Abstract
Objective
To examine the hypothesis that apathy is a core feature of Parkinson disease (PD) and that apathy can be dissociated from depression.
Methods
Eighty patients with PD and 20 patients with dystonia completed depression and apathy measures including the Marin Apathy Evaluation Scale (AES), Beck Depression Inventory (BDI), and Centers for Epidemiologic Studies–Depression Scale (CES-D).
Results
There was a significantly higher severity and frequency of apathy in PD (frequency = 51%, 41/80) than in dystonia (frequency = 20%, 4/20). Apathy in the absence of depression was frequent in PD and did not occur in dystonia (PD = 28.8%, dystonia = 0%).
Conclusions
Patients with Parkinson disease (PD) experienced significantly higher frequency and severity of apathy when compared with patients with dystonia. Apathy may be a “core” feature of PD and occurs in the absence of depression.
Recent attention has focused on a “syndrome of apathy”: a primary loss of motivation, loss of interest, and loss of effortful behavior.1,2 It has been proposed that apathy can manifest in neurologic disorders as both a symptom and a syndrome.3 Specific diagnostic criteria for a syndrome of apathy include a primary lack of motivation that manifests itself in three domains. The behavioral domain includes lack of effort, lack of productivity, and dependence on others to structure activities. The cognitive domain includes loss of interest in new experience and lack of concern about one's problems. The affective domain includes flattened affect and lack of response to positive or negative events. Emphasized is the primary lack of motivation that is not purely accounted for by intellectual impairment, emotional distress, or diminished consciousness such as drowsiness or delirium.3
However, it remains unclear whether apathy is a distinct syndrome or merely a symptom of depression. Depressed patients often have symptoms of apathy. Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) criteria for a major depressive episode demand at least five symptoms and require either a depressed mood or a markedly diminished interest or pleasure in activities. Loss of interest is also a cognitive symptom of apathy. Thus, depression includes symptoms of apathy. However, it has been suggested that apathy can occur in the absence of depression, and depression can occur in the absence of apathy.2,4
At least six studies have investigated apathy in PD.1,2,4-7 Several used informant-based rating scales. Because motivation is an internal state that may be difficult for informants to assess accurately, especially given the difficulties patients with PD have in expressing emotions (e.g., “masked facies”). It may be more accurate to allow patients with PD to answer questions. Other studies did not use a movement disorder control group. Without such, there is no way to know if rates of apathy are uniquely higher in PD. Comparison with another subcortical disorder would be a strong test of whether apathy is a “core feature” of PD. Therefore, we chose primary idiopathic adult-onset dystonia as a clinical control group. Dystonia, like PD, is a movement disorder that is progressive and disabling. It is characterized by involuntary, sustained muscle contractions often resulting in twisting movements and abnormal postures. The pathophysiology of dystonia, like PD, involves basal ganglia circuitry.8,9 PD has been hypothesized to disrupt nonmotor basal ganglia connections to mesial frontal–anterior cingulate cortex (putatively involved in apathy), whereas dystonia has been hypothesized to disrupt basal ganglia connections to prefrontal cortex and the supplementary motor area.8,10 In the present study, we hypothesized the following: 1) Apathy will occur more frequently and severely among patients with PD than those with dystonia (≥14 apathy scale). This difference may be due to the involvement of the mesial frontal cortex in PD. 2) A larger proportion of patients with PD than with dystonia will have apathy in the absence of depression (≥14 apathy scale without ≥14 Beck Depression Inventory [BDI]).
Methods
Consecutive patients with PD and dystonia from the University of Florida Movement Disorders Center Clinics were invited to participate during their routine medical appointment. To be included, participants had to meet diagnostic criteria for idiopathic PD or idiopathic adult-onset dystonia and be between ages 40 and 90. Exclusion criteria were co-morbid movement disorders, secondary causes of PD or dystonia, and neurosurgical treatments of deep brain stimulation or pallidotomy. Patients were not selected for any known cognitive or psychiatric state. Patients with PD were given the Unified Parkinson's Disease Rating Scale motor examination and a modified Hoehn–Yahr Scale11 to stage the severity and course of their disorder. Dystonia patients received the Unified Dystonia Rating Scale.12 Ninety-four patients with PD and 25 patients with dystonia were screened for entry into the study; 14 patients with PD and 5 patients with dystonia did not meet inclusion/exclusion criteria. The final sample included 80 patients with PD and 20 patients with adult-onset dystonia. Prior to participation, informed consent was obtained according to university and federal guidelines.
