Abstract
Human adolescents consume alcohol largely to enhance social interactions. Adolescent, but not adult rats likewise exhibit ethanol-induced social facilitation under low-stress circumstances. Since the relationship between stress and ethanol sensitivity across ontogeny still has yet to be well explored, the present study sought to characterize possible age-associated differences in the influence of stressor exposure on ethanol-induced changes in social behavior in adolescent [postnatal days (P) 30–36] and adult (P65-71) male and female Sprague-Dawley rats. Animals were repeatedly restrained (90 min/day) for 5 days, followed by examination of ethanol-induced (0, 0.25, 0.5, 0.75, or 1.0 g/kg) alterations in social behaviors on the last day. Results revealed typical age-related differences in sensitivity to ethanol among controls, with adolescents being uniquely sensitive to low-dose ethanol stimulation of social investigation and play fighting, but less sensitive than adults to the social suppression emerging at higher doses. At both ages, stressor exposure decreased sensitivity to social inhibitory effects of ethanol, while augmenting expression of ethanol’s social facilitatory effects. Ethanol also attenuated the stress-related suppression of social motivation at both ages. These results suggest that repeated stressor exposure diminishes age-related differences in the social consequences of ethanol, with stress enhancing ethanol-induced social facilitation across age.
Keywords: adolescent, ethanol, rat, social behavior, social interaction test, restraint, repeated stress, blood ethanol levels
Of the many drugs that have been reported to be used by adolescents, alcohol is by far the most commonly and excessively abused substance, with as many as 72% of 12th graders reporting lifetime alcohol use and approximately 25% reporting binge-like consumption in the last month (Johnston et al., 2007). Survey and self-report data have shown that adolescents drink alcohol for a variety of reasons; among these reasons social factors appear to be an especially salient contributor. Expectancy of social facilitation from drinking is an important predictor of current and longitudinal drinking (Mackintosh et al., 2006; Smith et al., 1995), with adolescents strongly believing that alcohol will make them more confident, socially assertive, relaxed in a social setting and sexually enhanced (Brown et al., 1987; Mackintosh et al., 2006; Smith et al., 1995).
Elevated levels of ethanol intake and ethanol-induced social facilitation are not restricted to human adolescents but can be seen using a simple model of adolescence in the rat (see Spear and Varlinskaya, 2005 for references and review). Similarities across species in neural, hormonal and behavioral attributes of adolescence (e.g., Adriani and Laviola, 2004; Doremus-Fitzwater et al., 2009a; Spear, 2000, 2007b) provide reasonable face and construct validity (Spear, 2007a) for the use of animal models to investigate adolescent responding to ethanol, investigations that are generally ethically inappropriate in underage youth. In rats, the period of adolescence can be conservatively defined as postnatal days (P) 28 to 42, during which subjects of both sexes exhibit adolescent-typical neurobehavioral characteristics (Adriani and Laviola, 2004; Spear, 2000; Spear and Brake, 1983), with this age range sometimes being subdivided into early (around P28), mid (around P35), and late (around P42) adolescence (Varlinskaya and Spear, 2006a, 2008b).
Adolescent rats ingest more ethanol than adults under various testing paradigms (Brunell and Spear, 2005; Doremus et al., 2005; Lancaster et al., 1996; Vetter et al., 2007; Vetter-O’Hagen et al., 2009) and differ markedly from adults in the impact of ethanol on their social behavior. Adolescent rats tested under familiar, non-anxiogenic circumstances show a facilitation in social behavior following acute exposure to relatively low doses (0.5 – 0.75 g/kg) of ethanol (Varlinskaya and Spear, 2002, 2006a). These doses produce blood ethanol concentrations (BECs) from approximately 40 to 80 mg/dl – BECs that are within the moderate consumption range in humans (see Eckardt et al., 1998 for references and review). Social facilitation induced by ethanol is seen in both male and female adolescent rats and is evident not only in terms of increases in play fighting, an adolescent-characteristic form of social interactions in rats, but also via increases in social investigation, a more adult-typical social behavior (Vanderschuren et al., 1997; Varlinskaya and Spear, 2008b).
Higher doses of ethanol induce social inhibition, an effect to which adults are more sensitive than adolescents (Varlinskaya and Spear, 2002, 2007). For instance, adult rats usually exhibit reduced levels of overall social activity, social investigation and play behavior following 0.75 and 1.0 g/kg doses of ethanol, whereas adolescents typically require doses in excess of 1 g/kg to demonstrate reductions in social behavior. Importantly, these age differences in ethanol’s stimulatory or inhibitory effects on social behavior have been shown to be unrelated to simple alterations in activity levels (ethanol-induced locomotor stimulation or hypoactivity) or pharmacokinetic factors (Varlinskaya and Spear, 2002).
