Abstract
Five genera of potentially zoonotic bacteria and parasites were detected in environmentally collected fecal samples from a remote indigenous community in Northern Saskatchewan, Canada. Organisms identified include Toxocara canis, Echniococcus granulosus, Giardia duodenalis, Cryptosporidium spp., and Campylobacter spp. The prevalence and intensity of Giardia spp. and Campylobacter spp. in fecal samples was particularly remarkable. Three-quarters of samples tested contained at least one zoonotic species of Campylobacter, and C. jejuni-containing feces had an average of 2.9 × 105 organisms/g. Over one-half of samples tested contained Giardia spp. with an average of 9,266 cysts/g. Zoonotic G. duodenalis Assemblage A was the only Giardia spp. genotype identified. These data suggest that canine feces have the potential to pose a significant health risk to Canadians in rural and remote indigenous communities.
Domestic dogs have long been recognized to be a potential source of zoonoses for people.1–4 In particular, zoonotic bacteria and parasites harbored in the canine intestine have been shown to pose a significant risk to human health.1–4 People are exposed to these pathogens through direct or indirect contact with infected dogs or their feces, and they may become infected after inadvertent ingestion of a zoonotic agent.2–4
In Canada, indigenous people living in rural and remote communities seem to have an increased risk of exposure to and infection with certain canine fecal zoonoses compared with other Canadians.5–7 This may be related to the fact that many of these communities have large populations of free-roaming domestic dogs and little access to veterinary care. These dogs have frequent contact with one another, canine feces, and a variety of refuse and foodstuffs that potentially contain zoonotic agents, all of which promote intestinal infection with a variety of zoonoses and subsequent human exposure.
Despite the apparent zoonotic risks that domestic dogs may pose to indigenous Canadians, there are very few contemporary studies that characterize the microbial and parasite content of canine feces in these communities. This is problematic, because people infected with canine fecal zoonoses often exhibit non-specific clinical signs6,8,9 that can be misdiagnosed if health care workers are unaware of the presence of these pathogens in their jurisdictions. Also, until the health risk posed by domestic dogs is better understood, it will not be possible to institute effective strategies to prevent human infection.
It is also important to consider that exposure to canine fecal zoonoses could present a more significant health problem in indigenous communities compared with other Canadian populations. Indigenous peoples seem to be at increased risk for certain infectious diseases, including those caused by zoonotic pathogens,5,7 likely because of traditional practices as well as risk factors associated with poverty, including poor nutrition and substandard housing.5,10 Also, infectious diseases may have a more significant impact on the health of indigenous people compared with other Canadians because of concurrent health problems and decreased access to health care.10
In 2008, a 6-year-old girl from a remote indigenous community in Northern Saskatchewan was diagnosed with an Echinococcus granulosus parasitic infection that was most likely acquired through contact with canine feces.11 The ensuing, community-based epidemiologic investigation revealed widespread exposure to and infection with E. granulosus in humans and dogs, respectively,11 creating concern that other zoonotic pathogens might be harbored by dogs in the community. To investigate this possibility, environmentally collected canine fecal samples were screened for a variety of bacterial and parasitic zoonoses.
One block was randomly selected within each of the three distinct neighborhoods that comprise the main community. All yards on that block were surveyed on foot, and any canid feces found were collected in individual plastic bags. Fecal samples were also collected from around the community landfill based on the researchers' suspicion that domestic dogs might frequent the landfill to scavenge on garbage. A total of 155 samples were collected from the four study sites. During the fecal-collection procedure, researchers observed numerous free-ranging dogs throughout the community, despite recent depopulation attempts. A numerical estimate of past or present dog populations could not be obtained.
Samples were subdivided, and subsamples were sent to the World Health Organization Collaborating Center for the Molecular Epidemiology of Parasitic Infections (Murdoch University, Murdoch, Australia) and the University of Saskatchewan (Saskatoon, Canada) where they were analyzed for the presence of E. granulosus as previously described.11
At the University of Saskatchewan, subsamples were also analyzed using quantitative fecal-flotation12 and sucrose-gradient13 techniques to concentrate and enumerate parasite eggs and Giardia spp. cysts/Cryptosporidium spp. oocysts, respectively.