Participants completed mood questionnaires including a modified Apathy Evaluation Scale (AES14 as modified),1 the BDI,13 and the Center for Epidemiologic Studies–Depression Scale (CES-D).15
Modified AES
The modified AES is a 14-item scale measuring cognitive, emotional, and behavioral symptoms of apathy. Sample items include the following: “Are you interested in learning new things?” “Are you indifferent to things?” Items are rated on a 0-to-3 Likert Scale. The scale is abridged from the original 18-item version developed previously.14 The original scale was validated on subjects ages 55 to 85 with either stroke, AD, or major depressive disorder. These groups were compared with normal elderly control subjects. Internal consistency reliability average was 0.86; test–retest reliability (mean = 25.4-day interval) was 0.76. Convergent and divergent validity with anxiety and depression scales was established with the multitrait–multimatrix method.14 Interestingly, predictive validity and external validity were investigated by observing participants in various scenarios (e.g., playing video games, examining novelty gadgets). Self-reported apathy scores increased as total score on the video games and difficulty level at which participants chose to play decreased. Thus, there was a behavioral correlate with self-reported symptoms. The original scale was shortened by four items, and wording was simplified in 1992.1 This modified AES was reported to have excellent psychometric properties in PD (internal consistency reliability = 0.76, test–retest 1-week r = 0.90).
BDI
The BDI assesses symptoms of depression experienced over the last week. Literature has referred to the following classification of depression: minimal ≤13, mild = 14 to 19, moderate = 20 to 28, severe ≥29.16 This classification is used in the current study. The BDI can be divided into items that focus on depressive ideations (Items 1 to 13) and items that focus on somatic symptoms (Items 14 to 21). Ideational sample items include the following: “I feel guilty a good part of the time,” “I feel sad,” and “I feel I have failed more than the average person.” Somatic sample items include the following: “I get tired more easily than I used to” and “I don't sleep as well as I used to.” Differences between ideational and somatic subscales are useful to examine because medically disabled patients may endorse somatic complaints not necessarily related to depression (e.g., fatigue caused by medical disorder vs depression). The BDI is thought to have excellent reliability and validity in use with Parkinson patients.17
CES-D Scale
The CES-D also assesses depressive symptoms experienced over the last week. A score of ≥16 has typically been used to classify significant depressive symptoms in the general population. Yet, this has been criticized as producing a high false-positive rate and poor specificity in medical outpatients and inpatients.18,19 Therefore, the recommended cut-score of 20 was used.
The frequency of mood symptoms between the PD and dystonia patients was analyzed via two-way χ2 test for independence, whereas severity of mood symptoms was examined via independent sample t tests.
Results
Comparison of demographic variables for patients with PD and dystonia are presented in table 1.
Table 1.
Patient characteristics
| Characteristic | PD patients, n = 80 |
DYS patients, n = 20 |
p Value |
t Value |
|---|---|---|---|---|
| Disease subtype | 91.2% tremor; 8.8% akinetic | 50% focal; 50% segmental | — | — |
| Age, y | 68.9 (9.5) | 60.9 (11.9) | <0.01 | 3.22 |
| Men/women | 55:25 | 6:14 | <0.01 | —3.32 |
| Education, y | 14.8 (3.0) | 15.1 (2.1) | NS | — |
| Dopa meds, % | 98.8* | — | — | — |
| Antidepressants, % | 51 | 20 | <0.01 | 2.47 |
| Duration of symptoms, y | 6.4 (5.7) | 8.0 (7.8) | NS | — |
| Hoehn–Yahr stage | 2.4 (0.67) | — | — | — |
| Parkinson motor score (UPDRS) | 29.5 (10.8) | — | — | — |
Approximately 99% of PD patients were taking dopaminergic medications (e.g., levodopa/carbidopa, dopamine agonists, with 1 patient prescribed levodopa/carbidopa on the day of evaluation).
PE = Parkinson disease; DYS = dystonia; UPDRS = Unified Parkinson's Disease Rating Scale.
