Abstract
The Organic Solute Transporter (OST)(alpha)-OST(beta) is an unusual heteromeric carrier expressed in a variety of tissues including the small intestine, colon, liver, biliary tract, kidney, and adrenal gland. In polarized epithelial cells, OSTα-OSTβ protein is localized on the basolateral membrane and functions in the export or uptake of bile acids and steroids. This article reviews recent results including studies of knockout mouse models that provide new insights to the role of OSTα-OSTβ in the compartmentalization and metabolism of these important lipids.
Keywords: Bile acids, Transporter, Enterocyte, Hepatocyte, Enterohepatic circulation, Malabsorption, Cholestasis, Adrenal, Steroid Hormones
1. Introduction
Many of the transporters important for maintenance of the enterohepatic circulation of bile acid have been identified over the past 2 decades. Notably absent from that list was the major transporter responsible for export of bile acids across the basolateral membrane of the enterocyte, cholangiocyte, and renal proximal tubule cell. Despite numerous attempts over the past 3 decades using protein purification [1], photoaffinity labeling [2], or candidate gene approaches [3, 4], the identity of the basolateral membrane bile acid transporter remained an important missing link in our understanding of the enterohepatic circulation of bile acids. This mystery was recently solved with the identification and characterization of a novel Organic Solute Transporter (OST), OSTα-OSTβ [5].
The previously identified Solute Carrier (SLC) and ATP-binding cassette (ABC) transporter family members important for maintaining the enterohepatic circulation of bile acids are thought to function as monomers or homo-multimers. In contrast, OST activity requires coexpression of multiple subunits. OST consists of a larger polytopic membrane protein (OSTα) and a smaller type 1 single-pass membrane protein (OSTβ), a paradigm more similar to the heteromeric amino acid transporters [6–8]. Since OSTα-OSTβ was first identified and cloned from the little skate in 2001 [5], much has been learned about the properties, regulation, and function of this novel transporter [9, 10]. This review highlights our current understanding of the physiological roles of OSTα-OSTβ in bile acid and steroid transport and also identifies important questions that remain to be answered.
2. Introduction to the enterohepatic synthesis of bile acids and regulation of hepatic bile acid synthesis
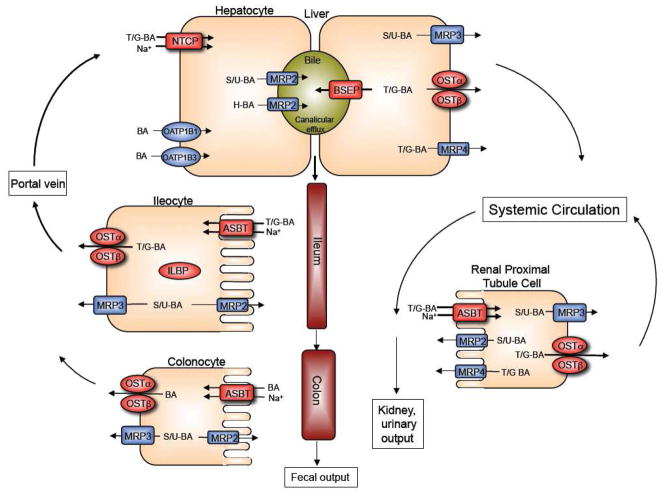
This section briefly highlights the major transporters and mechanisms involved in the enterohepatic circulation of bile acids and regulation of their hepatic synthesis, subjects that have recently been reviewed in detail [11–15]. The tissue expression and function of OSTα-OSTβ and other major transporters involved in the metabolism and enterohepatic circulation of bile acids are summarized in Figure 1. Bile acids are synthesized from cholesterol in the liver, conjugated (N-acyl amidated) to taurine or glycine, secreted into bile, and stored in the gallbladder. After entering the small intestine, bile acids facilitate absorption of fat-soluble vitamins and cholesterol [16]. Most of the bile acids (> 90%) are reabsorbed from the intestine and returned to the liver via the portal venous circulation. They are then taken up by the hepatocyte and resecreted across the canalicular membrane into bile [17]. Since these processes, i.e. intestinal absorption, return to the liver in the portal circulation, and hepatic extraction of bile acids, are so efficient, the majority of the bile acids secreted by the hepatocyte are derived from the recirculating bile acid pool with less than 10% from new de novo hepatic synthesis.
Figure 1.
Enterohepatic circulation of bile acids showing the individual transport proteins in hepatocytes, ileocytes (ileal enterocytes), and renal proximal tubule cells. Overall, this integrated transport system minimizes fecal and urinary bile acid loss and functions to largely restrict these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments. (BA, bile acids; T/G; taurine or glycine-conjugated bile acids; sulfate or glucuronide (S/U)-conjugated bile acids; H, tetrahydroxylated bile acids).
After their synthesis or reconjugation in the hepatocyte, taurine and glycine conjugated bile acids are secreted into bile by the canalicular membrane bile salt export pump (BSEP; gene symbol ABCB11). The small amount of bile acids that have been modified by the addition of sulfate or glucuronide are secreted into bile by the multidrug resistance-associated protein-2 (MRP2; gene symbol ABCC2) and possibly the breast cancer related protein (BCRP; gene symbol ABCG2). Bile acids can also be modified by additional hydroxylation and these species are secreted into bile by MRP2 and possibly P-glycoprotein (MDR1; gene symbol ABCB1A). The divalent or tetrahydroxylated bile acids are present in very small quantities under normal physiological conditions, but may accumulate in disease states such as cholestasis. After their secretion, bile acids are stored in the gallbladder and empty into the intestinal lumen in response to a meal. Bile acids are poorly absorbed in the proximal small intestine, but efficiently taken up by the apical sodium-dependent bile acid transporter (ASBT; gene symbol SLC10A2) in the ileum. After entering the ileal enterocyte, bile acids bind to the cytosolic ileal lipid binding protein (ILBP; gene symbol FABP6) and are efficiently exported across the basolateral membrane into the portal circulation by the more recently discovered heteromeric transporter OSTα-OSTβ. The multidrug resistance-associated protein-3 (MRP3; gene symbol ABCC3) is a minor contributor to basolateral membrane export of native bile acids from the enterocyte, but may have a more significant role in export of any modified (glucuronidated or sulfated) bile acids that may be formed. MRP2 may also serve to export modified bile acids, across the apical brush border membrane back into the intestinal lumen. The small fraction of bile acids that escape absorption in the small intestine spill into the colon, where they are extensively deconjugated and dehydroylated by the endogenous bacterial flora. The unconjugated bile acids can be absorbed passively or actively and returned to the liver, where they are efficiently reconjugated and mix with newly synthesized bile acids to be resecreted into bile. This process of intestinal deconjugation and hepatic reconjugation is a normal part of bile acid metabolism. Colonocytes express very low levels of ASBT, but appreciable levels of MRP3 and OSTα-OSTβ. These carriers may be contribute to the absorption of unconjugated bile acids from the lumen of the colon. After their absorption from the intestine, bile acids travel in the portal circulation back to the liver where that are cleared by the Na+-taurocholate cotransporting polypeptide (NTCP; gene symbol SLC10A1). Members of the Organic Anion Transport Protein family, OATP1B1 (gene symbol SLCO1B1) and OATP1B3 (gene symbol SLCO1B3) also participate in sinusoidal membrane bile acid uptake, and are particularly important for unconjugated bile acids. Under cholestatic conditions, unconjugated, conjugated, or modified (divalent or tetrahydroxylated) bile acids can be effluxed across the basolateral (sinusoidal) membrane of the hepatocyte by OSTα-OSTβ, MRP3, or multidrug resistance-associated protein-4 (MRP4; gene symbol ABBC4) into the systemic circulation. Under normal physiological conditions, a fraction of the bile acid escapes first pass hepatic clearance enters the systemic circulation. The free bile acids are filtered by the renal glomerulus, efficiently reclaimed by the ASBT in the proximal tubules, and exported back into the systemic circulation, thereby minimizing their excretion in the urine. This efficient renal reabsorption occurs even under cholestatic conditions for unconjugated and conjugated bile acids, when serum bile acid concentrations are dramatically elevated. Overall, this integrated transport system minimizes fecal and urinary bile acid loss and functions to largely restrict these potentially cytotoxic detergents to the intestinal and hepatobiliary compartments.
