Abstract
Background
Biofilm formation is an important component of vascular catheter infections caused by Candida albicans. Little is known about the interactions between human phagocytes and antifungal agents on Candida biofilms.
Materials and Methods
The interactions of C. albicans biofilms with human phagocytes alone and in combination with anidulafungin or voriconazole were investigated and compared with their corresponding planktonic counterparts using an in vitro biofilm model with clinical intravascular and green fluorescent protein (GFP) expressing strains. Phagocyte- and antifungal agent-mediated damages were determined by 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]2H-tetrazolium-5-carboxanilide assay and structural effects visualized by confocal microscopy. Oxidative burst was evaluated by flow cytometric measurement of dihydrorhodamine (DHR)-123 oxidation and cytokine release measured by EIA.
Results
Phagocytes alone or in combination with antifungal agents induced less damage against biofilms as compared to planktonic cells. However, additive effects occurred between phagocytes and anidulafungin against Candida biofilms. Confocal microscopy demonstrated absence of phagocytosis within biofilms, but marked destruction caused by anidulafungin and phagocytes. Anidulafungin but not voriconazole elicited a TNF-α release from phagocytes compared with untreated biofilms.
Conclusions
Candida albicans within biofilms are more resistant to phagocytic host defenses but are susceptible to additive effects between phagocytes and an echinocandin.
Keywords: Polymorphonuclear leukocyte, monocyte, Candida albicans, biofilm, voriconazole, anidulafungin, confocal laser scanning microscopy, cytokines, oxidative burst
Candida albicans is the most common cause of vascular catheter-related candidemia [1, 2]. Implanted medical devices, such as intravascular catheters are highly vulnerable to infection [3, 4]. Biofilms are a critical virulence determinant in such infections. Recently published guidelines by the Infectious Diseases Society of America recommend catheter removal in case of infection of central venous catheters with Candida [5]. However, this is not always effective or feasible [6].
Candida albicans biofilms constitute complex, three-dimensional ultrastructures, with distinctive developmental phases. Fully established Candida biofilms consist of a dense network of yeasts, hyphae and pseudohyphae embedded in a matrix of polysaccharides, proteins and other as yet undefined components [7, 8]. One of the principal characteristics of biofilms is their resistance to commonly used antifungal agents [9, 10]. By comparison to their planktonic (free-floating) counterparts, Candida biofilms are particularly resistant to azoles and amphotericin B but remain susceptible to the newly introduced echinocandins that target cell wall β-glucan biosynthesis [9, 11, 12].
While previous studies have examined the interactions between human phagocytes and planktonic Candida spp. [13], the corresponding phagocyte-biofilm interactions, are largely unknown. Chandra et al first addressed the role of host immune cells in the growing Candida biofilm [14]. However, it is unknown how Candida within the established biofilm responds to phagocytes. Similarly, it is unknown how antifungal agents interact with phagocytic cells against Candida biofilms. Whether the differential antifungal drug class activity could influence the host-cell interactions with biofilms is also unclear.
To address these questions, we investigated the interactions between C. albicans biofilms and polymorphonuclear leukocytes (PMNs) as well as monocytes (MNCs) alone and in combination with voriconazole (VRC) or anidulafungin (ANID).
MATERIALS AND METHODS
Organisms
The intravascular catheter isolate C. albicans-M61 and C. albicans-CAI4 (ura3::λimm434/ura3::λimm434) transformed with a green fluorescence (GFP)-producing plasmid were used [15]. The GFP-Candida system is based on the plasmid pACT1-GFP, which contains the codon-optimized yeast enhanced green fluorescent protein (yEGFP) cloned upstream of the C. albicans actin gene promoter on an integrating vector. Candida strains were maintained in 25% glycerol and 75% peptone solution at -35°C.
C. albicans-M61 and the GFP-tagged Candida were grown overnight in yeast-nitrogen-base (YNB) broth (Scharlau Chemie SA, Spain) supplemented with 50 mM glucose and in yeast-peptone-dextrose (Merck, Darmstadt, Germany) supplemented with 50 mg/L uridine, respectively, at 37°C. Before their use for biofilm formation, blastoconidia were suspended in 0.15 M phosphate-buffered saline (PBS; pH 7.2, Ca2+ and Mg2+ free; Biochrom KG, Germany), standardized to 106 or 107 blastoconidia/mL and used immediately [12, 16].
Biofilm formation
Biofilms were grown in vitro on the surface of disks placed in 96- or 12-well culture plates [12, 16]. For metabolic assays, the C. albicans-M61 was used. Specifically, disks (diameter, 6 mm) cut from silicone elastomer sheets (Bioplexus Corp., Saticoy, CA) were placed in 96-well flat-bottomed plates. Disks were pretreated with heat-inactivated fetal bovine serum (FBS; Gibco) at 37°C for 24 h on a rocker table. The FBS-coated disks were subsequently immersed in 300 μL of a standardized C. albicans suspension (1×106 blastoconidia/mL) in RPMI-1640. Blastoconidia were allowed to adhere and form biofilms at 37°C for 48 h in a humidified CO2 incubator under constant linear shaking for blood stream flow simulation. For microscopy, where the GFP-tagged C. albicans was used, biofilms were formed on the surface of disks (diameter, 12 mm) placed in 12-well plates, as previously described [14]. Planktonic conditions were grown identically but without silicone disks.
