Abstract
OBJECTIVE
Insulin-induced phosphatidylinositol 3-kinase (PI3K)/Akt signaling and interleukin-6 (IL-6)-instigated JAK/STAT3-signaling pathways in the liver inhibit the expression of gluconeogenic genes to decrease hepatic glucose output. The insulin receptor (IR) and JAK1 tyrosine kinases and STAT3 can serve as direct substrates for the T-cell protein tyrosine phosphatase (TCPTP). Homozygous TCPTP-deficiency results in perinatal lethality prohibiting any informative assessment of TCPTP's role in glucose homeostasis. Here we have used Ptpn2+/− mice to investigate TCPTP's function in glucose homeostasis.
RESEARCH DESIGN AND METHODS
We analyzed insulin sensitivity and gluconeogenesis in chow versus high-fat–fed (HFF) Ptpn2+/− and Ptpn2+/+ mice and insulin and IL-6 signaling and gluconeogenic gene expression in Ptpn2+/− and Ptpn2+/+ hepatocytes.
RESULTS
HFF Ptpn2+/− mice exhibited lower fasted blood glucose and decreased hepatic glucose output as determined in hyperinsulinemic euglycemic clamps and by the decreased blood glucose levels in pyruvate tolerance tests. The reduced hepatic glucose output coincided with decreased expression of the gluconeogenic genes G6pc and Pck1 and enhanced hepatic STAT3 phosphorylation and PI3K/Akt signaling in the fasted state. Insulin-induced IR-β–subunit Y1162/Y1163 phosphorylation and PI3K/Akt signaling and IL-6–induced STAT3 phosphorylation were also enhanced in isolated Ptpn2+/− hepatocytes. The increased insulin and IL-6 signaling resulted in enhanced suppression of G6pc and Pck1 mRNA.
CONCLUSIONS
Liver TCPTP antagonises both insulin and STAT3 signaling pathways to regulate gluconeogenic gene expression and hepatic glucose output.
Type 2 diabetes has reached epidemic proportions, afflicting roughly 170 million people worldwide. Although the underlying genetic causes and the associated pathologic symptoms are heterogenous, a common feature is high blood glucose due to peripheral insulin resistance. Circulating insulin released from β-cells in the pancreas serves to lower blood glucose by triggering the translocation of the facilitative GLUT4 to the plasma membrane in muscle and adipose tissue (1). Insulin also acts in the liver to promote glycogen synthesis and lipogenesis and to suppress hepatic glucose production (HGP) by inhibiting gluconeogenesis and glycogenolysis (1). Elevated HGP caused by defective suppression of gluconeogenesis is one of the primary defects contributing to fasting hyperglycemia in patients with type 2 diabetes (2–4).
Glucose-6-phosphatase (G6Pase; encoded by G6pc) and phosphoenolpyruvate carboxykinase (PEPCK; encoded by Pck1) are key enzymes involved in the rate-limiting steps of gluconeogenesis (1). The overexpression of PEPCK or G6Pase in rodent models results in hyperinsulinaemia, insulin resistance, and glucose intolerance (5–7), and in at least one instance, PEPCK overexpression has been shown to promote weight gain (8). PEPCK catalyzes the conversion of oxaloacetate to phosphoenolpyruvate, whereas G6Pase catalyzes the dephosphorylation of glucose 6-phosphate to free glucose, the final step of both gluconeogenesis and glycogenolysis. The expression of these key gluconeogenic enzymes is controlled by signaling pathways that are activated by insulin, glucagon, and IL-6. Although insulin and IL-6 suppress G6pc and Pck1 expression, glucagon stimulates their expression (1,9–11). Insulin exerts its effects via the PI3K/Akt pathway. Insulin binds to its cell surface receptor to stimulate intrinsic protein tyrosine kinase (PTK) activity, resulting in the phosphorylation of the insulin receptor (IR) and several IR substrates (IRS), such as IRS-1. IRS-1 tyrosine phosphorylation allows for the recruitment of PI3K, which catalyzes the formation of lipid phosphatidylinositol-3,4,5-triphosphate (PIP3) at the plasma membrane (1). Increases in PIP3 activate several Ser/Thr protein kinases, including Akt, which phosphorylates and prevents the translocation of the transcription factor Foxo1a to the nucleus, where it otherwise functions in concert with peroxisome proliferator-activated receptor g coactivator 1a (PGC1a) to increase the transcription of the gluconeogenic genes G6pc and Pck1 (1,12,13). Several studies have also implicated signal transducer and activator of transcription-3 (STAT3) in the PGC1a-independent suppression of hepatic gluconeogenic gene expression (11,14). In particular, hypothalamic control of hepatic IL-6 generation and JAK (Janus activated kinase)/STAT3 signaling has emerged as an important mechanism for the regulation of HGP (11,15–17).
Several protein tyrosine phosphatases (PTPs) have been implicated in the modulation of glucose homeostasis in vivo, including the prototypic protein tyrosine phosphatase 1B (PTP1B) (18–22). PTP1B dephosphorylates the IR PTK in liver and muscle to regulate glucose homeostasis (18,19,21,22). PTP1B also dephosphorylates and inactivates the JAK2 PTK in the hypothalamus to antagonize leptin-induced JAK2/STAT3 signaling and thus leptin's effects on body mass and peripheral insulin sensitivity (20,23). PTP1B dephosphorylates the IR-β subunit Y1162/Y1163 autophosphorylation site, which is necessary for IR activation, as well as the Y972 site that contributes to IRS-1 recruitment (24). Muscle or liver-specific PTP1B knockout mice exhibit increased insulin-induced IR Y1162/Y1163 phosphorylation and PI3K/Akt signaling and concomitant improved glucose tolerance associated with enhanced glucose uptake and decreased HGP respectively (21,22).
