Abstract
The entomological features of Chagas disease in two western Mexican villages were analyzed through triatomines collection by the inhabitants and active research in the peridomicile. The inhabitant collections have the following comparable characteristics: 1) Meccus longipennis was the dominant species (> 91%), 2) around 43% of the insects were collected indoors, 3) about 70% of triatomines were adults, 4) cumulated rates of infestation of the dwellings reached 40–50%, 5) the triatomine infection rate by Trypanosoma cruzi was > 50%, and 6) the indoor triatomines frequently feed on humans (range 38.5–56.2%). However, the collection was twice as abundant in the first village and the peridomicile infestation, evaluated by the active collection, reached up to 60% and only 4.9% in the other village. Furthermore, females predominated in the first village, whereas males in the other. The current results allow discussing the course of action to prevent Chagas disease in this region.
Introduction
The three principal domesticated vector species of Chagas disease, Triatoma infestans (Klug, 1834), Rhodnius prolixus (Stäl, 1859), and Triatoma dimidiata (Latreille, 1811), have received great attention over the last decades. Sub-regional vector control initiatives in the Southern Cone and Andean regions of South America, and in Central America, have undoubtedly diminished the transmission of Trypanosoma cruzi, the agent of Chagas disease, in countries where programs have been active for several years.1 However, less attention has been given to other regions, including Mexico, where other triatomine species (i.e., sylvatic) are present, even though the seroprevalence and clinical data confirmed that Chagas disease was endemic in the majority of Mexican regions.2 Moreover, between 1986 and 2006, the Mexican national epidemiologic surveillance system reported 1,814 cases of human chagas infection, having the majority of cases detected in the last 5 years (77%).† It is unclear whether the observed increase in Chagas disease incidence was caused by biotic and abiotic modifications of the environment, or whether the Public Health problem had not been adequately considered before.
In general terms, the transmission model linked to domestic vectors is coming out and is substituted by new features of vectorial transmission mainly associated with sylvatic triatomines.3–6
One exception is the insecticide resistance that has been recently documented in cases of the T. infestans domestic populations in Argentina.7 Three domestic transmission scenarios can typically be observed: 1) re-infestation by sylvatic populations (same or different species) after vector control of domestic populations7,8; 2) expansion of human settlement in areas where environmental modifications favor the domestication of sylvatic autochthonous species9; and 3) low endemic regions that have not yet gained adequate attention, but where vectors are concurrently sylvatic and domestic.10–13 The Mexican situation reflects the last two profiles. However, the study of emerging vector transmission systems is difficult because of the great diversity of triatomine species involved whose biological properties are poorly known. Biotic and abiotic modifications of the environment have become more and more important. The areas where sylvatic triatomines transmit disease caused by small-scale habitat colonization or temporary incursions are typically areas of low endemicity. Finally, low endemic areas may present specific challenges to seroprevalence surveys, diagnosis, and pathology. Human infection by sylvatic parasite strains needs to be better documented as unexpected clinical manifestations are possible.
In Mexico, at least 31 triatomine species have been described, 18 of them have been found naturally infected by T. cruzi.14,15 All of these species are sylvatic, and except for T. dimidiata, they are only endemic to Mexico.16,17 The most remarkable factor of these investigations with Mexican species is the high rate of infection by T. cruzi, also observed for most of the main South American vectors, such as T. infestans and Triatoma brasiliensis (Neiva, 1911).11,16 Although several Mexican species have been found in human habitats, it remains difficult to measure the true role of each species in the transmission of disease as there exist few studies on the ecology, geographical distribution, behavior in the human habitat, behavior in the natural medium, and relationship with the prevalence of human infection in corresponding areas. Furthermore, regarding the Meccus species complex, which includes Meccus longipennis, the species involved in the current study, controversy exists regarding their taxonomic status as species or subspecies because of their close genetic relationships.17,18
The basic assumption of this work is that a better understanding of Chagas disease in Mexico should consider the zooanthroponotic systems (reservoir-vector-human), which occur in shared spaces. As analyses of entomological features on the community scale are undoubtedly primary to evaluate transmission risk, two rural communities in western Mexico, Los Guerrero (Jalisco) and Cacalután (Nayarit), were studied. The current study showed that M. longipennis, originally a sylvatic species, is the principal vector in these two communities, but shows different patterns of distribution and abundance. This implies a likelihood of different transmission risks and requires specific control strategies for each location.
Materials and Methods
Study areas.
The study was conducted in two villages. The village of Los Guerrero (20°26′56.4²N, 103°53′87.2²W, 1295 m a s) is a rural community of the San Martín de Hidalgo municipality located in the state of Jalisco, whose characteristics have been previously described.19 Briefly, Los Guerrero is situated in a valley characterized by a deciduous seasonal forest (semi-arid region), which had been cleared to provide land for cultures around the village. Average temperatures are 20°C high and 28°C low, respectively. Annual rainfall averages range from 987.6 to 1,349 mm; the dry season extends from October until June. The village is composed of 314 dwellings, where 151 (48%) of them were closed because people were living and working in other places. The total surface of the village is 79.8 acres (323,000 m2).
