Abstract
West Nile virus (WNV) invaded Los Angeles in September 2003, and during the subsequent five-year period followed a pattern of amplification, subsidence, and resurgence. Enzootic transmission was tracked by abundance and infection incidence in Culex pipiens quinquefasciatus and Cx. tarsalis and by seroprevalence in peridomestic passerine birds, infection in dead birds, and seroconversions in sentinel chickens. Culex p. quinquefasciatus served as the primary vector of WNV, with gravid traps serving as the best sampling method and the most consistent indicator of viral activity. Spatial scan statistics applied to mosquito infection and positive dead bird data delimited three major clusters of WNV transmission, with introduction occurring in the Los Angeles Basin, and amplification and dispersal events carrying transmission to the San Fernando and Santa Clarita valleys. Los Angeles experienced major epidemics in 2004 and 2008, providing a unique opportunity to investigate specific patterns of enzootic amplification preceding epidemics.
Introduction
Enzootic West Nile virus (family Flaviviridae, genus Flavivirus, WNV) activity was first documented in Los Angeles, California, in September 2003.1,2 A human case had been reported in October 2002. However, autochthonous infection is still disputed. The emergence of WNV in urban Los Angeles was focused along the San Gabriel River corridor, with dead birds and infected Culex pipiens quinquefasciatus Say detected from September through October 20033 rapidly after initial detection in the Imperial Valley in California during July 2003.2
Historically, the endemic mosquito-borne encephalides in California, St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus, SLEV) and western equine encephalomyelitis virus (Togoviridae, Alphavirus, WEEV), were considered to be rural health problems, mostly of the Central Valley.4 However, during the 1980s, Los Angeles experienced intermittent enzootic transmission and outbreaks of SLEV.5 The last outbreak occurred in 1984, with 26 human cases, and the enzootic indicators were infected sentinel chickens, followed by infected pools of Cx. tarsalis Coquillett.6 The lack of detectable mosquito infection prior to human cases was likely caused by limited and focal mosquito surveillance at that time, with only 81 mosquito pools tested during 1984 from Los Angeles County.7 After discovery of urban SLEV transmission, the Greater Los Angeles County Vector Control District (GLACVCD) retained a small-scale arbovirus surveillance program monitoring mosquito abundance and infection and sentinel chicken seroconversions for WEEV and SLEV.
During 2002, prior to WNV reaching the West Coast, GLACVCD further expanded surveillance to include WNV testing for more sites and sampling frequency, additional sentinel chicken flocks, and the monitoring of wild bird seroprevalence. This multi-disciplinary surveillance approach captured the patterns of WNV introduction, amplification, and dispersal in this heavily urbanized maritime environment. Surveillance variables included human and equine case reports, mosquito abundance and infection, dead bird reports and test results, sentinel chicken seroconversions, wild bird seroprevalence, and climate data. The current report describes temporal and spatial patterns within our six-year data set and addresses our over-arching hypothesis that careful monitoring of antecedent WNV enzootic activity can help predict the timing and intensity of tangential transmission to humans and improve current risk models. A comparison of predictive risk models will be addressed in a companion report. Spatial and temporal patterns among these data and risk models enabled us to determine which surveillance indicators were most effective in predicting human cases reported by passive surveillance from densely populated and ethnically diverse Los Angeles.
Methods
Study area.
Los Angeles County in southern California is the most populous county in the United States. It consists of 88 incorporated cities and 140 unincorporated communities8 and contains approximately 10 million persons or 27% of the California state population.9 Landscape ranges from coastal chaparral in the Los Angeles Basin, San Fernando, and San Gabriel valleys to desert in the Santa Clarita Valley. The coastal chaparral is characterized by two forces; the south facing slopes of the San Gabriel and San Bernardino mountain ranges create a dry environment, and coastal fog creates humidity.10 Rainfall in this region is typically between November and April, with monthly precipitation rarely exceeding 10 cm. Daily high temperatures range from 20°C to 30°C, and lows range between 9°C and 20°C.11 There are four major river systems, the Los Angeles River, the San Gabriel River, Santa Clara River, and the Rio Hondo River. Juxtaposed on this natural landscape are 805 km of open concrete channels, 4,023 km of underground storm drains, and > 70,000 catch basins.12
Population estimates from the 2000 deci-centennial census for Los Angeles County were 9,519,331 persons, with a population density of 905 persons/km2.9 Los Angeles County covers 10,518 km2, of which 3,445 km2 are within the GLACVCD jurisdiction that serves approximately six million residents.13
Climate.
Daily high and low temperature data were made available by the National Aeronautics and Space Administration Terrestrial Observation and Prediction System,14 a combination of ground and satellite data with modeling that provides 1-km2 grid precision. These data were averaged for all grid cells across Los Angeles County in biweekly intervals for crude risk assessment estimates, stratified by cells falling within each of the three study zones, and averaged in biweekly intervals. Data aggregation and calculations were performed by using PostgreSQL 8.3.7.15
Human case monitoring.
Human cases were monitored by the Los Angeles County Department of Health and Human Services, Acute Communicable Disease Control, through passive case detection and reporting. Additional cases were discovered through blood donor programs and noted as asyptomatic blood donors (ASDs) unless symptoms developed after donation, at which time the person was included as a case. Cases fit the Centers for Disease Control and Prevention (Ft. Collins, CO) definition for neuroinvasive or febrile illness and were laboratory confirmed, typically by demonstration of IgM in serum samples or in spinal fluid by enzyme immunoassay (EIA).16
Mosquito abundance and infection.