Bile acids are synthesized from cholesterol via 2 major pathways, the “classical” neutral pathway (Cholesterol 7α-hydroxylase, CYP7A1, pathway) that favors cholic acid biosynthesis, and an “alternative” acidic pathway (Sterol 27-hydroxylase pathway) that favors chenodeoxycholic acid (in humans) or muricholic acid (in mice) [18]. Of the two major biosynthetic pathways, the neutral pathway is quantitatively more significant in the adult [19, 20], and CYP7A1 is the rate-limiting enzyme for this pathway [18]. The control of CYP7a1 expression is complex [21, 22], reflecting the need to carefully regulate the body’s bile acid load. Under physiological conditions, CYP7a1 transcription is under negative feedback regulation by bile acids [23]. While a role for the farnesoid X receptor (FXR) in the negative feedback regulation of CYP7A1 has been recognized for almost a decade [24, 25], a critical role for gut-liver signaling via Fibroblast Growth Factor (FGF) 15 (human ortholog: FGF19) has only recently gained appreciation [26–28]. In that pathway, bile acids act as ligands for FXR in ileal enterocytes to induce synthesis of the endocrine hormone FGF15/19. After its release by the enterocyte, FGF15/19 acts on hepatocytes through its cell surface receptor, a complex of the βKlotho protein and fibroblast growth factor receptor-4 (FGFR4), to repress CYP7A1 expression and bile acid synthesis [26, 28, 29]. These complex molecular titrations link bile acid synthesis to changes in intestinal as well as hepatic bile acid levels.
3. Identification of OSTα-OSTβ – a historical overview
Elasmobranchs (sharks, rays, and skates) are thought to have evolved almost 400 million years ago, however despite their evolutionary distance from humans, these lower vertebrates retain many physiologic features of modern mammals including a central hepatic role in the clearance and metabolism of bile acids, steroids, and other endobiotics or xenobiotics. These physiological properties have made the little skate, Leucoraja erinacea, a useful biomedical model [30]. In 2001, Dr. Ned Ballatori and coworkers set out to identify novel hepatic steroid and organic anion transporters from the little skate using an expression cloning strategy and the Xenopus laevis oocyte system. In a heroic effort that involved repeatedly subfractionating and complementing skate liver cDNA library pools that were positive for [3H]taurocholate uptake activity, this group simultaneously identified both subunits of Ostα-Ostβ [5]. In 2003, Ballatori subsequently cloned and expressed the human and mouse orthologues of the skate Ostα and Ostβ proteins [31]. When expressed in Xenopus laevis oocytes, the skate and mammalian Ostα-Ostβ proteins complemented one another and transported taurocholate as well as a variety of steroids. The physiologic function was unclear at this point, although based on the substrate specificity OSTα-OSTβ was thought to function as a transporter for steroids and eicosanoids to regulate the entry and/or exit of these compounds [31].
4. Sequence analysis and phylogeny
The human and mouse OST orthologues are conserved and share approximately 89% and 63% amino acid identity for OSTα and OSTβ, respectively [31]. The human/mouse OSTα/Ostα genes encode a 340 amino acid protein with a predicted extracellular amino-terminus, seven potential transmembrane domains, and a cytosolic carboxyl-terminus; the human/mouse OSTβ/Ostβ genes encodes a 128 amino acid Type 1 membrane protein with a predicted extracellular amino terminus, a single-pass transmembrane domain, and a cytosolic carboxyl-terminus. Several lines of evidence support the predicted membrane topologies for OSTα and OSTβ. First, the pattern of glycosidase-sensitivity for mouse Ostα protein from ileal tissue or transfected HEK 293 or MDCK cells suggested that the predicted N-linked glycosylation site at the amino terminus is utilized [32]. Second, experiments using epitope tagged proteins, as well as bimolecular complementation, demonstrated that the carboxyl-terminal regions of Ostα and Ostβ lie on the cytosolic side of the membrane [33].
The OSTα and OSTβ genes are encoded on different chromosomes, positions 3q29/16B3 and 15q22/9C for human/mouse OSTα/Ostα and OSTβ/Ostβ, respectively. In contrast with many other transporters that reside within large gene families, no paralogues have been identified in the human or mouse genomes for OSTα or OSTβ. However a search of the Emsembl database, (http://uswest.ensembl.org/Homo_sapiens/Gene/Compara_Ortholog?g=ENSG00000163959), reveals that at least 50 putative OSTα orthologues have been identified, including genes from lower vertebrates (species; Ensembl indentifier) such as zebrafish (Danio rerio; ENSDARG00000045306), frog (Xenopus topicalis; ENSTGUG00000009499), and anole lizard (Anolis carolinensis; ENSACAG00000006992). A detailed phylogenetic tree for OSTα can be found at: (http://uswest.ensembl.org/Homo_sapiens/Gene/Compara_Tree?g=ENSG00000163959). It is important to note that the list of putative OSTα orthologues includes genes from invertebrates such as the sea squirt (Ciona intestinalis; ENSCING00000009741), fruit fly (Drosophila melanogaster; FBgn0036834), and roundworm (Caenorhabditis elegans; C18A3.4). Since bile acids (salts) have not been detected in invertebrate animals [34], these results suggest that the original function of OSTα was to transport non-bile acid (salt) substrates such as steroid hormones or eicosanoids. At least 30 putative OSTβ orthologs have also been identified, but the list does not include sequences from lower vertebrates at this time. A detailed phylogenetic tree for OSTβ can be found at: (http://uswest.ensembl.org/Homo_sapiens/Gene/Compara_Tree?g=ENSG00000186198). It is possible that an OSTβ orthologue is present in the genomes of these invertebrates, but was not readily evident due to OSTβ’s small gene/protein size and relatively weak sequence conservation. For example, although the human and skate OSTα orthologues share 41% amino acid identity, their corresponding OSTβ orthologues shares only 25% identity. Alternatively, the invertebrate OSTα orthologue may not require a partner protein or may utilize a different partner protein, and OSTβ evolved more recently as a cofactor for OSTα in vertebrate species.
5. Tissue expression and membrane localization of OSTα-OSTβ
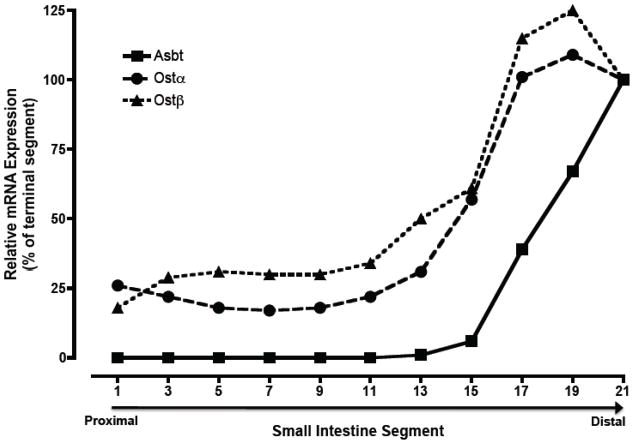
The tissue distribution of OSTα-OSTβ mRNA has been examined in a variety of species including skate [5], mouse [32, 35–37], rat [35], and human [31, 35, 38]. In humans, OSTα and OSTβ mRNA expression generally parallel one another with highest levels in small intestine, liver, kidney, and testis [31, 35]. Lower levels of OSTα and OSTβ mRNA expression are also detected by real time PCR in colon, adrenal gland, ovary, with lowest levels in heart, lung, brain, pituitary gland and prostate. In the mouse, Ostα and Ostβ mRNA expression is highest in distal small intestine followed by kidney, cecum, colon, and adrenal; Ostα and Ostβ mRNA expression was negligible in brain, heart, lung, muscle, skin, ovary, and testes [32, 37]. The gradient of Ostα and Ostβ expression along the cephalocaudal axis of the small intestine in mouse [32] or rat [35] is similar to that of the Apical sodium-dependent bile acid transporter (Asbt; Slc10a2) with highest levels in terminal ileum. But unlike the Asbt, Ostα-Ostβ is also expressed at appreciable levels in proximal small intestine. Figure 2 shows the small intestinal gradient of mRNA expression for Ostα, Ostβ, and Asbt in the nonhuman primate (African Green monkey). Similar to the rodent, Ostα and Ostβ mRNA are expressed at lower levels in duodenum and jejunum, and highest in the distal 20% of small intestine. Although directly comparable data are not available for humans, Balesaria et al. [39] reported that OSTα and OSTβ mRNA was detectable in human duodenum in addition to ileum.