The second village, Cacalután (21°06′98.8²N, 104°15′69.7²W, 920 m a s), is located in the municipality of Ixtlán del Río, in the state of Nayarit. The size of the village is approximately 74 acres (300,000 m2). Cacalután is located in a valley surrounded by mountains; the land is rough and steep, with a semi-dry climate. In winter temperatures range between 10 and 35°C, with an average of 13°C, and in summer between 20 and 40°C, with an average of 25°C and little precipitation. The population is dedicated to seasonal agriculture and cattle ranching. The village is composed of 134 dwellings, grouped along three principal streets, 30 (22%) of them were closed or abandoned. These villages have never been sprayed with insecticides before this study.
Perception of triatomines by the inhabitants.
Adults of both sexes were questioned on their own observations about triatomines in their house (indoor) and in the peridomicile (outdoor) areas during the visit of their dwellings. In Los Guerrero, 86 persons were interviewed and 78 in Cacalután.
Active collection of triatomines in peridomiciles.
At the beginning of the study, the villages were mapped, and all houses were drawn. One hundred occupied dwellings in Los Guerrero and 82 in Cacalután were randomly selected by an aleatory number assignment and visited during the study. The same team of well-trained health workers, assisted by an entomologist, searched vector insects manually with flashlights, with no insect repellent on them, during the day in the peridomestic area of Los Guerrero (July–August 2003) and of Cacalután (July 2005). A total of 369 sites in Los Guerrero and 300 sites in Cacalután were excavated: storage shelters, corrals, and chicken-coops were visited and the excavated sites consisted of piles of wood, rocks, brick, tile, or various goods and ends, which are in these structures and outdoors. All examined sites were numbered, characterized, and located on a map. Insects collected from each capture site were placed in separate labeled plastic flasks and transported to the laboratory for morphological identification. The mean time spent searching for insects at each dwelling was 1 hour and 15 min (± 40 min).
Collection of triatomines by the inhabitants.
After the research was carried out by the team of professional agents, inhabitants were asked to collect the triatomines using plastic bags to preserve them and later in flasks. They were not asked to make excavations but to keep triatomines they found during their daily activities. Two flasks were distributed per family to separate the insects collected inside the house from those collected outside. Each month the flasks were gathered and transported to the laboratory by the team of professional agents. The collection by the inhabitants lasted 24 months from October 2005 through September 2007 in Los Guerrero where 126 families participated in the collection and from January 2005 to December 2006 in Cacalután (92 families).
Processing of triatomines.
The identification of triatomines (adults and nymphs) was done according to the taxonomic keys.14 Sex and stages of development were also determined. Feces from each bug, obtained by abdominal pressure, were mixed with phosphate-buffered saline (PBS), and examined for the presence of trypanosomatids by direct microscopic observation at 400× magnifications. Parts of the bugs were dissected under a safety hood: the terminal part of the abdomen was cut and the abdominal contents (blood meal and intestinal part) collected in a microtube. All samples were kept at −20°C for further processing. Classical entomological indicators, infestation, and colonization were calculated according to The World Health Organization (WHO) standards.20
Host feedings of triatomines collected indoors.
The blood meal DNAs were extracted with the QIAamp DNA mini kit following the manufacturer's instructions (Qiagen, Courtaboeuf, France). The cytochrome b gene was then amplified with specific primers for the vertebrates from each DNA sample and heteroduplexes were produced with Sigmodon mascotensis amplified cytochrome b from a cloned gene in our laboratory as driver following the method previously described.21 The heteroduplex products were formed by denaturizing at 100°C for 2.5 min of cytochrome b mixtures (V/V) of sample and driver and slow cooling of the denatured solution at room temperature. Finally, the comparison of the heteroduplex patterns was done with 10% acrylamide electrophoreses (29 acrylamide: 1 bisacrylamide) in TBE buffer. Profiles were compared with different standards, including human. An absence of heteroduplex formation was equated to Sigmodon sp. blood meal after control of the profile using Dasypus novemcinctus as driver. Blood meal origin of part of the samples were also determined by direct sequencing of polymerase chain reaction (PCR) products or after cloning using the TOPO TA kit (Invitrogen, Cergy-Pontoise, France). The sequences were then blasted in gene banks.
Results
Inhabitant experiences with triatomines.
The inhabitants were questioned about their familiarity with triatomines. In the two villages, they were familiar with the well-known triatomines and could identify them by their local name of “chinche hocicona.” In fact, 47% and 30% of the questioned people in Los Guerrero and Cacalután, respectively, reported seeing triatomes indoors, most frequently in their bedroom (61% and 50%). They also reported having seen bugs outdoors in the peridomiciliary area with comparable occurrence to indoors (50% in Los Guerrero, 38% in Cacalután). Furthermore, 20% and 29% of the families in the two villages were able to identify triatomine bites as causing a large and painful chagome.