To determine the predominant Giardia spp. genotypes, polymerase chain reaction (PCR) was performed on selected samples as previously described14 to amplify a segment of the G. duodenalis β-giardin gene. Samples that contained > 10,000 Giardia spp. cysts/g were selected for analysis, because these feces had the potential to cause the greatest environmental contamination with Giardia spp. A total of 19 samples with > 10,000 cysts/g had sufficient material available for analysis. Four samples with 1,000–10,000 cysts/g were also tested to evaluate the sensitivity of the PCR assay at our institution. PCR was performed on DNA extracted from sucrose gradient concentrates using the QIAGEN DNeasy Blood and Tissue Kit (QIAGEN Inc., Valencia, CA). Because G. duodenalis is the only Giardia spp. known to infect dogs9,15 and all samples were observed to contain Giardia spp. cysts on microscopic examination, any failure to amplify product was interpreted to be the result of poor sample integrity or test sensitivity. Any product obtained by PCR was sequenced using the amplification primers. Sequencing of the β-giardin gene allows G. duodenalis samples to be classified into groups of genotypes called assemblages.14 This classification is essential when determining the zoonotic potential of Giardia spp. found in canine feces, because dogs may be infected with assemblages A, B, C, and D, of which only A and B are known to infect humans.14–16 It should be noted that the genus Cryptosporidium contains several species with zoonotic potential.15,16 The Cryptosporidium spp. identified in this study were not identified to the species level; however, domestic dogs have the potential to become infected with C. parvum and C. canis, both of which are known to cause disease in people.15–17
A subset of 60 fecal samples, which was comprised of 20 randomly selected samples from each of the three neighborhoods in the community, was selected for total bacterial DNA extraction (QIAGEN Stool Kit; QIAGEN Inc., Valencia, CA). These samples were tested for the presence of 14 known species of Campylobacter using a cpn60-based real-time quantitative PCR18 (also conducted at the University of Saskatchewan). Bacterial culture was not performed because of financial constraints and the degraded state of many of the samples.
Distribution of pathogen-containing fecal samples and relative intensity of infection were compared among study sites using the χ2, Wilcoxon rank sum, and Kruskall–Wallis tests.19 All calculations were performed using STATA/IC 10.0 (StatCorp LLP, College Station, TX) with a significance level of P < 0.05.
Five genera of potentially zoonotic pathogens were found in 155 canine fecal samples collected within a northern Saskatchewan indigenous community (Table 1). Of the 24 Giardia spp.-containing samples analyzed by PCR, 13 (56%) produced amplicons of the expected size. Sequencing revealed that the DNA amplified from all 13 (100%) samples belonged to the zoonotic group of G. duodenalis genotypes known as assemblage A.9,14–16 Of the 60 samples tested for Campylobacter spp., 28 (47%) contained one or both of the established zoonotic species C. jejuni and/or C. upsaliensis.8,20
Table 1.
Prevalence and intensity of multiple zoonotic organisms identified in environmentally collected canine fecal samples from an indigenous Canadian community
| Pathogen | Prevalence* | Intensity† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Neighborhood 1 | Neighborhood 2 | Neighborhood 3 | Landfill | Total | Minimum | Median | Mean | Maximum | |
| Helminths | |||||||||
| Toxocara canis | 12/48 (33%) | 6/43 (16%) | 6/25 (24%) | 2/39 (5%) | 26/155 (17%) | 3 | 80 | 826 | 6,250 |
| Echinococcus granulosus | 1/48 (2%) | 5/43 (12%) | 3/25 (12%) | 0/39 (0%) | 7/155 (5%) | 4 | 15 | 22 | 75 |
| Protozoa | |||||||||
| Giardia spp. | 28/48 (58%) | 32/43 (74%) | 15/25 (60%) | 20/39 (51%) | 95/155 (61%) | 33 | 2,200 | 9,266 | > 55,000 |
| Cryptosporidium spp. | 2/48 (4%) | 2/43 (5%) | 0/25 (0%) | 1/39 (3%) | 5/155 (3%) | 5 | 18 | 29 | 68 |
| Bacteria | |||||||||
| Campylobacter spp. | 17/20 (85%) | 15/20 (75%) | 13/20 (65%) | na | 45/60 (75%) | na | na | na | na |
| Campylobacter jejuni | 6/20 (30%) | 5/20 (25%) | 9/20 (45%) | na | 20/60 (33%) | 1.6 × 104 | 9.6 × 104 | 2.9 × 105 | 2.3 × 106 |
| Campylobacter upsaliensis | 8/20 (40%) | 7/20 (35%) | 5/20 (25%) | na | 20/60 (33%) | 6.0 ×103 | 5.0 × 104 | 8.6 × 105 | 1.3 × 107 |
Proportion of fecal samples tested that contained the zoonotic organism of interest.