Fig 2.
Expression of Ostα and Ostβ mRNA along the cephalocaudal axis of the small intestine from African Green monkey. Small intestine from an adult male African Green monkey was divided into 21 equal segments of ~10 cm in length and used for RNA isolation. Real-time PCR was used to determine the expression of Asbt, Ostα, and Ostβ mRNA expression. The threshold values (CT) are the means of triplicate determinations, and expression was normalized for mRNA expression of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase. The normalized threshold values are plotted as a percent of the terminal intestinal segment (terminal ileum). The CT values determine in terminal ileum were 19.84, 22.81, and 22.90 for the Asbt, Ostα, and Ostβ, respectively.
Despite being encoded on two different chromosomes, the expression of OSTα/Ostα and OSTβ/Ostβ mRNA appears to be coordinately regulated in the intestine under basal conditions. Studies of mouse small intestine, cecum, and colon, and human ileum showed that the mRNA expression of OSTα/Ostα is closely correlated with that of OSTβ/Ostβ, with r values of 0.93 (n = 30) and 0.76 (n = 53) in mouse [40] and human [41], respectively. The protein expression for OSTα/Ostα and OSTβ/Ostβ has also been measured in a limited number of studies and in most cases the protein levels correlated with mRNA expression. For example, in human ileal biopsy samples, a strong positive correlation was observed between the mRNA expression and protein levels for OSTα (r = 0.77) and OSTβ (r = 0.89) [41], suggesting that transcription is a major determinant of OSTα-OSTβ protein levels. Note that this coordination between the expression of OSTα/Ostα and OSTβ/Ostβ mRNA has not been a universal finding, particularly in the liver under pathophysiological conditions where the expression of OSTβ is dramatically induced relative to OSTα. The potential significance of these findings is unclear and is discussed further in the section on concluding remarks.
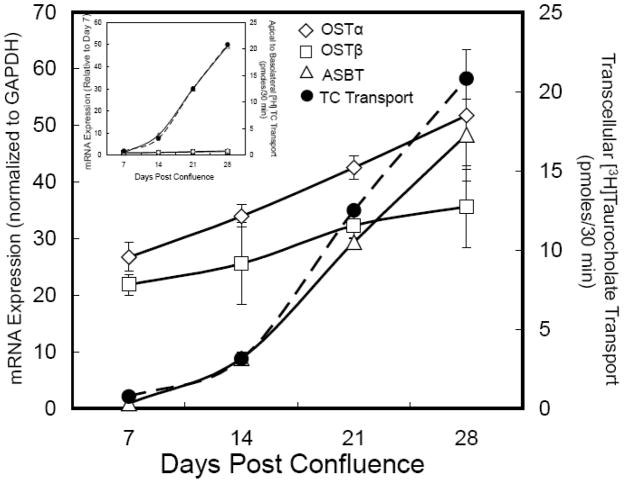
The expression of OSTα and OSTβ mRNA has also been measured in Caco-2 cells, a widely used model of a human small intestine polarized epithelial cell [42]. Previous studies have shown that bile acid transcellular transport activity is induced over time as Caco-2 cells differentiate when grown on porous membrane supports [43, 44]. Typically, bile acid transport activity is maximal about 30 days after reaching confluence and correlates with markers of enterocyte differentiation. Figure 3 shows the time course for induction of bile acid transcellular transport, and expression of ASBT, OSTα, and OSTβ mRNA in Caco-2 cells. Between post-confluent days 7 and 28, the apical to basolateral transcellular transport of taurocholate increases approximately 30-fold. This roughly parallels the increase in mRNA expression for ASBT, the rate-limiting step in ileal bile acid absorption. The expression of OSTα and OSTβ mRNA temporally precedes that of the ASBT and is already relatively high in day 7 post-confluent Caco-2 cells, increasing only 2 to 3-fold over the time period from day 7 to day 28. These findings are consistent with OSTα-OSTβ playing a role in basolateral bile acid transport (i.e., mRNA for these proteins is expressed in the cell line, which shows robust transcellular bile acid transport), although transport appears to be determined by expression of the ASBT in this model. The increase in ASBT mRNA over time is most likely related to expression changes in transcription factors important for terminal differentiation, cell fate, and jejunal-ileal identities such as Cdx, HNF-1α, HNF-1β, and GATA-4 [45–49]. For example, HNF-1α is essential for ASBT expression [50] and GATA-4 functions to restrict ASBT expression to the ileum by repressing synthesis in duodenal and jenunal enterocytes [45, 49]. The basal mRNA expression for OSTα and OSTβ appear to be regulated by a different complement of factors, in agreement with their broader gradient of expression along intestinal cephalocaudal axis. A potential rationale for tThese findings are consistent with OSTα-OSTβ playing a role in basolateral bile acid transport (i.e., mRNA for these proteins is expressed in the cell line, which shows robust transcellular bile acid tAn important species difference with regard to tissue expression is the significantly higher levels of OSTα and OSTβ mRNA expression in human versus rodent liver. Under basal conditions, Ostα and Ostβ mRNA expression is undetected or barely detectable in mouse [32], rat [35], or hamster liver (Rao and Dawson, unpublished), but is generally measurable in liver from human [35, 51] or monkey (Rao and Dawson, unpublished). As discussed below, both in rodents and humans, OSTα/Ostα and OSTβ/Ostβ expression is induced dramatically in response to bile acid feeding or cholestasis [52, 53].
Figure 3.
Ontogeny of OSTα-OSTβ mRNA expression and TC transport in Caco-2 cells. Caco-2 cells were seeded at high density onto transwell filters and maintained in DMEM plus 10% fetal calf serum. Two days prior to assaying bile acid transport at 7, 14, 21, and 28 days post-confluence, the cells were switched to DMEM plus 0.5% charcoal-stripped fetal calf serum to remove endogenous bile acids. At the indicated times, the cells were washed and incubated at 37°C for 30 min with 10 μM [3H]taurocholate in a Hanks balanced salt solution with sodium (137 mM) or without sodium (137 mM potassium) added to the apical chamber [32]. The transport is plotted as the pmoles of taurocholate transported corrected for the background transcellular transport measured in the Hanks balanced salt solution lacking sodium. The cells were harvested for RNA isolation to determine the mRNA expression for ASBT, OSTα and OSTβ by real time PCR. The CT values are the means of triplicate determinations and expression was normalized for GAPDH expression, which did not change between days 7 and 28 post-confluence. The results are plotted relative to the expression at day 7. The media from the basolateral chamber was collected to determine the transcellular transport of taurocholate. TC transport correlated with the appearance of ASBT mRNA at day 14 and increased ~30-fold between days 7 and 28. ASBT mRNA expression increased ~45-fold between days 7 and 28 post-confluence. OSTα and OSTβ mRNA are present at day 7 and increase ~2-fold between days 7 and 28 post-confluence. The CT values on day 28 were 22.15, 22.44, and 21.91 for ASBT, OSTα, and OSTβ mRNA, respectively. Inset, the mRNA expression is shown relative to the day 7 measurements.
The membrane localization for Ostα-Ostβ protein expression has been examined in ileum, kidney, and liver from human, rat and mouse [32, 35, 41, 54]. In ileum, expression of Ostα and Ostβ was largely restricted to the lateral and basal membranes of enterocytes. Like the Asbt, there was a gradient of Ostα and Ostβ expression along the intestinal crypt-to-villus axis, with highest levels in the mature villus enterocytes and little or no expression in the crypts. In the liver, OSTα/Ostα was readily detected on the basolateral membrane of human and mouse cholangiocytes, however basolateral (sinusoidal) membrane staining was detected for human but not mouse or rat hepatocytes [35, 54]. Overall, the gastrointestinal tissue distribution of OSTα-OSTβ mRNA expression and the cellular localization for OSTα-OSTβ protein are consistent with a major functional role as the basolateral membrane transporter responsible for export of bile acids and possibly other endobiotics from enterocytes into the portal circulation under normal physiological conditions and from hepatocytes into the sinusoidal compartment under conditions of cholestasis or hepatocyte injury.