Active manual search of triatomine in the peridomicile.
The manual search of triatomines by the professional team showed that abundance was radically different between the two villages. As previously reported in Los Guerrero, a total of 1,821 triatomine specimens were collected in 118 different sites, and the peridomestic infestation reached 60% among 100 visited units.22 There, M. longipennis was the dominant species (93.2% of the adults) and Triatoma barberi (Usinger, 1939) the second most frequently observed (6.6%). Furthermore, a high colonization rate of both species was observed (93.3% and 75%, respectively). In Cacalután, only seven triatomines were caught in four sites located in four different peridomestic units (two M. longipennis adults and five nymphs (fifth stage) of the Meccus complex), and the infestation index of the peridomiciliar units was 4.9% among the 82 visited.
Collection of triatomines by the inhabitants during 24 months.
Indoor and outdoor infestation patterns were followed by the longitudinal collection of triatomines by the inhabitants over 24 months (Table 1). In Los Guerrero, a total of 874 triatomines were collected, of which 43.6% were found indoors and 56.3% were found in peridomiciliar areas. The proportion of the different species calculated among adult specimens was as follows: M. longipennis (95.7%), T. barberi (2.5%), Meccus pallidipennis (Stål, 1945) (1.5%), and Meccus picturata (Usinger, 1939) (0.3%). A total of 269 nymphs belonging to the Meccus complex of species were captured (nymphs of Meccus species are not differentiated by morphology). The adult proportion reached 69.2% of the total population. In Cacalután, the inhabitants caught a total of 319 triatomines, of which 42.4% were indoors, and 57.6% outdoors. Meccus longipennis was the dominant species (91.7%), the secondary ones were Triatoma recurva (Stål, 1868) (2.5%), M. picturata (2.9%), and seven specimens remain to be determined (2.9%), but probably they belong to one species. A total of 78 nymphs were captured. Adult proportion reaches 75.5%. In Los Guerrero and in Cacalután no significant differences (P > 0.05) were found in the distribution of species and stages between indoor and outdoor collections (for the χ2 analysis, T. barberi, M. pallidipennis, and M. picturata were grouped in Los Guerrero and similarly T. recurva, M. picturata, and T. sp. in Cacalután). Further comparison between the two villages shows significant differences of the species and stages distribution (Table 1); the number of collected nymphs was also significantly lower in Cacalután than in Los Guerrero, and the number of adult specimens of other species than M. longipennis was higher in Cacalután (Table 1). Moreover the triatomines were twice as abundant in Los Guerrero (12.2 triatomines/month/100 dwellings) compared with Cacalután (5.1 triatomines/month/100 dwellings).
Table 1.
Triatomine species collected indoors and outdoors by the inhabitans in two Mexican villages during 24 months
| Villages in the occidental part of Mexico | χ2 statistic† | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Los Guerrero | Cacalután | ||||||||
| Indoor | Outdoor | Unknown‡ | Indoor | Outdoor | Unknown‡ | X2 value | df* | P value | |
| M. longipennis§ | 259 | 315 | 5 | 76 | 103 | 42 | 0.97 | 1 | > 0.05 |
| Other species | |||||||||
| T. barberi | 5 | 9 | 1 | 0 | 0 | 0 | 6.84 | 1 | < 0.01 |
| M. pallidipennis§ | 6 | 3 | 0 | 0 | 0 | 0 | |||
| M. picturata§ | 2 | 0 | 0 | 2 | 4 | 1 | |||
| T. recurva§ | 0 | 0 | 0 | 3 | 1 | 2 | |||
| T. sp.§ | 0 | 0 | 0 | 2 | 4 | 1 | |||
| Nymphs of Meccus complex | 103 | 157 | 9 | 26 | 36 | 16 | 4.53 | 1 | < 0.05 |
| Total | 375 | 484 | 15 | 109 | 148 | 62 | 10.11 | 2 | < 0.01 |
df = degrees of freedom.
Significant differences were evaluated between Los Guerrero and Cacalutan raw data.
The triatomines were collected indoors or outdoors.
Adults specimens.
Remarkably, the sex ratio of the triatomines collected indoors was significantly different between Los Guerrero and Cacalután, as shown in Figure 1 (X2 = 35, degrees of freedom [df] = 1, P < 10−4): whereas females predominate in Los Guerrero (X2 = 9.9, df = 1, P = 0.0019), males were more abundant in Cacalután (X2 = 9.7, df = 1, P = 0.0018). Moreover, these sex proportions were similar for the populations collected outdoors and indoors.
Figure 1.