The T-cell protein tyrosine phosphatase (TCPTP) (encoded by Ptpn2) is a ubiquitous tyrosine-specific phosphatase (25). The catalytic domains of PTP1B and TCPTP share a high degree of primary (72% identity) and tertiary structure similarity and have similar active sites. In particular, both PTPs share a second “phosphotyrosine-binding pocket” that allows for the selective recognition of tandem tyrosyl phosphorylated substrates (26,27), such as the IR (24,26,28) and JAK PTKs (JAK1–3 and TYK2) (29). Despite their similarity, TCPTP and PTP1B exhibit a high degree of substrate selectivity and cooperativity in a cellular context. PTP1B can dephosphorylate JAK2, but not JAK1/3, whereas TCPTP dephosphorylates JAK1/3, but not JAK2 (23,29). Moreover, using cell-based approaches, we previously identified the IR as a bona fide substrate for TCPTP (24,28). We reported that PTP1B and TCPTP could act in concert to regulate IR-β Y1162/Y1163 and Y972 phosphorylation and PI3K/Akt signaling (24). Additional substrates for TCPTP include STAT family members such as STAT-3 (25,30,31). Despite TCPTP's potential to regulate IR and JAK/STAT3 signaling, it remains unclear whether TCPTP regulates glucose homeostasis in vivo. This is due to the morbidity and lethality that is associated with a global deficiency in TCPTP (32); Ptpn2−/− mice develop inflammatory disease and hematopoietic defects and succumb at 2–3 weeks of age due to a bone marrow stromal cell defect (32,33). In this study we have explored the potential of TCPTP to regulate glucose homeostasis in Ptpn2+/− mice that have a normal life expectancy and an unaltered inflammatory response (32,33). Our studies point toward TCPTP acting as an integral negative regulator of gluconeogenesis and fasting blood glucose.
RESEARCH DESIGN AND METHODS
Antibodies and reagents.
JAK PTK inhibitor CMP6 (2-tert-butyl-9-fluoro-3,6-dihydro-7Hbenz[h]-imidaz[4,5-f]isoquin-oline-7-one) was from Calbiochem (San Diego, CA), and dexamethasone and insulin from Sigma-Aldrich (St Louis, MO). Rabbit α-phospho-Akt-S473, α-phospho-STAT3-Y705, α-Akt and α-STAT3 were from Cell Signaling (Beverly, MA); α-actin (sc-1616) was from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit α-phospho-IRβ-Y1162/Y1163, α-phospho-IRβ-Y972 and α-phospho-JAK1-Y1022/Y1023 from Biosource International (Camarillo, CA); mouse α-IRβ (Ab-5) and α-actin were from Thermo Scientific (Fremont, CA), and mouse α-tubulin from Sigma-Aldrich (St Louis, MO). The mouse IL-6 ELISA kit was from eBiosciences (San Diego, CA), and recombinant human and murine IL-6 from PeproTech (Rocky Hill, NJ).
Mice.
Mice were maintained on a 12-h light-dark cycle with free access to food and water. Age- and sex-matched mice were used for all experiments. Ptpn2+/− mice on a 129sv x BALB/c mixed background (32) were backcrossed onto BALB/c background for six generations and genotyped as described previously (32). Mice were fed a standard chow diet (19% protein, 4.6% fat, and 4.8% crude fiber; Specialty Feeds, Australia) or a high-fat diet (19% protein, 60% fat, and 4.7% crude fiber; Specialty Feeds, Australia) as indicated.
Metabolic measurements.
Insulin tolerance tests and pyruvate or glucose tolerance tests were performed on 4- and 6-h fasted mice, respectively, by injecting human insulin (0.75–1.5 mU/g body weight), d-glucose (1–2 mg/g body weight), or pyruvate (1–2 mg/g body weight) intraperitoneally and measuring glucose in tail blood as described previously (34). Euglycemic hyperinsulinemic clamps were performed on overnight-fasted and anesthetized mice as described previously (34). Fed and fasted blood glucose and corresponding plasma insulin levels were determined as described previously (34).
Cell culture.
The generation and culture conditions of control HeLa cells and those expressing TCPTP-specific shRNA have been described previously (31). Hepatocytes from 8- to 12-week-old Ptpn2−/− and Ptpn2+/+ mice were isolated by a two-step collagenase A (0.05% wt/vol; Roche Diagnostics, Germany) perfusion as described previously (34). Hepatocytes were cultured in M199 medium (Invitrogen, Carlsbad, CA) containing 10% (vol/vol) heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 nmol/l dexamethasone, 50 nmol/l insulin and 20 ng/ml EGF (R&D Systems, Minneapolis, MN) for no more than 3 days. Cells were starved in M199 medium alone for 4 h, and then stimulated with 10 nmol/l insulin or 1 ng/ml IL-6, as indicated.
Biochemical analyses.
Tissues were mechanically homogenized in ice cold RIPA lysis buffer (50 mmol/l Hepes [pH 7.4], 1% (vol/vol) Triton X-100, 1% (vol/vol) sodium deoxycholate, 0.1% (vol/vol) SDS, 150 mmol/l NaCl, 10% (vol/vol) glycerol, 1.5 mmol/l MgCl2, 1 mmol/l EGTA, 50 mmol/l sodium fluoride, leupeptin (5 μg/ml), pepstatin A (1 μg/ml), 1 mmol/l benzamadine, 2 mmol/l phenylmethysulfonyl fluoride, 1 mmol/l sodium vanadate) and clarified by centrifugation (100,000g for 20 min at 4°C). Tissue and cell lysates were resolved by SDS-PAGE and immunoblotted. Lipid analyses were performed as described previously (34).
RT-PCR.
Liver was dissected and immediately frozen in liquid N2, and RNA extracted using Trizol reagent (Invitrogen, Carlsbad, CA). mRNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and quantitative RT-PCR performed using the TaqMan Universal PCR Master Mix and Gene Expression Assays (Applied Biosystems) for G6pc, Pck1, Fbp1, Srebf1, Fasn, and Il6; Gapdh or 18S were used as internal controls. Reactions were performed in quadruplicate and relative quantification achieved using the DDCt method.
RESULTS
Decreased gluconeogenesis and hepatic glucose production in Ptpn2+/− mice.
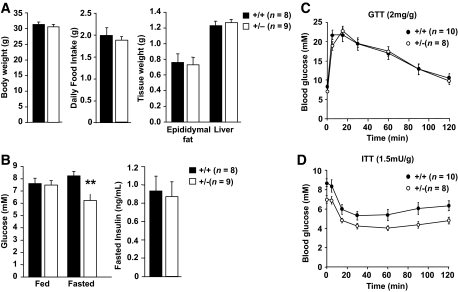
Ptpn2+/− (BALB/c) mice are healthy and fertile and do not show any overt histopathologies (32). To assess the impact of TCPTP heterozygous deficiency on glucose homeostasis, 8- to 10-week-old Ptpn2+/− versus +/+ littermate male mice were fed a standard chow diet for 20 weeks or a high-fat diet (60% fat; 74% energy from fat) for 15 weeks to induce insulin resistance and fasting hyperglycemia (Fig. 1; supplementary Fig. 1 which is available in the online appendix at http://diabetes.diabetesjournals.org). Food intake (high-fat diet) and body and tissue weights were determined, and insulin sensitivity and glucose homeostasis assessed in insulin tolerance tests (ITTs) and glucose tolerance tests (GTTs) and by monitoring blood glucose and insulin levels. Food intake (high-fat diet), body weights, and liver and fad-pad masses remained unaltered in +/+ versus +/− mice on either diet (Fig. 1A; supplementary Fig. 1A). Similarly, no significant differences were noted in ITTs or GTTs (Fig. 1C–D; supplementary Fig. 1C–D). However, fasted blood insulin levels were significantly reduced in chow-fed mice, and this trended with reduced fasted blood glucose (supplementary Fig. 1B). More importantly, fasted blood glucose levels were significantly reduced in high-fat–fed (HFF) Ptpn2+/− versus Ptpn2+/+ mice (Fig. 1B), approximating those seen in fasted chow-fed +/+ mice. Therefore, these results indicate that a reduction in TCPTP protein may be sufficient to prevent the fasting hyperglycemia that is associated with high-fat feeding-induced insulin resistance.
