Abstract
The hypothesis that nestlings are a significant driver of arbovirus transmission and amplification is based upon findings that suggest nestlings are highly susceptible to being fed upon by vector mosquitoes and to viral infection and replication. Several previous studies have suggested that nestlings are preferentially fed upon relative to adults in the nest, and other studies have reported a preference for adults over nestlings. We directly tested the feeding preference of nestling and adult birds in a natural setting, introducing mosquitoes into nesting boxes containing eastern bluebirds (Sialia sialis), collecting blood-fed mosquitoes, and matching the source of mosquito blood meals to individual birds using microsatellite markers. Neither nestlings nor adults were fed upon to an extent significantly greater than would be predicted based upon their relative abundance in the nests, although feeding upon mothers decreased as the age of the nestlings increased.
Introduction
Knowledge of the dynamics of arbovirus transmission requires an in-depth understanding of how vertebrate reservoirs and arthropod vectors interact. Given their immobility and lack of plumage and defensive behaviors,1 nestling birds have been proposed to be important hosts for bird-feeding (ornithophilic) mosquitoes2,3 and may even be preferentially selected in the presence of adult birds.2 In addition to their potential importance as sources of blood meals for questing mosquitoes, nestling birds may be important reservoirs for arboviruses because of their relatively naive immune systems.4 The combination of being targeted by questing mosquitoes and being immunologically susceptible to arboviruses may be of critical importance in the annual amplification of arboviruses in nature.2,5 If nestling birds serve as amplifying hosts (in which viruses rapidly multiply, providing an important source of pathogens for vectors), then the nesting periods of avian reservoirs should slightly precede the annual peak in the minimum infection rate observed in vector mosquitoes.6
Despite the potential importance of nestling birds in the ecology of arboviruses, whether nestling birds play a role in natural amplification cycles of arboviruses remains a matter of debate. Various experimental techniques have been devised to determine the attractiveness of nestling birds to questing mosquitoes and the rates at which nestlings are fed upon relative to adults. Observations resulting from these experimental studies have not consistently supported the hypothesis that mosquitoes show a feeding preference for nestling over adult birds.7 In most cases, these experiments were conducted under conditions that only vaguely resembled natural nesting conditions. In some of the earliest work investigating the relative attractiveness of nestling and adult birds to questing mosquitoes, Blackmore and Dow placed nestling and adult birds separately in open containers in cages where questing mosquitoes had access to them.2 Likely as a consequence of the immobility and sparse feathering of nestlings, mosquitoes fed much more successfully on nestlings than on adults.
In a more recent study designed to test relative attraction of mosquitoes to nestlings and adults, combinations of nestlings of various ages and adults were placed individually or in small groups in chambers in an experimental olfactometer. Questing mosquitoes could choose between nestlings and adults based on olfaction alone. Results indicated that mosquitoes were more attracted to adults and older nestlings than to hatchlings.5 Although these findings are interesting, they do not determine whether, under field conditions, mosquitoes feed upon nestling birds to a greater or lesser extent than adults. To understand the contribution of nestling birds to the annual amplification of arboviruses, it is necessary to know to what degree they are actually fed upon by vector mosquitoes.
More recent studies have investigated relative importance of nestlings as mosquito hosts under more natural conditions. In one such study, Griffing and others filmed the nests of American robins (Turdus migratorius) overnight and recorded the number of mosquitoes that landed on adults and nestlings.3 Landing rates by mosquitoes were higher for adults than for nestlings, but as pointed out by the authors, landing rates do not necessarily indicate feeding success. They merely indicate the frequency with which a questing mosquito makes contact with a host.3
To determine whether questing mosquitoes preferentially feed on nestling versus adults, and thus the potential importance of nestling birds as amplifying hosts of arboviruses, it is necessary to quantify the rates of relative blood feeding on nestlings and adult birds under natural conditions. In this study, we examined patterns of host feeding upon adult and nestling birds in the field. We introduced Culex quinquefaciatus mosquitoes into nest boxes containing nestling and adult eastern bluebirds (Sialia sialis) and used microsatellite loci to determine which birds were fed upon. Our goal was to elucidate the factors that influence mosquito feeding preferences in a nest environment and specifically to test the hypothesis that nestlings would be fed upon at a disproportionate rate compared with adult birds.