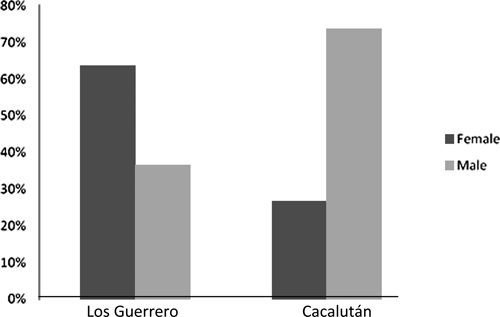
Histograms presenting the sex distribution of Meccus longipennis specimens collected indoors by the inhabitants of Los Guerrero and Cacalután villages, Mexico, over 2 years.
The seasonal examination of the indoor populations collected by the inhabitants show that they collected triatomines during the entire year in the two villages (Figure 2). The cumulated indoor infestation rates were also rather similar in the two villages, > 40% (Figure 3).
Figure 2.
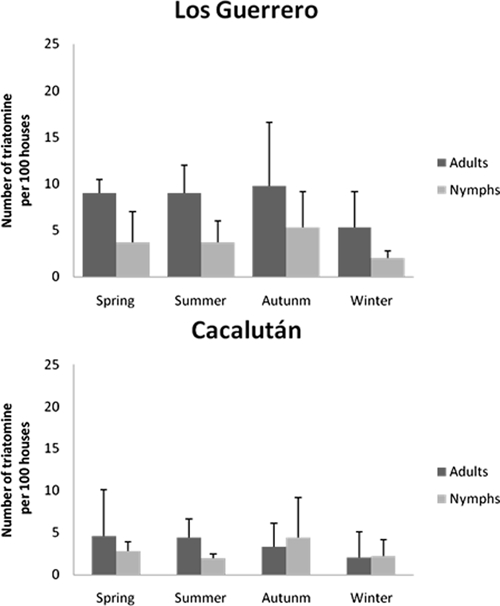
Seasonal variation of total numbers of triatomines harvested indoors by the inhabitants in Los Guerrero and Cacalután villages per 100 houses.
Figure 3.
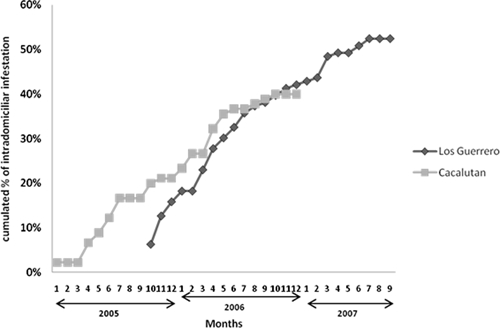
Cumulative rates of indoor infestation in Los Guerrero and Cacalután villages, Mexico, based on triatomine collection by the inhabitants per month, over 2 years.
Parasite infection in triatomines collected by the inhabitants.
The infection rates calculated from about 51% of M. longipennis adults and nymphs collected by the inhabitants in Los Guerrero and Cacalután, reached 55.1% and 49.4%, respectively (Table 2). Similar rates were observed for the triatomines collected indoors and outdoors and between sexes. The secondary species (M. pallidepennis, M. picturata, and T. recurva) were also found infected by T. cruzi but not the undetermined species (two T. sp. males analyzed).
Table 2.
Infection rates by Trypanosoma cruzi of Meccus longipennis adults and nymphs of Meccus complex collected by the inhabitants*
| Indoors | Outdoors | Total % positive | |||||
|---|---|---|---|---|---|---|---|
| N | Positive | % Positive | N | Positive | % Positive | ||
| Los Guerrero | |||||||
| N1 + N2 | 0 | 0 | – | 1 | 0 | – | – |
| N3 | 8 | 0 | 0.0 | 6 | 3 | 50.0 | 21.4 |
| N4 | 20 | 7 | 35.0 | 24 | 6 | 25.0 | 29.5 |
| N5 | 44 | 22 | 50.0 | 55 | 32 | 58.2 | 54.5 |
| F | 95 | 55 | 57.9 | 88 | 55 | 62.5 | 60.1 |
| M | 62 | 33 | 53.2 | 83 | 55 | 66.3 | 60.7 |
| Total | 229 | 117 | 51.1 | 257 | 151 | 58.8 | 55.1 |
| Cacalután | |||||||
| N1 + N2 | 1 | 0 | – | 0 | 0 | – | – |
| N3 | 0 | 0 | – | 2 | 0 | – | – |
| N4 | 11 | 3 | 27.3 | 6 | 2 | 33.3 | 29.4 |
| N5 | 8 | 4 | 50.0 | 18 | 5 | 27.8 | 34.6 |
| F | 13 | 9 | 69.2 | 17 | 7 | 41.2 | 53.3 |
| M | 30 | 17 | 56.7 | 48 | 29 | 60.4 | 59.0 |
| Total | 63 | 33 | 52.4 | 91 | 43 | 47.3 | 49.4 |
N1 to N5 = nymphs; F = adult female; M = adult male; N = total number of processed insects.
