Abstract
Tissue inflammation is a tightly regulated process that normally serves to recruit the immune system to sites of infection and injury and to facilitate tissue repair processes. When an inflammatory state is excessive or prolonged, local and systemic damage to host tissues can result in loss of normal physiological functions. Here, we briefly review recent studies that advance our understanding of signaling pathways involved in initiation of inflammatory responses at the level of transcription and counter-regulation of these pathways by selected members of the nuclear receptor superfamily. Studies of the intersection of nuclear receptors and inflammation have revealed mechanisms of positive and negative transcriptional control that may provide new targets for pharmacological intervention in chronic diseases such as atherosclerosis.
Inflammation Overview
Inflammation is a biological process that represents the initial response of an organism to infection and injury1. A disturbance that is successfully cleared results in a return to basal homeostatic set points. When conditions that induce inflammation are persistent, or resolution mechanisms fail, a state of chronic inflammation ensues that can lead to loss of normal physiological functions. The initiation and maintenance of immunity is a metabolically costly process. The interdependency of inflammatory responses and metabolic control systems are well-conserved evolutionarily. The two pathways share many signaling-mediator and responder molecules 2. Innate immune responses typically promote a transient decrease in insulin sensitivity that has been suggested to allow the redistribution of glucose from skeletal muscle to leukocytes and other cell types with increased energy demands 3. While malnutrition conditions impair immune functions, chronic metabolic overload and excess inflammation lead to immune imbalance and significantly contribute to chronic human diseases, including atherosclerosis, diabetes, fatty liver disease, airway inflammation, and cancers 2.
Local tissue inflammation involves four major components, including the inducers, the sensors, the responding mediators, and the effects of the mediators on the surrounding tissue (reviewed in 4). Tissue-resident macrophages, mast cells, endothelial cells, and barrier epithelial cells function to monitor tissue homeostasis, regulate tissue metabolism, and control inflammatory responses. These cells use extracellular and intracellular receptors to sense endogenous inducers of inflammation produced by stressed, damaged or malfunctioning cells and tissues, as well as exogenous inflammatory inducers that signal for infection.
Pattern-recognition receptors (PRRs) represent a class of receptors that sense both exogenous and endogenous inflammation stimulus. Four main families of PRRs have been described, including the nucleotide-binding oligomerization domain-like receptor family (NOD receptors and NALPs), Toll-like receptors (TLRs), C-type lectin-like molecules (including the mannose receptor and the β-glucan receptors), and a family of receptors with RNA-helicase and caspase-recruitment domains (RIG-1 and MDA5) 4, 5. PRRs detect exogenous inducers by recognizing structurally conserved lipid, carbohydrate, peptide and nucleic-acid molecules that are components of microbial and viral pathogens. Endogenous inducers, such as ATP, K+ ions, uric acid, HMGB1, and heat-shock proteins released from abnormal necrotic cell death commonly found in diabetic adipose tissue and atherosclerotic plaques, are also sensed by PPRs, including NALP3 and TLR4 (reviewed in 4). Furthermore, TLRs are also activated by fatty acids 6 and oxidized lipid-lipoproteins 7 in metabolically disturbed tissues, as well as heparin sulfates released from the extracellular matrix in response to infection and complement-coagulation cascades upon tissue injury 8.
Activation of the PRRs has diverse effects on the host, including alteration in metabolic states, protein production/secretion/processing, and induction of genes that function in innate and acquired immune responses 9. These effects are achieved by coupling receptor ligation to downstream signaling molecules that regulate the activities of several classes of signal-dependent transcription factors, including NFκB and AP-1 (reviewed in 10), illustrated in Figure 1. These transcription factors work in a combinatorial manner to recruit multiple transcriptional coregulators that remodel local nucleosomes, modify chromatin marks, and influence chromatin architecture required for initiating transcription and/or RNA polymerase elongation to rapidly alter the transcription program in the responding cells 11, 12 (Figure 2).