FIG. 1.
Decreased fasting hyperglycemia in HFF Ptpn2+/− mice. Eight to 10-week-old Ptpn2+/− and +/+ littermate male mice were fed a high-fat diet (60% fat) for 15 weeks and (A) body weights, daily food intake, and the indicated tissue weights determined. (B) Fed and fasted (6 h) blood glucose and fasted plasma insulin levels were measured. Mice were fasted for (C) 6 h and GTTs performed, or (D) for 4 h and ITTs performed. Results shown are means ± SE; **P < 0.01 by a two-tailed Student t test.
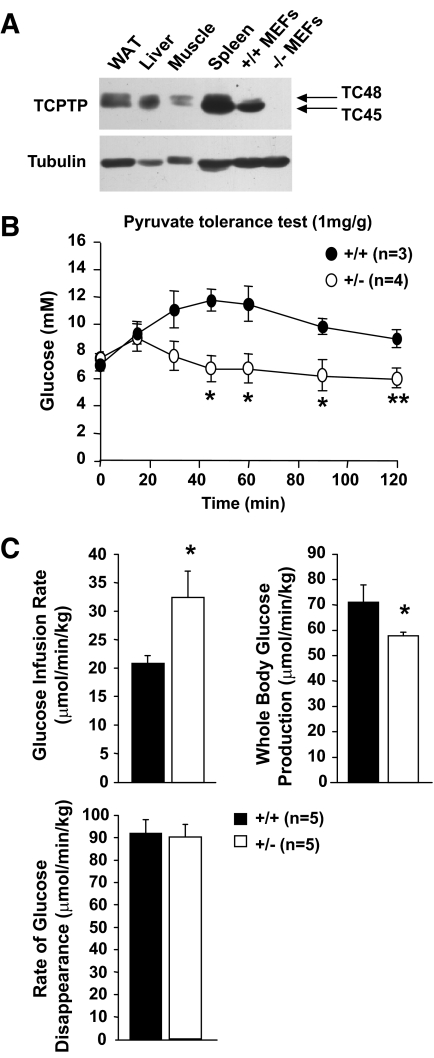
TCPTP is expressed in liver, white adipose tissue (WAT), and skeletal muscle (Fig. 2A), the key insulin responsive tissues responsible for the control of glucose homeostasis. TCPTP protein levels were not overtly altered in liver, WAT, or muscle in HFF (data not shown) or Ob/Ob obese mice (supplementary Fig. 2). The liver is the primary tissue responsible for the control of blood glucose levels in the fasted state, generating glucose from noncarbohydrate sources in a process known as gluconeogenesis during periods of fasting, starvation, or intense exercise (35). Fasting hyperglycemia in type 2 diabetes is linked to elevated gluconeogenesis and HGP (2–4). One possibility is that the lower fasted blood glucose levels in the HFF Ptpn2+/− mice may be caused by decreased gluconeogenesis. To assess this, we performed pyruvate tolerance tests (PTTs); administration of the gluconeogenic substrate pyruvate increases blood glucose levels by promoting gluconeogenesis in the liver. Administration of pyruvate (1 mg/g body weight) significantly enhanced blood glucose levels in Ptpn2+/+ mice, but this was attenuated in HFF Ptpn2+/− mice (Fig. 2B), indicating reduced gluconeogenesis; no differences were noted in PTTs in chow-fed mice (supplementary Fig. 3A). To further characterize the apparently reduced gluconeogenesis in HFF mice, whole-body glucose disappearance and production were measured in HFF Ptpn2+/− versus +/+ mice by performing hyperinsulinemic euglycemic clamps (Fig. 2C). The rate at which glucose was infused to maintain euglycaemia during the clamps was increased by ∼30% in Ptpn2+/− mice (Fig. 2C), indicative of enhanced insulin sensitivity. Although glucose disappearance (mainly in muscle and fat) remained unaltered, the ability of insulin to suppress whole-body (mainly hepatic) glucose production was increased in Ptpn2+/− mice (Fig. 2C). Taken together, these results indicate that insulin sensitivity was increased in HFF Ptpn2+/− mice and that this was ascribed to decreased HGP.
FIG. 2.
Decreased gluconeogenesis and hepatic glucose production in Ptpn2+/− mice. A: Expression of the 45-kDa (TC45) and 48-kDa (TC48) variants of TCPTP in white adipose tissue (epididymal; WAT), liver, muscle (gastrocnemius), and spleen, as well as immortalised Ptpn2−/− and +/+ mouse embryo fibroblasts (MEFs). B and C: Eight- to 10-week-old Ptpn2+/− and +/+ littermate male mice were fed a high-fat diet for 15 weeks and (B) pyruvate tolerance tests, or (C) hyperinsulinaemic-euglycemic clamps performed. Glucose infusion and disappearance rates were determined and whole-body glucose production determined by subtracting the glucose infusion rate from the glucose appearance rate. Results shown are means ± SE; *P < 0.05 by a two-tailed Student t test.
Decreased gluconeogenic and increased lipogenic gene expression in Ptpn2+/− mice.