Resuspended biofilm cells, used in oxidative burst and metabolic assays, originated from biofilms. Specifically, following biofilm formation and subsequent washing, biofilms were removed from disc surfaces by scraping with a sterile scalpel. Resuspended biofilm elements were added to PBS, vortexed for 10 min to dissolve fungal aggregates, recounted and adjusted to concentration of 1×106/mL in RPMI-1640.
Preparation of human phagocytes
A) Human PMNs
PMNs were isolated from heparinized whole blood of healthy adult volunteers by dextran sedimentation and ficoll centrifugation, as described elsewhere [17]. The cells were resuspended in HBSS–, counted on a hemocytometer and their concentration was adjusted to 1×107 cells/mL.
B) Elutriated human MNCs
Peripheral blood MNCs were isolated from healthy donors by a two-step procedure consisting of automated leukapheresis and counterflow elutriation (model J-6 centrifuge; Beckman Instruments, Fullerton, CA) [18]. For MNC visualization in microscopy studies, MNCs were stained with MitoTracker Deep Red 633 dye (Invitrogen, Eugene, Oreg.), a mitochondria-selective dye that stains live cells red. Following MNC isolation, cells were gently resuspended in a HBSS– solution containing 1% heat inactivated FBS to a final concentration of 3×106 cells/mL, and were incubated with the MitoTracker probe (10 μL from a 100 nM stock) at 37°C for 1 h. After staining MNCs were washed twice and adjusted to a concentration of 3×106 cells/mL prior to use.
C) Monocytic cell line
The THP-1 monocytic cell line (ATCC TIB202; American Type Culture Collection, Manassas, VA) was grown in a humidified CO2 incubator at 37°C in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (FCM). The cells were adjusted to 1×106 cells/mL and added to 12-well culture plates. THP-1 cells were differentiated to a macrophage phenotype with 10 ng/mL phorbol myristate acetate (PMA) at 37°C for 6 h [19]. Cells were then washed once with HBSS– and incubated with FCM at 37°C for 22 h prior to incubation with C. albicans [20]. Phagocyte viability was ≥95% as determined by trypan blue staining.
Incubation of Candida with antifungal agents and/or phagocytes
VRC and ANID (both from Pfizer Inc., Groton, CT) were tested alone or in combination with human phagocytes (PMNs or MNCs) against C. albicans biofilms and planktonic cells. A stock solution of VRC (6,400 mg/L) and of ANID (1,600 mg/L) were prepared in sterile distilled water with 10% dimethyl sulfoxide and methanol, respectively, and stored at -35°C. Working solutions were prepared in RPMI-1640 buffered to a pH of 7.4 with 0.165 M MOPS. Biofilms were incubated with human phagocytes at effector cell-target (E:T) ratios 1:1, 5:1 or 10:1 in the presence or absence of a range of clinically relevant concentrations 0.5, 2 and 32 mg/L VRC or 0.12 and 0.5 mg/L ANID at 37°C in a humidified 5% CO2 incubator for 2 or 22 h. For short (2 h) incubation, the phagocytes were suspended in RPMI-1640 only; whereas, for long (22 h) incubation, the phagocytes were suspended in FCM. Planktonic cells were treated in the same way as biofilms.
XTT-metabolic assay
After incubation, phagocytes were lysed hypotonically and phagocyte- or antifungal drug-induced damage were assessed by modification of the XTT (2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]2H-tetrazolium-5-carboxanilide; 0.25 mg/mL) metabolic assay using coenzyme Q0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone; 40 μg/mL) as the final electron acceptor agent [21]. Antifungal activities were expressed as % damage and were calculated by the formula: 100 × (1-X/C), where X is the average optical density of treated biofilms or planktonic cells and C is the average optical density of control biofilms or planktonic cells. Optical density was measured by spectrophotometer (Anthos 2000, Austria) at 450 nm with reference wavelength at 690 nm.
