Abstract
OBJECTIVE
Most knowledge on human β-cell cycle control derives from immunoblots of whole human islets, mixtures of β-cells and non-β-cells. We explored the presence, subcellular localization, and function of five early G1/S phase molecules—cyclins D1–3 and cdk 4 and 6—in the adult human β-cell.
RESEARCH DESIGN AND METHODS
Immunocytochemistry for the five molecules and their relative abilities to drive human β-cell replication were examined. Human β-cell replication, cell death, and islet function in vivo were studied in the diabetic NOD-SCID mouse.
RESULTS
Human β-cells contain easily detectable cdks 4 and 6 and cyclin D3 but variable cyclin D1. Cyclin D2 was only marginally detectable. All five were principally cytoplasmic, not nuclear. Overexpression of the five, alone or in combination, led to variable increases in human β-cell replication, with the cdk6/cyclin D3 combination being the most robust (15% versus 0.3% in control β-cells). A single molecule, cdk6, proved to be capable of driving human β-cell replication in vitro and enhancing human islet engraftment/proliferation in vivo, superior to normal islets and as effectively as the combination of cdk6 plus a D-cyclin.
CONCLUSIONS
Human β-cells contain abundant cdk4, cdk6, and cyclin D3, but variable amounts of cyclin D1. In contrast to rodent β-cells, they contain little or no detectable cyclin D2. They are primarily cytoplasmic and likely ineffective in basal β-cell replication. Unexpectedly, cyclin D3 and cdk6 overexpression drives human β-cell replication most effectively. Most importantly, a single molecule, cdk6, supports robust human β-cell proliferation and function in vivo.
While broadly similar, human pancreatic β- cells differ from their rodent counterparts in many ways. For example, rodent β-cells can be induced to replicate using many strategies, including partial pancreatectomy, induction of obesity and insulin resistance, infusion of glucose, administration of growth factors, and activation of signaling pathways downstream of these growth factors and nutrients (1–8). In contrast, while evidence suggests that human fetal and early neonatal β-cells are able to replicate (9–11), no investigator has been able to demonstrate or induce robust rates of proliferation in adult human β-cells using the same growth factors, nutrients, signaling pathways, and maneuvers that have been effective in rodents (12–19). For example, unlike events in rodents, obesity or type 2 diabetes in human adults is not associated with accelerated β-cell proliferation (19), and even partial pancreatectomy does not induce β-cell replication or regeneration in humans (17). This inability to induce substantial human β-cell replication is problematic, for it is now clear that β-cell replacement therapy for diabetes will require large numbers of human or xenogeneic β-cells (20).
While much has been learned from mouse genetic models regarding the molecular control of β-cell replication (21–35), little is known regarding the molecules that regulate the G1/S transition in the human adult β-cell. We have begun to explore these molecules in adult human islets, mapping the members of the G1/S proteome that are present in human cadaveric islets (12,36). Human islets are believed to contain most of the key molecules that govern the G1/S transition in other cell types (12,36,37). These include the three members of the pocket protein family (pRb, p107, and p130); cyclins D1 and D3; cdks 1, 2, 4, and 6; the four members of the INK4 family (p15, p16, p18, and p19); the three members of the KIP/CIP family (p21, p27, and p57); several E2F family members; and p53 and its E3 ligase, HDM2 (supplementary Fig 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1776/DC1). Upstream of these G1/S molecules are other regulatory molecules such as skp2, menin, FoxM1, bmi1, and ezh2 (26–29,34). We and others have also shown that human islets differ from their rodent counterparts in that they express cdk6, a cdk that is not detectable in mouse islets (12,35). We have also shown that overexpression of cdk6 in association with a D-cyclin, cyclin D1, markedly increases the replication of adult human β-cells (12), and it does so in a way that does not lead to dedifferentiation or accelerated β-cell death but instead leads to enhanced engraftment and function of adult human cadaveric islets in vivo (12). We also have shown that cdk4 (36) and cdk6 (12) in conjunction with cyclin D1 can enhance pRb phosphorylation and replication in human β-cells. Despite these advances, it is important to underscore that, of the 30+ molecules that regulate the human β-cell G1/S transition (supplementary Fig. 1), the therapeutic potential has been explored for only three: cdk4, cdk6, and cyclin D1. For example, nothing is known about the efficacy of the other two D-cyclins, cyclins D2 and D3, in driving proliferation of human β-cells.
Another difference between human and rodent islets may involve cyclin D2. In mice, excellent evidence indicates that cyclin D2 is indispensible for β-cell growth and islet development and function: animals that lack cyclin D2 develop islet hypoplasia, hypoinsulinemia, and diabetes (32,33). In contrast to mouse studies, in humans, cyclin D2 has been reported to be difficult to detect or undetectable, despite the use of multiple antisera from multiple vendors and appropriate positive controls (12,37).
Here, we asked four questions. 1) Which D-cyclins are definitively present in human islets? 2) Which of the three D-cyclins might be the optimal partner for cdk4 or cdk6 for enhancing adult human β-cell replication? 3) Are cdk4, cdk6, and the three D-cyclins actually present in β-cells [in contrast to immunoblot studies of whole islet extracts described previously (12,37)], and if so, in which subcellular compartment? 4) From a therapeutic standpoint, is it essential to deliver both a D-cyclin and a cdk4/6 family member to enhance human islet engraftment, or can this be simplified by using only a single member of the cdk4/6–D-cyclin complex?
We report here that 1) cyclin D1 is variably present, D2 is only marginally detectable, and D3 is readily detectable in human islets; 2) cyclin D3 appears to be surprisingly effective in inducing human β-cell proliferation and, in combination with cdk6, may be the most effective in driving human β-cell replication; 3) cdks 4 and 6, and cyclins D1 and D3, are located principally in the cytoplasm and not the nucleus; and 4) a single cdk, cdk6, is surprisingly as effective in driving human islet engraftment as the previously reported combination of cdk6 plus cyclin D1.