6. Biogenesis, subunit interaction, and cellular trafficking of Ostα-Ostβ
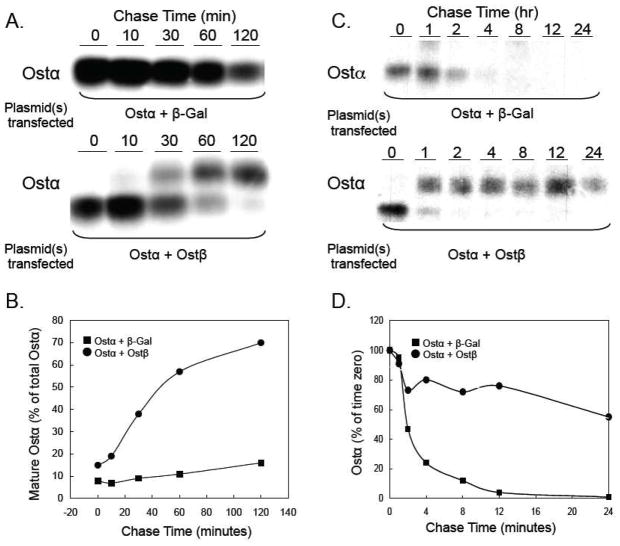
Although the initial cloning and transport studies of skate, mouse, and human OST/Ost proteins revealed that expression of both OSTα and OSTβ are required for transport activity [5, 31], many questions remained regarding the mechanism. Does OSTβ function as an activator of OSTα transport activity at the plasma membrane or as a chaperone to facilitate OSTα folding and exit from the ER? Early studies in Xenopus oocytes suggested that coexpression of both OSTα and OSTβ may not be necessary for trafficking of the individual subunits to the plasma membrane [31]. However there were several possible interpretations of those observations, including co-trafficking of OSTα and OSTβ with endogenous oocyte proteins, or mistargeting of a small fraction of the OSTα and OSTβ proteins being expressed in the oocytes. More extensive studies of OST/Ost in HEK 293, MDCK, and COS cells subsequently demonstrated that coexpression of both subunits is essential for egress from the ER and delivery to the plasma membrane [32, 33, 55, 56]. This subunit requirement for trafficking was readily evident from changes in the pattern of N-linked glycosylation for the mouse Ostα protein. When coexpressed with Ostβ in HEK 293 or MDCK cells, the apparent molecular weight of Ostα protein increased from approximately 35 to 40 kDa, as demonstrated by immunoblotting [32] or by immunoprecipitation in pulse-chase experiments where HEK 293 cells were transfected with Ostα alone or Ostα plus Ostβ (Figure 4A, 4B). When coexpressed with Ostβ, the Ostα N-linked carbohydrate is processed to an Endo H-resistant form, indicating that Ostα had exited the ER and trafficked through the Golgi complex. The changes in Ostα glycosylation correlated with changes in cellular localization, as demonstrated by indirect immunofluorescence and confocal microscopy. When expressed alone, Ostα or Ostβ displayed an ER-staining pattern with little detectable plasma membrane staining. However when coexpressed, Ostα and Ostβ were localized primarily on the plasma membrane [32]. Similar results were obtained for human OSTα and OSTβ in studies of transfected OST subunits in COS cells and of the endogenous OST subunits in HepG2 cells [56]. Surprisingly, although the human OSTα subunit lacks the consensus sequence, Asn-X-Ser/Thr, Soroka et al [56] provided convincing evidence for N-glycosylation of OSTα. This evidence included a decrease in the apparent molecular weight of OSTα after treatment of cells with tunicamycin and susceptibility of the human endogenous or transfected OSTα protein to the glycosidases, Endo H and PNGase. Although apparently rare, there are other examples of proteins being modified by the addition of N-linked oligosaccharide to non-consensus sites [57]. Notably, Soroka et al. show that the plasma membrane localization of OSTα and OSTβ in HepG2 or transfected COS cells was not affected by tunicamycin, indicating that glycosylation is not required for interaction of the subunits or their trafficking.
Figure 4.
Ostβ is required for the maturation and stability of Ostα. (A) HEK 293 cells were transfected with plasmids expressing mouse Ostα and β-galactosidase or mouse Ostα and mouse Ostβ. After 24 h, the cells were pulse labeled with [35S]-methionine/cysteine for 30 min at 37°C. Following the labeling period, cells were washed and incubated at 37° C in fresh media containing unlabeled methionine and cysteine. At the indicated times, cell lysates were prepared, subjected to immunoprecipitation using an anti-Ostα antibody, and analyzed by SDS-PAGE as described [110]. (B) The Ostα bands were quantified using a using a Molecular Dynamics 445 SI Phosphorimager. The amount of mature Ostα protein (upper band) is shown as the percent of total cellular Ostα protein for each time point. In the presence of Ostβ, approximately 70% of the newly synthesized Ostα is in the mature form after 2 h. (C) HEK 293 cells were transfected with plasmids expressing mouse Ostα and β-galactosidase or mouse Ostα and mouse Ostβ. After 24 h, the cells were pulse labeled with [35S]-methionine/cysteine for 30 min at 37°C. Following the labeling period, cells were washed and incubated at 37° C in fresh media containing unlabeled methionine and cysteine. At the indicated times, cell lysates were prepared, subjected to immunoprecipitation using an anti-Ostα antibody, and analyzed by SDS-PAGE as described [110]. (D) The Ostα bands were quantified using a using a Molecular Dynamics 445 SI Phosphorimager. The results indicate that the half-life of Ostα is ~2 hours versus greater than 24 h in the absence and presence of cotransfected Ostβ, respectively.
These observations raised additional questions as to the nature of the interaction between OSTα and OSTβ. Is the interaction between OSTα and OSTβ stable or transitory? Is the interaction required only for the initial folding of the subunits and their exit from the ER or is a stable association between the 2 subunits required for transporter function? Does OSTβ function as a general trafficking factor to promote egress of other proteins besides OSTα from the ER, or is OSTβ a dedicated partner of OSTα? Results from studies of transfected HEK 293 cells and Ostα−/− mice are beginning to answer these questions. Li et al. examined the stability of OST subunits in transfected HEK 293 cells incubated in the presence and absence of the protein synthesis inhibitor, cycloheximide [33]. When coexpressed, the OSTα and OSTβ subunits exhibited a long half-life in excess of 24 h. However, when individually expressed, there was little or no accumulation of the individual subunits. Similar results were obtained from pulse-chase analysis where HEK 293 cells were transfected with mouse Ostα or Ostα plus Ostβ. (Figure 4C, D). In the absence of co-transfected Ostβ, Ostα is rapidly degraded with a half-life of approximately 2 h. However when co-expressed Ostβ, Ostα protein is stabilized with a half-life in excess of 24 h. Analysis of Ostβ protein expression in Ostα−/− mice provided further in vivo insights into the consequences of dysregulated expression of the individual OST subunits. As predicted, targeted inactivation of the mouse Ostα gene resulted in loss of Ostα mRNA and protein expression. However, Ostβ protein levels were also reduced to almost undetectable levels in small intestine, cecum, colon, and kidney of Ostα −/− mice, despite persistent high levels of Ostβ mRNA expression [33, 36]. These in vivo results suggest that if unable to interact with Ostα, the Ostβ protein becomes labile and is rapidly turned over, most likely by an ER-associated degradation pathway [58]. Notably, the rapid turnover of Ostβ protein in Ostα −/− mice provides insight to the question of whether Ostβ functions as a general trafficking factor for other proteins. The results suggest that Ostβ is a dedicated partner of Ostα and unlikely to function as a general partner for other proteins.