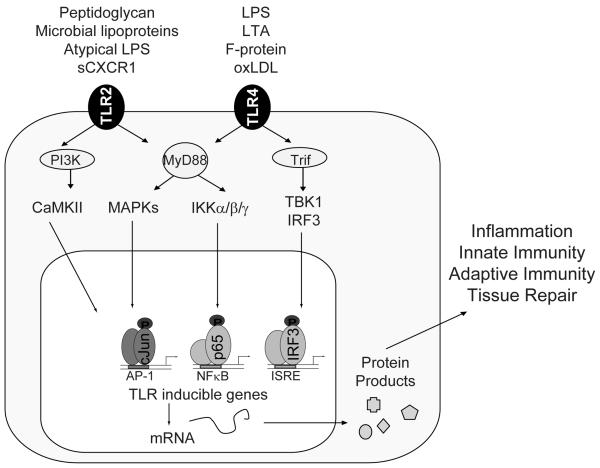
Figure 1. TLR signaling.
Together with its accessory proteins, TLRs recognizes both exogenous and endogenous inflammatory inducers, including lipopolysaccharides (LPS) found in all Gram-negative bacteria, LTA derived from Myocobacterium tuberculosis, fusion (F) protein of respiratory syncytial virus, peptidoglycan from Gram-positive bacteria, bacterial lipoproteins, atypic LPS produced by Leptospira interrogans and Porphyromonas gingivitis, as well as soluble CXCR1 from inflammatory sites in the lung. All TLRs, except TLR3, signal through the adapter molecule, MyD88, to activate the MAP Kinase cascades and the IκB kinase (IKK) complex. Activated IKKs rapidly phosphorylate IκBα to promote its ubiquitination and degradation, releasing the activated NF-κB into the nucleus. Activation of TLR3 or TLR4 allows a different adaptor molecule, TRIF, to associate with TBK1 and induce the phosphorylation and nuclear translocation of IRF3. TLR1, 2, and 6 have a conserved phosphatidyl inositol 3 kinase (PI3K) binding motif not found in other TLRs. Activation of PI3K and consequent calcium mobilization is particularly important for TLR2 signaling.
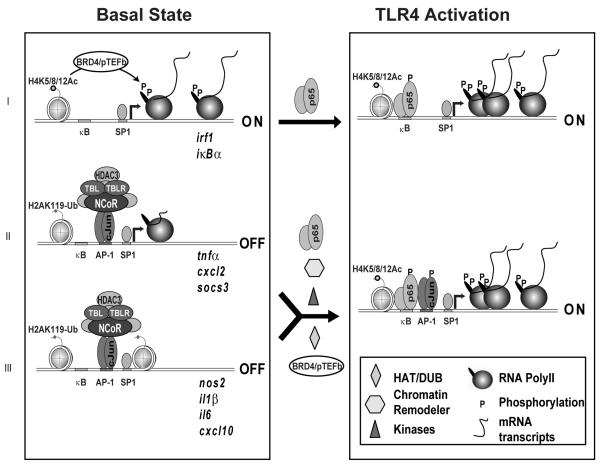
Figure 2. Three classes of inflammation responsive genes.
Class I inducible genes are basally expressed, and are further activated upon inflammatory signal. Class II inducible genes are “poised” with RNA Polymerase II positioned on the promoter in a paused state. Class III inducible genes are not decorated by RNA Polymerase II and are kept at a repressed state basally. Activation of Class II and Class III inflammatory response genes require removal of the basal corepressor complexes (NCoR), recruitment of transcription activators (p65) and coactivators (various kinases for phosphorylating transcription factors and RNA Polymerase II), as well as additional histone modifiers (Histone acetylase, HAT, and deubiquitin enzymes, DUB) and chromatin remodeling machinery.
Many of these inducible genes encode for critical inflammatory cytokines, chemokines, major histocompatibility complex, and co-stimulatory molecules involved in innate and acquired immunity. Together, these mediator molecules help to promote vascular permeability, upregulate expression of cell-adhesion molecules on vascular endothelium, and allow plasma proteins and leukocytes to gain access to extravascular tissue at the site of insult. The recruited neutrophils have enhanced phagocytic abilities and can release reactive oxygen and nitrogen intermediates, as well as toxic contents of their granules to facilitate the killing of microorganisms 13. After the initial insult has been removed, tissue macrophages facilitate inflammation resolution and tissue repair, at least in part, through secretion of transforming growth factor beta (TGFβ), growth factors, and anti-inflammatory lipid mediators, including lipoxins, resolvins, and protectins 14. When the acute inflammatory response fails to eliminate the initial disturbance, the inflammatory process acquires new characteristics, including pathological tissue remodeling, fibrosis, reduced normal tissue functions, and even persistent tissue metaplasia.