Oxidative burst by Candida-stimulated PMNs
Induced production of H2O2 and H2O2-dependent intracellular intermediates (DIIs) by Candida-stimulated PMNs was evaluated by flow cytometric measurement of dihydrorhodamine (DHR)-123 oxidation as previously described with appropriate modifications [22]. This assay is based on the capacity of H2O2 and DII to induce oxidation of DHR-123 to rhodamine 1,2,3 (R-123) in the presence of a metal catalyst [22, 23]. Briefly, C. albicans-M61 blastoconidia at 106 cells/mL were grown in 2 mL YNB broth at 37°C with 5% CO2. After a 48-h growth period, the broth was centrifuged and the planktonic supernatant was recovered. A biofilm supernatant was generated by resuspended biofilm (see biofilm formation paragraph) by centrifugation. PMA was added as positive control (without fungal supernatant) to PMNs. PMNs were incubated with resuspended biofilm or planktonic supernatants for 2 h and 22 h. DHR-123 solution (20 μM) was added to samples 1 h prior to termination of the respective incubation period with the fungal supernatants to assess the oxidation of DHR-123 to R-123. The percentage of DHR-123 oxidation-positive cells was measured by flow cytometry (EPICS XL Flow Cytometer Coulter Beckman, Miami FL) using an argon laser emitting 15 mV at 488 nm.
Cytokine and chemokine release
After 22-h incubation of THP-1 with C. albicans-M61 biofilms or planktonic cells (E:T 5:1) in presence or absence of 0.5 mg/L ANID or 2 mg/L VRC at 37°C, culture supernatants were collected, centrifuged and stored at -35°C until testing for IL-6, IL-8 and TNF-α concentrations. Cytokine release was assessed using Quantikine ELISA (R&D Systems) according to manufacturer's instructions. Five independent experiments were performed. The minimum detectable limits for IL-6, IL-8 and TNF-α were 0.7, 3.5 and 0.106 pg/mL, respectively.
Confocal laser scanning microscopy (CLSM)
CLSM was used to visualize structural effects of VRC, ANID alone and in combination with phagocytes against Candida biofilm and planktonic cells. Biofilm and planktonic cells treated with VRC or ANID, as described above, were transferred to 12-well plates and incubated with 3 mL HBSS– containing the fluorescent stains FUN-1 (1 μL from 10 mM stock; Molecular Probes, Inc. Eugene, Oreg.) and concanavalin A-Alexa Fluor 488 conjugate (ConA; 15 μL from 5 mg/mL stock; Invitrogen, Eugene, Oreg.), for 45 min at 37°C. FUN-1 is converted to orange-red or yellow-orange fluorescent intravacuolar compounds by metabolically active cells [24]; whereas, the Alexa 488-conjugated ConA binds to α-mannopyranosyl and α-glucopyranosyl residues of cell wall polysaccharides, and emits green fluorescence. MitoTracker deep red stained MNCs were allowed to interact with GFP-tagged C. albicans biofilms and planktonic cells as described above. Stained biofilm and planktonic cells were finally transferred to 14-mm-diameter glass-bottom multiwell culture plates (MatTek Corp., Ashland, Mass) and were visualized by CLSM. Imaging was performed with a Zeiss LSM 510 META confocal microscope (Carl Zeiss Microimaging) equipped with 40x C-Apochromat (numerical aperture, 1.2) objective lens. Image z-stacks with 0.22-μm x-y pixel size, 1.0-μm z-axis step size, and 2.0-μm optical slice thickness were collected.
Statistical analysis
Comparisons between mean values of three or more groups were statistically evaluated by analysis of variance (ANOVA) followed by Dunnett post hoc analysis. The combined effect of phagocytes and antifungal agents was calculated as follows: the damage induced by the phagocytes alone and the antifungal drug alone was calculated and compared with the effect of treatment with the combination of phagocytes and drug. Synergism was defined as an antifungal effect (damage) caused by the combination that was significantly greater than the effect of phagocytes alone plus the effect of the drug alone. An additive effect was defined as an antifungal effect of the combination that was significantly greater than the effect produced by either phagocytes or drug alone but that did not reach synergism [25]. Differences between biofilm and planktonic conditions were analyzed by Student's t test. A two-sided P value of <.05 was considered statistically significant. All statistical analyses were performed using the SPSS (version 11.5; SPSS Inc, Chicago, IL) software.
RESULTS
Human phagocytes induce equal damage and oxidative burst in response to C. albicans resuspended biofilm and planktonic cells
The antifungal activity of phagocytes against resuspended biofilm and planktonic cells were initially studied using the XTT assay. PMN-induced damage followed a E:T ratio-dependent relationship observed in both resuspended biofilm and planktonic cells. Damage induced by PMNs did not significantly differ between resuspended biofilm and planktonic cells (figure 1A).
Figure 1.