The PD and dystonia groups did not significantly differ with respect to education or number of years with disease symptoms but did differ significantly with respect to age, gender, and percentage of patients prescribed antidepressants. Patients with PD were significantly older, more likely to be male, and more likely to be taking antidepressants.
Apathy and depression frequency and severity
The initial analysis examined whether patients with PD exhibited a significantly higher frequency of apathy than patients with dystonia (defined by ≥14 on the AES). The frequency of apathy was greater in patients with PD (frequency = 51%, 41/80) than dystonia patients (frequency = 20%, 4/20) (χ2 [1, N = 100] = 6.31, p = 0.012, φ correlation coefficient = 0.25). Moreover, the apathy scores of the patients with PD (mean = 13.06, SD = 7.28) were higher than those of patients with dystonia [mean = 9.4, SD = 7.25) (t[98] = 2.01, p = 0.041, d = 0.5). The effect size was moderate. Thus, apathy occurred more frequently and was more severe in PD.
The frequency of depression was examined between the two groups using BDI and CES-D scores (defined by ≥14 BDI, ≥20 CES-D). With the BDI, the frequency of depression was similar between patients with PD (frequency = 26.3%, 21/80) and patients with dystonia (frequency = 30%, 6/20) (χ2 [1, n = 100] = 0.114, p = 0.74, φ correlation coefficient = 0.034). A similar finding occurred when the CES-D scores were used to index frequency of depression (PD frequency = 31.2%, 24/77, dystonia frequency = 30.0%, 6/20, χ2 = 0.01, p = 0.92, φ correlation coefficient = 0.01). In terms of depression severity, differences between groups were not detected. However, results suggest moderate effects for patients with PD to be higher on BDI somatic items, though these did not reach significance. Other effect sizes of depression scores were weak to small, and none reached significance (table 2).
Table 2.
Means (SD) for depression scales
| Scale | PD patients, n = 80 |
DYS patients, n = 20 |
p Value |
t Value |
Cohen's d |
|---|---|---|---|---|---|
| BDI total | 10.2 (6.8) | 8.3 (5.5) | 0.25 | 1.15 | 0.29 |
| BDI ideational | 3.9 (3.8) | 3.5 (3.4) | 0.69 | 0.397 | 0.1 |
| BDI somatic | 6.4 (3.7) | 4.8 (3.3) | 0.09 | 1.7 | 0.43 |
| CES-D total | 12.8 (10.2)* | 11.3 (11.2) | 0.56 | 0.588 | 0.15 |
n = 77; CES-D data were missing from 3 PD patients.
PD = Parkinson disease; DYS = dystonia; BDI = Beck Depression Inventory; CES-D = Center for Epidemiological Studies–Depression.
Relationship between apathy and depression frequency
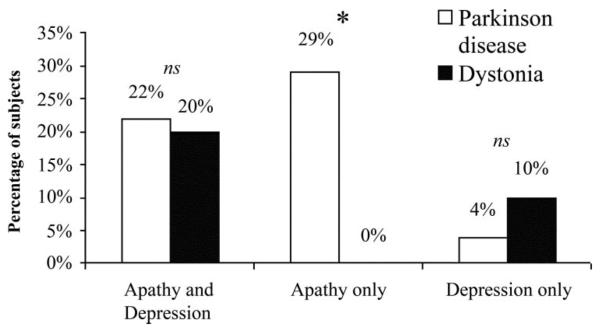
Another set of analyses tested the prediction that a larger proportion of patients with PD than patients with dystonia would exhibit apathy in the absence of depression. Patients were analyzed with respect to those who exhibited apathy only (≥14 on the AES without ≥14 on the BDI), depression only (≥14 on the BDI without ≥14 on the AES), and both apathy and depression (≥14 on the both scales). The frequency of patients exhibiting apathy alone was significantly higher in the PD group (frequency = 28.8%, 23/80) than in the dystonia group (frequency = 0%, 0/20) (Fisher exact significance = 0.006). The frequency of depression alone did not differ between PD (frequency = 4%, 3/80) and dystonia (frequency = 10%, 2/20) (Fisher exact significance = 0.261). Finally, the frequency of patients exhibiting both apathy and depression was not significantly different between PD (frequency = 22.5%, 18/80) and dystonia (frequency = 20.0%, 4/20) (Fisher exact significance = 1.0) (figure). Thus, approximately 30% of the PD sample exhibited apathy without depression, whereas none of the dystonia sample exhibited apathy without depression. The groups did not differ statistically in terms of depression alone or combined apathy and depression.