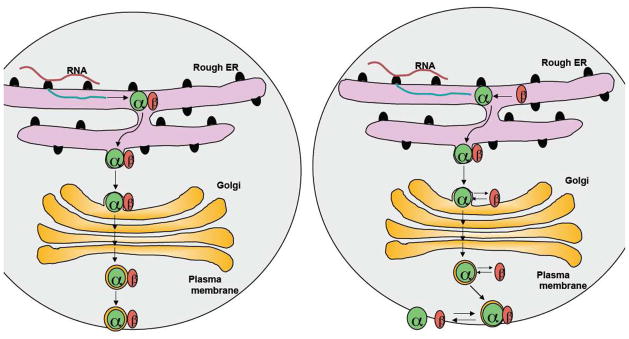
Potential models for the interaction of OSTα and OSTβ are shown in Figure 5. In the first model, OSTα and OSTβ form a stable complex in the ER and maintain this tight association during their movement through the Golgi complex and their residence at the plasma membrane. The second model proposes that the interaction between OSTα and OSTβ is transitory. OSTβ initially acts as a molecular chaperone for OSTα to ensure its proper folding and/or egress from the ER. However, after exiting the ER or after delivery to the plasma membrane, the OSTα-OSTβ complex reversibly dissociates, thereby establishing a new equilibrium between the individual subunits and the OSTα-OSTβ complex. What does the existing data tell us regarding these models and how OSTα and OSTβ interact? Sun et al. examined MDCK cells transfected with an epitope-tagged human OSTα and a human OSTβ-carboxyl terminal GFP fusion protein. The tagged subunits were functional and Sun et al. demonstrated co-immunoprecipitation of the two subunits [55]. Deletion of the N-terminal 50 amino acids of OSTα abolished transport activity, plasma membrane localization, and ability to coimmunoprecipitate OSTβ This region may be required for interacting with OSTβ, however another potential explanation for this finding is that deletion of the N-terminus affects OSTα membrane insertion and protein folding. Indeed, attempts to place epitope tags at the N-terminus of Ostα abolish transport activity and significantly reduces Ostα protein expression, suggesting that modification of the N-terminus renders the protein unstable ([33], and unpublished results). Sun et al. also reported that direct interactions between OSTα and OSTβ could be demonstrated in a mammalian but not a yeast 2-hybrid system [55]. The most convincing demonstration of direct interactions between the OSTα and OSTβ subunits were obtained in an elegant series of studies by Li et al. [33]. Both subunits were coimmunoprecipitated from membrane protein extracts prepared from mouse ileum as well as Ostα-Ostβ transfected HEK293 cells. Association of Ostα and Ostβ was also demonstrated in living cells using bimolecular fluorescence complementation analysis of HEK 293 cells transfected with Ostα and Ostβ tagged with portions of yellow fluorescent-protein (YFP). These studies demonstrated that Ostα and Ostβ are associated in the ER as well as at the plasma membrane. Furthermore, by tagging Ostα with different YFP fragments and co-expressing the two fusion proteins with Ostβ, Li et al. also showed the formation of Ostα homodimers in ER and plasma membrane in living cells.
Figure 5.
Models for the interaction of OSTα and OSTβ. (A) Model 1: OSTα and OSTβ form stable complexes and remain stably associated as they traffic through the ER and Golgi to the plasma membrane. (B) Model 2: The association of OSTβ ensures proper folding of OSTα and its appropriate targeting to the plasma membrane. After reaching the plasma membrane, the heterodimeric complex is in equilibrium with individual subunits.
These results demonstrate a clear and robust interaction between Ostα and Ostβ that generally supports Model 1. The results are consistent with association of the two proteins to mask an ER retention/retrieval motif or create a forward trafficking motif to promote ER exit and escape from the ER-associated degradation pathways. However, these studies do not indicate what proportion of the Ostα or Ostβ protein is present as a complex versus free subunit in the cell, particularly at the plasma membrane. The coimmunoprecipitation studies by Soroka et al. [56] provide some additional insight to this question. When OSTβ protein was immunoprecipitated from extracts of COS cells transfected with OSTα plus OSTβ, only the immature form of OSTα appeared to coimmunoprecipate. Attempts to coimmunoprecipitate the endogenous OSTα protein in HepG2 cells, which is primarily in the mature state, were not successful. These results raise the possibility that the strong association of OSTα and OSTβ may be only transitory and the mature subunits may reversibly dissociate at the plasma membrane (Model 2).
7. OSTα-OSTβ Transporter Activity
The mechanism for OSTα-OSTβ mediated transport has not been fully elucidated [35]. When expressed in Xenopus laevis oocytes, OSTα-OSTβ mediated transport was unaffected by depletion of intracellular ATP, by alterations in transmembrane electrolyte concentration gradients, or by changes in the pH gradient [35]. OSTα-OSTβ exhibits both uptake and efflux properties, and solute transport is trans-stimulated by known substrates [5, 31, 35]. The general consensus from these studies is that OSTα-OSTβ operates by facilitated diffusion and mediates solute uptake or efflux, depending on the solute’s electrochemical gradient. A systematic screening effort to identify OSTα-OSTβ transport substrates or careful comparison of the substrate specificity of OST from different species has not yet been published and this area remains largely unexplored. However, the existing list of OSTα-OSTβ substrates includes the major species of bile acids including glycine and taurine conjugates of cholic acid, deoxycholic acid, chenodeoxycholic acid, and ursodeoxycholic acid, as well as non-bile acid substrates such as estrone-3-sulfate, digoxin, prostaglandin E2, and dehydroepiandrosterone-3-sulfate (DHEAS) [35]. The list of inhibitors for OSTα-OSTβ mediated transport of taurocholate or estrone-3-sulfate is also broad and includes a variety of compounds such as spironolactone, bromosulfophthalein, probenecid, and indomethacin [31]. These preliminary results suggest that the substrate specificity for OSTα-OSTβ is relatively broad and is consistent with a direct role in the transport of other solutes including steroids or steroid sulfates.
8. Function in bile acid and steroid metabolism
8.1. Function of OSTα-OSTβ in intestinal bile acid transport
OSTα-OSTβ was identified as a candidate ileal basolateral membrane bile acid transporter using a transcriptional profiling approach [32]. In that study, the mRNA expression for a group of 180 known and orphan solute transporters was measured in ileum, colon, and liver of wild type and Asbt null mice [59] using real-time PCR. It was hypothesized that the intestinal basolateral membrane bile acid transporter is positively regulated by bile acids. As such, its expression would be decreased in ileum and increased in the colon of mice with impaired ileal bile acid absorption. Among the most regulated transporter genes was Ostα, whose expression was significantly reduced in ileum and increased more than 5-fold in colon of Asbt null mice [32]. Support for a physiologically important role of OSTα-OSTβ in bile acid transport came from a number of subsequent studies, and includes: 1) intestinal expression of OSTα and OSTβ mRNA that generally follows that of the ASBT, with highest levels in ileum [32, 35, 39], 2) appropriate cellular localization on the lateral and basal plasma membranes of ileal enterocytes [32], 3) expression of OSTα-OSTβ on the basolateral plasma membrane of hepatocytes, cholangiocytes, and renal proximal tubule cells, other cells important for bile acid transport [35], 4) efficient transport of the major bile acid species [32, 35], and 5) positive regulation of OSTα-OSTβ expression by bile acids via activation of the farnesoid X-receptor (FXR) [37, 38, 40, 53]. No inherited defects have been reported for the OSTα or OSTβ genes in humans, however targeted inactivation of the Ostα gene in mice has provided a clearer picture of the in vivo function of OSTα-OSTβ [36, 60, 61]. As predicted for a major intestinal basolateral membrane bile acid transporter, studies using everted gut sacs [36] or intra-ileal administration of [3H]taurocholate [60] demonstrated a significant reduction in trans-ileal bile acid transport in Ostα null mice. However fecal bile acid excretion was not increased in Ostα null mice, as had been observed in Asbt (Slc10a2) null mice [59, 62] or in patients with ASBT mutations [63]. These results were particularly perplexing since the whole body bile acid pool size was significantly decreased in Ostα −/− mice [36], a hallmark of intestinal bile acid malabsorption [59, 63]. Examination of the FGF15/19 signaling pathway [26] provided a possible solution to this conundrum. In the Ostα null mice, bile acids are taken up by the ileal enterocyte but their efflux across the basolateral membrane is impaired. As a result, there is increased activation of FXR and increased expression of FGF15. The intestinally derived FGF15 is then thought to signal at the hepatocyte to down-regulate hepatic CYP7A1 expression and bile acid synthesis. The net result is that hepatic bile acid synthesis is paradoxically repressed rather than induced, the normal physiological response to a block in intestinal bile acid absorption [36, 64].