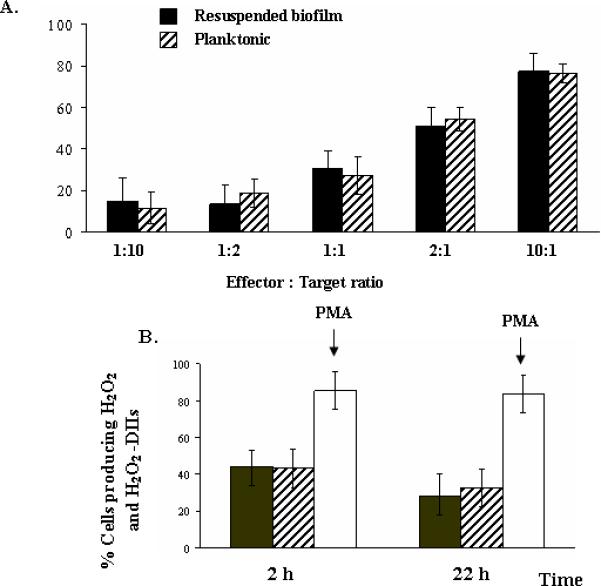
Percentage of damage induced by PMNs on C. albicans resuspended biofilms (dark bars) and planktonic cells (stripped bars). PMNs were added at effector cell: target ratios (E:T) ranging from 1:10 to 10:1 and were incubated with resuspended biofilms or planktonic cells for 2 h. Columns represent means ± SEs (error bars) of values derived from 5 experiments performed on different days. Differences in damage between resuspended biofilms and planktonic cells at each E:T ratio, as evaluated with Student t test, were not significant (panel A).
Oxidation of DHR-123 of PMNs in response to culture supernatants of C. albicans resuspended biofilms (dark bars) and planktonic cells (stripped bars) after incubation for 2 or 22 h (panel B). PMA stimulated phagocytes for >80% DHR-123 oxidation (open bars). Differences in oxidation between resuspended biofilms and planktonic cells after incubation for 2 or 22 h, as evaluated with Student t test, were not significant.
DHR-123 oxidation of PMNs was stimulated to the same extent by supernatants of resuspended biofilm and planktonic cells after incubation for 2 and 22 h (figure 1B).
Human phagocytes induce decreased damage against C. albicans biofilms as compared to planktonic cells
Phagocyte-induced damage, as studied by means of XTT assay, followed a dose-response relationship observed in both biofilm and planktonic cells and in all of the conditions studied (table 1). The damage induced by phagocytes was significantly lower in biofilms than in planktonic cells.
Table 1.
Comparative effects of human phagocytes (PMNs and MNCs) on damage of C. albicans biofilms and planktonic cells as determined by XTT assay.
| PMNs | MNCs | |||
|---|---|---|---|---|
| 2-h treatment | Biofilm | Planktonic | Biofilm | Planktonic |
| E:T ratio 1:1 | 20.9 ± 3.9 | 40.4 ± 8.8a | 22.8 ± 3 | 48.4 ± 22.9b |
| E:T ratio 5:1 | 40.3 ± 6.1 | 86.6 ± 1a | 27.6 ± 5.9 | 66.2 ± 7.6b |
| E:T ratio 10:1 | 50.2 ± 4.8 | 84.0 ± 1.6a | 43.9 ± 9.7 | 73.8 ± 1.3b |
| 22-h treatment | ||||
|---|---|---|---|---|
| E:T ratio 1:1 | 13.0 ± 3.7 | 30 ± 5.5a | 12.4 ± 3.1 | 26.5 ± 5.8a |
| E:T ratio 5:1 | 29.9 ± 5.4 | 52.7 ± 3.6a | 28.2 ± 4.9 | 47.3 ± 3.9a |
Results are expressed as percentages of damage of C. albicans biofilms and planktonic cells induced by human phagocytes (PMNs and MNCs) as compared with untreated controls (damage considered to be 0%). Data are presented as means of 7 experiments performed on different days.
P <.001
P <.01 indicate significant differences among biofilm and planktonic cells.
Human phagocytes in combination with antifungal agents induce differential damage against C. albicans biofilms and planktonic cells
ANID alone, at both concentrations tested, significantly inhibited growth of biofilm and planktonic cells when compared to drug-free controls. The ANID-induced damage was also significantly lower for biofilms than for planktonic cells (figure 2, panels A and B). While VRC alone, at all concentrations tested, significantly inhibited growth of planktonic cells (figure 2, panels C and D), the inhibition was not statistically significant for biofilms when compared to drug-free controls. Taken together, ANID but not VRC showed substantial potency against biofilms. ANID (at 0.12 mg/L) and PMN (at 1:1 and 5:1 ratios) combinations exhibited an additive effect against biofilms.
Figure 2.

Damage induced by human PMNs and/or ANID (panel A), MNCs and/or ANID (panel B), PMNs and/or VRC (panel C) or MNCs and/or VRC (panel D) after incubation at 37°C for 22 h against C. albicans biofilms (open bars) or planktonic cells (stripped bars) at different E:T ratios. The values are means ± standard error (SE) of 6-8 experiments. Each experiment with PMNs was conducted with PMNs of one donor and by use of triplicate or quadruplicate wells for each condition. The mean value of the replicate wells was considered as the value of that particular donor and experiment. The means of the replicate wells of each experiment were then used in the data analysis to calculate the mean ± SE for all the experiments conducted under the same conditions.