Number of infectious units per gram of feces (1 infectious unit = 1 egg for T. canis and E. granulosus, 1 cyst for Giardia spp., 1 oocyst for Cryptosporidium spp., and 1 bacterium for Campylobacter spp.)
There was no significant difference in the prevalence of Toxocara canis-, Giardia spp.-, Cryptosporidium spp.-, C. jejuni-, or C. upsaliensis-containing fecal samples between study sites. The prevalence of E. granulosus was significantly greater in neighborhoods 2 and 3 compared with neighborhood 1 and the landfill (P = 0.005). This could be the result of differences in the prevalence of E. granulosus infection among the different canid groups that frequent and/or populate the four sites, although the reason for these differences could not be determined.
The results of this study show that canine feces within this community contain a variety of zoonotic organisms that could pose a health risk to people coming into contact with dogs or their excrement. Zoonotic agents identified include bacteria, protozoa, and helminths known to cause both systemic and gastrointestinal disease in people. T. canis and E. granulosus are the causative agents of larval migrans and cystic hydatid disease, respectively,2,4,6 whereas G. duodenalis assemblage A, Cryptosporidium spp., and Campylobacter spp. are responsible for diarrheal diseases in people.9,16,20 Previous reports have implicated domestic dogs as a potential source of these zoonoses.2,6,9,11,15,21
In this study, the prevalence of T. canis in fecal samples was greater than that previously identified in owned dogs in the United States and Canada,17,22,23 although it was within the range reported for stray dogs24,25 and dogs in northern Canadian aboriginal communities.1,26 This variation in prevalence of infection could be the result of differences in anthelmintic treatment among the different groups of dogs. The prevalence of E. granulosus-containing feces in this study was also within the range reported for dogs in northern Canadian aboriginal communities.1,26 However, the prevalence of E. granulosus-infected dogs in these communities is highly variable among geographic locations and over time, likely as a result of variation in the dietary composition of dogs. The prevalence of Cryptosporidium spp. in fecal samples was similar to that identified in dogs in other North American studies.17,22
Of particular note is the prevalence and intensity of Giardia spp. and Campylobacter spp. in these fecal samples. The prevalence of Giardia spp. was much higher than expected given that the reported prevalence of Giardia spp. infection in stray and owned dogs in Canada and the United States is usually less than 10%,17,22–25,27 and the reported prevalence of infection in dogs from two northern Canadian aboriginal was not greater than 33%.1 In this study, well over one-half of the samples collected contained Giardia spp. cysts, and PCR results indicated that all samples in which product could be amplified contained zoonotic G. duodenalis assemblage A. On average, the fecal samples in this study contained over 9,000 cysts/g (mean) with 25% and 3% of samples containing > 10,000 and > 50,000 cysts/g, respectively. Because the infectious dose for G. duodenalis in humans is thought to be as low as 10 cysts,28 it is reasonable to consider that canine feces have the potential to be a significant source of Giardia spp. for people in this community.
This may also be the case for Campylobacter spp., because three-quarters of the fecal samples tested contained potentially zoonotic species of this bacterium. A previous study in Ontario, Canada did not identify Campylobacter spp. in a group of healthy dogs using PCR.22 However, studies of healthy dogs in the United Kingdom and Ireland, also using PCR, have shown a high prevalence of infection (upwards of 40% in some cases) with both C. jejuni and C. upsaliensis,29,30 similar to what was found in this study. The potential Campylobacter spp.-related zoonotic risk associated with canine feces is also supported by the intensity of infection in many of the samples. For example, the infectious dose of C. jejuni for people is thought to be approximately 800 organisms,20 and the C. jejuni-positive samples in this analysis contained 20–30,000 times that many organisms per gram of feces.