Social behavior is highly sensitive to stressors and anxiogenic stimuli (Doremus-Fitzwater et al., 2009b; File, 1993; File and Seth, 2003) and stress reduction and alleviation of anxiety is one of the frequently mentioned reasons for which human adolescents and adults report drinking alcohol (Beck and Treiman, 1996; Cooper et al., 2000). Adolescents who expect alcohol to alleviate their anxiety and to relieve their personal problems are especially likely to engage in heavy and problem drinking (Bates and Labouvie, 1997; Kuntsche and Kuendig, 2005; Montgomery et al., 1993). Although the association between alcohol use and stressful events has been extensively investigated in adult humans (Brady and Sonne, 1999; Pohorecky, 1991; Sayette, 1999) and laboratory animals (Chester et al., 2004; Lynch et al., 1999), this relationship has been shown to be quite complex, and still not completely understood. Limited evidence has suggested that, in humans, stressor exposure results in a —sobering effect to some of the aversive consequences of alcohol consumption (Breslin et al., 1995). Alternatively, evidence with animal models has shown that prior exposure to stressors results in sensitization to some alcohol effects, particularly those involving the positive/stimulatory consequences of ethanol (Phillips et al., 1997). Effects of stressors on ethanol sensitivity during ontogeny have been little explored, despite evidence that adolescence may be a relatively stressful phase characterized not only by elevated exposure to stressful life events (Arnett, 1999), but also by potentially enhanced stress reactivity (Spear, 2000; Walker et al., 2001). Given the magnitude and speed of adolescent-associated changes, it is not unlikely that these challenges could overburden the capacity of some adolescents to cope with different environmental and social challenges (Collins, 2001; Davis, 2003; Jessor, 1993) and potentially alter their responsiveness to drugs and alcohol.
The purpose of the present study, therefore, was to assess the impact of previous stress history on the acute effects of ethanol on social behavior of adolescent and adult male and female rats. Restraint was used for the repeated stressor since it is a primarily psychological stressor that does not cause physical pain (Herman and Cullinan, 1997; Weinberg et al., 2007). Animals were exposed to restraint for 90-min each day over a 5 day period, a chronic stress paradigm shown previously to induce significant anxiogenesis in the social interaction test (Doremus-Fitzwater et al., 2009b). After the final restraint session, animals were acutely challenged with ethanol, and ethanol-induced changes in social behavior were assessed using a modified social interaction test under familiar circumstances.
Methods
Subjects
A total of 240 adolescent and adult Sprague-Dawley male and female rats were used as experimental subjects, with the same number of animals assigned to serve as social partners. Animals were obtained from our breeding facilities, housed in a temperature-controlled (22°C) vivarium, and maintained on a 14:10 hr light:dark cycle (lights on at 0700 hr) with ad libitum access to food (Purina rat chow) and water. Litters were culled to 10 pups (5 males and 5 females) within 24 hr after birth on P0 and reared until weaning with their mothers in standard plastic opaque maternity cages with pine shavings as bedding material. Rats were weaned on P21 and housed with their same-sex littermates. At all times, rats used in the current experiments were produced, maintained and treated in accordance with the guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental Design
The design of this study was a 2 (age: adolescent, adult) × 2 (sex) × 2 (stress condition: non-manipulated or repeated restraint stress) × 5 (dose: 0, 0.25, 0.5, 0.75 or 1.0 g/kg ethanol) factorial, with 6 experimental animals tested per group. Males and females were tested either on P35 (adolescents) or on P70 (adults). To avoid the possible confounding of litter with stress condition and ethanol dose, no more than one male and one female animal from a given litter was placed into a particular experimental group (Holson and Pearce, 1992; Zorrilla, 1997).
Stressor Procedures
Beginning at P31 for adolescents or P66 for adults, animals from the repeated stress group were removed from their home cage between 1000 – 1200 hr and then restrained in an age/sex size-adjusted restraint tube for 90 min in a novel holding cage. Restraint tubes (Braintree Scientific, Braintree, MA) were round slotted Plexiglas cylinders with sliding plugs to allow adjustment of the tube length for each animal’s size. Cylinders measured 18.0 × 4.7 cm for adolescent males and females, 20.5 × 7.0 cm for adult females, and 23.0 × 8.0 cm for adult males (length × diameter). For animals in the stress group, this restraint procedure was repeated each day for 5 days. Animals placed in the control condition were non-manipulated throughout the 5-day stressor phase until the time of ethanol challenge, with all animals in each home cage assigned to the same stressor condition (restrained or non-manipulated), but to different ethanol challenge dose conditions.
One day prior to the testing (i.e. on the fourth day of the repeated stressor period), and at least 2 hr after the completion of the restraint stress procedure on that day for animals in the stress group, all experimental animals were individually exposed to the test apparatus for 30 min. This pretest familiarization was conducted to increase baseline levels of social interaction on the test day, hence making potential anxiogenic effects of the repeated stressors easier to observe (File, 1993; File and Seth, 2003).