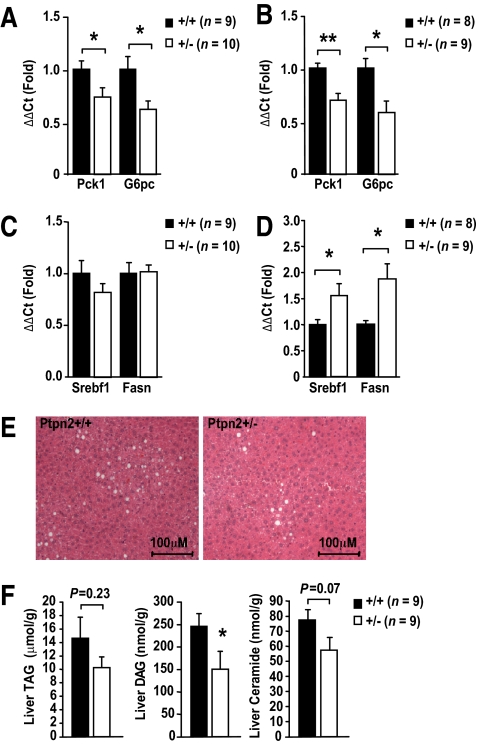
To further assess the potential of TCPTP to regulate hepatic gluconeogenesis, we examined the expression of the rate-limiting gluconeogenic genes G6pc and Pck1 in livers from fasted HFF Ptpn2+/− mice and from those subjected to clamps by quantitative RT-PCR (ΔΔCt) using Gapdh (Fig. 3) or 18S (data not shown) for normalization. We also measured the expression of genes encoding the lipogenic enzymes SREBP-1c (sterol regulatory element-binding protein 1c; encoded by Srebf1) and Fas (fatty acid synthase; encoded by Fasn) that are normally increased in expression in response to insulin (1). We found that G6pc and Pck1 were reduced in both fasted (Fig. 3A) and clamped HFF Ptpn2+/− mice (Fig. 3B), whereas Fasn and Srebf1 were increased in clamped (Fig. 3D), but not fasted mice (Fig. 3C); hepatic G6pc and Pck1 were not altered in chow-fed Ptpn2+/− versus +/+ mice (supplementary Fig. 3B). Given the increased lipogenic gene expression in clamped HFF Ptpn2+/− mice, we monitored for hepatic steatosis by histologic means and by measuring ceramide, diglyceride (DAG), and triglyceride (TAG) levels in HFF Ptpn2+/− versus Ptpn2+/+ mice. Histologically, steatosis appeared to be decreased in HFF Ptpn2+/− mice (Fig. 3E), and this coincided with a trend for reduced hepatic ceramides, TAGs, and significantly reduced DAGs (Fig. 3F), consistent with the overall enhanced insulin sensitivity evident in hyperinsulinemic euglycemic clamps. Taken together, these results indicate that hepatic insulin signaling was enhanced, in line with repressed gluconeogenesis and HGP in HFF Ptpn2+/− mice.
FIG. 3.
Altered gluconeogenic and lipogenic gene expression in Ptpn2+/− mice. Eight to 10-week-old Ptpn2+/− and +/+ male mice were fed a high-fat diet for 15 weeks. Livers were harvested from (A and C) 4-h fasted mice, or (B and D) at the end of hyperinsulinaemic-euglycemic clamps and processed for quantitative (ΔΔCt) RT-PCR to measure the expression of (A and B) gluconeogenic genes Pck1 and G6pc, or (C and D) lipogenic genes Srebf1 and Fasn, with Gapdh used for normalization; similar results were attained when 18S was used for normalization. E: Ptpn2+/− and +/+ male mice were fed a high-fat diet for 15 weeks, livers extracted, fixed in formalin, paraffin embedded, and processed for histology (hematoxylin and eosin). F: Mice were fasted for 4 h, livers isolated, and triglyceride (TAG), diglyceride (DAG), and ceramide extracted and quantified. Results shown are means ± SE; *P < 0.05, **P < 0.01 by a two-tailed Student t test. (A high-quality digital representation of this figure is available in the online issue.)
Enhanced hepatic STAT3 phosphorylation and PI3K/Akt signaling Ptpn2+/− mice.
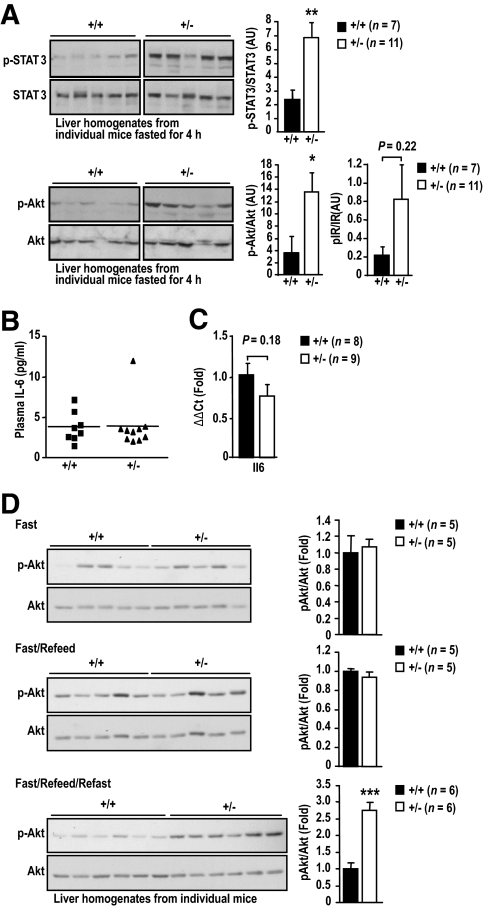
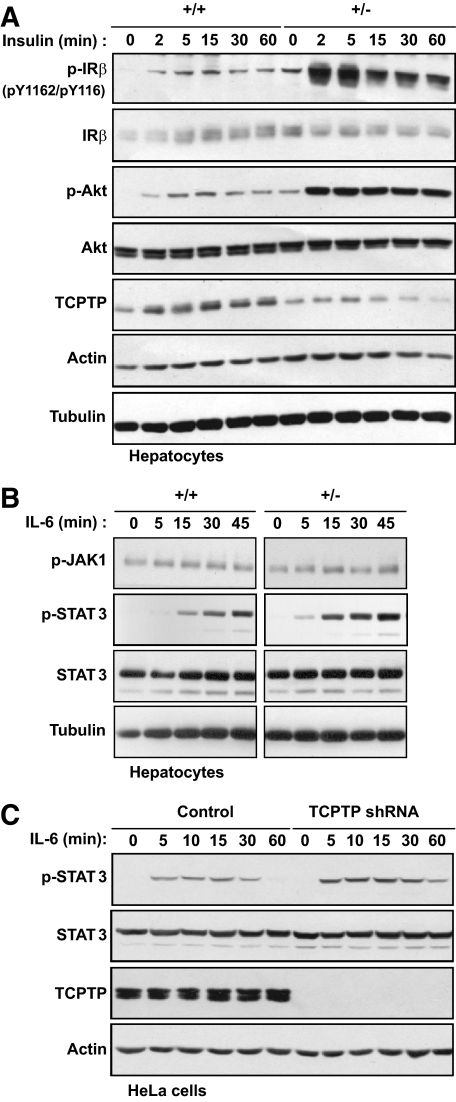
Next we examined the molecular basis for the decreased fasting blood glucose levels and decreased gluconeogenic gene expression and HGP in HFF Ptpn2+/− mice. We reported previously that TCPTP can dephosphorylate the IR PTK to suppress insulin signaling (24,28,36), whereas others have used overexpression approaches to identify STAT3 as a putative TCPTP substrate (30). Insulin-instigated PI3K/Akt signaling and IL-6-induced STAT3 pathways suppress gluconeogenic gene expression and HGP (1,10,11). Accordingly, we assessed the activation of these pathways in the livers of 4-h fasted HFF Ptpn2+/− versus +/+ mice by immunoblot analysis. We found that STAT3 Y705 phosphorylation was significantly enhanced in livers from fasted Ptpn2+/− mice (Fig. 4A). Importantly, IL-6 in blood or liver was not altered in HFF Ptpn2+/− mice (Fig. 4B and C). We also noted that PI3K/Akt signaling, as monitored by Akt Ser-473 phosphorylation, was elevated in livers from fasted HFF Ptpn2+/− mice, and this coincided with a trend for elevated IR-β subunit Y1162/Y1163 phosphorylation (Fig. 4A; supplementary Fig. 4A) and IRS-1 tyrosine phosphorylation (supplementary Fig. 4B). There were no significant increases in STAT3 or Akt phosphorylation in muscle or WAT from HFF Ptpn2+/− versus +/+ mice (supplementary Fig. 4C). Moreover, neither STAT3 phosphorylation nor PI3K/Akt were elevated in the livers of fasted chow-fed Ptpn2+/− mice (supplementary Fig. 3C). Interestingly, although hepatic