RESEARCH DESIGN AND METHODS
All of the methods and procedures have been described previously (5–7,12,18,24,36). Supplementary detailed methods are available in an online appendix. The University of Pittsburgh Institutional Review Board approved in advance both receipt of, and work with, human islets.
RESULTS
Human islets contain easily detectable cyclin D3, but only variable and marginal quantities of cyclins D1 and D2.
The cyclin D family includes three D-cyclins, shown schematically in supplementary Fig. 2A. As shown in supplementary Fig. 3 (available in an online appendix), mRNA encoding each of the three D-cyclins is present in human islets, as assessed by conventional PCR as well as by quantitative PCR.
Immunoblots were performed for each cyclin in adult human islets and were compared with the expression of the putative D-cyclin to expression in the same human islet preps transduced with adenoviruses encoding the corresponding D-cyclin (Fig. 1A). Human islets contain measurable but variable amounts of cyclin D1, which was present in 7 of 12 human islet extracts. This band was confirmed as being cyclin D1 because it was enhanced by overexpression of cyclin D1. Importantly, no cross-reactivity of this antibody was observed when cyclins D2 and D3 were overexpressed. These results were obtained using antibody #2,926 (Cell Signaling Technology, Danvers, MA), and are comparable to results described by Lavine et al. (37). Cyclin D1 was also present in several human cell lines (HK2, SaOS2, and HKC8) and at higher levels than in human islets in two of these (Fig. 1B).
FIG. 1.
D-cyclins in the human islet and β-cell. A: Western blot for cyclin D1, D2, and D3 in human islets. H1–H3 are three different human islet preparations; H3 was transduced with the adenoviruses overexpressing cyclin D1 (Ad.D1), cyclin D2 (Ad.D2), or cyclin D3 (Ad.D3) to examine cross-reactivity. Each Western blot is representative of three different blots. Islets were extracted between 24 and 48 h of arrival or after infection with adenoviruses. B: Immunoblots for cyclin D1, D2, and D3 in human cell lines and human islets. HK2, SaOS2, and HCK8 are three human cell lines, used for more physiological levels of expression, as compared with the adenoviral overexpression. Each blot is representative of at least three different blots. C: Subcellular localization of cyclin D1, D2, and D3 in human β-cells. Human islets were dispersed as described in Materials and Methods and stained for cyclin D1, D2, or D3 and insulin. Staining with no primary antibody is shown as a negative control. D: Transduced dispersed human islets. Human islets were dispersed and transduced with Ad.D1, Ad.D2, or Ad.D3 and stained for each cyclin D and insulin as described in Materials and Methods. (A high-quality digital representation of this figure is available in the online issue.)
Cyclin D3 was also easily detectable (Fig. 1A) in all human islets, being present in 11 of 11 human islet extracts, and was enhanced by adenoviral overexpression of human cyclin D3. Again, the band was specific, because it was intensified by overexpression of cyclin D3, and because overexpression of the other D-cyclins did not alter the intensity of the band. This blot was performed using antiserum #ab28283 (Abcam, Cambridge, MA). Cyclin D3 was also easily detectable in each of the three human cell lines (Fig. 1B).
In contrast to immunoblots for cyclins D1 and D3, and in contrast to results observed in PCR experiments, cyclin D2 was barely detectable using multiple cyclin D2 antisera in 3 of 6 human islet extracts and undetectable in 3 others, with 1 example shown in Fig. 1A using antiserum #C7339 (Sigma, St. Louis, MO). Cyclin D2 also was difficult to detect in SaOS2 and HKC8 human cell lines, but readily apparent in HK2 cells (Fig. 1B). Again, the antiserum was specific, for it detected cyclin D2 when overexpressed but not cyclins D1 or D3. These results suggest that cyclins D1 and D3 are present in human islets, but that cyclin D2, if present, is present at very low abundance. Further, because transcripts encoding cyclin D2 are present in human islets, the apparent lack of cyclin D2 protein may reflect inhibition of translation or accelerated degradation of cyclin D2 in human islets (31).
Cyclins D1, D2, and D3 are predominantly cytoplasmic in human β-cells.
To confirm the immunoblot observations, to determine the cell type within the islet that expresses the D-cyclins, and to determine the subcellular locations of the D-cyclins, we performed immunofluorescent laser confocal microscopy of single-cell suspensions of adult human cadaveric islets. Faint cytoplasmic (and little or no nuclear) staining was observed for cyclins D1, D2, and D3 in most β-cells (Fig. 1C). As was observed by immunoblot, cyclin D3 was more apparent than the other two D-cyclins.
Overexpression of the D-cyclins leads to nuclear expression in adult human β-cells.
To provide positive controls, we overexpressed the three D-cyclins and repeated these experiments. Bright immunofluorescence was observed when each of the three D-cyclins was overexpressed (Fig. 1D). Interestingly, all three D-cyclins were still primarily in the cytoplasmic compartment when overexpressed, but nuclear staining was now easily discernable. Importantly, as shown in Fig. 1D, there was no cross-reactivity among the three D-cyclin antisera.
cdk4 and cdk6 are easily detectable in adult human β-cells and are principally cytoplasmic.
cdk4 and cdk6 are homologous members of the cdk family (supplementary Figs. 1 and 2B, available in an online appendix). To define the subcellular localization of cdk4 and cdk6 in human adult islets, we performed immunofluorescent studies in human islets (Fig. 2A). Both cdk4 and cdk6 were readily detectable in human β-cells, both principally in the cytoplasm, with little or no nuclear presence. As a positive control, overexpression of cdk4 and cdk6 resulted in increased intensity of staining, without cross-reactivity, but staining remained principally cytoplasmic, although nuclear staining in a few cells is visible (Fig. 2B). As can also be seen in this figure, immunostaining for cdk4 and cdk6 was specific. Finally, to independently confirm that cdk4, cdk6, and cyclins D1 and D3 are principally cytoplasmic in human islet cells, we performed subcellular fractionation studies in human islets (Fig. 3A, B). These studies demonstrated that all four molecules appear in the cytosolic fraction with the cytosolic marker, Hsp90, and not in the nuclear fraction defined by the nuclear marker, histone 3 (H3).