The result of the damage induced by the combination of human phagocytes (PMNs or MNCs) and ANID was compared to the result of human phagocytes (PMNs or MNCs) or ANID alone by analysis of variance with Dunnett test.
† denotes that the combined effect of PMNs (at 1:1 and 5:1 ratio) with ANID (at 0.12 mg/L) on biofilms was additive on biofilms (P < .001 vs the two components) (panel A).
* Differences between biofilm and planktonic conditions (P <.05).
Differential cytokine and chemokine release from human phagocytes exposed to antifungal agents and C. albicans biofilms or planktonic cells
As shown in figure 3, significantly lower levels of TNF-α were released by monocytes stimulated by biofilms than by planktonic cells while treatment with ANID caused elicitation of comparable amounts of TNF-α. Comparable amounts of IL-8 were released when monocytes were stimulated by both biofilm and planktonic cells. Of note, IL-8 release in response to ANID-treated biofilms was significantly lower than that in response to ANID-treated planktonic cells. The IL-6 levels were undetectable in all of the conditions tested.
Figure 3.
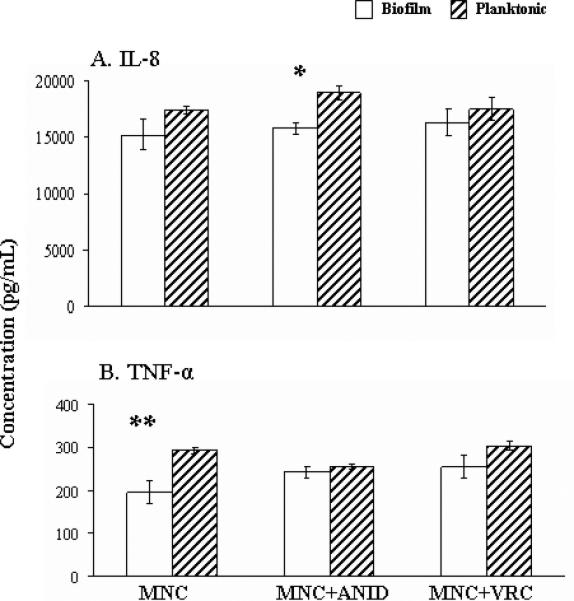
Profiles of IL-8 (panel A) and TNF-α (panel Β) release after incubation of THP-1 cells (MNCs) alone or in combination with ANID (0.5 mg/L) or VRC (2.0 mg/L) and C. albicans biofilms (open columns) or planktonic cells (stripped columns) at 37°C for 22 h. Data are presented as means ± standard errors of the means derived from five experiments. Comparisons between biofilm and planktonic conditions were performed with Student's t test. * P = .007, ** P = .001.
Structural features of C. albicans biofilms and planktonic cells treated with human phagocytes alone and in combination with antifungal agents
Figure 4 (panels A, B and C) shows the interactions of planktonic GFP-tagged cells with MNCs over time, where phagocytosis is clearly evident. Panels D and E show three-dimensional images of biofilms interacting with MNCs from 2 up to 22 h. From these serially taken images, it appears that MNCs progressively penetrated biofilm until the middle layers without being able to exhibit any phagocytic function.
Figure 4.
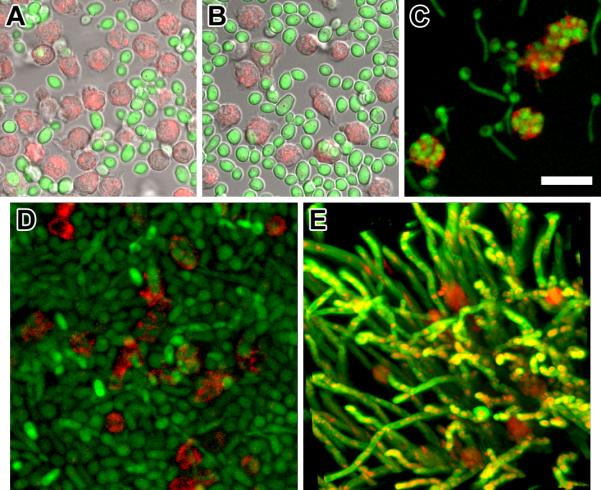
Confocal laser scanning microscopy (CLSM) of human phagocytes interacting with a green fluorescent protein (GFP) tagged C. albicans planktonic or biofilm cells. The top row of images are single confocal optical slices of A, the initiation of the interaction between planktonic C. albicans cells (green conidia) and MNCs (red cells), overlaid on brightfield DIC image (grey). B, 30 min following MNC addition to planktonic culture (green) where MNCs (red) exhibit a polarization or irregular morphology characteristic of stimulation, overlaid on bright field DIC image (grey). C, 2 h following MNC addition to planktonic culture the majority of the free-floating pseudohyphae (green) are either surrounded or engulfed (fully or partly) by MNCs (red) Scale bar, 20-μm. The second row of images is three-dimensional maximum intensity projections representative of 2 up to 22 h following MNC addition to 48 h biofilms. D, accumulation of MNCs primarily in the middle layers (2 h). E, MNCs appear trapped within the dense network of the biofilm three-dimensional structure without being able to move or change shape. Scale bar, 20-μm. All experiments were performed three times on different days.