Sensors of inflammatory stimuli have well-documented roles in chronic inflammatory diseases, including atherosclerosis, diabetes mellitus, arthritis, inflammatory bowel diseases, and neurodegenerative diseases in human patients and animal models 15-19. Elevated PRRs expression and their activation by local endogenous and exogenous mediators correlate with the pathological states of obesity, diabetes, and coronary artery diseases in human 20-23. Single nucleotide polymorphisms in genetic loci coding for various PRRs have been linked to differential risks for the development and progression of these inflammatory diseases in human population studies 24. Animal models revealed that deletion of PRRs are protective against diet induced insulin resistance 25 and artherosclerosis progression 17, 26. In contrary, administration of PRR agonists enhances local and systemic inflammation, increasing disease burden 18, 27.
In addition to PRRs, local tissue metabolic stresses, such as excess saturated fatty acids and free cholesterol, are sensed by intracellular lipid chaperone proteins, and cellular organelles, including the endoplasmic reticulum (ER) and mitochondria 28 . ER stress and mitochondrial activation can lead to increased inflammatory ROS production. ROS oxidation of high-density lipoproteins and low-density lipoproteins can convert these molecules into secondary inflammatory inducers. Malfunctioning of fatty acid chaperone proteins, ER, and mitochondria have been implicated in chronic inflammatory diseases, including type 2 diabetes and cardiovascular diseases in human 2, 29. Overall, the emerging picture suggests that receptors for inflammation inducers play quantitatively important roles in the initiation and progression of chronic inflammatory diseases.
Inflammation regulators: nuclear receptors
Tissue inflammation is a tightly regulated process. Given the need to resolve inflammation following eradication of the inciting stimuli and the importance of preventing excessive inflammation and the resulting tissue dysfunction, it is not surprising that inflammation is subject to counter-regulation at multiple levels. Signaling molecules downstream of TLR, Trif/MyD88, IRAKs/TRAFs, and NFκB, are negatively regulated in the cytoplasm by SARM/sMyD88, CYCLD/A20/Trim30a, and BCL1/ATF3 respectively (reviewed in 30). Members of the nuclear receptor superfamily of ligand-dependent transcription factors play diverse roles in the regulation of development, homeostasis and immune responses by positively and negatively regulating gene expressions. Many are found to crosstalk with the inflammatory signaling pathways and regulate the innate and adaptive immune system, contributing to inflammatory diseases in vivo 31, 32. We highlight below the roles of the ligand-binding glucocorticoid receptor (GR), peroxisome proliferator-activated receptors (PPARs), liver x receptors (LXR), and the orphan receptor, Nurr-1, in the physiology and pathology of inflammation and some of the recent advances in our understanding of the molecular mechanisms underlying their anti-inflammatory functions (illustrated in Figure 3). Several other members of the nuclear receptor family also contributed significantly to inflammatory processes, their roles in inflammatory diseases and the underlying mechanisms are briefly summarized in Table 1.
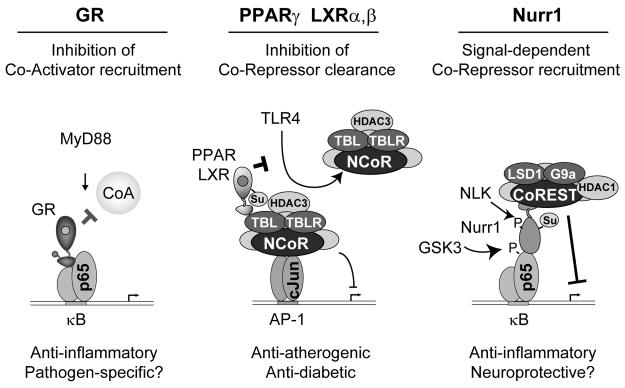
Figure 3. Nuclear receptor transrepression mechanisms.