Testing Procedures
Immediately after the 90-min stressor exposure on day 5 (or upon removal from the home cage for non-stressed animals), each subject was injected with one of the 5 doses of ethanol (0, 0.25, 0.5, 0.75 or 1.0 g/kg), given intraperitoneally (i.p.) as a 12.6% (v/v) solution in saline (0.9%, w/v). Ethanol challenge dose was varied by altering the volume of the 12.6% ethanol solution to avoid concentration-induced differences in ethanol absorption rate (see Linakis and Cunningham, 1979). Control animals were injected with isotonic saline at a volume equal to that of the highest dose of ethanol administered. All solutions were injected at room temperature.
Immediately after ethanol administration, each experimental animal was marked by a vertical line on the back and isolated in an opaque plastic holding cage (30 × 20 × 20 cm) for 30 min prior to testing (e.g., File, 1993). Thereafter, each animal was placed into the testing chamber simultaneously with a same age and sex test partner. Partners were always non-exposed animals that had not been socially isolated prior to testing and who were unfamiliar with both the test apparatus and the experimental animal with which they were paired for testing. Weight differences between test subjects and their partners were minimized as much as possible, with this weight difference not exceeding 10 g for animals at P35 and 20 g at P70, and test subjects always being heavier than their partners. The order of testing was counterbalanced for all treatment groups.
Apparatus
Testing was conducted in Plexiglas (Binghamton Plate Glass, Binghamton, NY) test chambers (30 × 20 × 20 cm for adolescents; 45 × 30 × 30 cm for adults) that contained clean pine shavings. The test apparatuses were divided into two compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for adolescents; 9 × 7 for adults) to allow movement of animals between compartments (Varlinskaya et al., 2001, 1999).
Each 10-min social interaction test session was conducted under dim light (15–20 lux) between 1200 and 1600 hr, with a white noise generator used to attenuate extraneous sounds during testing. The behavior of each pair was recorded by a video camera mounted above the apparatus.
Behavioral Measures
Frequencies of a number of social activities were scored and analyzed (Meaney and Stewart, 1981; Thor and Holloway, 1984; Vanderschuren et al., 1997; Varlinskaya and Spear, 2008b) by a trained experimenter without knowledge of the experimental condition of any given animal. Play fighting was scored as the sum of the frequencies of the following behaviors: pouncing or playful nape attack (experimental subject lunges at the partner with its forepaws extended outward); following and chasing (experimental animal rapidly pursues the partner); and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). Play fighting can be distinguished from serious fighting in the laboratory rat by the target of the attack—during play fighting, snout or oral contact is directed towards the partner’s nape, whereas during serious fighting the partner’s rump is the object of the attack (Pellis and Pellis, 1987). Aggressive behavior (serious fighting) was not analyzed in these experiments, since subjects did not exhibit serious attacks or threats. Social investigation was defined as the sniffing of any part of the body of the partner.
Social preference/avoidance was analyzed by separately measuring the number of crossovers demonstrated by the experimental subject towards as well as away from the social partner. Social motivation was assessed by means of a coefficient of preference/avoidance [coefficient (%) = (crossovers to the partner – crossovers away from the partner)/(total number of crosses both to and away from the partner) × 100]. Social preference was defined as positive values of the coefficient, while social avoidance was associated with negative values (Varlinskaya et al., 1999).
The total number of crossovers (movements between compartments through the aperture) exhibited by each experimental subject was used as an index of general locomotor activity in the social context (Varlinskaya et al., 1999).
Blood Ethanol Concentration
Blood ethanol concentrations (BECs) were determined in a separate set of animals (N = 192) using a 2 (age: adolescent or adult) × 2 (sex: male or female) × 2 (stress: no stress or repeated restraint) × 4 (dose: 0.25, 0.5, 0.75 or 1.0 g/kg ethanol) between subjects factorial design. Adolescents and adults were either non-manipulated or exposed to the 5-day repeated restraint procedure described above. On the fifth day of stressor exposure, animals were injected with the appropriate dose of ethanol immediately after removal from the restraint tubes. Following injection, animals were isolated in a novel holding cage for a 30-min period that was immediately followed by collection of tail blood samples for analyses of BECs. Thus, BECs were collected at a time that coincided with the onset of social interaction testing for the experimental animals.
Blood samples were obtained from the tail vein using a heparinized tube, rapidly frozen and stored at −80 °C. Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-sampler, which heated each individual vial for 8 min, and then extracted and injected a 1.0 ml sample of the gas headspace into the HP 5890 series Gas Chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analysis
Data were checked for outliers before analysis with mixed-factor analyses of variance (ANOVA) at each age, with a score > 2.0 standard deviations from the mean of a particular experimental group being considered an outlier. Since significant main effects of age emerged in the overall ANOVAs for most of the behavioral measures, separate analyses were conducted at each age using separate 2 (stress: no stress or repeated restraint) × 4 (dose: 0.25, 0.5, 0.75 or 1.0 g/kg ethanol) × 2 (sex: male, female) ANOVAs. Main effects and interactions involving repeated stress and ethanol exposure on social interactions were further explored using Fisher’s least significant difference (LSD) post hoc tests.