In addition to terminal ileum, OSTα-OSTβ is also expressed in the proximal small intestine, cecum, and colon where it may function to export bile acids that entered the cell by non-ionic diffusion [65–67] or facilitative transport [68]. While mice have almost exclusively taurine-conjugated bile acids (pKa~1), the circulating bile acid pool in humans and many other species is more hydrophobic and includes glycine-conjugated (pKa~4) and unconjugated (pKa~5) bile acids [34, 69]. Furthermore, in the distal ileum, cecum, and colon, bile acids are converted to more hydrophobic species by the intestinal flora through extensive deconjugation and dehydroxylation [70], processes that increase the pKa and significantly reduces the water solubility and critical micellar concentration of bile acids [16, 69]. Since the pH of the intestinal lumen or microclimate overlaying the mucosal cells is sufficiently low (pH ~6.5 to 7.5 in the small intestine and ~5.5 to 7 in the colon) [71, 72], a greater proportion of the glycine-conjugated and unconjugated bile acids will be protonated and uncharged, thereby capable of gaining entry to enterocytes or colonocytes by nonionic diffusion across the apical plasma membrane. Once inside the cytoplasmic compartment, these weak acids will ionize at the neutral intracellular pH, potentially trapping the bile acid in the cell. Export of this bile acid anion across the basolateral membrane would likely require a carrier such as OSTα-OSTβ.
8.2. Function of OSTα-OSTβ in renal and hepatic bile acid transport
OSTα-OSTβ is expressed on the basolateral membrane of human and rodent renal proximal tubule cells [35] where it functions in conjunction with the apically-expressed ASBT as a salvage mechanism to conserve bile acids [73, 74]. A small fraction of the bile acids returning from the intestine in the portal circulation escapes hepatic extraction and spills into the systemic circulation. Although binding to plasma proteins reduces glomerular filtration, the urinary excretion of non-sulfated bile acids is further minimized by an efficient sodium-dependent tubular reabsorption [75–77]. As a result in healthy humans, less than 5% of the approximately 100 μmol of bile acids filtered by the kidney each day is excreted in the urine [78]. Even in patients with cholestatic liver disease, in whom plasma bile acid concentrations are elevated, the 24-hour urinary excretion of non-sulfated bile acids is significantly less than the quantity that undergoes glomerular filtration [78–81].
OSTα-OSTβ mRNA expression is generally detectable in human but not rodent liver under non-cholestatic conditions [51, 52]. However, variable but dramatic increases are observed for Ostα and Ostβ mRNA expression in mice following bile acid feeding or bile duct ligation [52, 53]. Similar increases are observed for OSTα and OSTβ mRNA expression in liver from patients with Primary Biliary Cirrhosis [52] or extra-hepatic cholestasis [51]. An area of active investigation is the identification and analysis of transporters responsible for sinusoidal efflux of bile acids and other organic solutes from the interior of the hepatocyte into the space of Disse [82]. Whereas sinusoidal membrane bile acid transport is overwhelming in the direction of uptake under normal physiological conditions, bile acids can also be effluxed as part of the adaptive response to minimize bile acid accumulation under cholestatic conditions [83]. Much of this work has focused on members of the MRP (ABCC) family of ATP-dependent transporters, in particular MRP3 and MRP4 [84–86], and on OSTα-OSTβ [51, 52]. Expression of these transporters is induced under cholestatic conditions to promote bile acid efflux [52, 87–92], thus lowering bile acid concentration in the hepatocyte and decreasing the likelihood of apoptosis or necrosis [93]. MRP4 may be particularly important, as Mrp4 but not Mrp3 null mice develop more severe liver damage under cholestatic conditions [94, 95]. In order to directly examine the contribution of OSTα-OSTβ to the adaptive response to cholestatic liver injury, the effects of bile duct ligation were recently examined in wild type and Ostα null mice [61]. Based on the hypothesis that OSTα-OSTβ functions to reduce hepatic injury by promoting sinusoidal membrane export of cytotoxic bile acids [52], one would predict that the Ostα null mice develop more severe liver injury. Surprisingly, Ostα deficiency resulted in a substantial attenuation of cholestatic hepatic injury. This hepatoprotective effect was thought to be secondary to increased hepatic metabolism of bile acids to more hydrophilic tetrahydroxylated and sulfated species, and to increased urinary excretion of bile acids [61]. These results suggest that the sinusoidal transporters, Mrp3 and Mrp4, compensate for the loss of Ostα-Ostβ in hepatocytes and continue to export bile acids and modified bile acid species into the systemic circulation. A particularly important finding was that inactivation of Ostα leads to increased urinary excretion of bile acids, creating a shunt for elimination of hepatotoxic bile acids that can no longer be excreted efficiently via the biliary route.
8.3. OSTα-OSTβ and adrenal steroid transport
In addition to tissues of the gastrointestinal tract, OSTα and OSTβ were also identified as FXR target genes in adrenal gland [37, 96, 97]. In human adrenal, cells within the zona reticularis of the cortex abundantly express the steroid sulfotransferase SULT2A1 and synthesize significant amounts of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) [98]. This enzyme catalyzes the sulfonation of a broad range of substrates including DHEA, pregnenolone, and 17α-hydroxyprenenolone [99, 100]. Although the mouse does not synthesize DHEA or DHEAS [101, 102], the mouse adrenal gland still expresses steroid sulfotransferase (Sult2a1) [103], which sulfonates other endogenous steroid substrates [104]. The enzymes responsible for adrenal steroid synthesis have been identified and characterized [101], however the transport mechanism(s) responsible for cellular export of DHEAS and other hydrophilic steroid conjugates are not well understood. Support for a role of OSTα-OSTβ in the secretion of conjugated steroid intermediates into the circulation includes: 1) an efflux transport specificity that includes steroid sulfates such estrone-3-sulfate and DHEAS [35], 2) abundant expression of OSTα/Ostα and OSTβ/Ostβ mRNA in human and mouse adrenal [37, 97], 3) expression of FXR in zona reticularis cells in human and mouse adrenal cortex [97, 105], and 4) induction of OSTα/Ostα and OSTβ/Ostβ mRNA expression in mouse adrenal and the human adrenocortical cell line H295R in response to FXR ligands [37, 96, 97]. Notably, DHEAS production and SULT2A1 expression is high in human fetal adrenal gland, falls rapidly at birth, and remains low until about 5 years of age. DHEAS production and SULT2A1 expression is then induced at the onset of adrenache, maturation of the adrenal cortex, which typically occurs between ages 6 and 10 [98]. Whether expression of OSTα-OSTβ, the potential export mechanism for DHEAS, follows a similar pattern of expression is an important question that remains to be answered.
9. Concluding remarks
The long search for the basolateral membrane bile acid transporter appears to be over. The task proved to be more difficult than for the other bile acid transporters for several reasons related to the biology of OSTα-OSTβ. First, the transporter functions as a heterodimer, greatly complicating attempts to identify the transporter by expression cloning. Transport activity depends on co-expression of the two different cDNAs in the same transfected cell or injected oocyte, a low probability event, and activity is lost upon subfractionation of any positive pool of cDNA clones. Second, OSTα-OSTβ is not a member of the major ABC or SLC (solute carrier) transporter families, greatly complicating candidate gene approaches. OSTα or OSTβ would not stand out as potential candidate genes for the basolateral bile acid transporter by sequence homology to known transporters. Third, stable expression of OSTα and OSTβ requires coexpression of both subunits, greatly complicating attempts to analyze the function of the individual subunits in transfected cell systems. As such, cell culture-based attempts to analyze the properties of OSTα or OSTβ alone would not have been feasible.
A great deal has been learned about the expression, trafficking, regulation, and function of OSTα-OSTβ, however important questions remain to be answered [9, 10]. With regard to the cellular and biochemical properties, the individual functions of the subunits have not been resolved. As a polytopic membrane protein, OSTα is assumed to be the essential subunit for translocating substrates across the lipid bilayer. Whether OSTβ plays a direct role in solute transport or serves primarily to regulate OSTα maturation, membrane targeting, or activity is not known. The primary sequence of OSTα and OSTβ includes a number of potential phosphorylation sites that may be involved in regulating activity [31]. Phosphorylation of either subunit has not yet been demonstrated, but the characteristic doublet migration of OSTβ/Ostβ upon SDS-PAGE is consistent with such a post-translational modification. Results from studies in transfected cells and the Ostα null mouse suggest that coexpression of both subunits is required for stable expression of the proteins and maturation of the transporter complex, but the nature of the association and identity of other potential interacting proteins remain to be determined.