Nuclear receptors can interfere with numerous mechanisms required for signal-dependent gene activation so as to suppress inflammatory responses. Representative examples of these mechanisms are illustrated for Glucocorticoid Receptor (GR), PPARγ/LXRs and Nurr1. GR represses inflammatory responses by blocking coactivator (CoA) recruitment to inflammatory response genes. Upon ligand binding, PPARγ and Liver X Receptors (LXRs) are SUMOylated and inhibit induction of inflammatory response genes by preventing signal-dependent clearance of NCoR co-repressor complexes that maintain basal repression. SUMOylated Nurr-1 represses inflammatory gene induction by recruiting the CoREST corepressor complex to NFκB target promoters.
Table 1.
| Nuclear Receptor |
Known Ligand | Disease Association |
Known Molecular Mechanisms |
|---|---|---|---|
|
Estrogen Receptors (NR3A1-2) |
17b-estradiol | Vascular injury 75, chemical induced intestinal inflammation 76 |
Decrease NFκB activation and increase TGFβ signaling 76 to modulate inflammation, growth factor expression, and oxidative stress 77. |
|
Vitamin D Receptor (NR1I1) |
Vitamin D metabolite: 1,25- dihydroxyvitamin D3 |
SLE 78 79, 80, type 1 diabetes 81 82, rheumatoid arthritis 83, inflammatory bowel disease 84, EAE 85 |
Inhibits T cell proliferation and cytotoxicity, induces differentiation and expansion of regulator T cells, decreases the expression of chemokines and subsequent monocyte infilatration 85. |
|
Retinoic acid receptors (NR1B1-3) |
Vitamin A: metabolite – all- trans-retinoic acid and 9-cis- retinoic acid |
Asthma 86, psoriasis, acne, photoaging and cancer 87 |
Enhance cytotoxity and T cell proliferation, low concentration allows for Th17 cell differentiation, while high concentration favors the induction of regulator T cells 88. |
|
LRH-1 (NR5A2) |
Unknown | Inflammatory bowel disease 89, lipid absorption 90 |
SUMO and NCoR dependent anti- inflammatory mechanism 62. |
Glucocorticoid Receptor
GR is prototypic of a subset of the ligand-dependent nuclear receptors that integrate host immune responses with physiological circuits that are required for maintenance of necessary organ functions. Glucocorticoids have potent anti-inflammatory effects and have been used clinically to treat inflammatory diseases since mid 1900s (reviewed in 33). Similarly, animal studies have supported its protective role against cholesterol induced atherosclerosis 34. The ability of GR to repress inflammatory responses is thought to result, at least in part, from its ability to interfere with the activities of other signal-dependent transcription factors, including those of NF-κB and activator protein-1 (AP-1) 35, by direct interactions with NF-κB components 36, induction of negative regulators that target signaling molecules involved in activating NF-κB and AP-1, including IL-10, GILZ, MKP-1, and IκBα 37, 38, disruption of activator/coactivator complexes 35, blockage of transcriptional elongation 39, and/or alteration of the epigenetic states of chromatins on target gene promoters through MSK1 and GRIP-1 40-42.
PPARs
PPARs play important roles in regulating metabolism, cell differentiation, and tissue inflammation that contributes to metabolic disorders and cardiovascular diseases 43. Two classes of clinical drugs for increasing insulin sensitivity in type II diabetes and lowering circulating fatty acids and triglycerides, thiazolidinediones (TZDs) and fibrates, target PPARγ and PPARα respectively. In animal models, deletion of PPARγ from macrophages results in insulin resistance in lean animals and a loss of the full antidiabetic effects of synthetic PPARγ agonists in obese and insulin resistant mice 32. These findings are consistent with important functions of PPARγ in macrophages in controlling the production of pro-inflammatory mediators that help promote the insulin-resistant state. Deletion of PPARα from macrophages results in elevation of NFκB expression and increased atherosclerotic lesion formation 44. Similarly, PPARδ has been suggested to negatively regulate inflammatory responses implicated in chemical induced colitis, experimental autoimmune encephalomyelitis, and atherosclerosis 45.
