Abstract
The tuberculin skin test (TST) quantifies cell-mediated immunity to tuberculosis antigens. Helminths suppress cell-mediated immunity, so we studied the effect of helminth infection and deworming on the TST in a randomized, double-blind, placebo-controlled study in an indigenous Amazon community (N = 195). Stool microscopy diagnosed helminths in 98% and co-infection with multiple species in 24% of study subjects. The TST was positive (≥ 10 mm) for 49%, and responses increased with age (P < 0.001), Bacille Calmette Guerin (BCG) vaccination (P = 0.01), and tuberculosis contact (P = 0.05). TST results had no association with helminth-egg concentrations, species, or co-infections (all P > 0.1). One month after deworming with albendazole (three daily 400-mg doses), helminths were reduced, but 63% remained infected with helminths. Albendazole did not cause a change in TST size (P = 0.8) or positivity (P = 0.9) relative to placebo. Thus, TST reactions were unaffected by albendazole therapy that partially cured intestinal helminth infections, and TST interpretation was unaffected by high-burden helminth infections and co-infection with multiple helminth species.
Introduction
Tuberculosis and helminths have similar geographic distributions, and both principally affect socio-economically disadvantaged populations. One-third of the world's population is infected with Mycobacterium tuberculosis, and 9.2 million new cases of tuberculosis occur worldwide annually.1 More than 1 billion people are infected with helminths.2
Only a minority of people inhaling M. tuberculosis becomes infected, and less than 10% of those infected develop tuberculosis disease. Cellular immunity is believed to be the principal immune defense against tuberculosis infection and disease. Helminth infections may suppress and modulate cellular immunity.3 For example, helminths are associated with impaired cellular immune response against human immunodeficiency virus (HIV),4 malaria, and tuberculosis.5 Chronic helminth infections are characterized by T-helper type-2 responses that may be permissive to helminth long-term survival. Intestinal helminth infections tend to modulate the immune response cytokine profile to a T-helper type-2 response, which may cause T-helper type-1 imbalance and consequently, diminished cellular immune response against tuberculosis.6
Measurement of the cellular immune T-helper type-1 response to tuberculosis antigens is used to diagnose latent tuberculosis infection in vivo in the tuberculin skin test (TST) and recently, also in vitro with the interferon-gamma release assays.7,8 Specifically, the TST quantifies the cutaneous induration caused by the localized type IV hypersensitivity reaction 48 hours after an intradermal injection of purified tuberculosis antigens (purified protein derivative [PPD]). The results may be interpreted as continuous data quantifying the strength of the reaction or qualitatively as positive or negative depending on if the reaction is larger or smaller than a locally defined threshold (10 mm in Peru). Suppression of cellular immunity by HIV,9 malnutrition,10 or immunotherapy11 increases tuberculosis risk and may cause a reduction in the size of the TST; this may cause a false-negative TST result. Similarly, helminth infections can suppress cellular immunity.3 We have previously shown that TST results were unaffected by sporadic, occasional helminth infections in a community where most individuals were not infected by helminth.12 Here, we extend this finding to assess if TST interpretation should be modified in the presence of high-burden helminth infections or co-infections with multiple helminth species. We tested the hypothesis that TST results may be suppressed in individuals heavily infected with helminths13 and that deworming with albendazole treatment may reverse this effect, modifying the results of the TST.14
Methods
Study design.
We recruited healthy volunteers in an area believed to have frequent and heavy helminth infections. We first investigated if the severity of endemic intestinal helminth infections was associated with the baseline anti-mycobacterial immune response that we assessed in vivo with the TST. We then did a randomized placebo-controlled study to determine if attempted deworming with albendazole treatment affected repeat TST results 4 weeks later in the same individuals. In this double-blind study, the participants and researchers were unaware of the results of all tests and the drug (albendazole or placebo) administered until the codes were broken at the end of the study (Figure 1).
Figure 1.