It is interesting to note that, for all organisms identified in this study, the mean intensity of infection was consistently greater than the median (Table 1). This could reflect an aggregated distribution of infectious organisms within this dog population. In other words, a small number of dogs may harbor the majority of the organisms, and the remainder of the population has a much lower intensity of infection. This would increase the mean intensity of infection relative to the median. Although the aggregated distribution described above is most commonly associated with parasitic metazoa,31 similar variation between the median and mean were observed for all organisms identified in this study, suggesting that a similar phenomenon could occur with protozoa and bacteria. This seems to be the case for Giardia spp. in this study (Figure 1). Although some feces contained over 50,000 Giardia spp. cyst/g, over one-half of the samples contained < 5,000 cysts/g, suggesting that certain dogs with a heavier pathogen burden are responsible for a greater degree of environmental contamination compared with others. This suggestion is further supported by the fact that, although there was no significant difference in distribution of T. canis-containing fecal samples among study areas, the average intensity of infection was significantly higher in neighborhoods 1 and 3 compared with the landfill (P = 0.009 and 0.02, respectively). The apparently aggregated distribution of canine fecal zoonoses in this community has future research and management implications, because it highlights the importance of identifying the most heavily infected animals to properly assess and manage the risk of human exposure.
Figure 1.
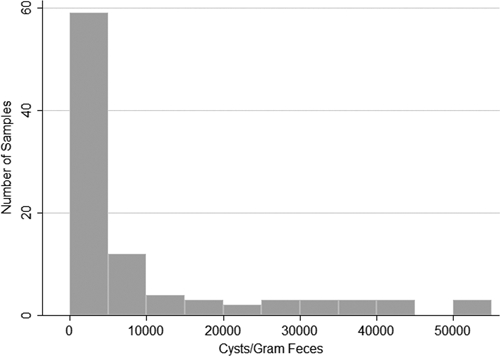
Summary of the intensity of infection with Giardia spp. in environmentally collected canine fecal samples from an indigenous Canadian community.
A limitation of this study is the fact that prevalence of infection in dogs could not be definitively determined with the sampling methodology used (i.e., environmentally collected canine fecal samples), because feces could not be traced back to the animal of origin and multiple samples may have originated from a single dog. For this reason, it is difficult to compare these results with those of studies that that describe the prevalence of fecal zoonoses in dogs. However, this study does confirm that dogs in this community are infected with a number of zoonoses and provides a crude evaluation of the degree of environmental contamination with these organisms. In this situation, the prevalence and distribution of zoonosis-containing fecal samples (versus prevalence of infection in dogs themselves) may, in fact, provide a more accurate assessment of the potential for human exposure to canine fecal zoonoses. Previous studies have indicated that soil contamination with zoonotic parasites is a risk factor for human infection,32 and there is reason to believe that, in this community, people are most likely to be exposed to canine fecal zoonoses in the environment because of limited direct contact between people and free-roaming dogs.11
Overall, this study revealed the presence of a number of zoonotic bacteria and parasites in environmentally collected canine feces from a remote Canadian indigenous community. There is evidence to suggest that, in this community, contact with canine feces has resulted in human exposure to and infection with at least one zoonotic pathogen (E. granulosus).11 To date, no other cases of infection with canine fecal zoonoses have been definitively identified; however, other than the Echinococcus investigation,11 no studies have been undertaken to determine the prevalence of these organisms and/or their associated diseases in people from this community. Given the generally non-specific clinical signs caused by infection with canine-fecal zoonosis,6,8,9 it is possible that human infection with these organisms has occurred and gone undiagnosed. Because the pathogens identified in this study pose a potential threat to human health, animal and human health-care professionals working in rural and remote indigenous Canadian communities should be aware of the significant and ongoing public-health risks associated with domestic dogs.