FIG. 2.
Subcellular localization of cdk4 and cdk6 in human β-cells. A: Uninfected dispersed human islets. Human islets were dispersed as described in Materials and Methods and stained for cdk4 or cdk6 and insulin. Staining with primary antibody and blocking peptide is shown as a negative control. B: Dispersed human islet cells transduced with Ad.cdk4 or Ad.cdk6. Human islets were dispersed and transduced with Ad.cdk4 or Ad.cdk6 and stained for cdk4 or cdk6 and insulin as described in Materials and Methods. As can be seen, overexpressed cdk4 and cdk6 are easily detectable, and cdk4 and cdk6 staining is specific. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 3.
Subcellular fractionation of human islets. Human islets were subcellularly fractionated into cytoplasmic fractions (C), as marked with heat shock protein 90 (Hsp90), and nuclear fractions (N) marked with histone 3, and compared to the initial prep of whole (W) human islets. The three components were then immunoblotted for cyclins D1 or D3 (A) or cdk6, cdk4 (B). As can be seen, all four cdk–cyclins are enriched in the cytosolic compartment and not detected in the nuclear compartment. These experiments are representative of a minimum of three human islet preparations.
Overexpression of cdk6 in combination with cyclin D3 yields robust β-cell replication.
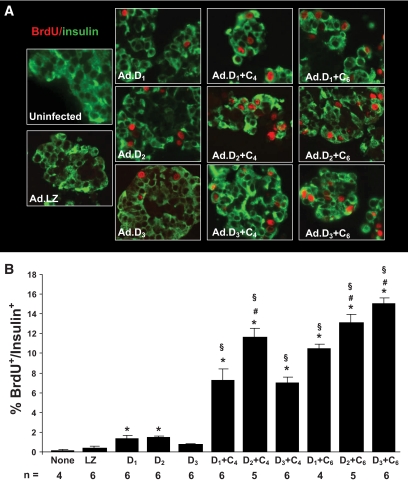
We next queried which individual cdk4/6 or D-cyclin and which combinations were most effective in driving human β-cell replication. As described previously by ourselves and others (12–19), no appreciable β-cell replication was observed in control human islets, nor in those transduced with a control adenovirus (Ad.lacZ) (Fig. 4A). Overexpression of the individual D-cyclins caused an obvious increase in BrdU incorporation, and this was augmented when combinations of D-cyclins and cdk4 or cdk6 were used. Not shown here, but reported previously (12,36), overexpression of cdk4 alone did not increase human β-cell replication, but cdk6 overexpression was able to induce both β-cell replication as well as pRb phosphorylation.
FIG. 4.
β-cell proliferation in vitro. A: This panel shows examples of isolated whole human islets, embedded in paraffin, sectioned, and stained for insulin (green) and BrdU (red) 72 h after transduction with adenoviruses encoding cdks and D-cyclins. B: Quantification of the BrdU-positive β-cells under each of the conditions. Bars indicate mean ± SEM. n refers to the numbers of human pancreatic islet samples examined. None refers to uninfected islets, LZ refers to Ad.lacZ, D1 refers to Ad.cyclin D1, D2 refers to Ad.cyclin D2, D3 refers to Ad.cyclin D3, C4 refers to Ad.cdk4, and C6 refers to cdk6. (A high-quality digital representation of this figure is available in the online issue.)
These experiments were quantified (Fig. 4B). Combinations of cdk4 and/or cdk6 with a D-cyclin were more effective in inducing proliferation than a single D-cyclin alone. Surprisingly, cyclin D3 in combination with cdk6 appeared to provide the largest increase in proliferation. These results were confirmed with Ki-67 (supplementary Fig. 4A, B, available in an online appendix). Importantly, these experiments were performed using intact human islets, in which the inner cells within the islet were likely not transduced, so these estimates of proliferation suggest that further increases in proliferation would be possible if individual β-cell preparations or more efficient delivery systems were used.
cdk6 and cyclin D1 translocate to the nucleus when overexpressed.
It was unanticipated that cdks 4 and 6, and the D-cyclins, would prove to be cytosolic. To begin to explore the mechanisms responsible for their ability to drive β-cell replication, we examined and quantified the nuclear presence of cdk6 when overexpressed alone, or in combination with cyclin D1. As shown in Fig. 5A, B, when overexpressed, cdk6 can be observed readily in the nucleus, and this nuclear presence is enhanced by co-overexpression of cyclin D1.
FIG. 5.
Nuclear translocation of cdk6 in response to adenoviral overexpression of cdk6 alone or in combination with cyclin D1. A: (Upper panel) Human β-cells contain cdk6, but it is largely cytoplasmic. (Middle panel) When cdk6 is overexpressed adenovirally, cdk6 is more abundant and can be observed in the nucleus in some cells. (Lower panel) When cdk6 is adenovirally overexpressed with cyclin D1, cdk6 is more abundant in nuclei. B: Quantification of the percentage of β-cells that contain cdk6 in each of the three conditions shown in A. (A high-quality digital representation of this figure is available in the online issue.)
A single G1/S molecule, cdk6, is capable of inducing adult human β-cell replication and enhancing β-cell transplantation in vivo.