Culex quinquefasciatus is the major vector of West Nile virus (WNV) and St. Louis encephalitis virus in the South Central United States.8,9 Eastern bluebirds have tested positive for antibodies to WNV in Alabama,10 Georgia,11 and Kansas,12 suggesting that bluebirds are exposed to WNV through the bite of infected vectors in nature. Our methods, which used field strains of Cx. quinquefasciatus and a proven avian host for WNV, enabled us to test our hypotheses under conditions that approached those that occur naturally in the transmission ecology of WNV in the South Central United States.
Materials and Methods
Mosquito introductions and blood meal identifications were performed essentially as described.13 Briefly, egg rafts of Cx. quinquefasciatus were collected from oviposition traps in the field and then reared in the laboratory by using standard methods.14 Adult mosquitoes (mean ± SD = 22.1 ± 3.8/nest) were then introduced into nest boxes of eastern bluebirds at dusk, after the mother bird returned to the nest to brood the nestlings for the night. Mosquitoes and birds were sealed inside the nest box (with a screened hole to allow air exchange and natural light) until just before dawn (Figure 1). At that time (approximately 4:00 am) the mother bird was allowed to leave the box, and mosquitoes were collected with a battery-powered vacuum aspirator. Mosquitoes were introduced into 31 total nest boxes during the bluebird nesting season (April–July) in 2008 and 2009. Blood-engorged female mosquitoes were identified, and processed for individual bluebird host determination by using three dinucleotide microsatellite loci, with three amplifications carried out for each sample.13 In 2009, we visually scored mosquitoes for engorgement (1–5, with 5 being fully engorged) to determine whether level of engorgement had an effect on amplification success.
Figure 1.

Eastern bluebird nest box with apparatus for introducing mosquitoes. The apparatus consisted of a plastic chamber (containing the mosquitoes), which fits into a plastic funnel inserted into the entrance hole of the best box.
Blood samples from bluebirds were collected from adult bluebirds by brachial venipuncture early in the spring, before nesting attempts, and from nestlings when they were eight days of age. After collection, blood samples were processed as described.13 Briefly, samples were transported on ice to the laboratory, centrifuged to separate erythrocytes from serum, then resuspended in TNE buffer solution (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 200 mM NaCl) before storage in a −20°C freezer for later DNA extraction and microsatellite genotyping.13
To determine whether mosquitoes specifically target nestlings we calculated the feeding index (blood meals from nestlings/total blood meals in nest)/(number of nestlings in nest/total animals in nest) for each nest and then averaged the indices from all nests with more than one blood meal identification. The resulting mean was compared with the null hypothesis (feeding index = 1) by using the t-test (PROC TTEST; SAS Institute, Cary, NC). Feeding indices infer host preference through a ratio of the relative abundance of a host and the fraction of the total blood meals derived from that host.15,16 A feeding index value of 1 is considered no preference. Values greater than one and less than one indicate preference and avoidance, respectively.17
We investigated predictors of host selection within the nest by using stepwise logistic regression and multiple regression (PROC LOGISTIC and PROC REG; SAS Institute). Variables examined for their effect on host use included the age of nestlings, number of nestlings in the nest, Julian date, and overnight low temperature. Temperature data was obtained from the National Climate Data Center (http://www7.ncdc.noaa.gov/CDO).
The study was reviewed and approved by the Internal Animal Care and Use Committee (IACUC 2008-1492) of Auburn University.