Acknowledgments
The authors would like to thank C. Fernando of the Department of Veterinary Microbiology, University of Saskatchewan as well as C. Covacin and A. Ash of the School of Veterinary and Biomedical Sciences, Murdoch University for technical support.
Footnotes
Financial support: This study was supported by the Division of Infectious Diseases, Royal University Hospital and the Western College of Veterinary Medicine Interprovincial Graduate Fellowship for Veterinarians.
Authors' addresses: Chelsea G. Himsworth, N. Jane Harms, and Frederick A. Leighton, Department of Veterinary Pathology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada, E-mails: chelsea.himsworth@usask.ca, naomi.harms@usask.ca, and ted.leighton@usask.ca. Bonnie Chaban, Emily Jenkins, Brent A. Wagner, and Janet E. Hill, Department of Veterinary Microbiology, University of Saskatchewan, Saskatoon, Saskatchewan, Canada, E-mails: bonnie.chaban@usask.ca, emily.jenkins@usask.ca, brent.wagner@usask.ca, and janet.hill@usask.ca. R. C. Andrew Thompson, World Health Organization Collaborating Centre for the Molecular Epidemiology of Parasitic Infections, School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, Western Australia, Australia, E-mail: a.thompson@murdoch.edu.au. Stuart Skinner, Division of Infectious Diseases, Royal University Hospital, Saskatoon, Saskatchewan, Canada, E-mail: Stuart.Skinner@saskatoonhealthregion.ca.
References
- 1.Salb AL, Barkema HW, Elkin BT, Thompson RC, Whiteside DP, Black SR, Dubey JP, Kutz SJ. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canada. Emerg Infect Dis. 2008;14:60–63. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaut M, Zimmerman EM, Goldstein RA. Health hazards to humans associated with domestic pets. Annu Rev Public Health. 1996;17:221–245. doi: 10.1146/annurev.pu.17.050196.001253. [DOI] [PubMed] [Google Scholar]
- 3.Traub RJ, Monis PT, Robertson I, Irwin P, Mencke N, Thompson RC. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitology. 2004;128:253–262. doi: 10.1017/s0031182003004505. [DOI] [PubMed] [Google Scholar]
- 4.Cook GC. Canine-associated zoonoses: an unacceptable hazard to human health. Q J Med. 1989;70:5–26. [PubMed] [Google Scholar]
- 5.Hotez PJ. Neglected infections of poverty among the indigenous peoples of the arctic. PLoS Negl Trop Dis. 2010;4:e606. doi: 10.1371/journal.pntd.0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Saghier M, Taylor MC, Greenberg HM. Canadian-acquired hydatid disease: a case report. Can J Infect Dis. 2001;12:178–182. doi: 10.1155/2001/302738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somily A, Robinson JL, Miedzinski LJ, Bhargava R, Marrie J. Echinoccocal disease in Alberta, Canada: more than a calcified opacity. BMC Infect Dis. 2005;5:1–7. doi: 10.1186/1471-2334-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkin GA, Tee W. Campylobacter upsaliensis-associated diarrhea in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:816–821. doi: 10.1086/514957. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RC. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. 2000;30:1259–1267. doi: 10.1016/s0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 10.MacMillan HL, MacMillan AB, Offord DR, Dingle JL. Aboriginal health. Can Med Assoc J. 1996;155:1569–1578. [PMC free article] [PubMed] [Google Scholar]
- 11.Himsworth CG, Jenkins E, Hill JE, Nsungu M, Ndao M, Thompson RCA, Covacin C, Ash A, Wagner BA, Leighton FA, Skinner S. The emergence of sylvatic Echinococcus granulosus as a parasitic zoonosis of public health concern in an indigenous Canadian community. Am J Trop Med Hyg. 2010;82:643–645. doi: 10.4269/ajtmh.2010.09-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox DD, Todd AC. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J Am Vet Med Assoc. 1962;141:706–709. [PubMed] [Google Scholar]
- 13.Olson ME, Thorlakson CL, Deselliers L, Morck DW, McAllister TA. Giardia and Cryptosporidium in Canadian farm animals. Vet Parasitol. 1997;68:375–381. doi: 10.1016/s0304-4017(96)01072-2. [DOI] [PubMed] [Google Scholar]
