WAT-associated immune reactions. As proinflammatory signaling is typically increased in sepsis, insulin resistance, and obesity-related type 2 diabetes (46), the cytokine-inducible TRB1 coactivator function in WAT might provide a molecular rationale for the amplification of systemic inflammation in these subjects.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (He3260/3-1), a Marie Curie Excellence Grant (European Union), and the Thyssen Foundation (to S.H.).
No potential conflicts of interest relevant to this article were reported.
A.O. researched data, prepared figures, and reviewed the manuscript. A.J., A.J.R., M.L., S.K., A.R., A.V., M.B., D.S., M.Y., T.S., and S.A. researched data and contributed to the discussion. S.H. designed, researched, and wrote the manuscript.
We thank K. Du, Tufts College, Boston, MA; M.O. Hottiger, University of Zurich, Zurich, Switzerland for providing reagents; E. Chichelnitskiy, DKFZ Heidelberg, Heidelberg, Germany; S. Kersten, University of Wageningen, Wageningen, Netherlands; P. Narvekar, DKFZ Heidelberg, Heidelberg, Germany; F. Mattijssen, University of Wageningen, Wageningen, Netherlands; S. Scherneck, German Institute of Human Nutrition, Potsdam, Germany; and I. Zschiedrich, DKFZ Heidelberg, Heidelberg, Germany for experimental advice and sample preparation.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Gesta S, Tseng YH, Kahn CR: Developmental origin of fat: tracking obesity to its source. Cell 2007;131:242–256 [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, Maillard I, Saltiel AR: T-ing up inflammation in fat. Nat Med 2009;15:846–847 [DOI] [PubMed] [Google Scholar]
- 3.Galic S, Oakhill JS, Steinberg GR: Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129–139 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Erbay E: Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier SF, Watkins LR, Fleshner M: Psychoneuroimmunology: the interface between behavior, brain, and immunity. Am Psychol 1994;49:1004–1017 [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM: Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK: Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 2006;147:5340–5351 [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R: Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil GS: Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 11.Seher TC, Leptin M: Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol 2000;10:623–629 [DOI] [PubMed] [Google Scholar]
- 12.Du K, Herzig S, Kulkarni RN, Montminy M: TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003;300:1574–1577 [DOI] [PubMed] [Google Scholar]
- 13.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M: PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 2004;10:530–534 [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Wu X, Franklin JL, Messina JL, Hill HS, Moellering DR, Walton RG, Martin M, Garvey WT: Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am J Physiol Endocrinol Metab 2010;298:E565–E576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi XP, Tan HW, Xing SS, Wang ZH, Tang MX, Zhang Y, Zhang W: Overexpression of TRB3 gene in adipose tissue of rats with high fructose-induced metabolic syndrome. Endocr J 2008;55:747–752 [DOI] [PubMed] [Google Scholar]
- 16.Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M: TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 2006;312:1763–1766 [DOI] [PubMed] [Google Scholar]
- 17.Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A: TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. J Biol Chem 2007;282:24075–24082 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Ohoka N, Hayashi H, Sato R: TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity. J Lipid Res, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Uematsu S, Okamoto T, Matsuura Y, Sato S, Kumar H, Satoh T, Saitoh T, Takeda K, Ishii KJ, Takeuchi O, Kawai T, Akira S: Enhanced TLR-mediated NF-IL6 dependent gene expression by Trib1 deficiency. J Exp Med 2007;204:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eder K, Guan H, Sung HY, Ward J, Angyal A, Janas M, Sarmay G, Duda E, Turner M, Dower SK, Francis SE, Crossman DC, Kiss-Toth E: Tribbles-2 is a novel regulator of inflammatory activation of monocytes. Int Immunol 2008;20:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang YY, Wang ZH, Zhang LP, Zhong M, Zhang Y, Deng JT, Zhang W: TRB3, upregulated by ox-LDL, mediates human monocyte-derived macrophage apoptosis. FEBS J 2009;276:2752–2761 [DOI] [PubMed] [Google Scholar]