Data were generated by monthly collections of mosquitoes over the six-year study period (2003–2008) in quarter mile (0.42 km) grids within the GLACVCD. Fixed trap sites were geocoded using a global positioning system device (Magellan MiTAC Digital Corp., Santa Clara, CA). Five core sites were operated on a bi-monthly schedule in conjunction with sentinel chicken and wild bird bleeding. Three trap types were used: Center for Disease Control and Prevention style dry ice-baited encephalitis virus surveillance (EVS) traps,17,18 Reiter-Cummings gravid traps19 baited with alfalfa-yeast media, and underground storm drain system traps that were unbaited EVS traps placed under manhole covers in storm drains. Mosquitoes were enumerated to species, sex, and trophic status. Infection was monitored by aggregating samples of Cx. p. quinquefasciatus, Cx. stigmatosoma, and Cx. tarsalis mosquitoes from the same time period and location into pools of 2–50 mosquitoes of each species and trap type. Pools were screened for WNV, SLEV, and WEEV RNA using real-time multiplex reverse transcription–polymerase chain reaction (RT-PCR) with an ABI Prism 7900 TaqMan (Applied Biosystems, Foster City, CA) and published20 and unpublished primers from the envelope gene. All multiplex RT-PCR-positive samples were confirmed by virus isolation on Vero cell culture, in situ EIA,21 and/or a second singleplex RT-PCR using primer sets from the nonstructural protein gene region.22 The predictive value of a positive multiplex RT-PCR was excellent if the critical threshold scores were < 30. Therefore, during 2006–2008, confirmation by a second method was conducted only on samples with screening cycle threshold values > 30 and < 40. Mosquito infection incidence was calculated using the PooledInfRate v2.0 Microsoft Excel® add-in (Microsoft, Redmond, WA).23
Dead birds.
Dead birds found by the public were reported to the California Department of Public Health, Vector Borne Disease Section, Dead Bird Hotline.24 If birds were considered to be in a testable condition, they were forwarded to the California Animal Health and Food Safety laboratory at the University of California Davis for necropsy. Oral swabs (American crows only) or kidney tissues were submitted to Center for Vectorborne Diseases for WNV testing by RT-PCR using previously published primers.25 Previous studies indicated a low case-fatality rate of birds infected with WEEV or SLEV.26 Therefore, dead birds were not tested for these viruses. From 2004 onwards the Dead Bird Hotline ceased testing birds from zip codes with a previously reported positive dead bird, but continued to collect dead bird reports. Throughout, bird common names follow those approved by the American Ornithologist's Union (http://www.aou.org/checklist/north/index.php).
Sentinel chickens.
Flocks of 10 White Leghorn hens were maintained at 7 sites each surveillance year. Blood samples (0.1 mL) were collected from all birds at each site every two weeks by brachial venipuncture and placed on filter paper strips.27 The strips were sent to the California Department of Public Health, Viral and Rickettsial Disease Laboratory in Richmond, California for testing by EIA and immunofluorescent antibody assay for antibodies to WNV, WEEV, and SLEV.28
Free-ranging avian serologic testing.
Free-ranging birds were collected by grain-baited Australian crow traps with inlet apertures reduced to limit ingress to small birds.29 Traps were placed at each of five core sites and were set for 24 hours biweekly. As modified, traps collected primarily peridomestic house sparrows (Passer domesticus) and house finches (Carpodacus mexicanus). Birds were banded pursuant to Federal Banding permit 22763, and 0.1 mL of blood was collected by 28-guage needle syringe from each bird by jugular venipuncture and expelled into 0.9 mL of sterile saline. The bird serum was tested by EIA for antibodies to WEEV and flavivirus.30 Because antibodies against WNV cross-react with SLEV in our EIA, positive results were confirmed and the infecting virus identified by end point plaque-reduction neutralization test using the NY99 strain of WNV and the KERN217 strain of SLEV. Wild bird serologic data were used to calculate seroprevalence proportion, defined as the total number of positive birds/total birds bled for each bleed date.
Statistical analysis.
A retrospective time-space scan for high prevalence was performed in SatScan™31 on the mosquito pool and tested dead bird datasets separately for each year of the study period.32,33 The data were binary. Therefore, a Bernoulli model was selected for the iterative scans for high rates. Cluster centers were not allowed within an overlapping cluster. Statistical significance of a cluster in either the dead bird or mosquito pool datasets was identified by a P value < 0.05, rejecting the null hypothesis of random dispersion of WNV-positive test results in time and space. The maximum time period permitted was 10% of the study period to ensure that all mosquito sites trapped on a monthly cycle were included in the scan. The maximum proportion of the observations allowed in any single cluster was 0.10 to reduce cluster size. Parameter restrictiveness for clustering was evaluated by determining if the number of observations within a cluster neared or equaled the maximum permissible proportion. Mapping of surveillance indicators and significant clusters was performed using ArcMap version 9.3 software (Environmental Systems Research Institute, Redlands, CA).
Trap efficacy for collecting Cx. tarsalis and Cx. p. quinquefasciatus was compared in EVS trap collections for the entire study period. These data were transformed by ln(y + 1) to adjust for skewness and control the variance34 and analyzed by analysis of variance (ANOVA) with least squared means.
Trap types were compared using data collected in 2004, when each site was sampled using paired EVS and gravid traps. These data were transformed by ln(y + 1) to adjust for skewness and control the variance,34 and tested by an ANOVA with Tukey's comparisons of transformed mean females per trap night (F/TN). Underground storm drain system trap collections were not compared in this fashion because the sampling scheme was not comparable. An additional non-parametric Wilcoxon rank sum test was performed on the trap type data for the Santa Clarita stratum because of the low sample size. Pearson correlation analysis was performed to determine the correlation between females per trap night by both trap methods with the month and site of collection. Analyses were conducted using SAS version 9.2 software (SAS Institute Inc., Cary, NC).
Ethics.
Collection, banding, and bleeding of wild birds was done under Protocols 11184 and 12889 that were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Davis, Master Station Federal Bird Banding Permit 22763 issued by the U.S. Geological Survey, California Resident Scientific Collection Permits by the State of California Department of Fish and Game, and Federal Fish and Wildlife Permit No. MB082812-0. Husbandry and bleeding of sentinel chickens was conducted according to Protocols 11186 and 12878 that were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Use of arboviruses was approved under Biological Use Authorizations #0554 and #0873 issued by the Environmental Health and Safety Committee of the University of California, Davis, and USDA Permit #47901.