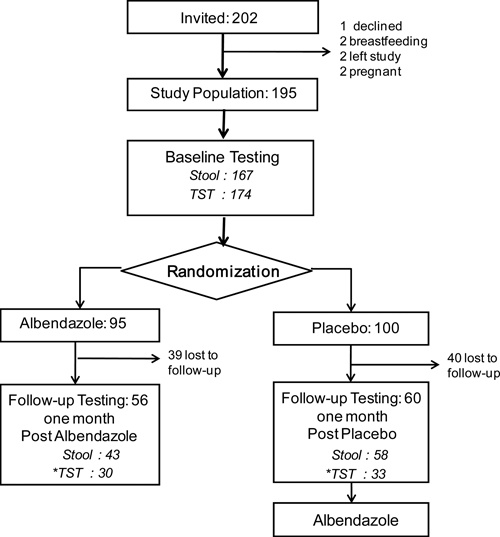
Study flowchart. TST indicates tuberculin skin test, and stool indicates stool parasitology diagnostic tests. * Follow-up TST was only done if the baseline TST was negative (< 10 mm).
Participants.
Adults (≥ 18 years old based on the Peruvian legal constitution) in Pevas, an indigenous rural community in the Peruvian Amazon, were randomly selected. Because of the lack of a town map or census, households were pseudo-randomly selected by visiting the first dwelling on the first housing block, the second dwelling on the second block etc. throughout the entire community. All eligible members of each randomly selected household were then invited to participate. Exclusion criteria were current pregnancy or lactation or in the past 6 months, tuberculosis treatment, TST, or deworming. The recruitment data are shown in Table 1 and included anthropometry as reported previously,12 socio-demographic data, and socio-economic data.
Table 1.
Population characteristics at the time of recruitment
| Feature | All participants (N = 195) | Albendazole (N = 95) | Placebo (N = 100) | P value* |
|---|---|---|---|---|
| Age, median years (IQR) | 40 (29–53) | 39 (29–52) | 43 (28–53) | 0.6 |
| Male gender, n (%) | 67 (34) | 26 (27) | 41 (41) | 0.05 |
| Schooling, median years, (IQR) | 6 (3–10) | 6 (3–9) | 6 (4–10) | 0.4 |
| Number of children in the household, median (IQR) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 0.6 |
| Anthropometry | ||||
| Body mass index, median (IQR) | 23 (21–25) | 23 (21–25) | 23 (22–25) | 0.6 |
| Percent body fat, median (IQR) | 30 (22–36) | 30 (20–36) | 31 (26–36) | 0.2 |
| Living conditions | ||||
| Earth floor, n (%) | 118/191 (62) | 65/93 (70) | 53/98 (54) | 0.07 |
| River water source, n (%) | 99/191 (52) | 49/93 (53) | 50/98 (51) | 0.5 |
| Lack of bathroom, n (%) | 93/191 (49) | 45/93 (48) | 48/98 (49) | 0.5 |
| Socio-economics | ||||
| Weekly money spent on food, median (IQR) | 20 (10–40) | 15 (10–35) | 20 (10–50) | 0.3 |
| Food spending per day per person, median (IQR)† | 0.14 (0.06–0.34) | 0.12 (0.05–0.29) | 0.12 (0.06–0.36) | 0.2 |
| Number of household residents, median (IQR) | 6 (4–8) | 6 (4–8) | 6 (4–8) | 0.9 |
| Parasites history | ||||
| Known past intestinal helminth infection, n (%) | 145/183 (80) | 69/90 (77) | 76/93 (82) | 0.4 |
| Tuberculin skin test | ||||
| Tuberculin skin-test size, median mm (IQR) | 9 (5–16) | 9 (5–16) | 9 (5–16) | 0.9 |
| Tuberculin skin-test positivity (≥ 10 mm), n (%) | 85/174 (49) | 42/86 (49) | 41/88 (47) | 0.8 |
| TB history | ||||
| Known past TB disease, n (%) | 12/192 (6) | 5/94 (5) | 7/98 (7) | 0.8 |
| Known past TB contact, n (%) | 34/191 (18) | 20/95 (21) | 14/96 (15) | 0.2 |
| BCG | ||||
| Absence of BCG scar, n (%) | 33/188 (18) | 18/92 (20) | 15/96 (16) | 0.5 |
| One BCG scar, n (%) | 64/188 (34) | 34/92 (37) | 30/96 (31) | 0.4 |
| Two or more BCG scar, n (%) | 91/188 (48) | 40/92 (43) | 51/96 (53) | 0.2 |
IQR = inter-quartile range; TB, tuberculosis.
Albendazole vs. placebo.
Money calculated in United States dollars.
Tests of anti-mycobacterial immunity.