Multiple mechanisms have been described to account for the anti-inflammatory action of PPARγ. In endothelial cells and vascular smooth muscle cells, activation of PPARγ inhibits the phosphorylation of NFκB, decreasing its transcriptional activities 46, 47. In the adaptive immune system, PPARγ activation decreases the capacity of dendritic cells to prime naïve T lymphocytes and its ability to interact with critical transcription factor, NFAT, reduces the production of pro-inflammatory molecules in T lymphocytes 48, 49. In cells of the innate immune system, PPARγ activation promotes expression of anti-inflammatory mediators, including IL-10 and LXR, and contributes to the phenotype of alternatively activated macrophages that exert suppressive effects on inflammation 50. In classically activated macrophages, PPARγ inhibits the transcription of genes coding for pro-inflammatory molecules, including MCP-1, NOS2, IL-1β, IL-12, and MMP9. The molecular mechanism underlying the repression of these inflammatory genes has been recently identified. In macrophages, many of the inflammatory responsive genes are kept at a “repressed but poised state” by the Nuclear Receptor Corepressor (NCoR/SMRT) checkpoint, (reviewed in 9), summarized in Figure 2. The dismissal of these corepressor complexes from inflammatory response genes is a prerequisite for their transcriptional activation by PRRs 51-53. Ligand activation of PPARγ induces an allosteric change that enables PIAS1-dependent SUMOylation of PPARγ by SUMO1 54. SUMOylated PPARγ binds to NCoR complexes on PPR-inducible gene promoters and prevents the signal-dependent turnover of NCoR. As a consequence, NCoR complexes continue to exert repression functions, resulting in attenuated transcription activation and dampened subsequent inflammatory responses 54.
Similar to PPARγ, PPARα activation also decreases NFκB and AP-1 activities in liver and endothelial cells 55. Three major mechanisms have been described for the anti-inflammatory actions of PPARβ/δ, including the induction of anti-inflammatory co-repressor BCL6 protein, inhibition of NFκB, and induction of anti-inflammatory mediators, such as TGFβ and RSG4 and RSG5 45.
LXRs
LXRs are sensors of cholesterol metabolites in vivo 56. In animal models, administration of synthetic LXR ligands can reduce atherosclerosis, while deficiencies in LXRs result in disturbed cholesterol homeostasis, promoting exaggerated inflammatory responses and accelerated diseases pathology 31, 57. LXRs play a role in regulating immunologic synapse formation in dendritic cells and have anti-proliferative effects on T cells 58, 59. In murine macrophages, ligand binding of LXRs promotes SUMOylation by SUMO2/3, using HDAC4 as the SUMO E3 ligase 60. Similar to PPARγ, SUMOylated LXRs exert transcription repression activities by directing their interaction with corepressor complexes, NCoR and SMRT, to inhibit a set of inflammatory genes in macrophages and other cell types 54, 60-62. Studies of primary macrophages derived from genetic knockout mice indicate that the NCoR/SMRT corepressors are required for nearly all of the transrepression functions of LXRs in macrophages 52.
NR4A Family
Three members of the NR4A family, Nurr77, Nor1, and Nurr1, have been found to play important roles in regulating inflammatory diseases. These receptors are induced by atherogenic stimuli in macrophages and smooth muscle cells and are found in atherosclerotic plaques (reviewed in 63). Overexpression of these receptors decreases inflammatory cytokine and scavenger receptor expression, lowering LDL accumulation in macrophages and formation of foam cells 64. Nurr77 also inhibits smooth muscle cell proliferation and lowers inflammatory gene expression in smooth muscle cells, macrophages and endothelial cells 64, 65. In contrast, Nor1 induces VCAM-1 and ICAM-1 expression in endothelial cells, promote monocytes adhesion, and its deficiency decreases neointima formation in response to vascular injury66. Molecular mechanisms accounting for these divergent effects have not been established. Mutations in Nurr1 are linked to familial Parkinson's disease, and this association has been suggested to be due, at least in part, to the diminished negative regulation of inflammatory responses by Nurr1 in microglia and astrocytes resulting in neurotoxicity in the brain 67. Upon TLR activation, PIAS4 conjugates Nurr1 to SUMO2/3 67. SUMOylated Nurr1 and its associated CoREST corepressor complex interact with phosphorylated NFκB and dislodge it from gene promoters, attenuating the expression of NFκB-dependent inflammatory genes to help protect against Parkinson's disease in animal model 67.