A particular vexing question is the apparent breakdown of coordinate mRNA expression for OSTα and OSTβ in liver. As discussed above, the mRNA expression levels for OSTα and OSTβ generally parallel one another in intestine. However, discordant precipitous increases in hepatic OSTβ/Ostβ mRNA levels associated with small changes in OSTα/Ostα mRNA expression have been observed under pathophysiological conditions in humans and mice [51, 52]. This includes cholestatic models where Ostα mRNA expression is decreased such as hepatotoxicant-induced acute intrahepatic cholestasis [106] or genetic models such as the Bsep and mdr1a/b null mice [107]. It should be noted that these studies typically measured mRNA but not protein levels, so the functional significance of these findings is unclear. One possible explanation for this pattern of expression is differential recognition of FXR binding sites for the two genes. A very recent chromatin immunoprecipitation study showed that FXR bound the upstream regions of both Ostα and Ostβ in mouse small intestine. However in liver, FXR bound the regulatory region of Ostβ but not Ostα[108]. These findings may provide a molecular explanation as to why hepatic OSTβ/Ostβ mRNA expression is particularly sensitive to changes in bile acids levels. But it does not provide an obvious explanation as to the physiological benefit of preferentially inducing OSTβ/Ostβ mRNA expression. One possibility is that an excess of OSTβ is synthesized to ensure that any nascent OSTα protein will be stabilized and trafficked from the ER. Alternatively, these findings could suggest an expanded role for OSTβ under certain conditions.
Although their role in bile acid transport is best characterized, it should be emphasized that OSTα-OSTβ is likely to have other important physiological roles. OSTα-OSTβ mRNA expression is detected in a wide range of human tissues [31], and the substrate specificity encompasses a broader range of organic solutes including steroids and the eicosanoid prostaglandin E2 [35]. The range of other physiological substrates has not yet been determined, and a targeted metabolomics approach, such as that recently carried out to identify major in vivo substrates of Mrp3 [109], is needed to help answer this question. OSTα-OSTβ may be important for conjugated steroid export in the adrenal [37], particularly in humans and old world primates that produce significant amounts of DHEAS [98], and a broader role in conjugated steroid production and metabolism should be explored. Finally, OSTα-OSTβ’s broad substrate specificity and bi-directional transport properties raises the possibility that this carrier may be involved in the disposition of drugs or drug metabolites.
Acknowledgments
We thank Dr. Larry Rudel for providing the African Green monkey tissue samples for the real time PCR analysis in Figure 2. This project was supported by NIH DK47987 (to P.A.D.) and an American Heart Association Grant in Aid (to P.A.D.). M.L.H. was supported by National Institutes of Health Training Grant HL 07115. A.R. is supported by a National Research Service Award (F32 DK079576).
Abbreviations
- ABC
ATP-binding cassette
- ASBT
apical sodium-dependent bile acid transporter
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone-3-sulfate
- ER
endoplasmic reticulum
- Endo H
endoglycosidase H
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FXR
farnesoid X-receptor
- GFP
green fluorescent protein
- HEK
human embryonic kidney
- MDCK
Madin-Darby canine kidney
- MRP
multidrug resistance-associated protein
- OST/Ost
organic solute transporter
- PNGase
peptide;N-glycosidase F
- SLC
Solute Carrier
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberg SL, Burckhardt G, Wilson FA. Taurocholate transport by rat intestinal basolateral membrane vesicles. Evidence for the presence of an anion exchange transport system. J Clin Invest. 1986;78:44–50. doi: 10.1172/JCI112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin MC, Weinberg SL, Kramer W, Burckhardt G, Wilson FA. Identification and comparison of bile acid-binding polypeptides in ileal basolateral membrane. J Membr Biol. 1988;106:1–11. doi: 10.1007/BF01871762. [DOI] [PubMed] [Google Scholar]
- 3.Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, LaRusso NF. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci U S A. 2000;97:11092–11097. doi: 10.1073/pnas.200325297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci U S A. 2001;98:9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Kuba K, Danilczyk U, Skovby F, Kleta R, Penninger JM, Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacin M, Nunes V, Font-Llitjos M, Jimenez-Vidal M, Fort J, Gasol E, Pineda M, Feliubadalo L, Chillaron J, Zorzano A. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005;20:112–124. doi: 10.1152/physiol.00051.2004. [DOI] [PubMed] [Google Scholar]
- 8.Verrey F, Singer D, Ramadan T, Vuille-dit-Bille RN, Mariotta L, Camargo SM. Kidney amino acid transport. Pflugers Arch. 2009;458:53–60. doi: 10.1007/s00424-009-0638-2. [DOI] [PubMed] [Google Scholar]
- 9.Ballatori N. Biology of a novel organic solute and steroid transporter, OSTalpha-OSTbeta. Exp Biol Med (Maywood) 2005;230:689–698. doi: 10.1177/153537020523001001. [DOI] [PubMed] [Google Scholar]
- 10.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front Biosci. 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009 doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz M, Lund EG, Setchell KD, Kayden HJ, Zerwekh JE, Bjorkhem I, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem. 1996;271:18024–18031. doi: 10.1074/jbc.271.30.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitro N, Godio C, De Fabiani E, Scotti E, Galmozzi A, Gilardi F, Caruso D, Vigil Chacon AB, Crestani M. Insights in the regulation of cholesterol 7alpha-hydroxylase gene reveal a target for modulating bile acid synthesis. Hepatology. 2007;46:885–897. doi: 10.1002/hep.21819. [DOI] [PubMed] [Google Scholar]
- 22.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilardi F, Mitro N, Godio C, Scotti E, Caruso D, Crestani M, De Fabiani E. The pharmacological exploitation of cholesterol 7alpha-hydroxylase, the key enzyme in bile acid synthesis: from binding resins to chromatin remodelling to reduce plasma cholesterol. Pharmacol Ther. 2007;116:449–472. doi: 10.1016/j.pharmthera.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Pandak WM, Heuman DM, Hylemon PB, Chiang JY, Vlahcevic ZR. Failure of intravenous infusion of taurocholate to down-regulate cholesterol 7 alpha-hydroxylase in rats with biliary fistulas. Gastroenterology. 1995;108:533–544. doi: 10.1016/0016-5085(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 28.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 29.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballatori N, Boyer JL, Rockett JC. Exploiting genome data to understand the function, regulation, and evolutionary origins of toxicologically relevant genes. EHP Toxicogenomics. 2003;111:61–65. [PubMed] [Google Scholar]
- 31.Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278:27473–27482. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- 32.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem J. 2007;407:363–372. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res. 2010;51:226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 36.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 39.Balesaria S, Pell RJ, Abbott LJ, Tasleem A, Chavele KM, Barley NF, Khair U, Simon A, Moriarty KJ, Brydon WG, Walters JR. Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoea. Eur J Gastroenterol Hepatol. 2008;20:413–422. doi: 10.1097/MEG.0b013e3282f41b82. [DOI] [PubMed] [Google Scholar]
- 40.Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G912–922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- 41.Renner O, Harsch S, Strohmeyer A, Schimmel S, Stange EF. Reduced ileal expression of OSTalpha-OSTbeta in non-obese gallstone disease. J Lipid Res. 2008;49:2045–2054. doi: 10.1194/jlr.M800162-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Chow EC, Liu S, Du Y, Pang KS. The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol. 2008;4:395–411. doi: 10.1517/17425255.4.4.395. [DOI] [PubMed] [Google Scholar]
- 43.Hidalgo IJ, Borchardt RT. Transport of bile acids in a human intestinal epithelial cell line, Caco-2. Biochim Biophys Acta. 1990;1035:97–103. doi: 10.1016/0304-4165(90)90179-z. [DOI] [PubMed] [Google Scholar]
- 44.Chandler CE, Zaccaro LM, Moberly JB. Transepithelial transport of cholyltaurine by Caco-2 cell monolayers is sodium dependent. Am J Physiol. 1993;264:G1118–1125. doi: 10.1152/ajpgi.1993.264.6.G1118. [DOI] [PubMed] [Google Scholar]