Results
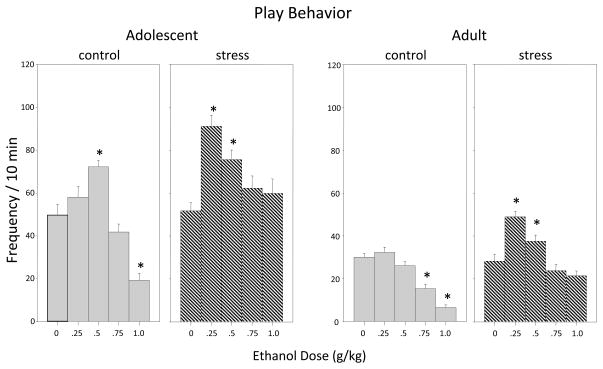
Play Fighting (Figure 1)
Figure 1.
The impact of repeated restraint stress on ethanol-related alterations in play behavior in both adolescent and adult male and female rats during a 10-min social interaction test. Ethanol-induced alterations are shown for both control (non-stressed) (gray bars) and stressed (hatched bars) animals, with data shown collapsed across sex. In this and subsequent figures, bars represent the mean for a given condition, with error bars representing the standard error of the mean. Asterisks (*) indicate significant differences from the corresponding saline control group (p ≤ .05).
The analysis of play fighting revealed a stress × dose interaction for adolescents (F(4,100) = 6.36, p ≤ .001) and for adults (F(4,100) = 5.52, p ≤ .001). Exposure to the repeated stressor did not impact baseline levels of play in either adolescents or adults administered saline relative to their non-stressed counterparts. When repeatedly stressed adolescents and adults given ethanol were compared to those injected with saline, animals that received the 0.25 and 0.5 g/kg doses exhibited increased play fighting at both ages, an effect similar to, but even more pronounced than ethanol-induced facilitation of play seen in non-stressed adolescents (at the 0.5 g/kg dose of ethanol). Additionally, stressed adolescents and adults did not demonstrate the ethanol-induced inhibition of play seen among non-stressed animals after the 1.0 g/kg dose of ethanol for adolescents and after doses of 0.75 and 1.0 g/kg among adults.
In adults, dose also interacted with sex in the analysis of this behavioral measure (F(4,100) = 3.12, p ≤ .05) with adult females being less sensitive to ethanol-related reductions in play at higher doses when compared to their adult male counterparts, regardless of stressor condition (see Table 1). No significant sex effects were observed in adolescents.
Table 1.
Sex-related differences in sensitivity to the social consequences of ethanol among adult (P70) rats, with data collapsed across stress condition (n=12 per group)
| Ethanol Dose (g/kg) | Play Fighting | Social Preference/Avoidance | ||
|---|---|---|---|---|
| male | female | male | female | |
| 0 | 31.1 ± 3.5 | 28.1 ± 1.8 | 27.9 ± 6.2 | 24.0 ± 5.9 |
| 0.25 | 41.7 ± 3.2 * | 37.8 ± 4.4 * | 49.0 ± 6.3 * | 33.8 ± 3.8 |
| 0.5 | 27.2 ± 3.0 | 34.8 ± 3.4 | 42.2 ± 6.2 | 32.7 ± 6.9 |
| 0.75 | 15.3 ± 2.9 * | 23.8 ± 2.5 | 29.0 ± 6.0 | 19.2 ± 6.3 |
| 1.0 | 10.4 ± 2.1 * | 17.5 ± 3.2 * | −10.8 ± 8.6 * | 10.0 ± 8.0 |
indicate significant differences from corresponding saline controls within each sex.
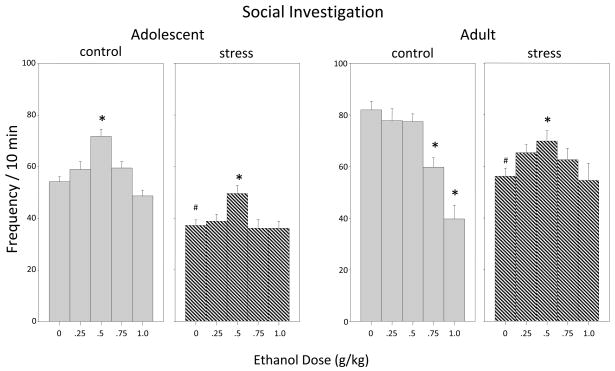
Social Investigation (Figure 2)
Figure 2.
The impact of repeated restraint stress on ethanol-related alterations in social investigation in both adolescent and adult male and female rats during a 10-min social interaction test. Ethanol-induced alterations are shown for both control (non-stressed) (gray bars) and stressed (hatched bars) animals, with data shown collapsed across sex. Asterisks (*) indicate significant differences from the corresponding saline control group, whereas the pound signs (#) indicate significant reductions in behavior among stressed animals relative to non-stressed saline controls within that age group (p ≤ .05).