Perspectives
Investigation of the intersection between an as yet small subset of nuclear receptors and inflammation pathways has led to new insights into basic transcriptional control mechanisms that are required for immunity and homeostasis. Systematic expression profiling experiments have documented the expression of 28 members of the nuclear receptor family in primary mouse macrophages, many of which exhibit dramatic changes in response to inflammatory stimuli 68. Corresponding studies in other immune cells have not as yet been performed, but are likely to yield similarly complex patterns of nuclear receptor expression. In addition, the biological roles and mechanisms of action of most members of the nuclear receptor family in regulating inflammation and immunity remain poorly understood. Even for the most intensively studied receptors, such as GR and PPARγ, many questions remain regarding the relative importance of positive and negative regulation of gene expression. Mechanistically, how are these receptors recruited to their respective gene target promoters to exert repression in ‘trans’ How are concentrations of endogenous ligands controlled at the local level in normal and disease states? Additional questions include whether post-translational modifications and corepressor/coactivator interactions modulate nuclear receptor functions and whether chronic inflammatory signals inactivate their protective effects. The respective contribution of each of the above molecular mechanism in inflammatory conditions in vivo remains to be elucidated in future studies.
Recent technological advances in performing genetic association studies, genome-wide localization studies (ChIP-seq), and transcriptome sequencing (GRO-Seq, RNA-Seq) will likely catalyze rapid progress in our understanding of how nuclear receptors re-engineer nuclear chromatin architectures, modulate expression of inflammatory genes and non-coding small RNAs with immuno-regulatory roles, and contribute to human inflammation-related diseases. Of note, many of the previously described molecular mechanisms of nuclear receptor function are studied in particular cell types, including macrophages, microglia, and endothelial cells. This begs the question of the generality of the described mechanisms. It is tempting to speculate that tissue-specific mechanisms should exist in vivo to facilitate different immunological and metabolic needs of different tissues in the context of an inflammatory response. It will therefore be of interest to use tissue-specific knockout animals in physiological and pathological contexts to evaluate the potential contribution of specific nuclear receptors in particular cell types in chronic inflammatory diseases.
A challenge of therapeutic interventions aimed at reducing inflammation is to tune down inflammatory programs that promote chronic disease processes without disarming the ability of the immune system to respond to infection or altering the homeostatic metabolic states of the organism. Although current therapeutic approaches that target members of the nuclear receptor super family have potent anti-inflammatory effects, many are associated with adverse side effects. For instance, glucocoticoids alter glucose homeostasis and inhibit the bone-forming activities of osteoclasts, among other adverse effects, resulting in hyperglycemia and osteoporosis that limit their use in treating chronic conditions in human patients. Long term exposure to LXR ligands could potentiate inflammatory responses by upregulating TLR4 expression and increase in triglyceride synthesis that could contribute to hepatic steatosis 69. Therapeutic approaches that prevent activation of sensors of inflammatory signals, such as biologicals that specifically target inflammatory cytokines such as TNF and IL-1 70 remain costly with delivery constraints and pitfalls of disease recurrences when treatment ceases. Together, the needs for novel therapeutics for treating chronic inflammatory conditions remain substantial.
One potential level of intervention is at the level of corepressor function, as illustrated by the anti-inflammatory activities of the nuclear receptors, PPARγ and LXRs. As discussed earlier, inflammation promoting genes are tightly regulated under basal conditions by the NCoR corepressor complexes that are recruited to broad sets of inflammatory response genes by members of the AP-1 transcription factor family member, c-Jun 51, 52, 71. NCoR/SMRT clearance from inflammatory response genes is a prerequisite for their transcription activation in response to external inflammatory stimuli 51-53. Recent studies identified several kinases, IKKα, JNK, IKKε and CaMKIIγ, downstream of cell surface receptor signaling that promote phosphorylation of components of the basal corepressor complex to facilitate corepressor turnover and activation of a subset of their respective target genes 72-74. These kinases represent a potentially important class of pharmacological targets as their inhibitors will likely mimic nuclear receptor anti-inflammatory effects by blocking corepressor turnover and inflammatory gene activation, yet bypassing the clinically significant side effects associated with therapies that target the nuclear receptors systemically. Recently, small-molecule inhibitor for JNK has been shown to be effective in treating arthritis in animal models 70. Better understanding of the process of inflammation and its natural regulatory pathways along with recent development of kinome-wide screens and tissue-specific drug delivery strategies will facilitate the identification of new therapeutic strategies for treating chronic inflammatory diseases in humans.