insulin signaling in fasted HFF Ptpn2+/− mice appeared to be elevated, we found no significant difference in IR and IRS-1/2 phosphorylation or PI3K/Akt signaling in response to bolus insulin (2 mU/g, 10 min) administration (supplementary Fig. 4A–B), indicating that TCPTP heterozygous deficiency does not alter the acute response to insulin. To further assess the impact of TCPTP heterozygous deficiency on insulin signaling, we monitored for hepatic Akt Ser-473 phosphorylation in overnight-fasted (8 h) and refed (4 h), and thereon refasted (4 h) HFF Ptpn2+/− mice. Although we noted no overt difference in PI3K/Akt signaling in +/+ versus Ptpn2+/− mice after refeeding, Akt Ser-473 phosphorylation was significantly elevated in HFF Ptpn2+/− mice that were refed and subsequently refasted (Fig. 4D), consistent with TCPTP heterozygous deficiency prolonging the insulin signal; convincing increases in IR-β subunit Y1162/Y1163 phosphorylation in either +/+ or +/− mice after fasting and refeeding could not be detected with the reagents at hand (data not shown). Nevertheless, these results are consistent with TCPTP-deficiency enhancing insulin signaling.
FIG. 4.
Increased hepatic STAT3 and PI3K/Akt signaling in fasted Ptpn2+/− mice. 8- to 10-week-old Ptpn2+/− and +/+ male mice were fed a high-fat diet for 15 weeks. A: Livers were harvested from 4-h fasted mice, and processed for immunoblot analysis with antibodies to the phosphorylated (Ser-473) and activated Akt (p-Akt), phosphorylated (Y705) STAT3 (p-STAT3) and phosphorylated (Y1162/Y1163) IR-β subunit (p-IR) or the corresponding proteins. Representative blots and quantified results (arbitrary units: AU) are shown (means ± SE); *P < 0.05, **P < 0.01 by a two-tailed Student t test. B: Plasma IL-6 levels were determined using an ELISA kit (eBiosciences, San Diego, CA) according to the manufacturer's instructions. C: Livers were harvested from 4-h fasted mice and processed for quantitative (ΔΔCt) RT-PCR to measure the expression of Il6. Results shown are means ± SE. D: Mice were fasted overnight for 8 h, and at the beginning of the light cycle, refed for 4 h, or refed and refasted for 4 h. Livers were harvested from fasted, refed, and fasted/refed and refasted mice and processed for immunoblot analysis as indicated. Representative blots and quantified results are shown (means ± SE); ***P < 0.005 by a two-tailed Student t test. AU, arbitrary units.
Enhanced insulin and IL-6 signaling and decreased gluconeogenic gene expression in Ptpn2+/− hepatocytes.
Our results suggest that the lower fasted blood glucose levels and the decreased gluconeogenic gene expression and HGP in HFF Ptpn2+/− mice might result from elevated basal PI3K/Akt and STAT3 signaling. Although the liver is comprised primarily of hepatocytes, we cannot formally exclude the possibility that the elevated STAT3 phosphorylation may be attributed to altered hepatic cellularity. To determine whether the enhanced STAT3 phosphorylation was intrinsic to hepatocytes and to further assess TCPTP's potential to regulate hepatic IR activation and signaling, we isolated hepatocytes from Ptpn2+/− versus +/+ mice, and stimulated them either with insulin or IL-6 (Fig. 5). Basal and insulin-induced IR-β Y1162/Y1163 phosphorylation and downstream Akt Ser-473 phosphorylation were enhanced in +/− versus +/+ hepatocytes (Fig. 5A). Furthermore, IL-6-induced STAT3 phosphorylation was enhanced, but the activation of the upstream JAK1 (Y1022/Y1023) PTK was not altered (Fig. 5B), consistent with TCPTP acting directly on STAT3. Although we have previously established that TCPTP deficiency is associated with elevated IR phosphorylation and signaling in mouse embryo fibroblasts (MEFs) and HepG2 hepatoma cells (24,28,36), the impact of TCPTP deficiency on IL-6 signaling has not been previously examined. To establish an independent model by which to examine the role of TCPTP in IL-6 signaling, we stably knocked down TCPTP by RNA interference in HeLa cells (31). Knockdown of TCPTP resulted in enhanced IL-6–induced STAT3 phosphorylation (Fig. 5C). Taken together, these results affirm the capacity of TCPTP to negatively regulate STAT3 signaling, including that mediated by IL-6, which in hepatocytes contributes to the suppression of gluconeogenesis.
FIG. 5.