Feeding blood meal origin of triatomines collected by the inhabitants.
Blood meal origin was determined for 65 triatomines in Los Guerrero and 32 in Cacalután collected indoors (Table 3). Among these bugs, 38.5% and 56.2% in Los Guerrero and Cacalután, respectively, presented a heteroduplex profile corresponding to single or mixed human feedings. Remarkably, Dasypus novemcinctus blood meal origin was detected in 69.2% of the triatomines in Los Guerrero and in 53.1% in Cacalután; sequencing of two PCR products giving a D. novemcinctus heteroduplex pattern confirms the species (Y11832.1, 97% identity). Similarly, sequencing of PCR products confirmed the Gallus gallus (DQ512918.1, 99% identity) and Sigmodon sp. (AY041203.1, 89% identity) host origin identified by the heteroduplex assay. Two other PCR products (one from each village) with a heteroduplex multibanding were cloned; the heteroduplex profiles of 10 clones from each PCR sample were identical to standards: nine clones corresponded to D. novemcinctus, three to human, nine to Sigmodon sp., and one to Mus musculus. One triatomine contained four different blood meal hosts (Table 3).
Table 3.
Blood meal origin of 97 triatomines collected indoors in Los Guerrero and Cacalután villages
| Blood meal origin | Villages | |||
|---|---|---|---|---|
| Los Guerrero | Cacalután | Total | ||
| Single blood meal | Human | 11 | 13 | 24 |
| Dasypus novemcinctus | 32 | 12 | 44 | |
| Sigmodon sp. | 4 | 2 | 6 | |
| Gallus gallus | 1 | 0 | 1 | |
| Several blood meals | Human + D. novemcinctus | 9 | 4 | 13 |
| Human + Sigmodon sp. | 1 | 0 | 1 | |
| Human + other | 3 | 0 | 3 | |
| D. novemcinctus + Sigmodon sp. | 1 | 0 | 1 | |
| D. novemcinctus + other | 2 | 0 | 2 | |
| Human + D. novemcinctus + Sigmodon sp.* | 1 | 0 | 1 | |
| Human + D. novemcinctus + Sigmodon sp. + Mus musculus* | 1 | 1 | ||
Origin of blood meals was determined by cloning and sequencing.
Discussion
The earlier entomological works pointed out the large geographical distribution of various species of the Meccus complex: M. longipennis, Meccus mazzottii (Usinger, 1899), M. pallidipennis, Meccus phyllosoma (Burmeister, 1835), and M. picturata in Mexico, but as yet, basic entomological studies aimed at determining the microdistribution of the vectors, the population density, and the transmission risk are scarce.23–27 Previous entomological data of the triatomine species belonging to this complex at the community scale have informed epidemiological trends; the peridomestic area can be heavily infested with one of these species in some villages but not in others, whereas intra-domicile infestation is generally low or undetectable by active search during the day.11,28
The current results show clearly that in two villages located in the western part of México, there is a year-round indoor risk of Chagas disease transmission, because a high proportion of the insects collected in the house by the inhabitants displayed a high rate of infection by the parasite, and these insects frequently feed on humans. However, the abundance of the vectors was different between the villages, which lead to differential exposures and associated transmission risks. The analysis of the insect life stages suggests low indoor colonization as a high proportion of adults (> 70%) was observed in both villages. Furthermore, the inhabitants seldom collected several insects at the same time in their houses. Moreover, several blood meals taken from non-domestic animals were identified, showing that these insects had invaded domicile. These data suggest that triatomines collected indoors came from peridomestic and/or sylvatic areas.
In Los Guerrero, the triatomines that penetrate the houses could originate from peridomestic areas, which are heavily infested as previously reported.22 In this work we have reported a high number of sites with only one or two insects (adult and nymphs), which indicates intense dispersive activity. Several field observations and experiments show that the dispersal capacity of triatomines by walking is somewhat important. For example, artificial hen houses placed in tropical Petén forests of Guatemala are first infected with fifth-instar nymphs before they are infected by adults of the species T. dimidiata.28 Thus, walking incursion of nymphs and adults from the peridomestic sites to the houses must be considered. However, the assumption of incursion of sylvatic triatomines into the house is not excluded because it was observed wild mammal feeding origins in several triatomines were collected indoors. Indeed, in Los Guerrero 48% of the dwellings are closed because people were living and working in other places, these houses have peridomiciles that are not maintained for months and can act as refuges for wild mammals, thereby increasing colonization by triatomines.19 Consequently, some triatomines found in the houses can be from these peridomestic-sylvatic areas.