The preceding studies provide multiple combinations of cdk–D-cyclins candidates for enhancing human β-cell replacement. However, because immunocompromised diabetic mouse/human islet transplant studies—a more rigorous form of analysis—are difficult, time-consuming, and expensive, we wondered whether a single cdk or cyclin might be sufficient to enhance human β-cell engraftment and function: if one single molecule could be shown to be effective, this might facilitate and simplify attempts to expand human islet mass and function. We selected cdk6 for further study, because we had shown that the combination of cdk6 plus cyclin D1 was effective in vivo (12), and because cdk6 is effective alone in inducing human β-cell proliferation in vitro (12) and also led to retention of β-cell function in vitro (12). A marginal mass islet transplant of 1,500 adult human islet equivalents (IEQ) transduced with a control adenovirus expressing β-galactosidase (Ad.lacZ) afforded transient, marginal improvements in blood glucose in streptozotocin-diabetic immunoincompetent NOD-SCID mice (Fig. 6A). In contrast, 4,000 IEQ, a positive control, normalized postprandial glucose values. As observed previously (12), 1,500 IEQ transduced with the combination of Ad.cyclin D1 plus Ad.cdk6 performed far better than 1,500 IEQ transduced with Ad.lacZ, comparably to the 4,000 IEQ positive control group.
FIG. 6.
cdk6 alone enhances human islet function in vivo in streptozotocin diabetic NOD-SCID mice. Bars indicate mean ± SEM. A: Mice transplanted with 1,500 IEQ transduced with Ad.lacZ are shown in the black lines, as described in the key within the figure. Mice transplanted with 1,500 IEQ transduced with Ad.cdk6 alone (C6), with Ad.cyclin D1 alone (D1), or both Ad.cdk6 and Ad.cyclin D1 (D1+C6) are shown in blue, purple, and green, respectively, and compared with 4,000 normal, nontransduced IEQ. The numbers in the key refer to the number of experimental animals in each group. B: Intraperitoneal glucose tolerance testing (IPGTT) in normal (nondiabetic) NOD-SCID mice, diabetic NOD-SCID mice transplanted with 4,000 IEQ human islets, 1,500 human IEQ transduced with Ad.lacZ, or 1,500 IEQ transduced with Ad.cdk6 alone (C6), with Ad.cyclin D1 alone (D1) or both Ad.cdk6 and Ad.cyclin D1 (D1+C6). Studies were performed 21 days after transplantation. The numbers in parentheses indicate the numbers of animals studied. (A high-quality digital representation of this figure is available in the online issue.)
There are two key novel observations in Fig. 6A. First, 1,500 human IEQ transduced with Ad.cyclin D1 alone performed less effectively than either Ad.cdk6 alone or Ad.cdk6 plus cyclin D1. Most importantly, 1,500 human IEQ transduced with Ad.cdk6 alone performed as well as 1,500 IEQ transduced with the combination of Ad.cdk6 plus cyclin D1. Thus, a single cdk, cdk6, is as effective as the combination of cdk6 plus cyclin D1 in enhancing human islet graft function.
To more rigorously assess the function of the human islet grafts, we performed intraperitoneal glucose tolerance tests three weeks after transplant (Fig. 6B). Mice transplanted with 1,500 IEQ transduced with Ad.cdk6 displayed fasting glucose values and glucose tolerance comparable to normal NOD-SCID mice (not treated with streptozotocin) and to streptozotocin-diabetic NOD-SCID mice transplanted with 4,000 IEQ, or 1,500 IEQ transduced with Ad.cdk6 plus cyclin D1. Glucose tolerance in the Ad.cdk6 group was superior to that in the 1,500 IEQ Ad.lacZ or Ad.cyclin D1 groups.
Ad.cdk6 induces sustained human β-cell replication in vivo, with little cell death.
We previously demonstrated that Ad.cdk6 plus D1 induces replication in human β-cells in vivo three days after transplant (12). Here we explored whether this early proliferation could be sustained for longer periods of time. We harvested renal capsular grafts 28 days after transplant and examined proliferation in β-cells using combined insulin and Ki-67 immunohistochemistry (Fig. 7A). Whereas little Ki-67 staining is observed in β-cells exposed to Ad.lacZ, markedly increased β-cell Ki-67 staining is present in grafts harvested at 28 days from islets transduced with Ad.cdk6 alone, Ad.cyclin D1 alone, and Ad.cdk6 plus Ad.cyclin D1 in combination. These observations are quantified in Fig. 7B, which shows that β-cell replication rates are very low in control grafts and remain elevated for at least 28 days after transplant in human islets transduced with cdk6, cyclin D1, or the combination. We also examined the islet grafts for cdk6 immunostaining (Fig. 7C). These figures show that cdk6 is particularly abundant in human β-cells in vivo, even 28 days after transplant, and that it is abundant in the nuclear compartment.
FIG. 7.
Effects of cdk6, cyclin D1, or cdk6 and cyclin D1 on β-cell proliferation in vivo. A: Proliferation in human β-cells in vivo 28 days after transplantation. Ki-67 is shown in red, and β-cells are shown in green. Human islets grafts were removed at day 28 after transplantation, fixed in 4% paraformaldehyde, embedded in paraffin, and stained for Ki-67. Ki-67 staining in samples of intestine cofixed, coembedded, and cosectioned with the islet grafts were performed as a positive control in the lower right panel. B: Quantification of Ki-67-positive insulin-positive cells as a function of total insulin-positive cells. The numbers shown within the bars indicate the number of Ki-67-positive (red) β-cells and of insulin-positive (green) cells. The numbers below the bars indicate the numbers of animals studied, with two sections per animal. C: cdk6 staining in human β-cells in islet grafts at 28 days posttransplant. Note that cdk6 is still easily visible 28 days posttransplant and that, in many β-cells, it is nuclear as well as cytoplasmic. (A high-quality digital representation of this figure is available in the online issue.)