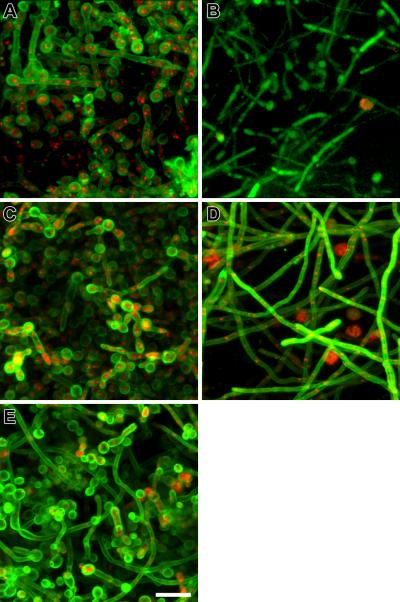
Figure 5 depicts biofilms exposed to ANID (at 0.5 mg/L, a concentration that equals the previously determined biofilm MIC [26]) alone or combined with MNCs (panels A and B) and to VRC (at 2 mg/L, a clinically relevant concentration, although exceedingly lower than biofilm MIC of >256 mg/L [26]) alone or combined with MNCs (panels C and D). In all the conditions tested, phagocytes exhibited a distinct non-reactive morphology being entangled within the biofilm while the morphology of the biofilm fungal elements varied according to the antifungal agent used. Videos, where these morphological changes are impressively shown, are available online as supplemetal files.
Figure 5.
CLSM images of C. albicans biofilms after interacting for 22 h with ANID (0.5 mg/L) (A), ANID (0.5 mg/L) in combination with MNCs (B), VRC (2 mg/L) (C) and VRC (2 mg/L) in combination with MNCs (D). E, Untreated control biofilm. Images utilize ConA (green stain highlighting blastoconidial cell walls) and FUN-1 (yellow stain highlighting nonviable cells) to directly visualize the effects of antifungal agents on biofilms (panels A, C and E). Images utilize green fluorescent protein (GFP)-tagged C. albicans and MNCs stained with MitoTracker red dye to visualize the fungal-host defense interactions (panels B and D). Note the substantial ultrastructural disruption of the MNC- plus ANID-treated biofilms (panel B, cell deformation or clumping, looser biofilm network, shorter hyphae and vacuoles on the cell wall) as compared with the ANID-alone treated biofilms or untreated control biofilm (panel A and E, respectively). The diminished GFP signal reflects damage by ANID plus MNCs. Minor abnormalities, like shorter hyphae, are noted in VRC-alone treated biofilm (panel C) and MNC- plus VRC-treated biofilm (panel D) appears looser and hyphal elements have an orthogonal arrangement. Images are single optical sections. Scale bar, 20-μm. All experiments were performed three times on different days.
DISCUSSION
The data presented here offer new insights into human phagocyte interactions with, medically relevant Candida biofilms under conditions that mimic catheter-related infections. Most available studies on the interactions between leukocytes and microorganisms have been performed in suspension (planktonic conditions); whereas, in biological environments these interactions occur on surfaces coated by biofilms [27]. We found that incubation of human phagocytes, for a relatively short or long duration, with C. albicans biofilms resulted in a decreased damage as compared with their planktonic counterparts. These data indicate that C. albicans biofilms display reduced susceptibility not only to certain antifungal agents, as has been previously shown, but also to immune cells [12, 26]. Using CLSM, it was depicted that human phagocytes retained their rounded morphology, characteristic of unstimulated cells, and were unable to internalize biofilm elements; whereas, they appeared entrapped within biofilm ultrastructure. These findings were in distinct contrast with the corresponding planktonic conditions where phagocytosis was clearly evident.
Chandra et al, using a similar in vitro biofilm model, evaluated the ability of C. albicans to form biofilms in the presence of host immune cells [14]. They demonstrated that co-culture of C. albicans with mononuclear cells enhanced the fungus ability to form biofilms. They also observed lack of monocyte-mediated phagocytosis of biofilm fungal elements underlying the immunosuppressive effect of biofilms. In aggregate, the findings of our study and that of Chandra et al are complementary, in that, together, they show the effects of human phagocytes on different stages of C. albicans biofilm development. These findings are of clinical relevance since they may explain the chronicity and recalcitrant nature of catheter-related infections. Additionally, we have not observed analogous phenomenon between resuspended biofilm and planktonic cells. Resuspended biofilm cells lacked the overall structure of biofilms consisting of a dense network of hyphae and blastoconidia entangled in matrix material and have, presumably, lost most of their matrix [28].