- 14.Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Caccio SM. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207–213. doi: 10.1016/j.ijpara.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Fayer R. Molecular characterization of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int J Parasitol. 2008;38:1239–1255. doi: 10.1016/j.ijpara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Caccio SM, Thompson RC, McLauchlin J, Smith HV. Unraveling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005;21:430–437. doi: 10.1016/j.pt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-central Colorado. J Am Anim Hosp Assoc. 2003;39:52–56. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- 18.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol. 2009;75:3055–3061. doi: 10.1128/AEM.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie A, Watson P. Statistics for Veterinary and Animal Sciences. 2nd ed. Ames, IA: Blackwell; 2006. pp. 55–173. [Google Scholar]
- 20.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 21.Tenkate TD, Stafford RJ. Risk factors for Campylobacter infection in infants and young children: a matched case-control study. Epidemiol Infect. 2001;127:399–404. doi: 10.1017/s0950268801006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefebvre SL, Waltner-Towes D, Peregrine AS, Reid-Smith R, Hodge L, Arroyo LG, Weese JS. Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: implications for infection control. J Hosp Infect. 2006;62:458–466. doi: 10.1016/j.jhin.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Little SE, Johnson EM, Lewis D, Jaklitsch RP, Payton ME, Blagburn BL, Bowman DD, Moroff S, Tams T, Rich L, Aucoin D. Prevalence of intestinal parasites in pet dogs in the United States. Vet Parasitol. 2009;166:144–152. doi: 10.1016/j.vetpar.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Blagburn B, Schenker R, Gagne F, Drake J, Johnson P, Bowles D, Wiedemann A, Ganjam V, Fucci V. Prevalence of intestinal parasites in companion animals in Ontario and Quebec, Canada, during the winter months. Vet Ther. 2008;9:169–175. [PubMed] [Google Scholar]
- 25.Blagburn BL, Lindsay DS, Vaughan BS, Rippey NS, Wright JC, Lynn RC, Kelch WJ, Ritchie GC, Hepler DI. Prevalence of canine parasites based on fecal flotation. Compend Contin Educ Pract Vet. 1996;18:483–509. [Google Scholar]
- 26.Unruh DHA, King JE, Eaton RDP, Allen JR. Parasites of dogs from Indian settlements in northwestern Canada: a survey with public health implications. Can J Comp Med. 1973;37:25–32. [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs SR, Forrester CPR, Yang J. A survey of the prevalence of Giardia in dogs presented to Canadian veterinary practices. Can Vet J. 2001;42:45–46. [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega YR, Adam RD. Giardia: overview and update. Clin Infect Dis. 1997;25:545–549. doi: 10.1086/513745. [DOI] [PubMed] [Google Scholar]
- 29.Acke E, McGill K, Golden O, Jones BR, Fanning S, Whyte P. Prevalence of thermophilic Campylobacter species in household cats and dogs in Ireland. Vet Rec. 2009;164:44–47. doi: 10.1136/vr.164.2.44. [DOI] [PubMed] [Google Scholar]
- 30.Parson BN, Porter CJ, Ryvar R, Stavinsky J, Williams NJ, Pinchbeck GL, Britles RJ, Christley RM, German AJ, Radford AD, Hart CA, Gaskell RM, Dawson S. Prevalence of Campylobacter spp. in a cross-sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Vet J. 2009;184:66–70. doi: 10.1016/j.tvjl.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Wobeser GA. Essentials of Disease in Wild Animals. Ames, IA: Blackwell; 2006. p. 37. [Google Scholar]
- 32.Thevenet PS, Nancufil A, Oyarzo CM, Torrecillas C, Raso S, Mellado I, Flores ME, Cordoba MG, Minvielle MC, Basualdo JA. An eco-epidemiological study of contamination of soil with infective forms of intestinal parasites. Eur J Epidemiol. 2004;19:481–489. doi: 10.1023/b:ejep.0000027352.55755.58. [DOI] [PubMed] [Google Scholar]