Results
Of 115 blood-engorged mosquitoes recovered from our experimental introductions, we were able to successfully amplify microsatellite loci and match the blood source to individuals for 80 (70%) of the blood meals. The size of the blood meal was positively correlated with successful amplification of host DNA from blood in mosquito guts (χ2 = 11.7840, degrees of freedom = 1, P = 0.0006). DNA from all small partial blood meals (i.e., those scored as 1, 2, or 3) was not successfully amplified. In contrast, DNA from 55% of blood meals (scored as 4) and 82% of complete blood meals (scored as 5) were successfully amplified at all three loci, which were then used to identify the source of the blood meal to the individual level.
Across all nests, 57% of blood meals were derived from nestling birds, which was not significantly different (T = −0.11, P = 0.91) from the proportion of nestling blood meals expected by chance on the basis of total birds available (mean ± SD feeding index of nestlings = 0.98 ± 0.45, range = 0.2–1.5, 31 observations). Nestlings accounted for 78.2% of birds within the nest boxes used for mosquito introductions. The mother was fed upon in just over half (54%) of all nests, whereas nestlings were fed upon in 84% of all nests. Seven (8.75%) of 80 mosquitoes fed upon multiple birds within a nest. Five of these (6.25% overall) fed upon both mother and nestling birds, and two mosquitoes (2.5% overall) fed on two nestlings.
Mother birds were fed upon more often in nests with younger nestlings than in nests with older nestlings (Figure 2). The probability that the brooding mother was fed upon decreased as nestling age increased (χ2 = 3.8997, degrees of freedom = 1, P = 0.0483). No other variable (number of nestlings in nest, Julian date, or overnight low temperature) was significantly associated with the feeding pattern.
Figure 2.
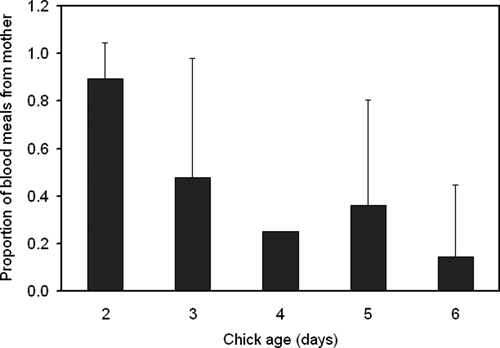
Proportion of mosquitoes introduced into eastern bluebird (Sialia sialis) nest boxes with mother and nestling birds (of different ages) that fed on the mother bird, Auburn, Alabama, 2008 and 2009.
Feeding on individual nestlings appeared to be somewhat stochastic. Within the nests for which a nestling blood meal was detected, the percentage of nestlings fed upon ranged from 25% to 100% and was not significantly associated with the number of nestlings in the nest (R2 = 0.16, F = 3.44, P = 0.09) or nestling age (R2 = 0.07, F = 1.98, P = 0.18).
Overall, mosquitoes were more likely to feed successfully in nests with younger nestlings than in nests with older nestlings (Figure 3). The proportion of mosquitoes that engorged was inversely related to the age of nestlings at the time of the experiment (R2 = 0.56, F = 17.76, P = 0.0012). The percentage of mosquitoes that successfully fed in each nest ranged from 5% to 60%.
Figure 3.
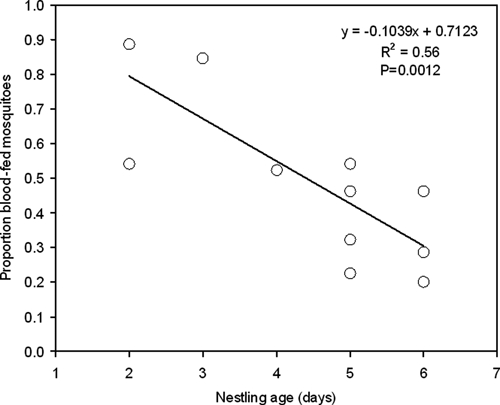
Proportion of mosquitoes introduced into eastern bluebird (Sialia sialis) nest boxes, which successfully obtained a blood meal, over a range of nestling ages, Auburn, Alabama, 2008 and 2009.