Repeated restraint stress reduced baseline levels of social investigation in adolescents [main effect of stress, F(1,100) = 128.98, p ≤ .0001]—a stress-induced anxiogenic effect observed previously (Doremus-Fitzwater et al., 2009b), with repeatedly stressed adolescents given saline at test demonstrating significantly less social investigation than their non-stressed counterparts. Typical ethanol-induced social facilitation reported earlier (e.g. Varlinskaya and Spear, 2002, 2006b) was also seen, with both non-stressed and stressed adolescents showing an ethanol-induced increase in social investigation at a low dose of ethanol (0.5 g/kg) [dose main effect for adolescents: F(4,100) = 13.55, p ≤ .0001].
The analysis of social investigation in adults revealed a significant stress × dose interaction (F(4,100) = 6.56, p ≤ .0001). Repeatedly stressed adults challenged with saline showed less social investigation than their non-stressed counterparts (i.e., stress-induced anxiogenesis). Among adults, exposure to the repeated restraint procedure eliminated the social inhibition that was observed at higher ethanol doses in non-stressed animals. Repeated restraint also resulted in the emergence of a significant increase in social investigation following the 0.5 g/kg dose of ethanol.
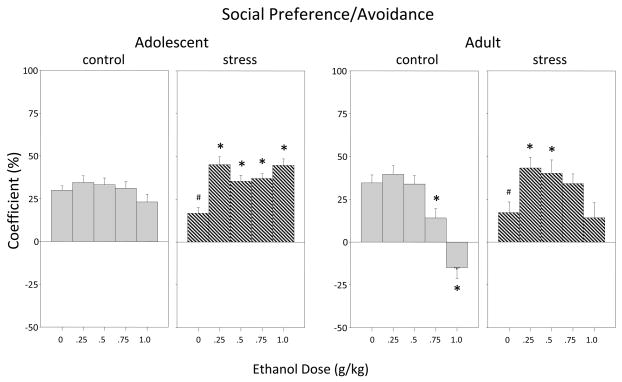
Social Preference/Avoidance (Figure 3)
Figure 3.
The impact of repeated restraint on ethanol-related alterations in coefficients of social preference/avoidance during the 10-min. social interaction test among adolescent and adult male and female rats. Data are collapsed across sex and are shown for control (gray bars) and repeatedly stressed (hatched bars) animals. Asterisks (*) indicate significant differences from the corresponding saline control group, whereas pound signs (#) indicate significant reductions in behavior among stressed animals relative to non-stressed saline controls within that age group (p ≤ .05).
Anxiogenic effects of repeated restraint also emerged in terms of social motivation when indexed via the social preference coefficient, with stressed adolescents and adults exposed to saline showing a reduction in social preference relative to their non-stressed, saline-treated counterparts. This reduction in social preference was reversed by ethanol at all doses in adolescents and at the 0.25 and 0.5 g/kg doses in adults [stress × dose interaction for adolescents: F(4,100) = 5.49, p ≤ .001; for adults: F(4,100) = 4.06, p ≤ .01]. Non-stressed adults showed the typical reduction in social motivation at higher ethanol doses—social preference was significantly reduced following 0.75 g/kg ethanol or even transformed into social avoidance by the 1.0 g/kg dose. These attenuating effects of higher ethanol doses on social motivation were not evident in stressed adults.
Although not interacting with the stressor condition, a significant sex × dose interaction (F(4,100) = 2.63, p ≤ .05) was observed among adults in the analysis of social preference, with adult males showing significant ethanol-induced increases and decreases in social preference at the 0.25 and 1.0 g/kg doses of ethanol, respectively, whereas no significant ethanol-induced alterations in social preference were seen in females (Table 1).
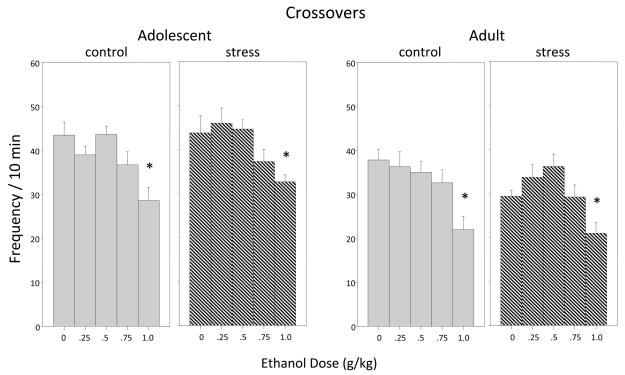
Locomotor Activity (Figure 4)
Figure 4.
The impact of repeated restraint and ethanol on number of crossovers exhibited during a 10-min social interaction test among adolescent and adult male and female rats that were either non-stressed (controls; gray bars) or repeatedly stressed (hatched bars). Data shown are collapsed across sex, with asterisks (*) indicating significant differences from the corresponding saline control group (p ≤ .05).
Overall locomotor activity within the social context (indexed via total crossovers) was not significantly affected by exposure to a repeated stressor in either adolescents or adults. Administration of the highest ethanol dose (1.0 g/kg) significantly decreased locomotor activity at both ages [dose main effect for adolescents: F(4,100) = 8.32, p ≤ .0001; for adults F(4,100) = 9.67, p ≤ .0001]. As is typically observed in adult rats, females (33.82 ± 1.09) were more active than males (28.80 ± 1.56) [sex main effect F(1,100) = 9.12, p ≤ .01], although this sex effect was not evident in the adolescents.