Results
A summary of surveillance effort for 2003–2008 is shown in Table 1.The intensity of surveillance was maintained throughout the study period, with the numbers of pools submitted for testing related to mosquito abundance. The first date of detecting a positive result by each surveillance indicator is shown in Table 2. Dead birds were consistently reported earliest each year. However, these reports appeared to be independent of amplification in avian or mosquito populations because they typically occurred over a month before a positive dead bird test result was detected. Positive dead birds also were detected prior to the mosquito season (typically May–October), which suggested either mosquito transmission at levels below our detection thresholds or bird-to-bird transmission.35 Sentinel chicken seroconversions were detected concurrently in time with infected Cx. stigmatosoma pools, and positive Cx. p. quinquefasciatus pools were detected consistently earlier in the season, followed by Cx. tarsalis pools. The magnitude of the Los Angeles epizootics and epidemics was considerable (Figure 1). Therefore, the area was stratified on the basis of time-space cluster findings in the sections that follow.
Table 1.
Summary of West Nile virus surveillance in Los Angeles County, 2003–2008*
| Surveillance method | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Total |
|---|---|---|---|---|---|---|---|
| Trap nights: CO2 trap | 1,078 | 2,056 | 2,027 | 1,542 | 1,934 | 1,779 | 11,088 |
| Gravid | 984 | 1,883 | 2,110 | 1,734 | 2,147 | 1,841 | 10,602 |
| USDS | 314 | 314 | 18 | 140 | 550 | 658 | 1,994 |
| Mosquitoes collected | |||||||
| Culex tarsalis | 6,957 | 6,829 | 8,090 | 2,042 | 2,956 | 5,862 | 32,736 |
| Cx. p. quinquefasciatus | 59,695 | 70,579 | 87,744 | 56,909 | 81,487 | 97,853 | 454,267 |
| Cx. stigmatosoma | 2,678 | 3,229 | 3,510 | 2,721 | 798 | 3,184 | 16,120 |
| Cx. erythrothorax | 97,029 | 133,423 | 129,142 | 32,167 | 47,554 | 74,404 | 513,719 |
| Total mosquitoes collected | 204,291 | 244,117 | 272,434 | 106,119 | 156,249 | 214,566 | 1,197,776 |
| No. mosquito pools | 1,621 | 2,456 | 2,920 | 1,614 | 2,288 | 2,558 | 13,457 |
| Pools positive for WNV | 6 | 345 | 181 | 78 | 89 | 472 | 1,172 |
| Mosquito infection prevalence per 1,000 mosquitoes | |||||||
| Cx. tarsalis | 0 | 3.91 | 3.11 | 0 | 0.41 | 1.48 | |
| Cx. p. quinquefasciatus | 0.15 | 6.20 | 1.89 | 1.56 | 0.66 | 3.87 | |
| Cx. stigmatosoma | 0 | 4.53 | 3.42 | 0 | 1.02 | 6.73 | |
| Cx. erythrothorax | 0 | 0.19 | 0 | 0 | 0 | 0 | |
| Sentinel chickens | |||||||
| Flocks | 6 | 7 | 7 | 7 | 7 | 7 | 41 |
| Seroconversions for WNV | 0 | 45 | 25 | 19 | 15 | 39 | 143 |
| No. wild bird serologic samples | 1,692 | 2,987 | 1,912 | 2,101 | 3,068 | 2,347 | 14,107 |
| WNV positive | 2 | 442 | 288 | 172 | 189 | 258 | 1,351 |
| WNV/SLEV (UI) positive | 0 | 44 | 28 | 14 | 19 | 0 | 105 |
| SLEV positive | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| No. LAC dead birds tested | 365 | 1,153 | 690 | 937 | 931 | 1,101 | 5,177 |
| No. LAC WNV-positive dead birds | 65 | 840 | 173 | 166 | 217 | 511 | 1,972 |
| Horse cases | 0 | 9 | 5 | 0 | 0 | 0 | 14 |
| Human cases | 0 | 168 | 29 | 10 | 28 | 83 | 310 |
CO2 trap = encephalitis virus surveillance trap baited with CO2; USDS = unbaited encephalitis virus surveillance trap set in an underground storm drain system; WNV = West Nile virus; SLEV = St. Louis encephalitis virus; UI = unidentified flavivirus; LAC = Los Angeles County.
Table 2.
Summary of first dates of detection of West Nile virus in Los Angeles County for each surveillance indicator by year*
| Indicator | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|
| First dead bird report | Jan 16 | Jan 4 | Jan 4 | Jan 3 | Jan 1 | Jan 1 |
| First positive dead bird | Sep 3 | Feb 24 | Feb 1 | Jan 9, Apr 6 | Apr 5 | Jan 29 |
| Sentinel chicken | ND | Jul 13 | Jun 18 | Jul 22 | Aug 11 | Jul 13 |
| Positive mosquito pools | ||||||
| Culex tarsalis | ND | May 24 | Jun 21 | ND | Aug 22 | Jun 25 |
| Cx. p. quinquefasciatus | Sep 26 | May 6 | Jun 1 | May 10 | Jan 11, May 15 | May 15 |
| Cx. stigmatosoma | ND | Jul 16 | Jun 22 | ND | Aug 3 | Aug 1 |
First positive dead bird = virus detection in a bird carcass by reverse transcription–polymerase chain reaction; ND = none detected in that surveillance year.
Figure 1.