Discussion
Based on the number of birds available in the nest boxes, the pattern of mosquito feeding was not significantly different than that expected if mosquitoes chose individuals at random, regardless of age. We therefore found no evidence to support the hypothesis that either nestlings or adults are preferred hosts. Using the proportion of nestling versus adult birds in the boxes as the basis for the null model is founded on the assumption that all individuals are equally accessible to questing mosquitoes. The proportion of blood meals from a given individual is a function of the attractiveness of the individual18 and the probability that a mosquito will successfully feed upon that individual once attracted.19 In this regard, we would predict that mosquito access to nestlings will be affected by the behavior of the mothers in the nest. In the case of bluebirds, mother birds actively attempt cover all of their nestlings at night. Thus, females leave their own dorsal surface exposed while attempting to leave no portion of any nestling exposed (Figure 4). Because the blood of nestlings was detected in 57% of blood meals, we know that the efforts of mothers to cover nestlings was only partly successful. Despite the increased exposure to questing mosquitoes resulting from her protective behavior, mother birds were successfully fed upon in only half of the nests. This leads us to believe that the plumage of mothers presents an effective barrier to questing mosquitoes.2,19 Mosquitoes likely gain access to the blood of mother birds on their faces where feathering is thin or absent.2,20
Figure 4.

Mother bluebird brooding two-day-old nestlings.
Mosquito feeding on the brooding mother decreased as nestling age increased, which suggested that as nestlings grow they become more difficult to cover and therefore become more accessible to questing mosquitoes. The youngest nestlings are almost completely covered by the mother and are thus relatively inaccessible to questing mosquitoes, which have no option but to feed on the brooding mother. Larger nestlings are more difficult to cover completely and a leg or wing sticking out from under the brooding mother would be sufficient to give questing mosquitoes an opportunity to feed upon a nestling.
Interestingly, overall mosquito feeding success decreased as nestling age increased. In nests with the youngest nestlings, a greater proportion of the mosquitoes placed into the nest box fed to engorgement than did in nest boxes with older nestlings. We can only speculate as to the nest dynamics that contribute to this pattern. Younger bluebird nestlings are incapable of thermoregulation and therefore require more consistent brooding during the night.21 It is conceivable that in providing this more consistent thermoregulation the mother bird must remain relatively motionless and thus tolerate more mosquito bites while brooding hatchlings. In nests with older nestlings, which are capable of thermoregulation,21 the mother may be more free to engage in more defensive behaviors and keep her face covered more of the time. Support for this idea was found in a study in which mosquito landings on nestlings was negatively correlated with the fraction of the night that parents fully brooded the nestlings.3
Previous results and those of this study suggest that patterns of feeding on nestling and adult birds at the nest are not the product of innate preferences of mosquitoes for one age class of birds versus another, but are a function of a mosquito's access to each animal and the physical and behavioral defenses of individual birds. Olfactory preferences of mosquitoes for adult birds versus nestlings are likely to influence long-range attraction to nests,5 but such preferences are not likely to be a critical factor in determining which animal is fed upon in the nest environment because olfactory cues are likely to be less important than visual cues for short-range host selection.22 One field study showed that mosquito landing rates are higher on adults than on nestlings, but that the ratio of mosquitoes landing on nestlings versus adults increases as brooding decreases,3 suggesting that landing rates on nestlings are mediated by the protective presence of the adult. Our findings that mothers are fed upon less in nests with older nestlings, when interpreted with respect to these other studies, suggest that the mother bird is better able to defend against questing mosquitoes than nestlings, which are easier targets for questing mosquitoes. Together, individual host attributes and vector-host interactions drive patterns of host use in the nest environment.