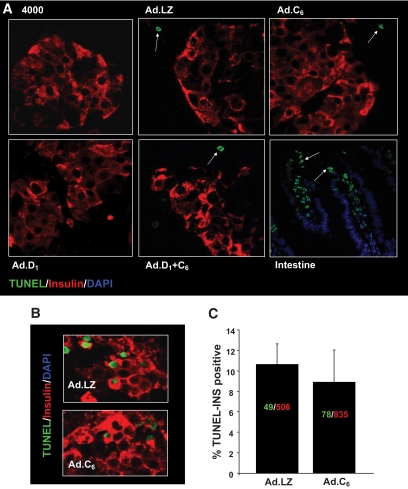
β-cell death, as assessed using transferase-mediated dUTP nick-end labeling (TUNEL) staining, was barely detectable in these grafts at 28 days, although it was easily detectable in the positive control (Fig. 8A). We had earlier shown that β-cell death was not increased at 3 days posttransplant (12). Because the immediate posttransplant period is associated with cell death, and because the engraftment response to cdk6 occurred very early posttransplant, we also examined TUNEL staining in β-cells at 24 h posttransplant (Fig. 8B, C). Again, no differences in β-cell apoptosis were observed in cdk6-expressing versus control β-cells.
FIG. 8.
Effects of cdk6, cyclin D1, or cdk6 and cyclin D1 on β-cell death in vivo. A: TUNEL assay was performed on each of the 2–6 fields from 3–4 human islet grafts shown in Fig. 7, 28 days after transplantation. No TUNEL-positive nuclei were observed. TUNEL-positive nuclei are shown in green, and β-cells are shown in red. Examples of TUNEL-positive nuclei are indicated by arrows. TUNEL staining in samples of intestine cofixed, coembedded, and cosectioned with the islet grafts were performed as a positive control in the lower right panel. B: Similar experiments were repeated, but grafts were removed 24 h after transplantation. As can be seen, β-cell apoptosis is similar in the Ad.cdk6-versus Ad.lacZ-transduced islets. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
We report several novel observations. First, we find that multiple cdk4/6 and D-cyclin combinations can robustly stimulate human β-cell replication in vitro. Surprisingly, among all of the possible cdk4/6–D-cyclin combinations, cyclin D3 appeared to be a particularly effective partner for cdk6 and cdk4. Second, we demonstrate that, although cyclin D2 is a very effective partner for both cdk4 and cdk6 in stimulating human β-cell replication, and despite its being both present and essential for rodent β-cell replication and function, it is only marginally detectable in human β-cells. Third, we observe that the D-cyclins and cdks 4 and 6 are principally cytosolic proteins in the human β-cell. Fourth, we report that a single member of the cdk4/6 D-cyclin complex, cdk6, is able to enhance human β-cell transplantation in vivo. Fifth, we demonstrate that human β-cell replication can be sustained in vivo for at least four weeks using cdk6.
The rapid proliferation induced by the D3 combinations (Fig. 4) was unanticipated. Of the three D-cyclins, cyclin D2 has repeatedly been demonstrated to be essential for mouse islet development and function, because its loss results in early-onset diabetes (32,33). Combined loss of both cyclins D1 and D2 accelerates this β-cell failure and diabetes, suggesting an additional or complimentary role for cyclin D1 in the β-cell (33). In human insulinoma, cyclin D1 overexpression has been implicated etiologically (38). In contrast, loss of cyclin D3 in mice has no apparent effect on β-cells (33,39), and no prior reports suggest a role for cyclin D3 in human β-cell physiology or therapy. Analogously, whereas cdk4 is well known to be essential in the rodent islet where its loss leads to diabetes and β-cell failure (23), genetic loss of cdk6 has no evident effect on the β-cell in mice (40). Indeed, normal mouse islets are essentially cdk6 deleted (12,35). Thus, one might have anticipated from mouse studies that cdk4 and cyclins D1 and D2 might have been most effective in driving human β-cell replication and engraftment. These observations should be interpreted with some caution, however, for no attempt was made to document that the actual concentrations of the several D-cyclins and cdk4/6 were identical when overexpressed, experiments which would be particularly challenging to perform. Thus, while we cannot definitively conclude that cyclin D3 is the optimal D-cyclin partner for cdks 4 and 6 in driving human β-cell replication, the cyclin D3–cdk6 combination is particularly attractive for future transplant studies.
Cyclin D2 is marginally detectable or nondetectable in human islets by immunoblot and barely detectable in human β-cells by immunofluorescence. These results corroborate those by Lavine et al. (37) and were confirmed by using appropriate controls (cyclin D2 was easily observed by immunoblot and immunohistochemistry in human islets transduced with cyclin D2 adenovirus and was also easily observed in at least one human cell line) and multiple different antisera. This observation is interesting for at least three reasons. First, the apparent indispensability of cyclin D2 in rodent islets, in combination with its apparent ability to markedly stimulate human β-cell replication, raises the possibility that the failure of adult β-cells to generate cyclin D2 may be relevant to their inability to replicate. Second, cyclin D2 is undetectable in human islets despite their containing easily measurable mRNA encoding cyclin D2. Together, these findings may indicate that lack of cyclin D2 could play an essential role in restraining human beta replication and suggest that cyclin D2 and its regulation warrant further investigation in human β-cell replication, particularly at the level of protein stability as suggested by Kushner and coworkers (31). For example, it would be interesting to examine whether cyclin D2 is present in β-cells in late embryonic or early neonatal human life when β-cells are actively replicating (9–11). Finally, they underscore the point that mouse islet studies do not predict with complete fidelity events that transpire in human β-cells. Other well documented examples are the far lower percentage of β-cells in the human islet as compared with the rodent (41,42), the absence of an endocrine cell mantle and more heterogeneous distribution of cell types in human islet (41,42), the use of different principal glucose transporters (Glut2 in the rodent, Glut1 in the human β-cell) (43–45), the lack of proliferative responses to obesity or partial pancreatectomy in the human β-cell (17,19), and the refractoriness of adult human β-cells to proliferative mitogens and growth factors that readily induce rodent β-cell replication (9,10,12–17,36).