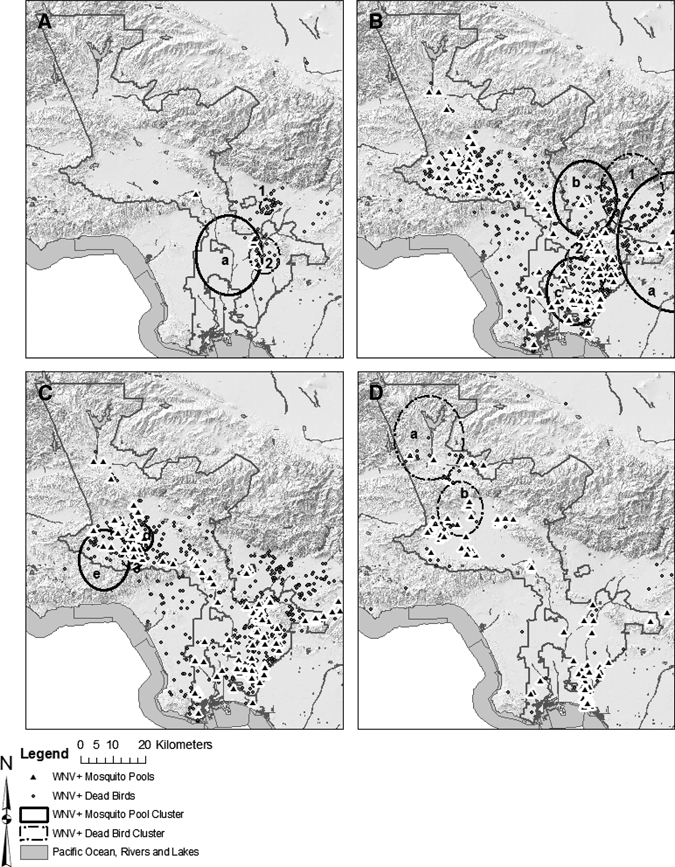
Time-space clusters of West Nile virus (WNV) plus dead birds (dots) and WNV+ mosquito pools (triangles) in Los Angeles County. The first evidence in the Los Angeles Basin in 2003 (A) with mosquito pool cluster a (10/9/2003–10/29/2003), and dead bird clusters 1 (9/13/2003–10/15/2003) and 2 (10/30/2003–12/3/2003). Amplification in the Los Angeles Basin in early 2004 (B) depicted by dead bird clusters 1 (5/8/2004–6/4/2004) and 2 (5/29/2004–7/2/2004) overlapping with mosquito pool clusters a–c (6/26/2004–7/30/2004, 7/3/2004–8/6/2004, and 7/10/2004–8/13/2004), then progressing into the San Fernando Valley in late 2004 (C) dead bird cluster 3 (7/10/2004–8/13/2004), and mosquito pool clusters d (7/24/2004–8/20/2004) and e (7/24/2004-8/20/2004). Amplification in Santa Clarita in 2005 (D) with dead bird clusters a (7/24/2005–6/27/2005) and b (9/11/2005–10/8/2005).
Introduction of WNV into the Los Angeles Basin occurred during 2003 (Figure 1A), and focal dead bird and mosquito pool activity were clustered at the confluence of the San Gabriel and Rio Hondo rivers near an enormous fall–winter American crow roost within the Whittier Narrows Nature Reserve (Figure 2) conservatively estimated to consist of approximately 50,000 birds. Analyses of time–space clustering showed significant (P < 0.05) dead bird and mosquito pool clustering in each study year. Amplification in the Los Angeles Basin commenced early and rapidly during 2004, with marked increases in numbers of WNV-positive dead birds and mosquito pools, followed by a rapid succession of significant clustering events (Figure 1B). By July 2004, significant activity moved into the San Fernando Valley near a second smaller American crow roost in Van Nuys (Figure 1B and C). In 2005, activity dispersed and subsided in the Los Angeles Basin. A significant bird cluster occurred in Santa Clarita Valley (Figure 1D) and completed the introduction of WNV into Los Angeles County. In 2006 and 2007, significant clustering was restricted to the San Fernando Valley [data not shown]. In 2008 clustering was centered again at the large corvid roost in the Whittier Narrows. Throughout, the proportion of total observations within a cluster never exceeded the imposed limit of 10% of the population (maximum = 7.7%). Therefore, this parameter was not considered overly restrictive.
Figure 2.
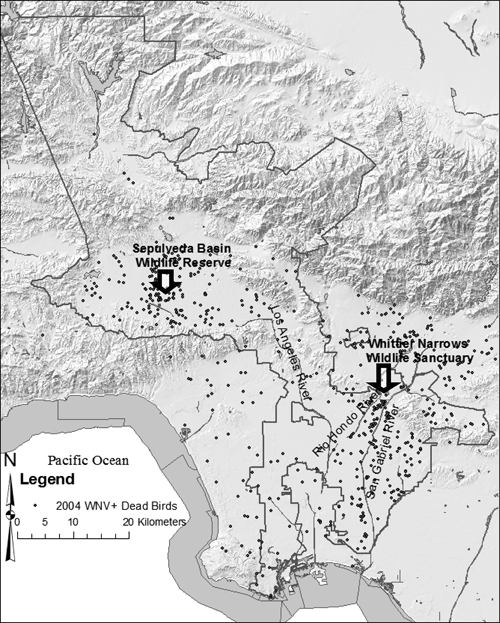
The 2004 WNV epizootic within the Greater Los Angeles County Vector Control District boundary (gray line), represented by positive dead birds (circles). Arrows indicate major corvid roosting sites.
Climate.
Maximum temperature in most study years exceeded the 15°C threshold permissive for WNV replication in Culex spp. by April 15, whereas in 2004 and 2007 this threshold was achieved in mid-March (Figure 3). A repeated measures ANOVA of mean temperatures blocked by year showed significant differences among years (F = 5.34, degrees of freedom [df] = 5, P = 0.012). When temperatures were stratified by region (Figure 3), there were significant differences in mean temperature among regions (F = 158.47, df = 2, P < 0.001), with no difference between Santa Clarita (mean = 16.77°C) and San Fernando Valleys (mean = 16.57°C), but significantly warmer mean temperatures in the Los Angeles Basin (mean = 19.26°C, Tukey's honestly significant difference test, df = 401, alpha = 0.05, minimum significant difference = 0.52°C). Warmer temperatures here could relate to increased urbanization creating heat island phenomena.36 The Los Angeles Basin had most of the epizootic and epidemic WNV activity. This finding could be related to the significantly higher mean temperatures enabling more rapid viral replication in the mosquito host and more frequent host–vector contact, even though mosquito abundance was lower in this region than in the Santa Clarita and San Fernando valleys.
Figure 3.