Figure.
Overlap between apathy and depression, apathy alone, and depression alone between groups.
Addressing demographic differences
The groups differed in terms of demographic variables. Patients with PD were more likely to be male, older, and taking antidepressant medication. Three post-hoc analytic approaches were used to examine demographic differences. As noted earlier, the percentage of patients who were prescribed antidepressants was greater in PD (51%) than in dystonia (20%). As such, the depression scores of the patients with PD may have been “artificially improved” due to the higher use of antidepressants. To address this possibility, subgroups of patients who had not been prescribed antidepressants were created, and this resulted in 38 PD and 14 dystonia patients. Group comparisons using independent t tests revealed that patients with PD tended to have higher total BDI scores than the dystonia patients (t[50] = 1.93, p = 0.059, d = 0.6). BDI ideational scores did not differ between groups, whereas BDI somatic symptoms were significantly higher in PD than dystonia. Further, the CES-D scores did not differ between the patients with PD and dystonia (table 3).
Table 3.
Means (SD) for patients not using antidepressants
| Scale | PD patients, n = 38 |
DYS patients, n = 14 |
p Value |
t Value |
Cohen's d |
|---|---|---|---|---|---|
| BDI total | 10.7 (7.6) | 6.4 (5.0) | 0.059* | 1.93 | 0.6 |
| BDI ideational | 4.1 (4.2) | 3.0 (3.2) | 0.403 | 0.844 | 0.26 |
| BDI somatic | 6.6 (3.8) | 3.4 (2.4) | 0.006† | 2.88 | 0.898 |
| CES-D total | 12.5 (11.0) | 10.0 (11.6) | 0.475 | 0.720 | 0.22 |
Values are significantly different at the level of a trend.
Values are significantly different.
PD = Parkinson disease; DYS = dystonia; BDI = Beck Depression Inventory; CES-D = Center for Epidemiological Studies–Depression.
To examine the unique effects of antidepressant usage, gender, and age on apathy and depression scores, these variables were used as covariates in parametric analyses. Antidepressant usage and gender were not related to apathy score (p = 0.79, p = 0.57). Age was found to be related to apathy score (F[1, 93] = 7.64, p < 0.01, r = 0.27). These factors (antidepressants, gender, age) were not related to the Beck depression score (p = 0.95, p = 0.85, p = 0.488). To further address the relationship between age and apathy, we created age-matched subgroups. These subgroups consisted of 18 patients with PD (mean = 63.2 years, SD = 10.24 years) and 18 dystonia patients (mean = 62.83 years, SD = 10.92 years). Results using these age-matched subgroups were in the expected direction; their significance was at trend level likely due to reduced power (e.g., n = 36). Patients with PD had higher mean apathy scores (mean = 12.16, SD = 6.7) than patients with dystonia (mean = 9.88, SD = 7.37) (t[34] = 0.97, p = 0.17, d = 0.32). Fifty percent (9/18) of patients with PD experienced apathy, whereas 22% (4/18) of patients with dystonia experienced apathy (χ2 [1, n = 36] = 3.01, p = 0.08, φ correlation coefficient = 0.29). The frequency of depression was similar across groups: 33% (6/18) of patients with PD and 33% (6/18) of patients with dystonia (p = 1.0). Finally, 22% of the patients with PD (4/18) showed apathy in the absence of depression, whereas no patients with dystonia showed this result (0/18) (Fisher exact significance = 0.05).
Discussion
We investigated two hypotheses. The first hypothesis was that apathy would occur more frequently and severely in patients with PD than in a movement-disordered control group (dystonia). This was based on the idea that certain frontal–subcortical systems putatively involved in apathy (e.g., anterior cingulate–mesial frontal cortex) are more disrupted in PD than in dystonia. The second hypothesis was that apathy is a core feature of PD and not simply a symptom of depression. Therefore, it was predicted that a larger proportion of patients with PD would exhibit apathy in the absence of depression than dystonia patients.