Five units of tuberculin in 0.1 mL solution (Tubersol; Aventis-Pasteur, Toronto, Canada) were injected intradermally in the ventral forearm. Two days later, any resultant induration was measured using the ball-point pen test by a field worker blinded to the subject's experimental allocation. A response with the average of the transverse and longitudinal induration diameters ≥ 10 mm was considered positive, as defined by guidelines in Peru15 and elsewhere.16
Parasitology.
A single stool sample was collected from each participant into a new plastic dry pot at the time of recruitment. The stool sample was kept at ambient temperature and was tested the same day by microscopy for the presence of helminth eggs. Each stool sample underwent three microscopy tests: unconcentrated direct stool testing, the Baerman concentration technique, and cup-gravity sedimentation tests. The concentration of helminth eggs of each species was graded according to local criteria as strongly positive (++) if an average of more than one helminth egg of that species was seen per microscopy field, weakly positive (+) if the infection was present at lower egg density, or negative (-) if no eggs were seen. All slides were read by one person on the study team (A.V.) within 24 hours of sample collection, and most were reread for quality control by a local laboratory technician within 72 hours.
Deworming/placebo.
The recruitment tests generally took 2–4 days, and immediately after these tests, the participants were given either 400 mg of albendazole as a broad-spectrum deworming treatment or inert placebo pills similar in appearance to the albendazole one time daily for 3 consecutive days. Pills were administered with direct observation of consumption of every pill.
Repeat analyses after deworming/placebo.
Stool parasitology, questionnaire, anthropometry, and TST were repeated 4 weeks after albendazole/placebo. To prevent large and potentially ulcerated boosted reactions, TSTs were only repeated if the initial reaction diameter was < 10 mm. Initially negative TSTs (< 10 mm) were considered to have converted to positive if the repeat test was ≥ 10 mm and had increased in size by 6 mm or more.16
Ethics.
At the end of the study, participants who had received placebo during the study were treated with albendazole to ensure that all participants were offered the potential benefits of this therapy. Thus, all participants received the same tests and interventions and only the timing of the albendazole administration differed. For this reason, the project was not a clinical trial according to regional and national regulations and was approved as a research study that received ethics committee approval from the Iquitos Directorate of Health, the Asociación Benefica PRISMA, Lima, Perú, and Imperial College, London, United Kingdom. All participants gave informed written consent. In Peru, approximately one-half of all adults have positive TST;12 evidence of latent tuberculosis infection is not considered to be an indication for treatment, but any participants with symptoms suggestive of possible tuberculosis disease were referred for medical assessment.
Data analysis.
All analyses were performed using STATA 10.1 (Stata Corp., College Station, TX), and all tests were two-tailed tests. Non-Gaussian data are summarized as medians with inter-quartile ranges (IQR) and were compared with the Wilcoxon signed rank test and Spearman correlation coefficient. Proportions are presented with their 95% confidence intervals (95% CI). Categorical data were compared with the McNemar test, the two-sample z test of proportions, and logistic regression. Scars from bacille Calmette-Guerin (BCG) vaccination were counted. Because very few participants had more than two scars, these data were analyzed for trend across the three groups: no scar, one scar, or two or more scars. For linear regression, the TST size data were non-Gaussian and therefore, were transformed to their base-10 logarithm for analysis. Initial multiple regression models included all variables with a significance level of P < 0.2 in univariate analyses. The log-likelihood ratio test was used to sequentially remove non-contributory variables from the model in a stepwise manner, except for the universal confounding variables of age and sex that were included in all regression models.
Results
Population.
The study flow chart is shown in Figure 1, and the population characteristics are in Table 1. From the 202 people invited to join the study, 195 healthy people provided recruitment data and were considered to be the study population. Our study population included few males (34%), because local fishing and forestry work practices involved many men leaving the community intermittently. According to national statistics,17 the community population is > 95% indigenous, 46% (948/2,066) are < 15 years old, and 51% are male.
Socio-demographics.
In our study population, 97% reported owning their own home, 62% of which had an earth floor; 50% had access to a shared public toilet, and only 19% had a private latrine. The river on which the community was built served as the sole water source for 52% of participants. There was an average of six persons per household, and 97% of the population reported spending less than $1 on food per person per day.
Helminth infections.