Increased insulin and IL-6 signaling in Ptpn2+/− hepatocytes. (A and B) Ptpn2+/− versus +/+ hepatocytes were serum starved for 4 h and stimulated with 10 nmol/l insulin or 1 ng/ml IL-6, as indicated, and processed for immunoblot analysis. C: Control HeLa cells or those expressing TCPTP-specific shRNAs were serum-starved for 4 h, stimulated with 10 ng/ml IL-6 for 10 min, medium replenished, and cells collected at the indicated times for immunoblot analysis. Results shown are representative of three independent experiments.
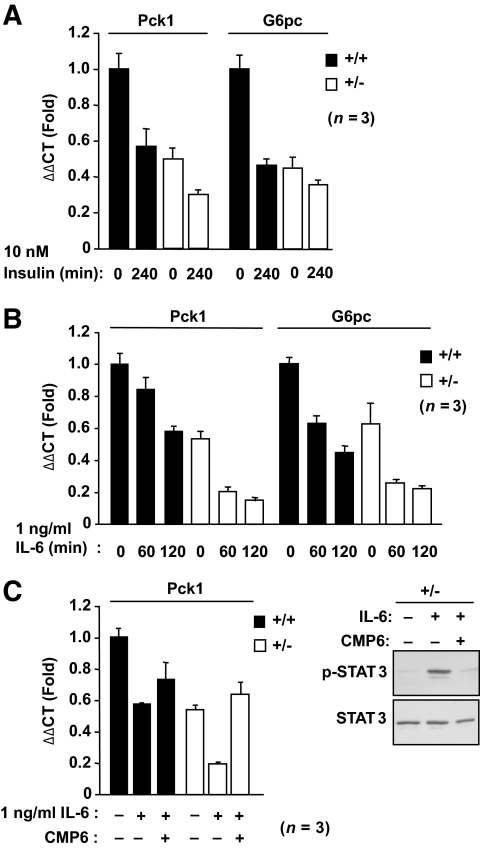
Next we assessed the impact of elevated insulin-instigated IR phosphorylation and PI3K/Akt signaling and IL-6–induced STAT3 signaling on the expression of gluconeogenic genes by quantitative RT-PCR. We found that the elevated basal IR/PI3K/Akt signaling in serum-starved +/− hepatocytes coincided with decreased G6pc and Pck1 expression that could be further suppressed by insulin (Fig. 6A). IL-6 also suppressed G6pc and Pck1 expression (Fig. 6B), and this could be prevented by pretreating cells with the JAK PTK inhibitor CMP6 (Fig. 6C). Pretreating serum-starved +/− hepatocytes with CMP6 did not revert the already reduced G6pc and Pck1 expression to that seen in +/+ cells (data not shown), indicating that the decreased basal gluconeogenic gene expression was independent of the JAK/STAT pathway and most likely attributable to elevated basal IR signaling. These results are consistent with TCPTP heterozygous deficiency promoting both IR and STAT3 signaling in hepatocytes to suppress gluconeogenic gene expression.
FIG. 6.
Increased insulin- and IL-6–induced Pck1 and G6pc suppression in Ptpn2+/− hepatocytes. Ptpn2+/− versus +/+ hepatocytes were serum starved for 4 h ± 2 μmol/l CMP6 (Calbiochem) for the last hour and stimulated with (A) 10 nmol/l insulin, or (B and C) 1 ng/ml IL-6 for the indicated times and processed for quantitative (ΔΔCt) RT-PCR to measure the expression of Pck1 and G6pc. C: Lysates from control and IL-6 (1 h) ± CMP6-treated hepatocytes were also processed for immunoblot analysis. Results shown are means ± SE of three independent experiments performed in quadruplicate.
DISCUSSION
An increased rate of hepatic gluconeogenesis is primarily responsible for the enhanced HGP and fasting hyperglycemia that is characteristic of patients with type 2 diabetes (2–4). The regulation of gluconeogenesis is dependent largely on the control of PEPCK and G6Pase expression. Although the absolute levels of HGP are only moderately increased in the diabetic state, PEPCK, G6Pase, and HGP are inadequately suppressed by glucose and insulin (2–4). In this study, we have identified TCPTP as a novel regulator of G6pc and Pck1 expression and HGP. Our studies indicate that a heterozygous deficiency in TCPTP in the liver may be sufficient to lower G6pc and Pck1 expression and consequently lower HGP and ameliorate the fasting hyperglycemia that is associated with high-fat feeding and the development of insulin resistance.
TCPTP's primary metabolic function may be in the regulation of glucose production since whole-body glucose production and gluconeogenesis, as assessed in hyperinsulinemic euglycemic clamps and pyruvate tolerance tests, respectively, were reduced in fasted-HFF Ptpn2+/− mice, whereas glucose disappearance, a measure of glucose uptake by muscle, remained unaltered. Furthermore, we found no difference in IR signaling in muscle or adipose tissue, and we see no overt difference in insulin signaling in adipocytes differentiated from Ptpn2+/− versus +/+ mouse embryo fibroblasts (Deng and Tiganis, unpublished observations). The liver is the primary tissue responsible for whole-body glucose production, with the kidney playing a smaller role (37). Although we cannot formally exclude the possibility that TCPTP may have a role in the kidney, several lines of evidence support the theory that the liver is an important site of action for TCPTP in the control of blood glucose. First, the STAT3 and PI3K/Akt signaling pathways that suppress gluconeogenesis were enhanced in the livers of fasted Ptpn2+/− mice. Second, this coincided with decreased hepatic gluconeogenic gene expression. And third, insulin and IL-6–induced signaling were increased and downstream gluconeogenic gene expression decreased in Ptpn2+/− hepatocytes. Although our analyses of 1) IR phosphorylation and PI3K/Akt signaling in fasted livers, 2) hepatic lipogenic gene expression in clamped mice, and 3) insulin signaling in isolated hepatocytes all indicate that TCPTP has the capacity to regulate insulin sensitivity, surprisingly, we found that insulin-induced IR phosphorylation and downstream PI3K/Akt signaling in response to bolus insulin administration were not overtly altered in Ptpn2+/− livers. Previously we reported that TCPTP serves to control the duration, rather than the intensity, of IR Y1162/Y1163 phosphorylation and downstream PI3K/Akt signaling, so that TCPTP-deficient fibroblasts exhibit prolonged, but not enhanced, insulin signaling (24). Therefore, one possibility is that TCPTP heterozygosity may result in prolonged insulin signaling in vivo. This would be evident in the livers of fasted mice, or after clamping, but not after the short periods of acute stimulation used to assess IR activation and signaling. Consistent with this possibility, we found that PI3K/Akt signaling remained significantly elevated in HFF Ptpn2+/− mice that were fasted, refed, and fasted once more.