Acknowledgement
W.H. was supported by NRSA 1F31DK083913. CKG was supported by a Leducq Transatlantic Network Grant and Grants from the National Institutes of Health (RO1 CA52599, PO1 HC088093, and PO1 DK074868).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 4.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 8.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 12.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 13.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 14.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 15.Greene CM, Carroll TP, Smith SG, Taggart CC, Devaney J, Griffin S, O'Neill SJ, McElvaney NG. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 16.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 17.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DM, Velloso LA, Carvalheira JB, Saad MJ. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol. 2008;199:399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 20.Shiraki R, Inoue N, Kobayashi S, Ejiri J, Otsui K, Honjo T, Takahashi M, Hirata K, Yokoyama M, Kawashima S. Toll-like receptor 4 expressions on peripheral blood monocytes were enhanced in coronary artery disease even in patients with low C-reactive protein. Life Sci. 2006;80:59–66. doi: 10.1016/j.lfs.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moazed TC, Campbell LA, Rosenfeld ME, Grayston JT, Kuo CC. Chlamydia pneumoniae infection accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 23.Laman JD, Schoneveld AH, Moll FL, van Meurs M, Pasterkamp G. Significance of peptidoglycan, a proinflammatory bacterial antigen in atherosclerotic arteries and its association with vulnerable plaques. Am J Cardiol. 2002;90:119–123. doi: 10.1016/s0002-9149(02)02432-3. [DOI] [PubMed] [Google Scholar]
- 24.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 25.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, Van Middelaar BJ, Pasterkamp G, Quax PH, De Kleijn DP. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–398. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 28.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci U S A. 2006;103:6970–6975. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 31.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickard AJ, Young MJ. Corticosteroid receptors, macrophages and cardiovascular disease. J Mol Endocrinol. 2009;42:449–459. doi: 10.1677/JME-08-0144. [DOI] [PubMed] [Google Scholar]
- 34.Poon M, Gertz SD, Fallon JT, Wiegman P, Berman JW, Sarembock IJ, Taubman MB. Dexamethasone inhibits macrophage accumulation after balloon arterial injury in cholesterol fed rabbits. Atherosclerosis. 2001;155:371–380. doi: 10.1016/s0021-9150(00)00605-5. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 37.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 38.Caelles C, Gonzalez-Sancho JM, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, De Bosscher K. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 2008;27:1682–1693. doi: 10.1038/emboj.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chinenov Y, Sacta MA, Cruz AR, Rogatsky I. GRIP1-associated SET-domain methyltransferase in glucocorticoid receptor target gene expression. Proc Natl Acad Sci U S A. 2008;105:20185–20190. doi: 10.1073/pnas.0810863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reily MM, Pantoja C, Hu X, Chinenov Y, Rogatsky I. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. Embo J. 2006;25:108–117. doi: 10.1038/sj.emboj.7600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan SZ, Usher MG, Mortensen RM. PPARs: the vasculature, inflammation and hypertension. Curr Opin Nephrol Hypertens. 2009;18:128–133. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- 44.Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ, Semenkovich CF, Fazio S, Linton MF. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;116:1404–1412. doi: 10.1161/CIRCULATIONAHA.106.684704. [DOI] [PubMed] [Google Scholar]
- 45.Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol Ther. 2009;124:141–150. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91:427–433. doi: 10.1161/01.res.0000031271.20771.4f. [DOI] [PubMed] [Google Scholar]
- 48.Szatmari I, Rajnavolgyi E, Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann N Y Acad Sci. 2006;1088:207–218. doi: 10.1196/annals.1366.013. [DOI] [PubMed] [Google Scholar]