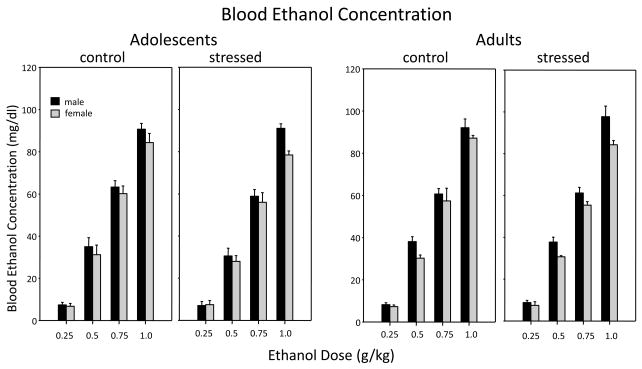
Blood Ethanol Concentration (Figure 5)
Figure 5.
Blood ethanol concentration (BEC) 30 min. following acute challenge with 1 of 5 doses of ethanol among non-stressed (control) and repeatedly restrained (stressed) adolescent and adult male (black bars) and female (gray bars) rats.
BECs increased in a dose-dependent fashion (F(3, 160) = 1085.96, p ≤ =.0001) but did not differ as a function of age and stress. However, while BECs were similar across age and stressor exposure, an effect of sex was observed that interacted with dose [sex × dose interaction: F(3,160) = 2.97, p ≤ .05]. Post hoc analysis of data collapsed across age and stressor to explore this interaction revealed significantly higher BECs in males than in females at the 0.50 and 1.0 g/kg doses of ethanol.
Discussion
Similar to our earlier findings (Varlinskaya and Spear, 2002, 2007), considerable age-related differences in ethanol-induced alterations in social behavior were observed among control animals, with non-stressed adolescents being uniquely sensitive to ethanol-induced facilitation of social investigation and play fighting and less sensitive to ethanol-induced social inhibition, demonstrating significantly attenuated decreases in all social behavioral measures at higher ethanol doses compared to their adult counterparts. Repeated restraint stress exacerbated this adolescent-typical responsiveness to the social consequences of acute ethanol, making adolescent animals even more sensitive to the stimulatory effects of ethanol on play fighting and even less sensitive to ethanol-induced social inhibition. These findings are in agreement with the results reported recently (Trezza et al., 2009), with adolescent animals tested under unfamiliar and hence more stressful circumstances being more responsive to the stimulatory effects of ethanol on play behavior than those tested in a familiar environment. Surprisingly, among adults, exposure to repeated stress induced an adolescent-typical pattern of social behavior to an acute ethanol challenge: prior stress precipitated the expression of ethanol-induced facilitation of social investigation and play fighting among adults and eliminated the social inhibition normally seen in adults at the higher doses tested. Therefore, repeated stress increased sensitivity of both adolescents and adults to the social-facilitating effects of ethanol and rendered them similarly insensitive to the socially suppressing effects of ethanol. Assessments of ethanol concentrations in trunk blood in the present study revealed no effects of age and pretest stress conditions, suggesting that observed age-related and stress-induced changes in sensitivity to the social consequences of ethanol were not simply due to pharmacokinetic factors.
We have shown previously that ethanol-induced social facilitation seen in adolescent animals under normal (i.e., non-stressful) circumstances is attenuated by a non-selective opioid antagonist naloxone and the selective mu antagonist CTOP (Varlinskaya and Spear, 2009). These earlier findings suggest that ethanol-induced social facilitation seen under normal circumstances among young adolescents but not adults (Varlinskaya and Spear, 2002, 2007) may be related to greater ethanol-associated activation of the endogenous mu opioid system in adolescence than in adulthood, although direct across-age comparisons of ethanol activation of this system have yet to be conducted. The induction of sensitivity to the social stimulatory effects of ethanol in adult animals following restraint stress may likewise be associated with stress-induced alterations within mu opioid systems. Indeed, repeated restraint stress not only makes adult animals sensitive to the stimulatory effects of ethanol but also to the socially activating effects of mu opioid receptor activation. The latter conclusion is based on other recent work where adolescent, but not adult rats were found to be sensitive to the socially stimulating effects of a selective mu agonist DAMGO under non-stressful circumstances, whereas adults then demonstrated DAMGO-induced social facilitation when stressed (Varlinskaya and Spear, 2008a). Taken together, these findings suggest that stress exposure may alter the endogenous mu opioid system of adult animals in a way that increases sensitivity of mu opioid receptors to the socially facilitating properties of mu opioid ligands.