On questioning, 79% of participants reported having been aware of previous intestinal helminth infections (Table 1), generally because they had seen parasites expelled in their stools spontaneously or after previous deworming treatment. The results of our stool parasitology tests are shown in Figure 2: intestinal helminths were detected in 98% of participants, Ascaris lumbricoides in 97%, Trichuris trichiuria in 14%, hookworms in 10%, and Strongyloides stercoralis in 4%. Co-infection with more than one helminth species was diagnosed in 24% of participants. The three participants without helminths detected in their stool were all health-care professionals. Quantitative helminth egg counts identified 36% of participants as having a high-burden helminth infection (Figure 2). Gastro-intestinal symptoms were rare and were not associated with the diagnosis of intestinal helminth co-infections or high helminth egg concentrations (all P > 0.1; data not shown).
Figure 2.
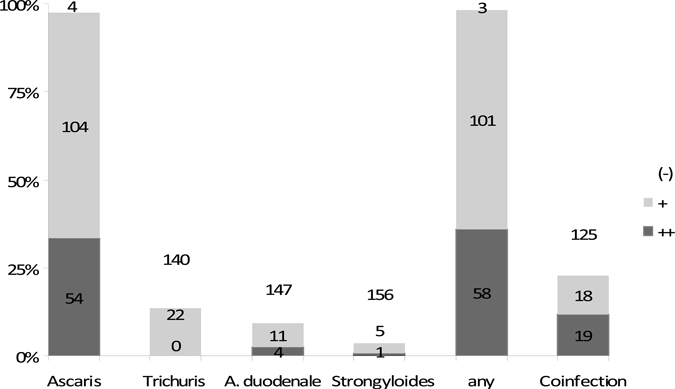
Intestinal helminth infections in study participants at recruitment. Stool parasitology was graded as strongly positive (++) if an average of more than one helminth egg was seen per microscopy field, weakly positive (+) if the infection was present at lower egg density, and negative (-) if no eggs were seen. For the co-infection with > 1 helminth bar, the highest helminth egg concentration data for either helminth species are shown. Five stool samples were excluded from this analysis, because they were sufficient only for helminth egg detection but not for quantification.
Anthropometry.
Body mass index calculation (Table 1) revealed no very low results (< 16 kg/m2) and that only 9% (17/191) of results were low (16–20 kg/m2). Regardless of if body mass index was assessed as a continuous or categorical variable, it was not associated with TST size or positivity (both P > 0.4). The percentage body fat was also not associated with TST size or positivity (all P > 0.08; Table 2)
Table 2.
Associations with tuberculin skin test (TST) size and positivity
| Recruitment TST size | Recruitment TST positivity | Follow-up increase in TST size | Follow-up TST conversion | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate regression (N = 174) | Multiple regression (N = 166) | Univariate regression (N = 174) | Multiple regression (N = 166) | Univariate regression (N = 63) | Multiple regression (N = 52) | Univariate regression (N = 63) | Multiple regression (N = 56) | |||||||||
| Co. | P value | Co. | P value | OR | P value | OR | P value | Co. | P value | Co. | P value | OR | P value | OR | P value | |
| Data collected at recruitment | ||||||||||||||||
| Age | 0.2 | 0.001 | 0.012 | 0.001 | 1.0 | 0.07 | 1.02 | 0.1 | 0.006 | 0.8 | −0.0079 | 0.8 | 1.0 | 0.4 | 1.03 | 0.3 |
| Gender | −0.001 | 1.0 | −0.0023 | 1.0 | 1.0 | 0.9 | 0.995 | 1.0 | −0.02 | 0.9 | 0.39 | 0.7 | 1.5 | 0.5 | 1.1 | 0.9 |
| BCG scars | 0.3 | 0.005 | 0.29 | 0.009 | 2.7 | 0.03 | 2.4 | 0.01 | −0.48 | 0.7 | −1.2 | 0.2 | 0.1 | 0.05 | 0.1 | 0.039 |
| Known TB contact | 0.28 | 0.05 | 0.30 | 0.03 | 2.0 | 0.07 | 2.4 | 0.041 | −0.3 | 0.2 | – | – | 0.6 | 0.6 | – | – |
| Body mass index | 0.003 | 0.8 | – | – | 1.0 | 0.7 | – | – | 0.02 | 0.2 | 0.21 | 0.1 | 1.2 | 0.09 | – | – |
| Percent body fat | 0.007 | 0.2 | – | – | 1.0 | 0.7 | – | – | 0.03 | 0.6 | – | – | 0.03 | 0.6 | – | – |
| Intestinal helminth infection* | −0.08 | 0.8 | – | – | 1.7 | 0.6 | – | – | 0.3 | 0.6 | – | – | – | – | – | – |
| Intestinal helminth co-infection | −0.11 | 0.4 | – | – | 0.6 | 0.2 | – | – | 0.09 | 0.9 | – | – | 2.1 | 0.3 | – | – |
| Data collected at follow-up | ||||||||||||||||
| Albendazole vs. placebo | NA | NA | NA | NA | NA | NA | NA | NA | 0.2 | 0.8 | – | – | 1.1 | 0.9 | – | – |
| Cure of helminths | NA | NA | NA | NA | NA | NA | NA | NA | −0.1 | 0.5 | – | – | 0.6 | 0.6 | – | – |
| Cure/reduction in egg concentration | NA | NA | NA | NA | NA | NA | NA | NA | −0.2 | 0.1 | – | – | 0.5 | 0.3 | – | – |
NA signifies that the analysis was not biologically appropriate, and – signifies that the variable did not feature in the multiple regression model (see Methods). Co = coefficient; OR = odds ratio.