To further enhance our understanding behind biofilm resistance we also evaluated the cytokine response of phagocytes interacting with C. albicans biofilms compared with planktonic cells. Our study shows that biofilms induced the release of TNF-α from monocytes at levels that were significantly diminished compared with planktonic cells. Accordingly, the production of IL-8 appeared to follow the same trend. Like TNF-α, IL-6 is an important activator of phagocytes and is highly expressed by monocytes infected with Candida. However, IL-6 was not detected in our experiments. This is consistent with previous data showing that the expression of genes encoding IL-6 peaks within the first 6 h of exposure to C. albicans [29].
The levels of different cytokines in supernatants from a biofilm-phagocyte co-culture were measured by Chandra et al. They demonstrated that biofilms induced decreased levels of TNF-α and IL-6 while IL-8 appeared unchanged [14]. These findings are consistent with ours suggesting that biofilms and phagocytes undergo multiple interactions mediated by different cytokines [14]
Echinocandins and triazoles are among the newest available antifungal agents introduced into clinical practice. Anidulafungin, like all echinocandins, acts by inhibiting biosynthesis of β-1, 3-glucan of the fungal cell wall leading to lower amounts of β-glucan [26, 30, 31]. However, the inhibition of β-glucan synthesis upsets the necessary equilibrium of fungal cell wall activating compensatory mechanisms, like upregulation of chitin synthesis [32, 33]. This cell wall remodeling can cause more exposure of β-glucan even in the presence of lower bulk levels of β-glucan. C. albicans has high levels of the structural molecule β-glucan in its cell wall, but the majority of its β-glucan is masked by a mannoprotein layer precluding recognition by immune system [34, 35]. Recent reports have demonstrated that exposure of β-glucan by drug treatment alters the way the fungi are recognized by immune cells. Particularly, it has been shown that unmasking the underlying β-glucan in the cell wall of C. albicans by subinhibitory concentrations of caspofungin, induced the exposed fungi to elicit a stronger immune response [35]. This ‘unmasking effect’ of caspofungin is class specific, since anidulafungin and micafungin also enhance phagocyte-mediated damage [36]. The studies to date have demonstrated that recognition of C. albicans β-glucan is mediated by mammalian innate immune receptors, such as dectin-1 [37]. Dectin-1 is expressed widely on phagocytes and contributes to the immunological response to β-glucans [38, 39].
To our knowledge, this is the first study to examine the immunopharmacological effects of anidulafungin and voriconazole against C. albicans biofilms. In our study, exposure of C. albicans biofilms to subinhibitory concentrations of anidulafungin (0.12 mg/L) was associated with a significant increase in phagocyte-mediated damage. These patches, like buds or scars, would be sufficient to activate dectin-1 and trigger potent antifungal inflammatory responses to macrophages [34]. Consequently, it is conceivable, that anidulafungin-treated biofilms have a heightened β-glucan exposure eliciting a larger proinflamatory response from phagocytes [35]. Echinocandins appear to exert their immunomodulating properties in the presence of damaged hyphal lesions unlike other antifungal agents that directly stimulate immune cells [20, 22, 40, 41].
We also have found that exposure of C. albicans biofilms to antifungal agents elicits a modified proinflammatory response from phagocytes compared with untreated biofilms. Specifically, while elicitation of the proinflammatory cytokine TNF-α from phagocytes was significantly lower in untreated biofilms as compared with untreated planktonic cells, treatment with anidulafungin caused elicitation of comparable amounts of TNF-α between the two Candida phenotypes. The elicitation of IL-8 appeared to follow another inflammatory response pattern. Untreated and voriconazole-treated biofilms and planktonic cells elicited comparable amounts of IL-8. However, the expression of IL-8 was down-regulated in anidulafungin-treated biofilms as compared with planktonic cells. Previous studies, carried out in planktonic conditions, have shown that subinhibitory doses of caspofungin caused high levels of TNF-α elicitation and this was due to caspofungin β-glucan unmasking effect [35].
In conclusion, our findings demonstrate significantly reduced susceptibility of C. albicans biofilms to host immune cells as compared with their planktonic counterparts. Together our observations suggest that a major contributor for this behavior could be the biofilm ultrasturcture per se. This is the first report that has investigated the immunopharmacological effects of antifungal agents against biofilms. We have shown that anidulafungin, unlike voriconazole, has an additive effect with immune cells against Candida biofilms. Further, this additive interaction results in a differential release of the proinflammatory cytokine TNF-α and the chemokine IL-8. The beneficial Th1 response observed after treatment of biofilms with anidulafungin could open up new therapeutic options, involving the inhibition of cytokines with deleterious effects and the induction of others with favorable effects.
ACKNOWLEDGMENTS
We thank Elpiniki Georgiadou for technical assistance and M. Ghannoum as well as A. Brown for kindly providing the isolates used in this study.