The first hypothesis was supported by the data. The frequency of apathy was significantly higher in PD than dystonia. Approximately half of the patients with PD experienced apathy compared with one-fifth of the patients with dystonia. Moreover, patients with PD had significantly more severe apathy scores than dystonia patients. The effect size was moderate, suggesting that the differences between the two groups are clinically meaningful. There were no statistical differences detected between groups for BDI depression scores or CES-D depression scores. As patients with PD were more likely to be prescribed antidepressant medications than the dystonia patients, it is possible that depression measures were “artificially” lowered in the PD group. This led us to carefully examine depressive symptoms among the subgroup of patients with PD (n = 38) dystonia patients (n = 14) who were not taking antidepressants. Scores on the CES-D did not differ between groups, although BDI scores tended to be higher in the patients with PD (p = 0.059). When further examined, it appeared that the somatic rather than the ideational symptoms of depression were driving this difference. Thus, it seems that the difference between groups on the BDI was due to patients with PD experiencing more somatic complaints than the patients with dystonia.
The second hypothesis that patients with PD had significantly greater frequency of apathy in the absence of depression than dystonia patients was supported. Twenty-nine percent of patients with PD showed apathy in the absence of depression, whereas no dystonia patients showed this result. This was the most dramatic and potentially important finding of the study. This finding suggests that apathy in PD, but not dystonia, manifests at a significant level independent of depression level. It is proposed that some of the differences in apathy may result from differing pathophysiology underlying each basal ganglia disorder. PD may disrupt mesial frontal–anterior cingulate cortex connections, which seem to be involved in apathy in other neurologic conditions.20 This area is also involved in the syndrome of extreme apathy: akinetic mutism.21 Carefully controlled neuroepidemiologic and neuroimaging studies may further our understanding about the brain areas involved in the expression of apathy.
A high prevalence of apathy in PD is consistent with the current literature. Three studies have examined self-reported apathy in PD, and each has reported the prevalence of apathy as 38, 42, and 43%.1,2,4 These rates are similar to that found in the current study (51%). Two of these studies broke down their prevalence figures into apathy alone, depression alone, and apathy and depression combined. A 1992 study reported 12% apathy alone (6/50 PD subjects), 21.6% depression alone (13/50 PD subjects), and 30% apathy and depression combined (15/50 PD subjects).1 The percentage of combined apathy and depression is similar to that found in the current study. The percentage of apathy alone was lower than the current study (12 vs 29%). A 2002 study reported 23.3% apathy alone (7/30 PD subjects), 13% depression alone (4/30 PD subjects), and 46.6% apathy and depression combined (14/30 subjects).2 These prevalence values are similar to those found in the current study. It should be noted that these values were based on an Italian translation of the scale. Turning to the severity of apathy in these two studies, the patients with PD showed significantly more severe apathy than normal elderly as well as osteoarthritis patients.
Although some problems surrounding the use of caregiver ratings of apathy in PD were presented earlier, it is of interest to examine the results from the three apathy studies that relied on the caregiver-rated Neuropsychiatric Inventory (NPI). Overall, these studies found lower rates of overall apathy than the current study. A comprehensive epidemiologic sample of 139 patients with PD found a prevalence rate for apathy of 16.5% (23/139 PD subjects), with 4.3% of the total PD subjects showing apathy in the absence of depression (6/139 PD subjects).5 A further study used a subset of the same data and produced similar results for apathy in PD (e.g., 16.5%, 17/103 patients).6 Finally, a 1998 study found that 5% of patients showed apathy alone (2/40 PD subjects), and 28% of patients showed a combination of apathy and depression (11/40 PD subjects).7 These values are lower than those from self-report scales (e.g., 38 to 51% total apathy in self-reported versions vs 16.5 to 33% in caregiver-rated versions). There may be several explanations for this discrepancy. First, the data from one of the studies with a lower frequency were based on a selected sample: that of surgery candidates.7 Participants are often screened for significant psychiatric symptoms before being deemed surgically appropriate; therefore, they may have a lowered rate of overall psychiatric symptoms. Second, the NPI caregiver scale assesses fewer symptoms of apathy (and depression) than rating scales such as the AES or BDI. For example, there is only one item assessing the presentation of apathy on the NPI. One question does not cover the full diagnostic criteria. Not covering as many symptoms as other versions of scales could artificially lower the apathy scores for many patients with PD.