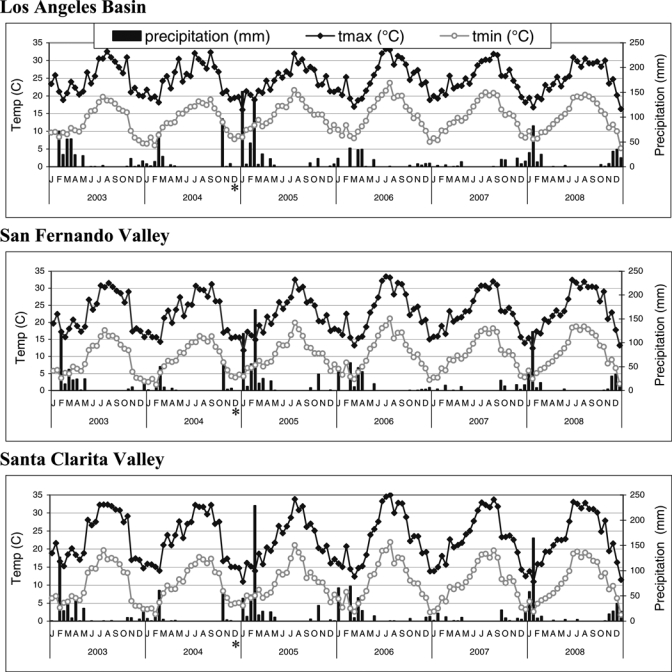
Biweekly temperature and rainfall for the three regions of Los Angeles County. Tmax is the average of the daily maximum temperatures for the two-week period, and tmin is the average of the daily minimum temperatures. *Temperature data for December 2004 were not available from the Terrestrial Observation and Prediction System weather datasets
Human cases.
Laboratory-confirmed human cases were observed during all six years of the study (Table 3), with most reported during the 2004 epidemic. A total of 168 cases with known onset date were found within the GLACVCD jurisdiction and 287 were found within Los Angeles County.37 During 2004, 56% of the 149 reported human cases with a clinical designation were diagnosed as West Nile neuroinvasive disease (WNND), the severe outcome of WNV infection. Cases decreased during 2005–2007, but increased again in 2008, with 83 cases reported (72% WNND). The percent of cases diagnosed as WNND in 2008 was significantly greater than the percent WNND in 2004 (χ2 = 5.73, df = 1, P = 0.017), perhaps indicating increased under-reporting of West Nile fever. Of interest were the ASDs because they represented infected persons who were detected during the viremia period while making a blood donation. Based on these persons, the 2008 outbreak with 15 ASDs may have been more severe than in 2004, which had 9 ASDs.
Table 3.
Summary of human cases by syndrome for the Greater Los Angeles Vector Control District, 2003–2008*
| Year | Total | WNND (%) | WNF (%) | ABD |
|---|---|---|---|---|
| 2003 | 1 | 1 (100) | ||
| 2004 | 160* | 84 (56) | 65 (43) | 9 |
| 2005 | 29 | 20 (69) | 9 (31) | 4 |
| 2006 | 9 | 2 (22) | 7 (78) | 0 |
| 2007 | 28 | 17 (61) | 11 (39) | 4 |
| 2008 | 83 | 60 (72) | 23 (28) | 15 |
| Total | 310 | 183 (61) | 116 (39) | 32 |
Eleven of these cases were missing clinical designation on the epidemiologic report. WNND = West Nile neuroinvasive disease; WNF = West Nile fever; ABD = asymptomatic blood donor not included in the case totals or calculations.
A time-series graph of human cases (Figure 4) showed that tangential transmission to humans was confined to June and October, and typically peaked concurrent with Cx. p. quinquefasciatus mosquito infection incidence per 1,000.
Figure 4.
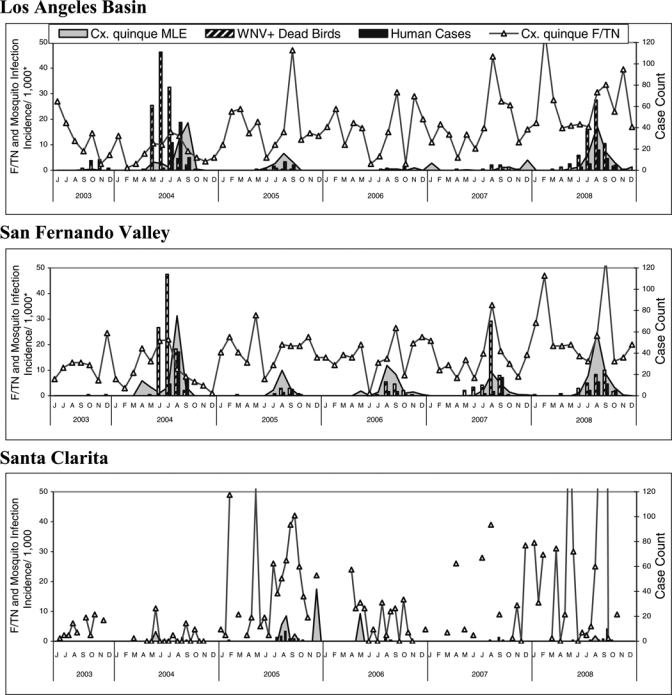
Summary of human cases, positive dead birds Culex quinquefasciatus abundance in females per trap night (F/TN) and infection incidence (maximum likelihood estimation [MLE]) reported in the Greater Los Angeles County Vector Control District during 2003–2008 by biweekly interval. *Mosquito infection incidence/1,000 mosquitoes was calculated biweekly by bias corrected MLE methods with skewness-corrected 95% confidence intervals using PooledInfRate software add-in.
Mosquito abundance.
Most Cx. tarsalis specimens were collected by dry ice-baited EVS traps, which had 1.90 females per trap night. Gravid traps had 0.81 females per trap night over the total study period. Mosquito collection efforts were similar throughout the study period. However, the number of mosquito pools tested differed because of abundance patterns. Transformed annual Cx. tarsalis abundance in Los Angeles was significantly less than that for Cx. p. quinquefasciatus when EVS trap collections were compared by ANOVA with least squares means (t = 16.83, df = 287, P < 0.001, back-transformed means were 1.24 and 6.02 females per trap-night, respectively). A similar comparison could not be drawn in gravid trap collections because of infrequent Cx. tarsalis samples by this method.