Sources of financial support
This study was partially supported by the Aristotle University, Thessaloniki, Greece and partially by the intramural research program of the National Cancer Institute, Bethesda, MD. A.K. was a recipient of a grant from the European Society of Pediatric Infectious Diseases (2006-2007 Small Grant Awards) and a grant from Pfizer (GA900095).
Footnotes
Potential conflicts of interest. E.R. has received research funding from Pfizer, Gilead, Enzon, Schering, has been a consultant for Schering, Gilead, Astellas, Pfizer, and has been on the speakers’ bureau for Gilead, Cephalon, Pfizer, Schering and Merck. T.J.W. has a co-operative research and development agreement with Astellas. A.K. has received research funding from Pfizer. The other authors report no conflict of interest.
Presented in part: 47th Annual Meeting of Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, IL, 17-20 September, 2007; 3rd Trends in Medical Mycology (TIMM), Torino, Italy, 28-31 October, 2007; 26th Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID), Graz, Austria, 13-16 May, 2008.
1REFERENCES
- 1.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000;19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- 4.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342–7. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin Infect Dis. 2002;34:591–9. doi: 10.1086/338714. [DOI] [PubMed] [Google Scholar]
- 7.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006;9:340–5. doi: 10.1016/j.mib.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 10.Nett J, Lincoln L, Marchillo K, et al. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–20. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:1127–32. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roilides E, Walsh T. Recombinant cytokines in augmentation and immunomodulation of host defenses against Candida spp. Med Mycol. 2004;42:1–13. doi: 10.1080/13693780310001631341. [DOI] [PubMed] [Google Scholar]
- 14.Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun. 2007;75:2612–20. doi: 10.1128/IAI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barelle CJ, Manson CL, MacCallum DM, Odds FC, Gow NA, Brown AJ. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast. 2004;21:333–40. doi: 10.1002/yea.1099. [DOI] [PubMed] [Google Scholar]
- 16.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–9. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185–93. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roilides E, Dimitriadou A, Kadiltsoglou I, et al. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–9. [PubMed] [Google Scholar]
- 19.Lagoumintzis G, Christofidou M, Dimitracopoulos G, Paliogianni F. Pseudomonas aeruginosa slime glycolipoprotein is a potent stimulant of tumor necrosis factor alpha gene expression and activation of transcription activators nuclear factor kappa B and activator protein 1 in human monocytes. Infect Immun. 2003;71:4614–22. doi: 10.1128/IAI.71.8.4614-4622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simitsopoulou M, Roilides E, Likartsis C, et al. Expression of immunomodulatory genes in human monocytes induced by voriconazole in the presence of Aspergillus fumigatus. Antimicrob Agents Chemother. 2007;51:1048–54. doi: 10.1128/AAC.01095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT). J Infect Dis. 1995;172:1153–6. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 22.Dotis J, Simitsopoulou M, Dalakiouridou M, et al. Effects of lipid formulations of amphotericin B on activity of human monocytes against Aspergillus fumigatus. Antimicrob Agents Chemother. 2006;50:868–73. doi: 10.1128/AAC.50.3.868-873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–55. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 24.Millard PJ, Roth BL, Thi HP, Yue ST, Haugland RP. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol. 1997;63:2897–905. doi: 10.1128/aem.63.7.2897-2905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gil-Lamaignere C, Roilides E, Mosquera J, Maloukou A, Walsh TJ. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob Agents Chemother. 2002;46:2234–7. doi: 10.1128/AAC.46.7.2234-2237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katragkou A, Chatzimoschou A, Simitsopoulou M, et al. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob Agents Chemother. 2008;52:357–60. doi: 10.1128/AAC.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–5. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Choi EH, Khan J, et al. Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect Immun. 2005;73:3714–24. doi: 10.1128/IAI.73.6.3714-3724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson MJ, Piper KE, Nguyen G, Steckelberg JM, Patel R. In vitro activity of anidulafungin against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:2242–3. doi: 10.1128/AAC.00211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–51. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 32.Angiolella L, Maras B, Stringaro AR, et al. Glucan-associated protein modulations and ultrastructural changes of the cell wall in Candida albicans treated with micafungin, a water-soluble, lipopeptide antimycotic. J Chemother. 2005;17:409–16. doi: 10.1179/joc.2005.17.4.409. [DOI] [PubMed] [Google Scholar]
- 33.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50:3160–1. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–86. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2:e35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis. 2008;47:226–35. doi: 10.1086/589290. [DOI] [PubMed] [Google Scholar]
- 37.Gow NA, Netea MG, Munro CA, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007;196:1565–71. doi: 10.1086/523110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 39.Lamaris GA, Lewis RE, Chamilos G, et al. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis. 2008;198:186–92. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem. 2003;278:37561–8. doi: 10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- 41.Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother. 2007;51:1078–81. doi: 10.1128/AAC.01268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]