Despite the above criticisms, it is important to mention that all studies reviewed found some proportion of their sample to present with apathy in the absence of depression. As such, it appears that apathy and depression may indeed be separable in PD and that apathy may have a key place in the mood profile of the disorder. It may be important, therefore, for clinicians to screen for both apathy and depression so that patients can be triaged into appropriate treatment groups. There are currently effective pharmacologic and psychotherapeutic treatments for depression. Development of treatments for apathy in neurologic disorders is currently under way.22 However, it should be noted that although there are empirically validated treatments for major depressive disorder, there are currently no published clinical trial results to determine the most effective treatments for depression specifically in patients with PD. Additionally, educating caregivers and spouses about apathy may help them to understand that it is a characteristic of this disorder and likely a direct result of disease pathology. Thus, apathetic behavior is not under the PD patient's voluntary control and is not oppositional behavior or laziness, but a symptom of PD.
More theoretically, it is of interest to understand whether separate neural systems underlie depression and apathy. It may be that orbitofrontal–subcortical connections underlie depression in PD, whereas mesial frontal–anterior cingulate subcortical connections underlie apathy. It may additionally be important to study subgroups of patients that show high levels of apathy. For example, the literature proposes a relationship between PD apathy and certain cognitive deficits. High-apathy PD groups showed decreased performance compared with low-apathy PD groups in verbal fluency (Category test and Controlled Oral Word Fluency), changing mental categories (Wisconsin Card Sort categories sorted and errors), speeded task performance (color naming and word naming on the Stroop and Trails B), and inhibition (Stroop color–word naming).2,4 The current study did not assess cognitive status and therefore cannot address the relationship between cognitive dysfunction and apathy. The continued examination of the relationship between cognitive abilities and apathy is an important area for future studies.
There are several limitations to the current study. First, although every attempt was made to obtain control patients that matched the PD group in terms of demographics, this was not possible. The dystonia group tended to be younger (dystonia mean = 60.9 years, SD = 11.9 years vs Parkinson mean = 68.9 years, SD = 9.5 years), composed of more women (dystonia 70% vs Parkinson 31%), and less likely to be on antidepressants (dystonia 20% vs Parkinson 51%). Therefore, post-hoc analytic approaches were used to examine how demographic factors may have affected the findings. We found that age was significantly related to apathy severity, whereas gender and antidepressant usage were not. When we created two age-matched subgroups of PD and dystonia patients (n = 18/group), the findings were consistent with the original results. Apathy mean scores were higher in PD than in dystonia, the frequency of apathy was higher in PD than dystonia, and only patients with PD showed apathy in the absence of depression. Effect sizes remained consistent, whereas results presented at trend level (p = 0.05 to p = 0.1), likely due to reduced power (n = 100 to n = 36). The literature has provided mixed findings with respect to the relationship between apathy and age. In stroke patients, apathy has been associated with age.23, 24 However, previous studies with PD have not detected a relationship between apathy and age.1,4 The finding that apathy and age were related in this study may warrant further investigation.
Another limitation is the use of depression symptom checklists rather than psychiatric interviews and DSM-IV diagnoses. This would have allowed for distinct diagnoses of major depressive disorder, dysthymia, and other depressive mood disorders. Individual interviews with each patient would also have allowed us to examine previous psychiatric history. Inability to compare current results with past psychiatric histories is a limitation of this study. Another limitation is that apathy and depression have overlapping symptoms, so the scales overlap in content. The BDI includes item content that overlaps with apathy (e.g., Item 4: “I don't enjoy things the way I used to,” Item 12: “I am less interested in other people than I used to be”). It is possible that a particular symptom endorsement on the BDI might better represent apathy but is being counted in the depression total score. One way to address the limitation of apathy and depression total scores is to use a different methodologic approach. Item factor analysis could be used to investigate whether apathy and depression factors can be identified. Items that are thought to relate more to depression or more to apathy would be delineated a priori, and data would be examined whether responses fall into proposed groups. Based on the requirements of factor analysis, this approach would require several hundred subjects. Efforts are currently under way to continue subject recruitment to meet this goal.