- 22.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H: TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 2005;24:1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M: CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001;413:179–183 [DOI] [PubMed] [Google Scholar]
- 24.Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M: CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature 2003;426:190–193 [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, Taguchi T: Experimental cancer cachexia induced by transplantable colon 26 adenocarcinoma in mice. Cancer Res 1990;50:2290–2295 [PubMed] [Google Scholar]
- 26.Berriel Diaz M, Krones-Herzig A, Metzger D, Ziegler A, Vegiopoulos A, Klingenspor M, Muller-Decker K, Herzig S: Nuclear receptor cofactor receptor interacting protein 140 controls hepatic triglyceride metabolism during wasting in mice. Hepatology 2008;48:782–791 [DOI] [PubMed] [Google Scholar]
- 27.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR: Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000;6:87–97 [PubMed] [Google Scholar]
- 28.Rodeheffer MS, Birsoy K, Friedman JM: Identification of white adipocyte progenitor cells in vivo. Cell 2008;135:240–249 [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 30.Du K, Ding J: Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta. Mol Endocrinol 2009;23:475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canettieri G, Morantte I, Guzman E, Asahara H, Herzig S, Anderson SD, Yates JR, 3rd, Montminy M: Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol 2003;10:175–181 [DOI] [PubMed] [Google Scholar]
- 32.Zschiedrich I, Hardeland U, Krones-Herzig A, Berriel Diaz M, Vegiopoulos A, Muggenburg J, Sombroek D, Hofmann TG, Zawatzky R, Yu X, Gretz N, Christian M, White R, Parker MG, Herzig S: Coactivator function of RIP140 for NFkappaB/RelA-dependent cytokine gene expression. Blood 2008;112:264–276 [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP: Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84:491–495 [DOI] [PubMed] [Google Scholar]
- 34.Tisdale MJ: Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–871 [DOI] [PubMed] [Google Scholar]
- 35.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S: Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999;162:3749–3752 [PubMed] [Google Scholar]
- 36.Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B: Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol 2002;61:953–963 [DOI] [PubMed] [Google Scholar]
- 37.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M: Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology 2007;148:2753–2763 [DOI] [PubMed] [Google Scholar]
- 38.Kiss-Toth E, Wyllie DH, Holland K, Marsden L, Jozsa V, Oxley KM, Polgar T, Qwarnstrom EE, Dower SK: Functional mapping and identification of novel regulators for the Toll/Interleukin-1 signalling network by transcription expression cloning. Cell Signal 2006;18:202–214 [DOI] [PubMed] [Google Scholar]
- 39.Ashton-Chess J, Giral M, Mengel M, Renaudin K, Foucher Y, Gwinner W, Braud C, Dugast E, Quillard T, Thebault P, Chiffoleau E, Braudeau C, Charreau B, Soulillou JP, Brouard S: Tribbles-1 as a novel biomarker of chronic antibody-mediated rejection. J Am Soc Nephrol 2008;19:1116–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung HY, Guan H, Czibula A, King AR, Eder K, Heath E, Suvarna SK, Dower SK, Wilson AG, Francis SE, Crossman DC, Kiss-Toth E: Human tribbles-1 controls proliferation and chemotaxis of smooth muscle cells via MAPK signaling pathways. J Biol Chem 2007;282:18379–18387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hedner T, Corella D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshuler DM, Orho-Melander M: Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 2008;40:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Ban MR, Zou GY, Cao H, Lin T, Kennedy BA, Anand S, Yusuf S, Huff MW, Pollex RL, Hegele RA: Polygenic determinants of severe hypertriglyceridemia. Hum Mol Genet 2008;17:2894–2899 [DOI] [PubMed] [Google Scholar]
- 43.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR: Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008;40:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glass CK, Witztum JL: Atherosclerosis: the road ahead. Cell 2001;104:503–516 [DOI] [PubMed] [Google Scholar]
- 45.Sung HY, Francis SE, Crossman DC, Kiss-Toth E: Regulation of expression and signalling modulator function of mammalian tribbles is cell-type specific. Immunol Lett 2006;104:171–177 [DOI] [PubMed] [Google Scholar]
- 46.Zeyda M, Stulnig TM: Obesity, inflammation, and insulin resistance: a mini-review. Gerontology 2009;55:379–386 [DOI] [PubMed] [Google Scholar]