This information could not be included in some analyses in which all participants with available data had intestinal helminth infection.
TSTs were positive for 49% of participants (85/174), and the median size was 9.0 mm (IQR = 5.0–16).
TST size and positivity were both significantly independently associated with age, BCG vaccination, and known past contact with a tuberculosis patient (Figure 3 and Table 2). TST size and positivity were both significantly associated with the presence of two or more BCG scars (Figure 3C and D; both P = 0.03) and with the number of BCG scars (Table 2; both P < 0.04). TST results (size, positivity, and anergic; 0-mm responses) all had no association with the diagnosis of helminth infection, infection with each specific helminth species, helminth egg concentrations, or diagnosis of helminth co-infections with multiple species (all P > 0.1).
Figure 3.
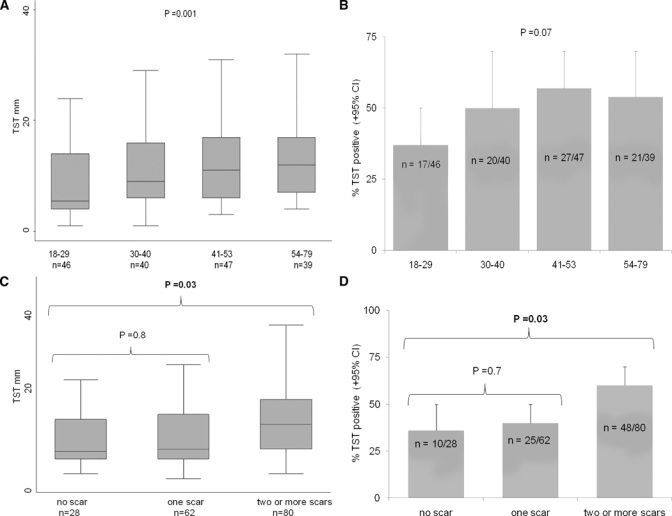
TST associations in univariate analyses at the time of recruitment. Box plots (A and C) show the median, inter-quartile range (shaded), and range, excluding outliers (whiskers), for the TST size; the bar graphs (B and D) show the percentage of positive TST reactions. (A) TST size and age quartiles. (B) TST positivity and age quartiles. (C) TST size and BCG vaccination. (D) TST positivity and BCG vaccination.
BCG vaccination was shown by the presence of one or more scars in 82% (155/188) of participants.
The presence of BCG scars was not associated with any species of helminth infection, helminth co-infections, or intensity of helminth infection (Table 1). No BCG scar was visible for 55% (5/11) of participants who reported past tuberculosis disease, significantly more than in people who did not report past tuberculosis disease (16%; 27/174; P = 0.01). Thus, the odds ratio of tuberculosis disease was 4.5 (95% CI = 1.3–16) if no BCG scar was visible (P = 0.02). Helminth infections were not associated with past tuberculosis (P = 0.5).
Follow-up.