It should also be noted that patients with PD and dystonia patients receive different pharmacologic treatments for their disorders. Patients with PD were treated with dopaminergic agonists and levodopa, whereas dystonia patients were not. Although this is an important difference to consider, it is unlikely that dopamine-containing drugs caused increased apathy in the patients with PD. This is because dopamine agents have shown preliminary support for the treatment of apathy. Currently, pharmacologic interventions for apathy in neurologic disorders have included four main approaches: dopaminergic agents, amphetamines, atypical anti-psychotics, and acetylcholinesterase inhibitors.24 Bromocriptine and amantadine are the dopaminergic agents that have been examined in the treatment of apathy. Preliminary support for the efficacy of bromocriptine and amantadine exists in traumatic brain injury and poststroke patients; however, most studies are case studies and small (n) studies.22 Carefully controlled randomized clinical trials are needed to continue examining treatment approaches for apathy.
Acknowledgments
Supported by a National Parkinson's Foundation Center of Excellence grant and NIH grant to M.S.O. (K23 NS044997-01A1) and NIH grants to D.B. (R01 MH62539 and R01 NS50633).
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Starkstein SE, Mayberg HS, Preziosi T, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 2.Isella V, Melzi P, Grimaldi M, et al. Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson's disease. Mov Disord. 2002;17:366–371. doi: 10.1002/mds.10041. [DOI] [PubMed] [Google Scholar]
- 3.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Pluck GC, Brown RG. Apathy in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:636–649. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarsland D, Litvan I, Larsen JP. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2001;13:42–49. doi: 10.1176/jnp.13.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- 8.Hallett M. Physiology of dystonia. In: Fahn S, Marsden CD, DeLong MR, editors. Dystonia 3: advances in neurology. Vol. 78. Lippincott–Raven; Philadelphia: 1998. pp. 11–18. [PubMed] [Google Scholar]
- 9.Jankovic J, Fahn S. Dystonic disorders. In: Jankovic JJ, Tolosa E, editors. Parkinson's disease and movement disorders. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 331–357. [Google Scholar]
- 10.Brown RG, Pluck G. Negative symptoms: the “pathology” of motivation and goal directed behaviour. Trends Neurosci. 2000;23:412–417. doi: 10.1016/s0166-2236(00)01626-x. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton RL, members of the UPDRS Development Committee . Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Lieberman A, editors. Recent developments in Parkinson's disease. Vol. 2. Macmillan Health Care Information; New Jersey: 1987. pp. 153–163. 1987. [Google Scholar]
- 12.Comella CL, Leurgans S, Wuu J, Stebbins GT. Chmura TDystonia Study Group. Rating scales for dystonia: a multicenter assessment. Mov Disord. 2002;18:303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT. Beck Depression Inventory. Psychological Corp.; San Antonio, TX: 1978. [Google Scholar]
- 14.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 16.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. Oxford University Press; New York: 2004. Tests of personal adjustment and emotional functioning; pp. 738–754. [Google Scholar]
- 17.Levin BE, Llabre MM, Weiner WJ. Parkinson's disease and depression: psychometric properties of the Beck Depression Inventory. J Neurol Neurosurg Psychiatry. 1988;51:1401–1404. doi: 10.1136/jnnp.51.11.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schein R, Koenig H. The Center for Epidemiological Studies–Depression (CES-D) Scale: assessment of depression in the medically ill elderly. Int J Geriatr Psychiatry. 1997;12:436–446. [PubMed] [Google Scholar]
- 19.Schulberg HC, Saul M, McClelland M, Ganguli M, Christy W, Frank R. Assessing depression in primary medical and psychiatric practices. Arch Gen Psychiatry. 1985;42:1164–1170. doi: 10.1001/archpsyc.1985.01790350038008. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JL. Frontal–subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 21.Tranel D. Functional neuroanatomy: neuropsychological correlates of cortical and subcortical damage. In: Yudofsky SC, Hales RE, editors. The American Psychiatric Press textbook of neuropsychiatry. American Psychiatric Press; Washington, DC: 1992. pp. 58–88. [Google Scholar]
- 22.van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci. 2005;17:7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, Kobayashi S, Yamagata S, Takahashi K, Yamaguchi S. Post-stroke apathy and regional cerebral blood flow. Stroke. 1997;28:2437–2441. doi: 10.1161/01.str.28.12.2437. [DOI] [PubMed] [Google Scholar]
- 24.Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson R. Apathy following cerebrovascular lesions. Stroke. 1993;24:1625–1630. doi: 10.1161/01.str.24.11.1625. [DOI] [PubMed] [Google Scholar]