Recent studies have shown that IRS-1 and IRS-2 can differentially contribute to the regulation of hepatic metabolism, with IRS-1 being more closely linked to glucose metabolism, and IRS-2 to lipid metabolism in the fasted state (38,39). In our studies, hepatic IRS-1, but not IRS-2 tyrosine phosphorylation, trended higher in fasted-HFF Ptpn2+/− mice in tune with the increased Akt phosphorylation and the trend for elevated IR Y1162/Y1163 phosphorylation. Although we cannot formally exclude any possible increase in basal IRS-1 tyrosine phosphorylation contributing to the selective suppression of gluconeogenesis in the fasted state, we suggest that G6pc and Pck1 may be primarily suppressed by the hyperphosphorylated STAT3, since further repression of G6pc and Pck1 expression was not evident under conditions of hyperinsulinaemia when Fasn and Srebf1 were otherwise induced. Previous studies have established the capacity of TCPTP to dephosphorylate STAT3 (25,30), whereas our studies demonstrate that TCPTP deficiency specifically enhances IL-6–induced STAT3 signaling in hepatocytes and HeLa cells. Several lines of evidence support the contribution of STAT3 to the control of gluconeogenesis. Liver-specific STAT3 knockout mice exhibit insulin resistance and elevated blood glucose levels that are associated with increased hepatic expression of G6pc and Pck1, whereas STAT3 overexpression in lean or obese mice decreases gluconeogenic gene expression and lowers blood glucose levels (11,40). STAT3 is tyrosyl (Y705) phosphorylated and activated by JAK PTKs downstream of all cytokines that act via the gp130 receptor, including IL-6. It is known that insulin signaling in AgRP neurons in the hypothalamus promotes IL-6 release from Kupffer cells in the liver that activates STAT3 in hepatocytes and thus suppresses gluconeogenesis and HGP (11,15–17). In our studies, we found that hepatic IL-6 levels in HFF Ptpn2+/− mice were not altered. In addition, food intake and body weight, which are also suppressed by central insulin action (41,42), were not altered in HFF Ptpn2+/− mice. Thus, the impact of TCPTP heterozygous deficiency on HGP is most likely attributable to the regulation of STAT3 phosphorylation in the liver, rather than the central control of insulin signaling. Recently, STAT3 in hepatocytes has also been shown to be controlled by sirtuin-1–mediated deacetylation (43). Sirtuin-1 is a NAD+ dependent deacetylase that is activated in response to fasting and caloric restriction (44). In the liver, sirtuin-1 activates the stimulatory effects of Foxo1 and PGC-1a on gluconeogenesis, while repressing the inhibitory effects of STAT3 (45,46). In particular, STAT3 deacetylation by sirtuin-1 coincides with STAT3 dephosphorylation (43). Previous studies have shown that STAT1 dephosphorylation by TCPTP can be regulated by STAT1 acetylation (47). It remains unknown whether changes in STAT3 acetylation affect its dephosphorylation status by TCPTP.
Previous studies have identified PTP1B as an important regulator of hepatic IR signaling and HGP, and these effects have been linked to the regulation of IR-β subunit Y1162/Y1163 phosphorylation (22). Interestingly, although liver-specific PTP1B knockout mice exhibited decreased gluconeogenic gene expression and HGP, fasted blood glucose levels were not overtly altered in liver-specific PTP1B heterozygous mice (22), as seen in TCPTP heterozygous mice. Thus, despite the high degree of similarity between the catalytic domains of PTP1B and TCPTP, it appears that the two PTPs may differentially contribute to the regulation of gluconeogenesis. We surmise that this may be attributable, at least in part, to the capacity of TCPTP to also regulate IL-6 signaling. Furthermore, despite the enhanced IR activation, liver-specific PTP1B knockout mice had diminished SREBP and Fas expression in the fed state, and decreased hepatic and serum triglyceride and cholesterol levels (22), consistent with the theory that PTP1B regulates additional, insulin-independent pathways pertinent to the control of lipogenesis. In HFF Ptpn2+/− mice, Srebf1 and Fasn were not altered under fasted conditions and increased after clamps, consistent with the idea that TCPTP deficiency enhances insulin sensitivity. Despite the increased insulin-induced expression of lipogenic genes, steatosis was not evident in HFF Ptpn2+/− mice, but rather decreased, which is consistent with the low hepatic lipid levels observed in insulin-sensitive phenotypes.
PTP1B's role in IR and leptin signaling has led to considerable attention being focused on PTP1B as a target for development of novel therapeutics for the treatment of both type 2 diabetes and obesity. Antisense oligonucleotides targeting PTP1B are in clinical trials, whereas drugs that inhibit PTP1B activity are in preclinical development (48–50). The lethality that is associated with TCPTP-deficiency (32) has meant that specific attention has been placed on generating PTP1B inhibitors that do not inhibit TCPTP. However, our studies suggest that the partial inhibition of TCPTP in the liver may be beneficial and contribute to the suppression of fasting hyperglycemia that is associated with high-fat-diet–induced insulin resistance, by enhancing not only IR-dependent, but also IR-independent STAT3-mediated pathways that may be particularly pertinent under conditions of severe insulin resistance. Therefore, we conclude that partial inhibition of TCPTP in the liver, either alone, or in the context of PTP1B inhibition, might be effective for the suppression of gluconeogenesis and the attenuation of fasting hyperglycemia in type 2 diabetes and obesity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia (to T.T., S.A., and M.J.W.). S.A. is a National Health and Medical Research Council R.D. Wright Fellow, and M.J.W and T.T are National Health and Medical Research Council Senior Research Fellows.
No potential conflicts of interest relevant to this article were reported.
A.F., K.L., S.G., B.F., B.S., and F.W. researched data. M.L.T provided reagents and edited the manuscript. M.J.W. researched data and edited the manuscript. S.A. contributed discussion and edited the manuscript. T.T. directed the research program, researched data, and wrote the manuscript.