The immunohistochemical subcellular localization studies were important for several reasons. First, they provide methodology for studying cdks 4 and 6 and the D-cyclins in human β-cells. Second, they confirm that this family, which had been described in immunoblots of whole islets previously, is present in adult human β-cells. Third, they show that all five of these molecules are principally located in the cytoplasm, a result that was confirmed by subfractionation studies. This is surprising, because these are generally thought to be in nuclear kinase complexes that function by phosphorylating nuclear substrates such as pRb. Of course, in contrast to human cancers and mouse embryonic fibroblasts, which are rapidly replicating cell types and which inform most studies on this class of molecules, adult human β-cells are typically quiescent, failing to enter the cell cycle. That the cdk–D-cyclin family is cytoplasmic in human β-cells may provide an explanation for this quiescence: their low abundance and inability to translocate to the nuclear compartment may be rate-limiting for adult human β-cell replication. Recently, in accord with these findings, He et al. have reported that cyclin D2, the key D-cyclin in the mouse β-cell, is also cytoplasmic (31). It is widely believed that cdk4/6–D-cyclins, which lack nuclear localization signals, require cytoplasmic preassembly or chaperoning by p21 and p27, which do contain nuclear localization signals, and are required for nuclear transit (46,47), such that inadequate quantities of p21 or p27 could prevent nuclear access for cdk4/6–D-cyclins. It is also possible that the Cip/Kip or INK4 family could sequester the D-cyclin–cdk4/6 complexes in the cytoplasm, preventing them from entering the nucleus and driving proliferation. Moreover, evidence suggests that GSK3β not only phosphorylates and targets D-cyclins for degradation, it also may prevent nuclear access of D-cyclins (14,31,48). Thus, it is also possible that this or other signaling events or lack thereof, unrelated to cell cycle inhibitors, could prevent nuclear access for cdk4/6–D-cyclin complexes in the β-cell. Equally importantly, it is clear that, when overexpressed, cdk6 can enter the nucleus and that cooverexpression of cyclin D1 can facilitate or enhance this process (Fig. 5A, B). Clearly, the questions as to how and why cdks4/6 and D-cyclins appear to remain stranded in the cytoplasm, where they are presumably unable to phosphorylate pRb, and what regulates their trafficking into the nucleus of the human β-cell provide fertile ground for future study.
The in vivo transplant studies make at least two additional important and novel points. First, human β-cells that overexpress only a single G1/S molecule, in this case, cdk6, replicate and function better than normal control human β-cells. Indeed, they also function as well as islets that overexpress both cdk6 and cyclin D1. Second, they replicate in vivo at high rates after transplantation, and this replication continues for at least four weeks. This is unaccompanied by increases in cell death and is accompanied by euglycemia and normal glucose tolerance, suggesting that β-cell differentiation is maintained despite the marked induction of proliferation. One incompletely addressed question is “what proportion of the rapid and sustained reduction in blood glucose in the cdk6-transduced islets, and in the cdk6+cyclin D1-transduced islets in our prior study (12), is due to proliferation and increased islet mass, versus enhanced β-cell function (glucose-sensing and insulin secretion) versus enhanced survival?” So far, we know that proliferation is elevated at days 3 (12) and 28 (herein). We also know that overexpression of cdk6 in human islets for 3 and 10 days resulted in full retention of glucose-stimulated insulin secretion (12). We also know that cdk6 overexpression was not associated with increases in cell death at day 3 (12) or days 1 or 28 posttransplant (Fig. 8). We also know that cdk6 differs from most cdks in that it appears to have differentiating activities in osteoblasts and prostate cells, mediated by direct binding to differentiating transcription factors such as Runx2 and the androgen receptor, respectively (49,50). Nonetheless, it remains possible that enhanced survival, for example, at 6–12 h after transplant, may be contributing to enhanced islet graft function. In addition, it is important to point out that no attempt was made to quantify β-cell mass in this model. Thus, it is unknown at present precisely how cdk6 alone or in combination enhances human islet engraftment. These observations make cdk6 a particularly attractive target for future longer studies in vitro and in vivo aimed at enhancing human β-cell proliferation, mass, and function, as well as at better understanding the mechanisms through which it acts.
These studies have limitations and raise additional new questions for future study. For example, as noted above, they are not quantitative with regard to the absolute levels of expression of the different D-cyclins and cdks: they reflect the affinity and specificity of available antisera, which were not directly compared in strict quantitative terms.