Trap collections for 2004 were assessed by ANOVA (n = 2,659 trap nights) and varied significantly by month of collection (F = 67.08, df = 11, P < 0.001), trap type (F = 582.95, df = 1, P < 0.001), and interaction between month and trap type (F = 3.11, df = 11, P = 0.0004). A comparison of mean females per trap type showed that gravid trap counts (n = 1,372, mean = 10.7 females per trap night) were significantly greater than EVS trap counts (n = 1,287, mean = 2.9 females per trap night) (P < 0.001, by Tukey's honestly significant difference test). Pearson correlation analysis showed females collected in EVS and gravid traps per night were correlated inversely over time and space during 2004 (r = −0.39, n = 2,659, P < 0.0001), but positively correlated over months (r = 0.11, n = 2,659, P < 0.001), with a modest effect of site (r = 0.08, n = 2,659, P < 0.001).
Analysis of variance of transformed paired gravid and EVS trap collections showed significant difference by region of Los Angeles (F = 34.74, df = 2, 1251, P < 0.001). Mean counts in the San Fernando Valley (back-transformed mean = 2.18 F/TN) were greater than in the Los Angeles Basin (back-transformed mean = 1.50 F/TN) (P < 0.001, by Tukey's multiple comparison), but not statistically different from mean counts in Santa Clarita (back-transformed mean = 1.29 F/TN). Mean trap counts were not statistically different in the Los Angeles Basin and Santa Clarita. Further analysis was performed to stratify the data by trap type. In the Los Angeles Basin, there were significant differences between collections made with gravid and EVS traps (n = 1,480, F = 155, df = 1, 1478, P < 0.001). Similarly, in the San Fernando Valley, there were significant differences in collections between the two trap types (n = 984, F = 198.59, df = 1, 982, P < 0.001). Interestingly, in more rural Santa Clarita Valley, collections were not significantly different (n = 44, F = 3.54, df = 1, 42, P = 0.066), even by non-parametric methods used to account for lower sampling effort (z = -1.45, P = 0.14, by Wilcoxon rank-sum test).
Mosquito infection.
Only a single pool of 25 Cx. p. quinquefasciatus from Griffith Park were positive for SLEV during 2003, despite continued testing of each pool for SLEV, WEEV, and WNV. Mosquito infection was monitored in all species in 2004 (Table 4). No infected Culiseta spp. were detected, despite a relatively large number of pools submitted for Cs. incidens. Of the Culex mosquitoes, Cx. p. quinquefasciatus exhibited the highest infection incidence, followed by Cx. stigmatosoma, Cx. tarsalis, Cx. thriambus, and Cx. erythrothorax. No infected Cx. restuans were detected. However, the sample size was limited.
Table 4.
Mosquito species tested for West Nile virus in Los Angeles County, 2004, with infection incidence per 1,000 mosquitoes as calculated by bias-corrected maximum likelihood estimation methods with skewness-corrected 95% confidence intervals using the PooledInfRate software add-in*
| Species | No. pools submitted | No. positive pools | MLE | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Anopheles hermsi | 43 | 1 | 0.74 | 0.04 | 3.58 |
| Culesita incidens | 133 | 0 | 0 | – | – |
| Cs. inornata | 7 | 0 | 0 | – | – |
| Cs. particeps | 7 | 0 | 0 | – | – |
| Culex erythrothorax | 440 | 4 | 0.19 | 0.06 | 0.47 |
| Cx. quinquefasciatus | 1,558 | 313 | 6.20 | 5.55 | 6.91 |
| Cx. restuans | 2 | 0 | 0 | – | – |
| Cx. stigmatosoma | 71 | 6 | 4.53 | 1.89 | 9.31 |
| Cx. tarsalis | 172 | 19 | 3.91 | 2.44 | 5.98 |
| Cx. thriambus | 22 | 2 | 2.87 | 0.52 | 9.58 |
MLE = maximum likelihood estimation; CI = confidence interval.
Biweekly Cx. p. quinquefasciatus maximum likelihood estimation of mosquito infection incidence at our study areas ranged from 0 to 32.3 per 1,000. Most specimens tested were from gravid traps, which represented 76%, 75%, 74%, 68%, 58%, and 69% of the total Cx. p. quinquefasciatus specimens tested for the years 2003–2008, respectively. The disparity in mosquito pool submission was caused by differences in the number of mosquitoes collected by each trap method, as described above. Biweekly mosquito infection incidence for Cx. tarsalis ranged from 0.94 to 16.04 per 1,000 mosquitoes.
On the basis of the time–space cluster analyses, the GLACVCD was stratified into three main areas (Figure 5) that experienced dramatically different Cx. p. quinquefasciatus abundance and infection incidence over time (Figure 4). The Los Angeles Basin experienced most of the 2004 and 2008 epidemics, starting earlier and lasting for a longer duration, than at the other strata. The San Fernando Valley experienced the 2004 epidemic a month later than the Los Angeles Basin, with a steep epidemic curve and cessation of activity in October, similar to the termination of activity in Los Angeles Basin. The intensity of activity during 2005–2007 was greater in the San Fernando Valley than in the Los Angeles Basin. The Santa Clarita Valley, which had the smallest population of persons at risk, experienced small epidemics in 2005 and 2008.
Figure 5.
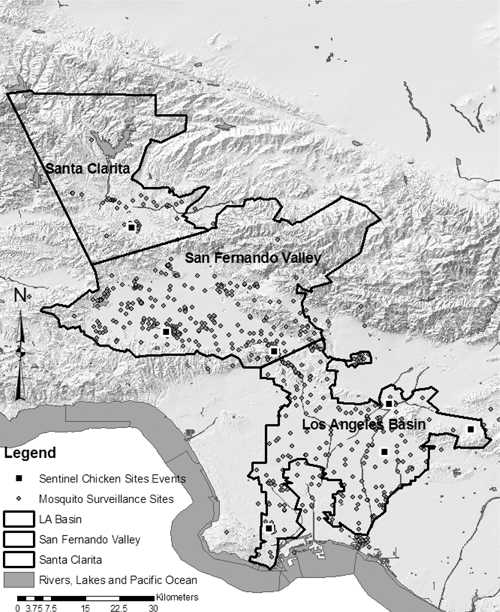
Three study areas in Los Angeles County defined by the time-space scan results with mosquito and chicken surveillance sites.