The authors thank Christine Yang, Teresa Tiganis, Amy Blair, and Jane Honeyman for technical support.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Saltiel AR, Kahn CR: Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806 [DOI] [PubMed] [Google Scholar]
- 2.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI: Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 1992;90:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A, Landau BR, Ferrannini E: Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes 2000;49:1367–1373 [DOI] [PubMed] [Google Scholar]
- 4.Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J: Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE: Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem 2002;277:23301–23307 [DOI] [PubMed] [Google Scholar]
- 6.Valera A, Pujol A, Pelegrin M, Bosch F: Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A 1994;91:9151–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seoane J, Trinh K, O'Doherty RM, Gomez-Foix AM, Lange AJ, Newgard CB, Guinovart JJ: Metabolic impact of adenovirus-mediated overexpression of the glucose-6-phosphatase catalytic subunit in hepatocytes. J Biol Chem 1997;272:26972–26977 [DOI] [PubMed] [Google Scholar]
- 8.Lamont BJ, Visinoni S, Fam BC, Kebede M, Weinrich B, Papapostolou S, Massinet H, Proietto J, Favaloro J, Andrikopoulos S: Expression of human fructose-1,6-bisphosphatase in the liver of transgenic mice results in increased glycerol gluconeogenesis. Endocrinology 2006;147:2764–2772 [DOI] [PubMed] [Google Scholar]
- 9.Jiang G, Zhang BB: Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E671–678 [DOI] [PubMed] [Google Scholar]
- 10.Metzger S, Goldschmidt N, Barash V, Peretz T, Drize O, Shilyansky J, Shiloni E, Chajek-Shaul T: Interleukin-6 secretion in mice is associated with reduced glucose-6-phosphatase and liver glycogen levels. Am J Physiol 1997;273:E262–267 [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M: Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 2004;10:168–174 [DOI] [PubMed] [Google Scholar]
- 12.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM: Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003;423:550–555 [DOI] [PubMed] [Google Scholar]
- 13.Nakae J, Kitamura T, Silver DL, Accili D: The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 2001;108:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramadoss P, Unger-Smith NE, Lam FS, Hollenberg AN: STAT3 targets the regulatory regions of gluconeogenic genes in vivo. Mol Endocrinol 2009;23:827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG, Jr, Rhodes CJ, Schwartz MW: Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 2006;3:67–73 [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M: Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006;3:267–275 [DOI] [PubMed] [Google Scholar]
- 17.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC: Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 [DOI] [PubMed] [Google Scholar]
- 18.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP: Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999;283:1544–1548 [DOI] [PubMed] [Google Scholar]
- 19.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB: Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 2000;20:5479–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB:: Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 2006;12:917–924 [DOI] [PubMed] [Google Scholar]
- 21.Delibegovic M, Bence KK, Mody N, Hong EG, Ko HJ, Kim JK, Kahn BB, Neel BG: Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol 2007;27:7727–7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK:: Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced ER stress. Diabetes 2008;50:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK: TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 2001;276:47771–47774 [DOI] [PubMed] [Google Scholar]
- 24.Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T: Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol 2005;25:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiganis T, Bennett AM: Protein tyrosine phosphatase function: the substrate perspective. Biochem J 2007;402:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D: Molecular basis for recognition and dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Molecular Cell 2000;6:1401–1412 [DOI] [PubMed] [Google Scholar]
- 27.Iversen LF, Moller KB, Pedersen AK, Peters GH, Petersen AS, Andersen HS, Branner S, Mortensen SB, Moller NP: Structure determination of T-cell protein tyrosine phosphatase. J Biol Chem 2002;20:20. [DOI] [PubMed] [Google Scholar]
- 28.Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK, Tiganis T: Regulation of insulin receptor signaling by the protein Tyrosine phosphatase TCPTP. Mol Cell Biol 2003;23:2096–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ: The T-cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol 2002;12:446–453 [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N, Matsuda T: The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun 2002;297:811–817 [DOI] [PubMed] [Google Scholar]
- 31.Shields BJ, Hauser C, Bukczynska PE, Court NW, Tiganis T: DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell 2008;14:166–179 [DOI] [PubMed] [Google Scholar]
- 32.You-Ten KE, Muise ES, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp WS, Tremblay ML: Impaired bone marrow microenvironment and immune function in T-cell protein tyrosine phosphatase-deficient mice. J Exp Med 1997;186:683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinonen KM, Nestel FP, Newell EW, Charette G, Seemayer TA, Tremblay ML, Lapp WS:: T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 2004;103:3457–3464 [DOI] [PubMed] [Google Scholar]
- 34.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T: Reactive oxygen species enhance insulin sensitivity. Cell Metab 2009;10:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proietto J, Andrikopoulos S: Molecular mechanisms of increased glucose production: identifying potential therapeutic targets. J Investig Med 2004;52:389–393 [DOI] [PubMed] [Google Scholar]
- 36.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK: Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem 2004;279:37716–37725 [DOI] [PubMed] [Google Scholar]
- 37.Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, Gerich J: Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol 1998;274:E817–826 [DOI] [PubMed] [Google Scholar]
- 38.Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, Sasako T, Moroi M, Sugi K, Kakuta S, Iwakura Y, Noda T, Ohnishi S, Nagai R, Tobe K, Terauchi Y, Ueki K, Kadowaki T: Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab 2008;8:49–64 [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi CM, Ueki K, Kahn R: Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest 2005;115:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kinoshita S, Ogawa W, Okamoto Y, Takashima M, Inoue H, Matsuki Y, Watanabe E, Hiramatsu R, Kasuga M: Role of hepatic STAT3 in the regulation of lipid metabolism. Kobe J Med Sci 2008;54:E200–208 [PubMed] [Google Scholar]
- 41.Woods SC, Lotter EC, McKay LD, Porte D, Jr.:: Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282:503–505, 1979 [DOI] [PubMed] [Google Scholar]
- 42.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR: Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 43.Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q: STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol 2009;11:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–118 [DOI] [PubMed] [Google Scholar]
- 45.Rodgers JT, Puigserver P: Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A 2007;104:12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frescas D, Valenti L, Accili D: Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 2005;280:20589–20595 [DOI] [PubMed] [Google Scholar]
- 47.Kramer OH, Knauer SK, Greiner G, Jandt E, Reichardt S, Guhrs KH, Stauber RH, Bohmer FD, Heinzel T: A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev 2009;23:223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, Kroeger PE, Reilly RM, Koterski S, Opgenorth TJ, Ulrich RG, Crosby S, Butler M, Murray SF, McKay RA, Bhanot S, Monia BP, Jirousek MR: PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci U S A 2002;99:11357–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rondinone CM, Trevillyan JM, Clampit J, Gum RJ, Berg C, Kroeger P, Frost L, Zinker BA, Reilly R, Ulrich R, Butler M, Monia BP, Jirousek MR, Waring JF: Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes 2002;51:2405–2411 [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Zhang ZY: PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today 2007;12:373–381 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.