Follow-up data were available for 59% of participants. Albendazole reduced helminth infections, egg density, and helminth co-infections (all P < 0.03). Consequently, at follow-up, the placebo group was more likely than the albendazole group to have helminth (P = 0.01) or a high-burden helminth infection (P < 0.0001). However, after albendazole, 63% of participants were infected with helminth. Follow-up TSTs were available for 71% of those eligible (i.e., with baseline TSTs < 10 mm), but this constituted only 63 individuals. The study was planned to test for a 33% effect of albendazole on TST conversion, but this partial follow-up resulted in the study having 80% power to detect only a > 66% effect of albendazole on TST conversion. In both the placebo and albendazole groups, the follow-up TST was significantly larger (both P < 0.004) and more likely to be positive (both P < 0.002) than the baseline TST. For the placebo group, the follow-up TST was, on average, 2.2 mm (standard deviation [SD] = 3.7 mm) larger than the baseline TST, and conversion occurred in 18% (6/30). For the albendazole group, the follow-up TST was, on average, 2.4 mm (SD 3.6 mm) larger than the baseline TST, and conversion occurred in 20% (6/33). Thus, the increase in TST size from baseline to follow-up and the frequency of TST conversion were similar for the placebo and albendazole groups (both P > 0.7). TST conversion was associated with BCG vaccination (Table 2; P < 0.05), but neither TST change in size nor conversion were associated with albendazole therapy, helminth cure, or change in egg concentration (Table 2; all P > 0.1).
Discussion
In this double-blind study, the severity of helminth infections and co-infections with several helminth species had no effect on TST results or their association with known risk factors for latent tuberculosis infection. Three consecutive daily doses of albendazole with directly observed therapy only caused partial deworming in this population that had hyperendemic intestinal helminths. In the absence of effective deworming, the albendazole therapy had no effect on the TST reactions.
As in some other villages in the Peruvian Amazon with a lack of clean water supply and sanitation, intestinal helminth infections were almost universal.18,19 Despite this, gastrointestinal symptoms were rare and were not associated with high helminth burden or helminth co-infections.20 The stool microscopy was performed by staff blinded to information about participants, and it was striking that only health-care professionals were free from intestinal helminths. This finding is probably explained by enhanced hygiene or frequent anti-helminthic administration in health-care staff. Because almost all participants had intestinal helminth infections, similar to other studies in this region,18,19 the current research could not meaningfully compare naturally infected versus uninfected individuals. However, our previous research in a nearby community12 and research in other settings19 have shown that TST results do not differ between individuals who are naturally infected versus naturally uninfected with intestinal helminths. The present research extended this knowledge by showing that there was no association between TST results and low versus high helminth egg burden or co-infections with multiple helminth species. Also, in contrast with other studies in communities with a high prevalence of intestinal helminths, we found no participants with completely unreactive TST,21,22 despite almost universal intestinal helminth infections and frequent co-infections with multiple intestinal helminths. Potential explanations for this include the likely lifelong helminth infections in the indigenous community in the present research.21
In previous studies, a single dose of albendazole cured intestinal helminths in more than 80% of participants.23,24 In contrast, in the current research, most participants remained heavily parasitized 1 month after three doses of albendazole. This could not be explained by incomplete adherence to the attempted deworming, because the albendazole was administered with directly observed therapy. Reinfection is unlikely to explain this observation, because the 1-month interval between attempted deworming and the presence of helminth eggs in the feces was probably too brief for gravid adult helminths to develop.13 Alternative potential reasons for the inadequate deworming in the current study include inadequate activity of the albendazole formulation used (although this was the regional standard formulation) and possible albendazole interactions with local foods or other host-related factors.24 With current living conditions and the treatment strategy that we used, sustained helminth eradication seems challenging. The inadequacy of repeated albendazole therapy was surprising and warrants comparison with the local use of indigenous plants such as OJE (Ficus insipid). Plants are popular in some native communities, because effective deworming is generally desired and drugs such as albendazole have limited availability.25
Albendazole treatment reduced parasite burden compared with the group that took placebo, but neither the administration of albendazole nor the associated decrease in the prevalence of intestinal helminths and egg concentrations affected TST size or positivity. This study, therefore, shows that three daily doses of albendazole had no effect on the TST 1 month later, which contrasts the anti-parasitic agent levimasole that does have direct immuno-modulatory effects.26 This is an important observation, because other studies have reported that TSTs increase in size after albendazole if the albendazole therapy cures intestinal helminth infections.14 The present study implies that if albendazole augments TST responses, then this action is mediated by deworming not by direct immunological effects of the drug albendazole.