Parental care by birds, in the form of brooding, may drive the early season peak in feeding on birds through increased tolerance to mosquito attack. During the nesting period, mother birds must remain on their eggs/young during the night, or risk losing their eggs and young nestlings incapable of thermoregulation.21 In effect, the mother is faced with the choice of tolerating mosquitoes or abandoning her offspring and losing her reproductive effort. Increased tolerance to questing mosquitoes during the breeding season would lead to an increase in mosquito feeding success, possibly driving the apparent seasonal shift in mosquitoes feeding on birds and mammals.23
These vector–host interactions in avian nests may have important implications for the pre-epidemic amplification of arboviruses such as WNV.24 One study concluded that recently fledged hatch-year birds play an important role in the amplification of WNV.25 The study found that WNV in mosquito vectors was tightly linked with abundance of recently fledged birds and that hatch-year birds exhibited high levels of WNV infection and seropositivity. However, a study published nearly concurrently (and by the same group of researchers) asserted that nestling passerines (perching birds) are not important hosts for amplification of West Nile virus.26 The general lack of seropositivity in nestling birds led these authors to the conclusion that nestling passerines experience low WNV exposure, which precludes them from serving as focal amplification hosts. However, our results and those of others3 indicate that nestling birds are fed upon by mosquitoes and in proportion to their abundance. At our own study site, nestlings outnumber adults more than two to one (assuming one mother and one father per nest). Therefore, although mosquitoes demonstrated no preference for feeding on nestlings, nestling birds represent an immunologically naive bolus of reservoir hosts that is renewed each year, which could contribute to late spring–early summer amplification of arboviruses. On the basis of our observations, nestlings can be treated as adults in terms of their likelihood of being exposed to an arbovirus. However, because of their immature immune systems, they may be prone to higher amplification of virus once they are exposed.
Acknowledgments
We thank V. Rebal, E. Dunavant, and A. Manoharan for their assistance in the field, M. Rios for assisting in DNA extraction, and J. Steffen for helpful discussions on data analyses.
Footnotes
Financial support: This study was supported by a grant from the National Institute of Allergy and Infectious Diseases, project no. R01AI049724 to Thomas R. Unnasch and Geoffrey E. Hill.
Authors' addresses: Nathan Burkett-Cadena, Department of Entomology and Plant Pathology, Auburn University, Auburn, AL. Russell A. Ligon, Mark Liu, and Geoffrey E. Hill, Department of Biological Sciences, Auburn University, Auburn, AL. Hassan K. Hassan and Thomas R. Unnasch, Global Infectious Disease Research Program, Department of Global Health, College of Public Health, University of South Florida, Tampa, FL, E-mail: tunnasch@health.usf.edu. Micky D. Eubanks, Department of Entomology, Texas A&M University, College Station, TX.
References
- 1.Kale HW, Edman JD, Webber LA. Effect of behavior and age of individual ciconiiform birds on mosquito feeding success. Mosq News. 1972;32:343–350. [Google Scholar]
- 2.Blackmore JS, Dow RP. Differential feeding of Culex tarsalis on nesting and adult birds. Mosq News. 1958;18:15–17. [Google Scholar]
- 3.Griffing SM, Kilpatrick AM, Clark L, Marra PP. Mosquito landing rates on nesting American robins (Turdus migratorius) Vector Borne Zoonotic Dis. 2007;7:437–443. doi: 10.1089/vbz.2006.0560. [DOI] [PubMed] [Google Scholar]
- 4.McLean RG, Crans WJ, Caccamise DF, McNelly J, Kirk LJ, Mitchell CJ, Calisher CH. Experimental infection of wading birds with eastern equine encephalitis virus. J Wildl Dis. 1995;31:502–508. doi: 10.7589/0090-3558-31.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Scott TW, Lorenz LH, Edman JD. Effects of house sparrow age and arbovirus infection on attraction of mosquitoes. J Med Entomol. 1990;27:856–863. doi: 10.1093/jmedent/27.5.856. [DOI] [PubMed] [Google Scholar]