The scenario in Cacalután could be different from Los Guerrero. In Cacalutan the peridomicile colonization by M. longipennis was extremely lower, because the active research for triatomines in 82 peridomiciles allowed collecting only seven triatomines, the infestation rate was 4.9%. In these conditions it is difficult to believe that triatomines entering the home are from triatomine colonies located in the peridomicile. Most remarkable was the high proportion of M. longipennis males collected by the inhabitants of Cacalután. This high proportion of males could explain the very low colonization load of the peridomicile, whereas the abundance of peridomestic structures (permanent or built structures such as storage shelters, animal shelters, corrals, chicken-coops, and temporary structures, like piles of wood, brick, tile, or various goods and ends), and domestic animals would suggest a favorable situation for colonization, such as in the village of Los Guerrero.
The analysis of the blood meals also showed that a high proportion of the triatomines found in houses of Cacalután were carrying wild mammal feeding origins. In addition, several inhabitants testified to see triatomines flying in the street during the evening, which indicates active dispersion of triatomines in this area. These data make it possible to hypothesize that in Cacalután, the infestation by M. longipennis would originate from a wild environment rather than from the peridomiciles. Likewise, the secondary species T. recurva, regarded as a sylvatic species, is also caught by the inhabitants indoors and outdoors.
Our study and others clearly show that in low endemic regions, longitudinal monitoring of the triatomine infestation by the inhabitants themselves made it possible to more accurately describe the risk patterns when compared with transversal active research.29,30 In the current work, several secondary species were collected only by the inhabitants (indoors and outdoors) and were infected by T. cruzi. Moreover the longitudinal collection by the inhabitants shows that, even if the peridomicile present a very low infestation (active collection in Cacalután); a risk of transmission exists because infected indoor triatomes are collected. The proposed index of triatomine abundance can be used to compare different regions. As an example, in the city of Merida, Yucatán, México, only 0.7 triatomines/month/100 dwellings were found, a significant smaller number than those observed in the villages examined during this study (Los Guerrero, 12.2 triatomines/month/100 dwellings; Cacalután, 5.1 triatomines/month/100 dwellings).29
In Yucatán, another region of México where the T. dimidiata species is the principal vector, a seasonal indoor incursion model occurs; a mathematical model predicted that the majority of the domestic population is comprised of immigrants and genetic population studies found a low genetic differentiation between domestic triatomines and wild populations.13,31 In the current region, the incursion of triatomines indoors was only slightly reduced during the coldest months, but the research in the sylvatic environment must be performed to clearly assess the origin of the populations that penetrate the dwellings using population genetics.
The vector systems related to the wild triatomine species are various and complex. They involve many species (about 30) whose wild habitat is terrestrial or arboricolous. Their distribution spreads over large geographic areas characterized by a wide variety of landscapes. In the current study, which focuses on the analysis of villages, we found that two different vector dynamic models of the same species could operate. In addition, we did not gather information or observe any factors that suggested an emerging process of transmission in any of the villages. Traditional control approaches (e.g., household insecticide spraying) are unlikely to be effective against vectors that have an incursion behavior. Novel strategies are urgently needed, and their development crucially depends on innovative research.
Acknowledgments
We extend our gratitude to the medical students of the “Unidad Académica de Medicina” of the “Universidad Autónoma de Nayarit” who participated in field work in the village of Cacalután: Roberto Altamirano Gallegos, Jose Cruz Cruz, Víctor Adauto Díaz Flóres, Jaime Díaz Valdivia, Fausto Guadarrama, Dulce Yaneth Romero Pérez, Claudia Rubí, and Gabriela Alejandra Ulloa Minjarez.
Footnotes
Financial support: This work received financial support from the UNDP/WorldBank/WHO Special program for Research and Training in Tropical Diseases (TDR) on Operational Research on Chagas disease, ID A30442 and from the “Institut de Recherche pour le Développement” (IRD) France.
Authors' addresses: Simone Frédérique Brenière and Marie-France Bosseno, Institut de Recherche pour le Développement (IRD), Representación en Bolivia, UR 016 Caractérisation et Contrôle des Populations de Vecteurs, Avenida Hernando Siles N° 5290, Esq. Calle 7, Obrajes, C.P. 9214, La Paz, Bolivia, E-mails: Frederique.Breniere@ird.fr and Marie-France.Bosseno@ird.fr. Ezequiel Magallón Gastélum, María Margarita Soto Gutiérrez, Marina de Jesús Kasten Monges, and Felipe de Jesús Lozano Kasten, Departamento de Salud Pública, Centro Universitario de Ciencias de la Salud, AP 2-136, Universidad de Guadalajara, Guadalajara, Jalisco, México, E-mails: mge28525@cucs.udg.mx, msg0059_2001@hotmail.com, m_kasten_m@yahoo.com.mx, and f_lozano_k@hotmail.com. José Horacio Barraza Salas and José Justo Romero Paredes, Universidad Autónoma de Nayarit, Unidad Académica de Medicina, Ciudad de la Cultura Amado Nervo, Nayarit, México, E-mails: barraza28@hotmail.com and jose1_justo@hotmail.com.