TST size at the time of recruitment was independently associated with age, BCG vaccination, and contact with tuberculosis, consistent with previous studies.22,27 This shows that these associations, which were largely characterized without testing for interactions with parasitic infections, were maintained in this population with hyperendemic intestinal helminth infections. TST conversion from negative to positive when repeated 1 month later, termed boosting, occurred in one in five participants.16 This shows the importance of using the two-step TST if repeated testing is anticipated.16 This boosting of the repeat TST was unaffected by attempted deworming with albendazole but was significantly associated with past BCG vaccination, consistent with previous research.14 It was also noteworthy that BCG vaccination was associated with significant protection against tuberculosis in this population. This observation was based on self-reported, unconfirmed ascertainment of tuberculosis disease, but it is consistent with previous research28 and supports the ongoing use of the BCG vaccine in this area.
Limitations of the current study include the use of locally standard criteria applied to only a single stool sample for quantifying helminth egg concentrations, which may be insensitive and less accurate than repeated Kato–Katz stool tests. Also, the lack of electricity in this remote jungle community prevented TST confirmation with interferon-gamma release assays, which may be less influenced by past BCG vaccination than the TST. Another limitation is the significant proportion of the study population that were lost to follow-up because of their migratory work patterns; this may have introduced bias and reduced the power of the study to detect an effect of albendazole on the TST. An important finding is the partial deworming efficacy of the albendazole therapy, although this also limits the interpretation of some results. Thus, this study had sufficient sample size at both recruitment and follow-up to show associations between TST results and known determinants of tuberculosis immunity and boosting, including BCG vaccination. In contrast, there was no association between TST results and intestinal helminth worm burden, helminth co-infections, or partial resolution after albendazole therapy. Future research would usefully extend these findings by increasing follow-up assessment after deworming and by achieving more effective deworming.
Conclusions
TST reactions were unaffected by albendazole therapy that partially cured intestinal helminth infections. TST interpretation was unaffected by high-burden helminth infections and co-infection with multiple helminth species.
Acknowledgments
This research and members of the study team were funded by the charity Innovation For Health And Development (IFHAD), The Wellcome Trust, the Foundation for Innovative New Diagnostics (FIND), the Civil Society Challenge Fund of the Department For International Development of the British Government, the Bill and Melinda Gates Foundation, and by a scholarship from the Fulbright Program, United States. We are grateful to Paula Maguina, Maribel Rivero, and Silvia Carrera for administrative support, Robert H. Gilman for advice, and the health staff and population of Pevas for their invaluable assistance in this research.
Footnotes
Authors' addresses: Karine Zevallos, Héctor H. García, and Carlton A. Evans, Laboratorio de Investigación y Desarrollo, Universidad Peruana Cayetano Heredia, Av. Honorio Delgado #430 - Urb. Ingeniería, San Martin de Porres, Lima, Perú, E-mails: karen_zevallos@hotmail.com, hgarcia@jhsph.edu, and Carlton.Evans@IFHAD.org. Carlos Vidal, Ministerio de Salud de Loreto, Av. 28 de julio s/n, Distrito Punchana, Provincia de Maynas, Loreto, E-mail: vidalcar2003@yahoo.com. Katherine C. Vergara and Antonio Vergara, Asociación Benéfica PRISMA, Av Carlos Gonzales Nro 251, Urbanización Maranga, San Miguel, Lima 32, Perú, E-mails: k8vergara@gmail.com and antoniodvergara@gmail.com.