- 6.Hayes RO, LaMotte LC, Holden P. Ecology of arboviruses in Hale County, Texas, during 1965. Am J Trop Med Hyg. 1967;16:675–687. doi: 10.4269/ajtmh.1967.16.675. [DOI] [PubMed] [Google Scholar]
- 7.Mahmood F, Chiles RE, Fang Y, Barker CM, Reisen WK. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- 8.Reisen WK, Meyer RP, Milby MM, Presser SB, Emmons RW, Hardy JL, Reeves WC. Ecological observations on the 1989 outbreak of St. Louis encephalitis virus in the southern San Joaquin Valley of California. J Med Entomol. 1992;29:472–482. doi: 10.1093/jmedent/29.3.472. [DOI] [PubMed] [Google Scholar]
- 9.Andreadis TG, Armstrong PM. A two-year evaluation of elevated canopy trapping for Culex mosquitoes and West Nile virus in an operational surveillance program in the northeastern United States. J Am Mosq Control Assoc. 2007;23:137–148. doi: 10.2987/8756-971X(2007)23[137:ATEOEC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Hill GE, Sieferman L, Liu M, Hassan H, Unnasch TR. The effects of West Nile virus on the reproductive success and overwinter survival of eastern bluebirds in Alabama. Vector Borne Zoonotic Dis. 2009;10:159–163. doi: 10.1089/vbz.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs SE, Allison AB, Yabsley MJ, Mead DG, Wilcox BR, Stallknecht DE. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006;6:57–72. doi: 10.1089/vbz.2006.6.57. [DOI] [PubMed] [Google Scholar]
- 12.Shelite TR, Rogers CM, Litzner BR, Johnson RR, Schneegurt MA. West Nile virus antibodies in permanent resident and overwintering migrant birds in south-central Kansas. Vector Borne Zoonotic Dis. 2008;8:321–329. doi: 10.1089/vbz.2007.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligon RA, Burkett-Cadena ND, Liu M, Hill GE, Hassan H, Unnasch TR. Assessing mosquito feeding patterns on nestling and brooding adult birds using microsatellite markers. Am J Trop Med Hyg. 2009;81:534–537. [PubMed] [Google Scholar]
- 14.Gerberg EJ, Barnard DR, Ward RA. Manual for Mosquito Rearing and Experimental Techniques. Mount Laurel, NJ: American Mosquito Control Association; 1994. p. 95. [Google Scholar]
- 15.Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- 16.Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, Unnasch TR. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–815. [PMC free article] [PubMed] [Google Scholar]
- 17.Kay BH, Boreham PFL, Edman JD. Application of the “feeding index” concept to studies of mosquito host-feeding patterns. Mosq News. 1979;39:68–72. [Google Scholar]
- 18.Mukabana WR, Takken W, Coe R, Knols BG. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar J. 2002;1:17. doi: 10.1186/1475-2875-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J Med Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Nasci RS, Edman JD. Culiseta melanura (Diptera: Culicidae): population structure and nectar feeding in a freshwater swamp and surrounding areas in southeastern Massachusetts, USA. J Med Entomol. 1984;21:567–572. doi: 10.1093/jmedent/21.5.567. [DOI] [PubMed] [Google Scholar]
- 21.Gowaty PA, Plissner JH. In: The Birds of North America Online. Poole A, editor. Ithaca, NY: Cornell Laboratory of Ornithology; 1998. http://bna.birds.cornell.edu/bna/species/381 (Eastern bluebird (Sialia sialis)). Available at. Accessed May 3, 2010. [Google Scholar]
- 22.Day JF. Host-seeking strategies of mosquito disease vectors. J Am Mosq Control Assoc. 2005;21:17–22. doi: 10.2987/8756-971X(2005)21[17:HSOMDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Edman JD, Taylor DJ. Culex nigripalpus: seasonal shift in bird-mammal feeding ratio in a mosquito vector of encephalitis. Science. 1968;161:67–68. doi: 10.1126/science.161.3836.67. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- 26.Loss SR, Hamer GL, Goldberg TL, Ruiz MO, Kitron UD, Walker ED, Brawn JD. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector Borne Zoonotic Dis. 2008 doi: 10.1089/vbz.2008.0042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