References
- 1.Moncayo A, Yanine MI. An update on Chagas disease (human American trypanosomiasis) Ann Trop Med Parasitol. 2006;100:663–677. doi: 10.1179/136485906X112248. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Reyes A, Pickering-López JM. Chagas disease in México: an analysis of geographical distribution during the past 76 years–a review. Mem Inst Oswaldo Cruz. 2006;101:345–354. doi: 10.1590/s0074-02762006000400001. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira AR, Monteiro PS, Rebelo JM, Argañaraz ER, Vieira D, Lauria-Pires L, Nascimento R, Vexenat CA, Silva AR, Ault SK, Costa JM. Emerging Chagas disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon. Emerg Infect Dis. 2001;7:100–112. doi: 10.3201/eid0701.700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrasco HJ, Torrellas A, García C, Segovia M, Feliciangeli MD. Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int J Parasitol. 2005;35:1379–1384. doi: 10.1016/j.ijpara.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Martín MJ, Feliciangeli MD, Campbell-Lendrum D, Davies CR. Could the Chagas disease elimination programme in Venezuela be compromised by reinvasion of houses by sylvatic Rhodnius prolixus bug populations? Trop Med Int Health. 2006;11:1585–1593. doi: 10.1111/j.1365-3156.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 6.Zeledón EB, Kelly NM. Understanding large-scale deforestation in southern Jinotega, Nicaragua from 1978 to 1999 through the examination of changes in land use and land cover. J Environ Manage. 2009;90:2866–2872. doi: 10.1016/j.jenvman.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Picollo MI, Vassena C, Orihuela PS, Barrios S, Zaidemberg M, Zerba E. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from northern Argentina. J Med Entomol. 2005;42:637–642. doi: 10.1093/jmedent/42.4.637. [DOI] [PubMed] [Google Scholar]
- 8.Diotaiuti L, Pereira AS, Loiola CF, Fernandes AJ, Schofield JC, Dujardin JP, Dias JC, Chiari E. Inter-relation of sylvatic and domestic transmission of Trypanosoma cruzi in areas with and without domestic vectorial transmission in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1995;90:443–448. doi: 10.1590/s0074-02761995000400002. [DOI] [PubMed] [Google Scholar]
- 9.Almeida CE, Folly-Ramos E, Peterson AT, Lima-Neiva V, Gumiel M, Duarte R, Lima MM, Locks M, Beltrão M, Costa J. Could the bug Triatoma sherlocki be vectoring Chagas disease in small mining communities in Bahia, Brazil? Med Vet Entomol. 2009;23:410–417. doi: 10.1111/j.1365-2915.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Magallón-Gastélum E, Lozano-Kasten F, Bosseno MF, Cárdenas-Contreras R, Ouaissi A, Brenière SF. Colonization of rock pile boundary walls in fields by sylvatic triatomines (Hemiptera: Reduviidae) in Jalisco State, Mexico. J Med Entomol. 2004;41:484–488. doi: 10.1603/0022-2585-41.3.484. [DOI] [PubMed] [Google Scholar]
- 11.Magallón-Gastélum E, Lozano-Kasten F, Gutiérrez MS, Flores-Pérez A, Sánchez B, Espinoza B, Bosseno MF, Brenière SF. Epidemiological risk for Trypanosoma cruzi transmission by species of Phyllosoma complex in the occidental part of México. Acta Trop. 2006;97:331–338. doi: 10.1016/j.actatropica.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Bosseno MF, García LS, Baunaure F, Gastelúm EM, Gutierrez MS, Kasten FL, Dumonteil E, Brenière SF. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am J Trop Med Hyg. 2006;74:303–305. [PubMed] [Google Scholar]
- 13.Dumonteil E, Tripet F, Ramírez-Sierra MJ, Payet V, Lanzaro G, Menu F. Assessment of Triatoma dimidiata dispersal in the Yucatán Peninsula of México by morphometry and microsatellite markers. Am J Trop Med Hyg. 2007;76:930–937. [PubMed] [Google Scholar]
- 14.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and significance as vectores of Chagas' disease. Bull Am Mus Nat Hist. 1979;163:125–520. [Google Scholar]
- 15.Galvão C, Carcavallo R, Rocha DS, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36. [Google Scholar]
- 16.Zárate LJ, Zárate RJ. A checklist of the Triatominae (Hemiptera, Reduviidae) of Mexico. Int J Entomol. 1985;61:257–271. [Google Scholar]