References
- 1.WHO . Global Tuberculosis Control—Surveillance, Planning, Financing. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 2.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, Bentwich Z. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–1060. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003;86:315–333. doi: 10.1016/s0001-706x(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira AP, Aguiar AS, Fava MW, Correa JO, Teixeira FM, Teixeira HC. Can the efficacy of bacille Calmette-Guerin tuberculosis vaccine be affected by intestinal parasitic infections? J Infect Dis. 2002;186:441–443. doi: 10.1086/341656. [DOI] [PubMed] [Google Scholar]
- 6.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi ME, Copello JA, Villalba-Galea CA, Velez P, Diaz Sylvester PL, Bolanos P, Marcano A, Fill M, Escobar AL. Differential Ca2+ and Sr2+ regulation of intracellular divalent cations release in ventricular myocytes. Cell Calcium. 2004;36:119–134. doi: 10.1016/j.ceca.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Reported HIV status of tuberculosis patients—United States, 1993–2005. MMWR Morb Mortal Wkly Rep. 2007;56:1103–1106. [PubMed] [Google Scholar]
- 10.Hernandez-Pando R, Orozco H, Aguilar D. Factors that deregulate the protective immune response in tuberculosis. Arch Immunol Ther Exp (Warsz) 2009;57:355–367. doi: 10.1007/s00005-009-0042-9. [DOI] [PubMed] [Google Scholar]
- 11.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen specific IFN-gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 12.Pelly TF, Santillan CF, Gilman RH, Cabrera LZ, Garcia E, Vidal C, Zimic MJ, Moore DA, Evans CA. Tuberculosis skin testing, anergy and protein malnutrition in Peru. Int J Tuberc Lung Dis. 2005;9:977–984. [PMC free article] [PubMed] [Google Scholar]
- 13.Buck AA, Anderson RI, MacRae AA. Epidemiology of poly-parasitism. III. Effects on the diagnostic capacity of immunological tests. Tropenmed Parasitol. 1978;29:145–155. [PubMed] [Google Scholar]
- 14.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministerio de Salud del Perú . Norma técnica de salud para el control de la tuberculosis. Lima; Peru: 2006. p. 36. [Google Scholar]
- 16.Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15–21. doi: 10.1164/ajrccm.159.1.9801120. [DOI] [PubMed] [Google Scholar]
- 17.INEI . Peru: Estimaciones de poblacion por departamentos, provincias, distritos 1995–2000. Pebas: Instituto Nacional de Estadística e Informática; 1999. rhttp://www1.inei.gob.pe/biblioineipub/bancopub/Est/Lib0004/INDICE.htm Loreto. Available at. [Google Scholar]
- 18.Chernela J, Thatcher V. The effects of settlement on the prevalence of Ascaris infection in two American populations of the Brazilian Amazon. Acta Amazon. 1993;23:25–35. [Google Scholar]
- 19.Cooper PJ, Chico ME, Gaus D, Griffin GE. Relationship between bacille Calmette-Guerin vaccination, Mantoux test positivity, and geohelminth infection. Trans R Soc Trop Med Hyg. 2003;97:473–476. doi: 10.1016/s0035-9203(03)90094-0. [DOI] [PubMed] [Google Scholar]
- 20.Sarinas PS, Chitkara RK. Ascariasis and hookworm. Semin Respir Infect. 1997;12:130–137. [PubMed] [Google Scholar]
- 21.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar AL, Coimbra CE, Jr, Camacho LA, Santos RV. Tuberculin reactivity and tuberculosis epidemiology in the Pakaanova (Wari') Indians of Rondonia, south-western Brazilian Amazon. Int J Tuberc Lung Dis. 2004;8:45–51. [PubMed] [Google Scholar]
- 23.Norhayati M, Oothuman P, Azizi O, Fatmah MS. Efficacy of single dose albendazole on the prevalence and intensity of infection of soil-transmitted helminths in Orang Asli children in Malaysia. Southeast Asian J Trop Med Public Health. 1997;28:563–569. [PubMed] [Google Scholar]
- 24.Bennett A, Guyatt H. Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitol Today. 2000;16:71–74. doi: 10.1016/s0169-4758(99)01544-6. [DOI] [PubMed] [Google Scholar]
- 25.Hansson A, Zelada JC, Noriega HP. Reevaluation of risks with the use of Ficus insipida latex as a traditional anthelmintic remedy in the Amazon. J Ethnopharmacol. 2005;98:251–257. doi: 10.1016/j.jep.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Argani H, Akhtarishojaie E. Levamizole enhances immune responsiveness to intra-dermal and intra-muscular hepatitis B vaccination in chronic hemodialysis patients. J Immune Based Ther Vaccines. 2006;4:3. doi: 10.1186/1476-8518-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipner EM, Gopi PG, Subramani R, Kolappan C, Sadacharam K, Kumaran P, Prevots DR, Narayanan PR, Nutman TB, Kumaraswami V. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am J Trop Med Hyg. 2006;74:841–847. [PubMed] [Google Scholar]
- 28.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31((Suppl 3)):S64–S67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]