This suggestion of a role for mu opioid receptors in stress-related induction of sensitivity to ethanol-induced social facilitation at maturity is consistent with other evidence for close interrelationships between stressors and endogenous opioid systems. Numerous studies have suggested that endogenous opioid systems play an important role in the mediation, modulation, and regulation of endocrine and behavioral components of stress responses (Drolet et al., 2001). Conversely, stress has been shown to influence the endogenous opioid systems as well, with reports of stress- induced alterations in opioid receptor binding, levels of opioid peptides and their mRNAs, as well as mRNAs encoding different types of opioid receptors (Dantas et al., 2005; Nikulina et al., 1999; Yamada and Nabeshima, 1995; Yamamoto et al., 2003). Although the nature of these stress-induced alterations depend on paradigm used, strain, and brain regions under investigation, a number of studies have reported stress-related down-regulation of mu opioid receptors, perhaps as a result of excessive release of endogenous ligands during repeated stressor exposure (Dantas et al., 2005; Stuckey et al., 1989).
Although the endogenous opioid systems play a substantial role in ethanol-induced social facilitation and stress responsiveness, other neural systems may also be involved. For instance, indirect cannabinoid agonists (Trezza & Vanderschuren, 2008a, b) and NMDA antagonists (Siviy et al., 1995), similar to ethanol, facilitate social behavior in adolescence, with endogenous cannabinoid and NMDA systems being implicated in ethanol intake and reinforcement (Vengeliene et al., 2008), as well as in stress responsivity (Covington et al., 2008; Marco and Viveros, 2009). Furthermore, ethanol-induced increases in play behavior can be blocked by CB1 receptor or dopamine receptor antagonists (Trezza et al., 2009). Therefore, endogenous cannabinoid, NMDA, and dopamine systems may contribute to stress-induced changes in sensitivity to the socially activating effects of ethanol.
Repeated stress not only influenced ethanol’s impact on social behavior in the present study, but also suppressed baseline levels of social investigation and social motivation in both adolescents and adults as well. These apparent anxiogenic effects of repeated restraint are similar to results reported previously (Doremus-Fitzwater et al., 2009b), with stressed adolescents and adults challenged with saline at test showing less social investigation and reduced social preference relative to their non-manipulated counterparts. Such anxiogenic consequences of restraint stress on social preference were reversed by acute ethanol regardless of age, whereas similar anxiolytic effects of ethanol were not evident under these familiar test circumstances in non-stressed controls. Stress-exposed animals not only are seemingly more sensitive to the anxiolytic effects of ethanol, but also are less sensitive to ethanol’s inhibitory effects on social behavior. Both of these consequences of repeated restraint may be related to alterations in GABAa receptor systems, given substantial evidence that GABAa receptors contribute to inhibitory and anxiolytic effects of ethanol (Eckardt et al., 1998). Under some circumstances, stress-induced alterations seen among adolescents may be long lasting. Animals stressed as early adolescents (P27–P29) and tested as adults at P60 became more sensitive to anxiolytic effects and less sensitive to sedative effects of benzodiazepines – compounds that target GABAa receptors (Jacobson-Pick et al., 2008).
GABAa receptors sensitive to ethanol traditionally are characterized as containing β2 and γ2 subunits in partnership with benzodiazepine-sensitive α1, α2, α3 and α5 subunits (Faingold et al., 1998), with non-selective benzodiazepine agonists and ethanol sharing many prominent effects, including anxiolysis, behavioral suppression, and sedation at higher doses (e.g., Blednov et al., 2003; Corbett et al., 1991). Given evidence for a role of receptors containing α1-subunits in impairing and sedative effects of ethanol (Blednov et al., 2003; Rudolph et al., 1999), and α2- and/or α3-subunits in ethanol-related anxiolysis (e.g., Atack et al., 2006), the results of the present study would be consistent with a potential stress-related shift toward lower α1-subunit and greater α2- and/or α3-subunit activity, especially among adolescents. Clearly the impact of repeated stressors on mu opioid and GABAa receptor systems and their sensitivity to ethanol during adolescence and in adulthood provides promising areas for future ontogenetic investigation of factors contributing to initiation of alcohol intake and its increased consumption during periods of stress.