- 17.Brenière SF, Taveira B, Bosseno MF, Ordoñez R, Lozano-Kasten F, Magallón-Gastélum E, Ouaissi A, Ramsey J. Preliminary results of random amplification of polymorphic DNA among Triatominae of the phyllosoma complex (Hemiptera, Reduviidae) Mem Inst Oswaldo Cruz. 2003;98:1033–1038. doi: 10.1590/s0074-02762003000800010. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Hernandez F, Martínez-Ibarra JA, Catalá S, Villalobos G, de la Torre P, Laclette JP, Alejandre-Aguilar R, Espinoza B. Natural crossbreeding between sympatric species of the phyllosoma complex (Insecta: Hemiptera: Reduviidae) indicate the existence of only one species with morphologic and genetic variations. Am J Trop Med Hyg. 2010;82:74–82. doi: 10.4269/ajtmh.2010.09-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter A, Lozano-Kasten F, Bosseno MF, Ruvalcaba EG, Gutiérrez MS, Luna CE, Baunaure F, Phelinas P, Magallón-Gastélum E, Brenière SF. Peridomicilary habitat and risk factors for Triatoma infestation in a rural community of the Mexican occident. Am J Trop Med Hyg. 2007;76:508–515. [PubMed] [Google Scholar]
- 20.WHO . Control of Chagas Disease: Second Report of the WHO Expert Committee. Geneva: WHO; 2002. [Google Scholar]
- 21.Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599–604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenière SF, Bosseno MF, Magallón-Gastélum E, Castillo Ruvalcaba EG, Gutiérrez MS, Montaño Luna EC, Basulto JT, Mathieu-Daudé F, Walter A, Lozano-Kasten F. Peridomestic colonization of Triatoma longipennis (Hemíptera, Reduviidae) and Triatoma barberi (Hemíptera, Reduviidae) in a rural community with active transmission of Trypanosoma cruzi in Jalisco state, México. Acta Trop. 2007;101:249–257. doi: 10.1016/j.actatropica.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Tay Zavala J, Sánchez Vega JT, Robert Guerrero L, Alonso Guerrero T, Romero-Cabello R. New sites with Triatominae infected by Trypanosoma cruzi in the Mexican Republic. Bol Chil Parasitol. 1996;51:49–53. [PubMed] [Google Scholar]
- 24.Magallón-Gastélum E, Magdaleno-Peñaloza NC, Kattahain-Duchateau G, Trujillo-Contreras F, Lozano-Kasten FJ, Hernández-Gutiérrez RJ. Distribución de los vectores de la enfermedad de Chagas (Hemíptera: Reduviidae: Triatominae) en el estado de Jalisco, México. Rev Biomed. 1998;9:151–157. [Google Scholar]
- 25.Bautista NL, De La Torre GSG, Arteaga ID, Schettino PMS. Importance of Triatoma pallidipennis (Hemiptera: Reduviidae) as a vector of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) in the state of Morelos, México, and possible écotopes. J Med Entomol. 1999;36:233–235. doi: 10.1093/jmedent/36.3.233. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey JM, Ordoñez R, Cruz-Celis A, Alvear AL, Chavez V, Lopez R, Pintor JR, Gama F, Carrillo S. Distribution of domestic triatominae and stratification of Chagas disease transmission in Oaxaca, Mexico. Med Vet Entomol. 2000;14:19–30. doi: 10.1046/j.1365-2915.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 27.Espinoza-Gómez F, Maldonado-Rodríguez A, Coll-Cárdenas R, Hernández-Suárez CM, Fernández-Salas I. Presence of triatominae (Hemiptera, Reduviidae) and risk of transmission of Chagas disease in Colima, Mexico. Mem Inst Oswaldo Cruz. 2002;97:25–30. doi: 10.1590/s0074-02762002000100002. [DOI] [PubMed] [Google Scholar]
- 28.Monroy MC, Bustamante DM, Rodas AG, Enriquez ME, Rosales RG. Habitats, dispersion and invasion of sylvatic Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) in Petén, Guatemala. J Med Entomol. 2003;40:800–806. doi: 10.1603/0022-2585-40.6.800. [DOI] [PubMed] [Google Scholar]
- 29.Guzmán-Tapia Y, Ramírez-Sierra MJ, Dumonteil E. Urban infestation by Triatoma dimidiata in the city of Mérida, Yucatán, México. Vector Borne Zoonotic Dis. 2007;7:597–606. doi: 10.1089/vbz.2007.0133. [DOI] [PubMed] [Google Scholar]
- 30.Dumonteil E, Gourbière S, Barrera-Pérez M, Rodríguez-Félix E, Ruíz-Piña H, Baños-López O, Ramírez-Sierra MJ, Menu F, Rabinovich JE. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatán Peninsula of México. Am J Trop Med Hyg. 2002;67:176–183. doi: 10.4269/ajtmh.2002.67.176. [DOI] [PubMed] [Google Scholar]
- 31.Gourbière S, Dumonteil E, Rabinovich JE, Minkoue R, Menu F. Demographic and dispersal constraints for domestic infestation by non-domicilated chagas disease vectors in the Yucatán Peninsula, México. Am J Trop Med Hyg. 2008;78:133–139. [PubMed] [Google Scholar]