- 45.Battle MA, Bondow BJ, Iverson MA, Adams SJ, Jandacek RJ, Tso P, Duncan SA. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686. e1671. doi: 10.1053/j.gastro.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–31917. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 47.Boudreau F, Zhu Y, Traber PG. Sucrase-isomaltase gene transcription requires the hepatocyte nuclear factor-1 (HNF-1) regulatory element and is regulated by the ratio of HNF-1 alpha to HNF-1 beta. J Biol Chem. 2001;276:32122–32128. doi: 10.1074/jbc.M102002200. [DOI] [PubMed] [Google Scholar]
- 48.D’Angelo A, Bluteau O, Garcia-Gonzalez MA, Gresh L, Doyen A, Garbay S, Robine S, Pontoglio M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137:1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- 49.Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL, Stoffel M. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet. 2001;27:375–382. doi: 10.1038/86871. [DOI] [PubMed] [Google Scholar]
- 51.Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49:1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 52.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 53.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 54.Khan AA, Chow EC, Porte RJ, Pang KS, Groothuis GM. Expression and regulation of the bile acid transporter, OSTalpha-OSTbeta in rat and human intestine and liver. Biopharm Drug Dispos. 2009;30:241–258. doi: 10.1002/bdd.663. [DOI] [PubMed] [Google Scholar]
- 55.Sun AQ, Balasubramaniyan N, Xu K, Liu CJ, Ponamgi VM, Liu H, Suchy FJ. Protein-protein interactions and membrane localization of the human organic solute transporter. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1586–1593. doi: 10.1152/ajpgi.00457.2006. [DOI] [PubMed] [Google Scholar]
- 56.Soroka CJ, Xu S, Mennone A, Lam P, Boyer JL. N-Glycosylation of the alpha subunit does not influence trafficking or functional activity of the human organic solute transporter alpha/beta. BMC Cell Biol. 2008;9:57. doi: 10.1186/1471-2121-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valliere-Douglass JF, Kodama P, Mujacic M, Brady LJ, Wang W, Wallace A, Yan B, Reddy P, Treuheit MJ, Balland A. Asparagine-linked oligosaccharides present on a non-consensus amino acid sequence in the CH1 domain of human antibodies. J Biol Chem. 2009;284:32493–32506. doi: 10.1074/jbc.M109.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 59.Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 60.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295:G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007;48:2693–2700. doi: 10.1194/jlr.M700351-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2) J Clin Invest. 1997;99:1880–1887. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis RA, Attie AD. Deletion of the ileal basolateral bile acid transporter identifies the cellular sentinels that regulate the bile acid pool. Proc Natl Acad Sci U S A. 2008;105:4965–4966. doi: 10.1073/pnas.0801194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aldini R, Montagnani M, Roda A, Hrelia S, Biagi PL, Roda E. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 1996;110:459–468. doi: 10.1053/gast.1996.v110.pm8566593. [DOI] [PubMed] [Google Scholar]
- 66.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24:545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 67.Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amelsberg A, Schteingart CD, Ton-Nu HT, Hofmann AF. Carrier-mediated jejunal absorption of conjugated bile acids in the guinea pig. Gastroenterology. 1996;110:1098–1106. doi: 10.1053/gast.1996.v110.pm8612999. [DOI] [PubMed] [Google Scholar]
- 69.Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25:1477–1489. [PubMed] [Google Scholar]
- 70.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Lucas M. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983;24:734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377–385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- 74.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 75.Wilson FA, Burckhardt G, Murer H, Rumrich G, Ullrich KJ. Sodium-coupled taurocholate transport in the proximal convolution of the rat kidney in vivo and in vitro. J Clin Invest. 1981;67:1141–1150. doi: 10.1172/JCI110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiner IM, Glasser JE, Lack L. Renal Excretion of Bile Acids: Taurocholic, Glycocholic, and Colic Acids. Am J Physiol. 1964;207:964–970. doi: 10.1152/ajplegacy.1964.207.5.964. [DOI] [PubMed] [Google Scholar]
- 77.Barnes S, Gollan JL, Billing BH. The role of tubular reabsorption in the renal excretion of bile acids. Biochem J. 1977;166:65–73. doi: 10.1042/bj1660065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stiehl A. Bile salt sulphates in cholestasis. Eur J Clin Invest. 1974;4:59–63. doi: 10.1111/j.1365-2362.1974.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 79.Stiehl A, Earnest DL, Admirant WH. Sulfation and renal excretion of bile salts in patients with cirrhosis of the liver. Gastroenterology. 1975;68:534–544. [PubMed] [Google Scholar]
- 80.Raedsch R, Lauterburg BH, Hofmann AF. Altered bile acid metabolism in primary biliary cirrhosis. Dig Dis Sci. 1981;26:394–401. doi: 10.1007/BF01313580. [DOI] [PubMed] [Google Scholar]
- 81.Rudman D, Kendall FE. Bile acid content of human serum. I. Serum bile acids in patients with hepatic disease. J Clin Invest. 1957;36:530–537. doi: 10.1172/JCI103450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyer JL. New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. J Hepatol. 2007;46:365–371. doi: 10.1016/j.jhep.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 84.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33:783–791. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. Characterization of inducible nature of MRP3 in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G438–446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- 86.Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, Moore DD, Borst P, Schuetz JD. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–22257. doi: 10.1074/jbc.M314111200. [DOI] [PubMed] [Google Scholar]
- 87.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundstrom R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 88.Akita H, Suzuki H, Sugiyama Y. Sinusoidal efflux of taurocholate is enhanced in Mrp2-deficient rat liver. Pharm Res. 2001;18:1119–1125. doi: 10.1023/a:1010918825019. [DOI] [PubMed] [Google Scholar]
- 89.Trauner M, Wagner M, Fickert P, Zollner G. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J Clin Gastroenterol. 2005;39:S111–124. doi: 10.1097/01.mcg.0000155551.37266.26. [DOI] [PubMed] [Google Scholar]
- 90.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 91.Zollner G, Wagner M, Fickert P, Silbert D, Gumhold J, Zatloukal K, Denk H, Trauner M. Expression of bile acid synthesis and detoxification enzymes and the alternative bile acid efflux pump MRP4 in patients with primary biliary cirrhosis. Liver Int. 2007;27:920–929. doi: 10.1111/j.1478-3231.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 92.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 2005;129:735–740. doi: 10.1016/j.gastro.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 94.Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40:585–591. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 96.Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 97.Xing Y, Saner-Amigh K, Nakamura Y, Hinshelwood MM, Carr BR, Mason JI, Rainey WE. The farnesoid X receptor regulates transcription of 3beta-hydroxysteroid dehydrogenase type 2 in human adrenal cells. Mol Cell Endocrinol. 2009;299:153–162. doi: 10.1016/j.mce.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 99.Falany CN, Comer KA, Dooley TP, Glatt H. Human dehydroepiandrosterone sulfotransferase. Purification, molecular cloning, and characterization. Ann N Y Acad Sci. 1995;774:59–72. doi: 10.1111/j.1749-6632.1995.tb17372.x. [DOI] [PubMed] [Google Scholar]
- 100.Strott CA. Steroid sulfotransferases. Endocr Rev. 1996;17:670–697. doi: 10.1210/edrv-17-6-670. [DOI] [PubMed] [Google Scholar]
- 101.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 102.Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 103.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garweg G, Kinsky I. Radioactive labeling of mouse adrenal cortex for study of steroid sulfation in inner cortex zone. Acta Histochem. 1973;(SUPPL 13):177–183. [PubMed] [Google Scholar]
- 105.Higashiyama H, Kinoshita M, Asano S. Immunolocalization of farnesoid X receptor (FXR) in mouse tissues using tissue microarray. Acta Histochem. 2008;110:86–93. doi: 10.1016/j.acthis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 106.Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology. 2009;50:948–956. doi: 10.1002/hep.23089. [DOI] [PubMed] [Google Scholar]
- 108.Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology. 2009;137:1725–1735. doi: 10.1053/j.gastro.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 110.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–27234. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]