As another example, while we demonstrate that cdk6 is particularly effective in vivo, we did not examine all of the other possible cdk4/6–D-cyclin combinations: there are some 25 potential single, double, and/or triple cdk4/6–D-cyclin combinations that we might have explored in the in vivo NOD-SCID diabetes model. Examining all of these possibilities would be prohibitive with regard to time, expense, and availability of human islets. Because we were limited with regard to experimental transplant paradigms, we elected to approach the human β-cell proliferation/engraftment issue with an eye toward simplification, selecting a head-to-head comparison of cdk6 versus cyclin D1. To our surprise, cdk6 alone proved to be as effective as the combination and superior to cyclin D1 alone. Thus, proliferation rates induced in vitro do not necessarily predict transplant efficacy in vivo: although we found previously that combined overexpression of cdk6 plus cyclin D1 in vitro produces far higher proliferation than either alone, we found here that cdk6 alone produced graft function that was comparable to the combination. This observation was unanticipated, for during cell cycle progression, cdks remain constant, awaiting a D-cyclin partner to activate their kinase activity. Thus, we had assumed that cyclin D1 might have been rate-limiting and that cdk6 overexpression alone might have had limited effects, but we observed the opposite. These observations raise a number of questions, such as “what is the D-cyclin partner that binds to cdk6 in β-cells when it alone is overexpressed and activates proliferation?” Also, as noted above, “is cdk6 regulation a normal checkpoint in human β-cell replication?” Another question is, “what exact cell in islet is the target of the adenoviral cdk6?” Because cdk6 delivery was driven by the CMV promoter, it is anticipated that many cell types in addition to the β-cell would be induced to proliferate, a prediction that is supported by our previous report (12). These cells do not appear to be endocrine cells (12), but may be endothelial, ductal stromal, mesenchymal, progenitor, or other cells. Thus, it will be important to learn whether cdk6 overexpression using a β-cell-specific promoter leads to the same enhancement of engraftment and function. From a therapeutic standpoint, the implications are unequivocal: cdk6 is a particularly attractive agent, both as a therapeutic molecule for enhancing β-cell replication and also as a “druggable” target for small-molecule therapeutics that might enhance human β-cell regulation. As noted, these findings also suggest that cdk6/cyclin D3 might also be a particularly useful combination therapeutically.
We demonstrate that adenoviral cdk6 can drive human β-cell replication for at least 28 days in vivo. This raises additional questions as well. For example, “how long will/can β-cell proliferation continue in this in vivo model?” For another example, “is sustained proliferation a good thing, or will it eventually lead to hypoglycemia or oncogenic transformation in β-cells?” Another question is, “how long is cdk6 expression required to ensure sustained β-cell engraftment?” “Will cdk6 be required indefinitely, or can transient proliferation during the critical period of engraftment suffice to ensure long-term engraftment?” A related question is, “can either regulated or sustained delivery of cdk6 produce sustained human β-cell replication in vitro, in a way that will permit expansion of human β-cells ex vivo with retention of differentiation?” These are questions that should be examined in future studies in which cdk6 is delivered using regulatable promoters.
With regard to the oncogenicity question, it is important to recall that the G1/S family is also likely responsible for normal human β-cell proliferation in embryonic and neonatal life (9,10) and that they are not necessarily oncogenes. Having said this, it would seem likely that unregulated, long-term expression of cdk6 might have oncogenic consequences. This concern of course applies not only to G1/S cell cycle-activating molecules such as the cdks and D-cyclins, but also to most mitogenic signaling pathways, from receptors (e.g., EGF receptor, G-protein receptors, etc.) to signaling pathways (PI3 kinase, MAP kinase, Ras/Raf, JAK-STAT pathways, etc.) as well. Thus, one principal goal of these studies would be to use these strategies to identify key cellular molecules and pathways that could be targets for small-molecule agonists. For example, now that it is clear that cdk6 is effective individually in driving human β-cell expansion, it would be reasonable to screen small-molecule libraries to identify molecules that activate cdk6 in human β-cells. An additional goal might be to develop tools that would permit regulated expression of G1/S molecules such as cdk6 and D-cyclins such that they can be transiently activated to drive β-cell expansion for a few days or weeks and then inactivated to avoid sustained β-cell proliferation (51).
In conclusion, the G1/S transition is regulated by many proteins in addition to the five studied here, as described in the Introduction and supplementary Fig. 1. Each of these merits future study as both a normal and a potential therapeutic regulator of human β-cell replication. The efficacy of these, alone or in combination, and whether they may prove to be as or more effective than cdk6 as a therapeutic target for ex vivo β-cell expansion or in vivo β-cell engraftment, warrants additional study. Finally, these studies emphasize that, while broadly similar, human and rodent β-cells display differences in G1/S repertoires. These studies provide experimental models and multiple targets exploiting human β-cell replication, replacement, and engraftment in patients with diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Juvenile Diabetes Research Foundation (Grants 1-2008-39 and 34-2008-630), the NIH/NIDDK (Grant R-01 DK55023), the NIH/NIDDK Beta Cell Biology Consortium (Grant U-01 DK072473), the NIH/NCRR- and NIDDK-supported Islet Cell Resource Consortium, its Administrative Bioinformatics Coordinating Center, The Spanish Ministry of Science and Innovation (CP08/00094), and the Pam and Scott Kroh and Don and Arleen Wagner Family Foundations. No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
We also thank Drs. Adolfo Garcia-Ocaña, Laura C. Alonso, and Rupangi C. Vasavada for helpful discussion during the development of this project.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 2.Flier SN, Kulkarni RN, Kahn CR: Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Nat Acad Sci 2001;98:7475–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocaña A: Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miettinen PJ, Ustinov J, Ormio P, Gao R, Palgi J, Hakonen E, Juntti-Berggren L, Berggren PO, Otonkoski T: Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes 2006;55:3299–3308 [DOI] [PubMed] [Google Scholar]
- 5.Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF: Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 2000;275:15399–15406 [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF: Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 2000;275:1226–1232 [DOI] [PubMed] [Google Scholar]
- 7.Fujinaka Y, Sipula D, Garcia-Ocaña A, Vasavada RC: Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic beta-cell survival. Diabetes 2004;53:3120–3130 [DOI] [PubMed] [Google Scholar]