Dead birds.
In 2003, the California Dead Bird Hotline was started by the California Department of Public Health, and 365 birds were reported from GLACVCD and tested, of which 65 (18%) were positive for WNV (Table 5). In 2004, the number of dead bird reports increased dramatically, but although the proportion tested decreased because of zip code closures and the high numbers of samples, the percent positive was highest (73%). The percent positive decreased during 2005–2006, then increased during 2007–2008. The percent tested (χ2 = 5915.19, df = 5, P < 0.001) and the percent of tested birds that were positive (χ2 = 987.65, df = 5, P < 0.001) differed significantly among years. The lowest observed percent of WNV-positive tested birds was 18% in 2003 and 2006. The percent of reported birds tested was highest in 2003 (100%) when the testing protocol included all reported birds. After the testing protocol was adapted in 2004 to include only birds dead less than 24 hours, the highest reported percent tested was in 2007 (30%) and the lowest was in 2004 (4%).
Table 5.
Dead birds reported and tested for West Nile virus, by year, in Los Angeles County, California 2003–2008
| Year | No. reported | No. tested (% of reported) | No. positive (% of tested) |
|---|---|---|---|
| 2003 | 365 | 365 (100) | 65 (18) |
| 2004 | 25,662 | 1,153 (4) | 840 (73) |
| 2005 | 4,534 | 690 (15) | 173 (25) |
| 2006 | 3,630 | 937 (26) | 166 (18) |
| 2007 | 3,146 | 931 (30) | 217 (23) |
| 2008 | 6,087 | 1,101 (18) | 511 (46) |
| Total | 43,424 | 5,177 (12) | 1,972 (38) |
The most frequently tested species was the American crow, followed by the house finch, common raven, and house sparrow (Table 6). Temporal patterns in the proportion tested (number of birds in testable condition from open zip codes/ total dead birds reported) and the proportion positive (number of dead birds with a positive test result/total tested birds) calculated biweekly are shown in Figure 6. The proportion positive increased seasonally from July through November in subsidence years 2005–2007. However, in the 2004 and 2008 outbreak years there were early season peaks.
Table 6.
Frequently tested dead birds submitted to the California Department of Public Health Dead Bird Hotline, 2004–2008, and percent positive for WNV RNA by RT-PCR*
| Species | No. tested (% positive, 0 if not indicated) | |||||
|---|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | 2008 | Total | |
| Acorn woodpecker | 1 | 4 (25) | 5 (20) | |||
| American coot | 2 | 3 (33) | 12 (17) | 2 | 19 (16) | |
| American crow | 979 (80) | 263 (49) | 275 (36) | 348 (43) | 672 (62) | 2,537 (62) |
| American goldfinch | 7 | 6 | 1 | 3 (33) | 17 (6) | |
| American kestrel | 1 | 1 | 1 | 2 | 1 (100) | 6 (17) |
| American robin | 1 | 9 (11) | 1 | 6 (17) | 17 (12) | |
| Anna's hummingbird | 2 | 4 | 3 | 7 (14) | 16 (6) | |
| Black phoebe | 2 (100) | 1 | 6 | 6 | 5 | 20 (10) |
| Black-headed grosbeak | 1 | 3 | 7 | 4 (25) | 15 (7) | |
| Brewer's blackbird | 2 (50) | 3 (33) | 9 | 7 (14) | 5 (20) | 26 (15) |
| Brown-headed cowbird | 1 | 6 (17) | 1 | 1 | 9 (11) | |
| Bullock's oriole | 3 (33) | 1 | 1 | 5 (20) | ||
| Burrowing owl | 3 (33) | 1 | 4 (25) | |||
| California quail | 5 | 8 | 4 (25) | 1 | 18 (6) | |
| California towhee | 1 (100) | 5 | 3 | 3 | 7 (14) | 19 (11) |
| Cliff swallow | 12 (8) | 1 | 13 (8) | |||
| Cockatiel | 6 | 5 | 2 (50) | 13 (8) | ||
| Common raven | 47 (21) | 62 (6) | 53 (8) | 47 (23) | 40 (18) | 249 (14) |
| Cooper's hawk | 13 (8) | 11 (18) | 18 (17) | 18 (17) | 12 (42) | 72 (19) |
| European starling | 3 | 13 | 13 (8) | 9 (11) | 13 | 51 (4) |
| Golden-crowned sparrow | 2 | 10 (30) | 1 | 13 (23) | ||
| Gouldian finch | 2 | 2 (50) | 4 (25) | |||
| Great blue heron | 1 | 3 | 2 (50) | 6 (17) | ||
| Great horned owl | 6 (33) | 6 (33) | 8 | 11 | 4 | 35 (11) |
| House finch | 11 (45) | 49 (14) | 44 (23) | 92 (21) | 66 (41) | 262 (26) |
| House sparrow | 15 (27) | 67 (4) | 47 (13) | 31 (13) | 56 (23) | 216 (14) |
| House wren | 1 (100) | 3 (33) | 4 (50) | |||
| Lesser goldfinch | 2 | 2 | 4 (25) | 4 | 12 (8) | |
| Mallard duck | 1 (100) | 1 | 13 | 4 | 14 (29) | 33 (15) |
| Mourning dove | 15 (7) | 44 | 10 (10) | 9 (33) | 78 (6) | |
| Northern mockingbird | 4 (75) | 10 | 27 (15) | 14 (7) | 16 (31) | 71 (18) |
| Orange-crowned warbler | 1 | 7 (14) | 8 (13) | |||
| Purple finch | 2 (100) | 1 | 3 (67) | |||
| Red-shouldered hawk | 3 | 7 (29) | 10 | 8 | 9 (33) | 37 (14) |
| Red-tailed hawk | 6 (33) | 7 (29) | 6 (50) | 4 | 11 (45) | 34 (35) |
| Rock dove | 18 (11) | 26 | 5 | 2 (50) | 51 (6) | |
| Sharp-shinned hawk | 2 | 1 | 4 (50) | 6 | 1 | 14 (14) |
| Spotted towhee | 1 | 3 | 3 (33) | 7 (14) | ||
| Western bluebird | 2 | 2 (50) | 4 (25) | |||
| Western scrub-jay | 36 (69) | 23 (65) | 17 (47) | 17 (18) | 12 (33) | 105 (52) |