Acknowledgments
Supported by grants R01 AA016887, R01 AA01735501 to Linda Spear and R01 AA012453 to Elena Varlinskaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–26. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluor ophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–22. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Bates ME, Labouvie EW. Adolescent risk factors and the prediction of persistent alcohol and drug use into adulthood. Alcohol Clin Exp Res. 1997;21:944–50. [PubMed] [Google Scholar]
- Beck KH, Treiman KA. The relationship of social context of drinking, perceived social norms, and parental influence to various drinking patterns of adolescents. Addict Behav. 1996;21:633–44. doi: 10.1016/0306-4603(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA. Deletion of the alpha1 or beta2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther. 2003;304:30–6. doi: 10.1124/jpet.102.042960. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health. 1999;23:263–71. [PMC free article] [PubMed] [Google Scholar]
- Breslin FC, Hayward M, Baum AS. Stress and alcohol: the moderating effect of chronic stress on the acute stress--intoxication relationship. J Stud Alcohol. 1995;56:546–52. doi: 10.15288/jsa.1995.56.546. [DOI] [PubMed] [Google Scholar]
- Brown SA, Christiansen BA, Goldman MS. The Alcohol Expectancy Questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48:483–91. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–93. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Collins ME. Transition to adulthood for vulnerable youths: A review of research and implications for policy. Soc Serv Rev. 2001;75:271–91. [Google Scholar]
- Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: the role of personality and affect regulatory processes. J Pers. 2000;68:1059–88. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Corbett R, Fielding S, Cornfeldt M, Dunn RW. GABAmimetic agents display anxiolytic-like effects in the social interaction and elevated plus maze procedures. Psychopharmacology (Berl) 1991;104:312–6. doi: 10.1007/BF02246029. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas G, Torres IL, Crema LM, Lara DR, Dalmaz C. Repeated restraint stress reduces opioid receptor binding in different rat CNS structures. Neurochem Res. 2005;30:1–7. doi: 10.1007/s11064-004-9679-2. [DOI] [PubMed] [Google Scholar]
- Davis M. Addressing the needs of youth in transition to adulthood. Adm Policy Ment Health. 2003;30:495–509. doi: 10.1023/a:1025027117827. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2009a;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009b;97:484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–41. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N’Gouemo P, Riaz A. Ethanol and neurotransmitter interactions--from molecular to integrative effects. Prog Neurobiol. 1998;55:509–35. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- File SE. The social interaction test of anxiety. Neuroscience Protocols. 1993;10:1–7. [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Elkobi A, Vander S, Rosenblum K, Richter-Levin G. Juvenile stress-induced alteration of maturation of the GABAA receptor alpha subunit in the rat. Int J Neuropsychopharmacol. 2008;11:891–903. doi: 10.1017/S1461145708008559. [DOI] [PubMed] [Google Scholar]
- Jessor R. Successful adolescent development among youth in high-risk settings. Am Psychol. 1993;48:117–26. doi: 10.1037//0003-066x.48.2.117. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. In: Monitoring the Future national results on adolescent drug use: Overview of key findings, 2006. Abuse NIoD., editor. Bethesda, MD: National Institute on Drug Abuse; 2007. p. 71. [Google Scholar]
- Kuntsche EN, Kuendig H. Do school surroundings matter? Alcohol outlet density, perception of adolescent drinking in public, and adolescent alcohol use. Addict Behav. 2005;30:151–8. doi: 10.1016/j.addbeh.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–9. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64:61–5. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–23. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Mackintosh MA, Earleywine M, Dunn ME. Alcohol expectancies for social facilitation: A short form with decreased bias. Addict Behav. 2006;31:1536–46. doi: 10.1016/j.addbeh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Marco EM, Viveros MP. The critical role of the endocannabinoid system in emotional homeostasis: avoiding excess and deficiencies. Mini Reviews in Medicinal Chemistry. 2009;9:1407–15. doi: 10.2174/138955709789957468. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15:197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Benedicto JA, Haemmerlie FM. Personal vs social motivations of undergraduates for using alcohol. Psychol Rep. 1993;73:960–2. doi: 10.2466/pr0.1993.73.3.960. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr, Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–9. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behav. 1987;13:227–42. [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav. 1997;57:487–93. doi: 10.1016/s0091-3057(96)00448-0. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–59. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Does drinking reduce stress? Alcohol Res Health. 1999;23:250–5. [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiology & Behavior. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Smith GT, Goldman MS, Greenbaum PE, Christiansen BA. Expectancy for social facilitation from drinking: the divergent paths of high-expectancy and low-expectancy adolescents. J Abnorm Psychol. 1995;104:32–40. doi: 10.1037//0021-843x.104.1.32. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol Teratol. 2007a;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The developing brain and adolescent-typical behavior patterns: An evolutionary approach. In: Walker E, Romer D, editors. Adolescent Psychopathology and the Developing Brain: Integrating Brain and Prevention Science. New York, NY: Oxford University Press; 2007b. pp. 9–30. [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- Stuckey J, Marra S, Minor T, Insel TR. Changes in mu opiate receptors following inescapable shock. Brain Res. 1989;476:167–9. doi: 10.1016/0006-8993(89)91552-7. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr Social play in juvenile rats: a decade of methodological and experimental research. Neurosci Biobehav Rev. 1984;8:455–64. doi: 10.1016/0149-7634(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008a;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. European Neuropsychopharmacology. 2008b;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Spear L. Social Facilitation induced by pharmacological activation of mu opioid receptors: Impact of age, sex and stress. Poster presented at the annual meeting of the Society for Neuroscience; Washington, DC. 2008a, November. [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006a;48:146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006b;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008b;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25:377–85. [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol Behav. 1999;67:475–82. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake andsensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–32. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150:478–86. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–45. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Komori T, Matsumoto T, Zhang K, Miyahara S, Shizuya K, Okazaki Y. Effects of single and repeated prolonged stress on mu-opioid receptor mRNA expression in rat gross hypothalamic and midbrain homogenates. Brain Res. 2003;980:191–6. doi: 10.1016/s0006-8993(03)02969-x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]