- 8.Fatrai S, Elghazi L, Balcazar N, Cras-Méneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E: Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006;55:318–325 [DOI] [PubMed] [Google Scholar]
- 9.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC: Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B: Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000;49:1325–1333 [DOI] [PubMed] [Google Scholar]
- 11.Hayek A, Beattie GM: Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab 1997;82:2471–2475 [DOI] [PubMed] [Google Scholar]
- 12.Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, Harb G, Selk K, Cozar-Castellano I, Stewart AF: Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes 2009;58:882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA: Proliferation of sorted human and rat beta cells. Diabetologia 2008;51:91–100 [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA, McDaniel ML: Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes 2009;58:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrberg B, Eizirik DL, Hellerström C, Pipeleers DG, Andersson A: Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology 1996;137:5694–5699 [DOI] [PubMed] [Google Scholar]
- 16.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC: A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995;59:817–820 [PubMed] [Google Scholar]
- 17.Menge BA, Tannapfel A, Belyaev O, Drescher R, Müller C, Uhl W, Schmidt WE, Meier JJ: Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes 2008;57:142–149 [DOI] [PubMed] [Google Scholar]
- 18.Rao P, Roccisana J, Takane KK, Bottino R, Zhao A, Trucco M, García-Ocaña A: Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic severe combined immunodeficient mice. Diabetes 2005;54:1664–1675 [DOI] [PubMed] [Google Scholar]
- 19.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 21.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocaña A, Vasavada R, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006;27:356–370 [DOI] [PubMed] [Google Scholar]
- 22.Heit JJ, Karnik SK, Kim SK: Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol 2006;22:311–338 [DOI] [PubMed] [Google Scholar]
- 23.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M: Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet 1999;22:44–52 [DOI] [PubMed] [Google Scholar]
- 24.Harb G, Vasavada RC, Cobrinik D, Stewart AF: The retinoblastoma protein and its homolog p130 regulate the G1/S transition in pancreatic beta-cells. Diabetes 2009;58:1852–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M: Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 2005;11:175–182 [DOI] [PubMed] [Google Scholar]
- 26.Zhong L, Georgia S, Tschen SI, Nakayama K, Nakayama K, Bhushan A: Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 2007;117:2869–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhawan S, Tschen SI, Bhushan A: Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes and Development 2009;23:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK: Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes and Development 2009;23:975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann Misfeldt A, Costa RH, Gannon M: Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 2008;57:3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Gaspard JP, Mizukami Y, Li J, Graeme-Cook F, Chung DC: Overexpression of cyclin D1 in pancreatic beta-cells in vivo results in islet hyperplasia without hypoglycemia. Diabetes 2005;54:712–719 [DOI] [PubMed] [Google Scholar]
- 31.He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA: Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol 2009;23:1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgia S, Bhushan A: Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004;114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF: Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005;25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK: Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A 2005;102:14659–14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martín J, Hunt SL, Dubus P, Sotillo R, Néhmé-Pélluard F, Magnuson MA, Parlow AF, Malumbres M, Ortega S, Barbacid M: Genetic rescue of Cdk4 null mice restores pancreatic beta-cell proliferation but not homeostatic cell number. Oncogene 2003;22:5261–5269 [DOI] [PubMed] [Google Scholar]
- 36.Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF: Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 2004;53:149–159 [DOI] [PubMed] [Google Scholar]
- 37.Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD: Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation: a cautionary note. Mol Endocrinol 2010;24:464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung DC, Brown SB, Graeme-Cook F, Seto M, Warshaw AL, Jensen RT, Arnold A: Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab 2000;85:4373–4378 [DOI] [PubMed] [Google Scholar]
- 39.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P: Mouse development and cell proliferation in the absence of D-cyclins. Cell 2004;118:477–491 [DOI] [PubMed] [Google Scholar]
- 40.Malumbres M, Sotillo R, Santamaría D, Galán J, Cerezo A, Ortega S, Dubus P, Barbacid M: Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004;118:493–504 [DOI] [PubMed] [Google Scholar]
- 41.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC: Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 42.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A: The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F: Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 1995;96:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrer J, Benito C, Gomis R: Pancreatic islet GLUT2 glucose transporter mRNA and protein expression in humans with and without NIDDM. Diabetes 1995;44:1369–1374 [DOI] [PubMed] [Google Scholar]
- 45.Schuit FC: Is GLUT2 required for glucose sensing? Diabetologia 1997;40:104–111 [DOI] [PubMed] [Google Scholar]
- 46.Malumbres M, Barbacid M: Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005;30:630–641 [DOI] [PubMed] [Google Scholar]
- 47.Sherr CJ, Roberts JM: CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999;13:1501–1512 [DOI] [PubMed] [Google Scholar]
- 48.Kida A, Kakihana K, Kotani S, Kurosu T, Miura O: Glycogen synthase kinase-3beta and p38 phosphorylate cyclin D2 on Thr280 to trigger its ubiquitin/proteasome-dependent degradation in hematopoietic cells. Oncogene 2007;26:6630–6640 [DOI] [PubMed] [Google Scholar]
- 49.Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, Waring P, McArthur GA, Walkley CR, Holloway AJ, Diyagama D, Grim JE, Clurman BE, Bowtell DD, Lee JS, Gutierrez GM, Piscopo DM, Carty SA, Hinds PW, McArthur G, Walkeley C, Holoway AJ, Diyagama D, Clurman B, Bowtell DDL, Lee J-S, Gutierrez G, Piscopo DM, Carty SA, Hinds PW: Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol 2004;167:925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim JT, Mansukhani M, Weinstein IB: Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc Nat Acad Sci 2005;102:5156–5161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takane KK, Kleinberger J, Salim F, Thomas S, Fiaschi-Taesch NM, Stewart AF: Regulated induction of human beta cell replication: tetracycline-inducible overexpression of cdk6 and cyclin D1 in human beta cells. In Proceedings of the 70th ADA Annual Scientific Sessions, Orlando, FL, June 2010 (submitted) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.