- 49.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 50.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose DW, Johnson RS, Rosenfeld MG, Glass CK. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci U S A. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–693. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 54.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 56.Barish GD. Peroxisome proliferator-activated receptors and liver X receptors in atherosclerosis and immunity. J Nutr. 2006;136:690–694. doi: 10.1093/jn/136.3.690. [DOI] [PubMed] [Google Scholar]
- 57.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- 59.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blaschke F, Takata Y, Caglayan E, Collins A, Tontonoz P, Hsueh WA, Tangirala RK. A nuclear receptor corepressor-dependent pathway mediates suppression of cytokine-induced C-reactive protein gene expression by liver X receptor. Circ Res. 2006;99:e88–99. doi: 10.1161/01.RES.0000252878.34269.06. [DOI] [PubMed] [Google Scholar]
- 62.Venteclef N, Jakobsson T, Ehrlund A, Damdimopoulos A, Mikkonen L, Ellis E, Nilsson LM, Parini P, Janne OA, Gustafsson JA, Steffensen KR, Treuter E. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 2010;24:381–395. doi: 10.1101/gad.545110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pearen MA, Muscat GE. Minireview: Nuclear Hormone Receptor 4A Signaling: Implications for Metabolic Disease. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 65.Bonta PI, Pols TW, de Vries CJ. NR4A nuclear receptors in atherosclerosis and vein-graft disease. Trends Cardiovasc Med. 2007;17:105–111. doi: 10.1016/j.tcm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Nomiyama T, Zhao Y, Gizard F, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Bruemmer D. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation. 2009;119:577–586. doi: 10.1161/CIRCULATIONAHA.108.822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19:2466–2477. doi: 10.1210/me.2004-0529. [DOI] [PubMed] [Google Scholar]
- 69.Fontaine C, Rigamonti E, Nohara A, Gervois P, Teissier E, Fruchart JC, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ Res. 2007;101:40–49. doi: 10.1161/CIRCRESAHA.106.135814. [DOI] [PubMed] [Google Scholar]
- 70.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 71.Weiss C, Schneider S, Wagner EF, Zhang X, Seto E, Bohmann D. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. Embo J. 2003;22:3686–3695. doi: 10.1093/emboj/cdg364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell. 2009;35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Wiesner P, C S, Almazan F, Benner C, Huang W, Butler S, Witztum JL, Glass CK, Miller YI. Minimally oxidized LDL and LPS cooperatively activate macrophages via AP-1 and NF-kappaB. Circulation Research. 2010 doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mendelsohn ME. Estrogen actions in the cardiovascular system. Climacteric. 2009;12(Suppl 1):18–21. doi: 10.1080/13697130903020291. [DOI] [PubMed] [Google Scholar]
- 76.Giroux V, Lemay F, Bernatchez G, Robitaille Y, Carrier JC. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int J Cancer. 2008;123:303–311. doi: 10.1002/ijc.23532. [DOI] [PubMed] [Google Scholar]
- 77.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 79.Abe J, Nakamura K, Takita Y, Nakano T, Irie H, Nishii Y. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha,25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 80.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 81.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol. 2004;173:2280–2287. doi: 10.4049/jimmunol.173.4.2280. [DOI] [PubMed] [Google Scholar]
- 82.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6–10. doi: 10.1177/0961203307085879. [DOI] [PubMed] [Google Scholar]
- 84.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 85.Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 86.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 87.Thacher SM, Vasudevan J, Chandraratna RA. Therapeutic applications for ligands of retinoid receptors. Curr Pharm Des. 2000;6:25–58. doi: 10.2174/1381612003401415. [DOI] [PubMed] [Google Scholar]
- 88.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 89.Coste A, Dubuquoy L, Barnouin R, Annicotte JS, Magnier B, Notti M, Corazza N, Antal MC, Metzger D, Desreumaux P, Brunner T, Auwerx J, Schoonjans K. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc Natl Acad Sci U S A. 2007;104:13098–13103. doi: 10.1073/pnas.0702440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J, Schoonjans K. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–8339. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]