| Western tanager | 1 | 2 | 6 (17) | 9 (11) | ||
| White-crowned sparrow | 1 | 4 | 6 | 24 (4) | 5 | 40 (3) |
| Wilson's warbler | 2 | 1 | 1 (100) | 4 (25) | ||
| Yellow-rumped warbler | 1 | 4 | 17 (6) | 3 | 25 (4) | |
| Total | 1,136 (74) | 606 (29) | 712 (22) | 746 (28) | 1,005 (50) | 4,205 (45) |
WNV = West Nile virus; RT-PCR = reverse transcription–polymerase chain reaction. Additional species that were collected and tested infrequently reported as number tested (% WNV positive) were black-crowned night heron 2 (50), cactus wren 2 (50), glaucous-winged gull 1 (100), oak titmouse 2 (50), osprey 1 (100), Pacific parrotlet 1 (100), savannah sparrow 2 (50), Stellar's jay 2 (50) and sulphur-crested cockatoo 1 (100). Birds missing species level identification and positive for WNV reported as no. tested (% positive) were unknown Psittacine 15 (13), unknown canary 16 (13), unknown duck 22 (9), unknown finch 22 (27), unknown goldfinch 9 (33), unknown goose 6 (17), unknown hawk 2 (50), unknown parakeet 22 (5), and unknown sparrow 47 (11). Additional birds tested but WNV negative reported as no. tested were African gray parrot (3), Allen's hummingbird (6), American wigeon (1), ash-throated flycatcher (1), band-tailed pigeon (4), barn owl (51), barn swallow (1), Bewick's wren (1), blue and yellow macaw (2), blue-crowned parakeet (1), brown pelican (7), budgerigar (3), California thrasher (2), Cassin's kingbird (1), cedar waxwing (32), common ground dove (1), common murre (1), common poorwill (2), common tern (3), common yellowthroat (3), dark-eyed junco (5), double-crested cormorant (5), downey woodpecker (2), eclectus parrot (5), fox sparrow (2), Goffin's cockatoo (2), great Egret (1), green-cheeked conure (1), green-winged teal (1), Harris's hawk (1), Hawaiian goose (1), hermit thrush (23), hooded oriole (4), Indian ring-necked parakeet (1), lesser nighthawk (1), lesser sulphur-crested cockatoo (1), Lincoln's sparrow (2), MacGillivray's warbler (3), merlin (2), Meyer's parrot (1), Nashville warbler (3), northern flicker (6), northern rough-winged swallow (2), northern shoveler (1), Nuttall's woodpecker (3), Pacific-slope flycatcher (3), pelagic cormorant (2), peregrine falcon (2), Pionus parrot (1), rainbow lorikeet (1), red-breasted sapsucker (3), red-crowned parrot (1), red-necked phalarope (3), red-winged blackbird (3), rock pigeon (4), ruddy duck (1), snowy egret (1), song sparrow (8), sora (1), sun conure (3), Swainson's hawk (1), Swainson's thrush (26), turkey vulture (7), turtle dove (1), umbrella cockatoo (3), unidentified (42), unknown bushtit (2), unknown caique (2), unknown chickadee (1), unknown cockatoo (2), unknown flycatcher (5), unknown gull (9), unknown hornbill (1), unknown hummingbird (13), unknown lovebird (3), unknown macaw (1), unknown myna (1), unknown owl (2), unknown parrot (10), unknown peacock (9), unknown pigeon (5), unknown starling (5), unknown thrush (2), unknown toucanet (1), unknown warbler (7), unknown woodpecker (1), varied thrush (1), violet-green swallow (3), Virginia rail (2), warbling vireo (1), western grebe (1), western gull (2), western kingbird (1), western meadowlark (1), white-breasted nuthatch (1), white-throated sparrow (1), willow flycatcher (1), yellow warbler (5), and zebra finch (3).
Figure 6.
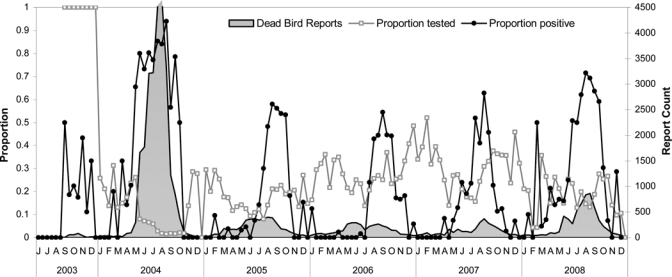
Summary of dead birds tested in Los Angeles County during 2003–2008, reported as proportion tested (birds that were in a testable condition/total birds reported) and proportion positive (birds that tested positive/total tested birds).
Sentinel chickens.
No sentinel chickens seroconverted during the 2003 surveillance year (Table 1). In 2004, 7 flocks of 10 chickens were monitored, and 45 seroconversions were detected. In 2005, the same numbers of flocks were monitored, but only 25 birds seroconverted. The number of seroconversions decreased further in 2006 and 2007 with 19 and 15 seroconversions, respectively, and then increased in 2008 with 39 seroconversions observed.
Free-ranging bird serologic results.
A total of 14,107 wild bird serum samples were collected during 2003–2008 (Table 1). Of these samples, 1,456 (9%) were positive for WNV (1,351) or unidentified flavivirus (105); i.e., were positive by EIA and plaque-reduction neutralization test, but titers were equivocal or < 4 times those for SLEV by end point titration. The most frequent species collected were house finches (8,249 collected, 0.113 proportion infected), followed by house sparrows (5,476, 0.083), white-crowned sparrows (724, 0.014), nutmeg manakins (342, 0.012), brown-headed cowbirds (320, 0.043), California towhees (202, 0.059), and mourning doves (137, 0.241). Additional species bled less frequently or with collaboration of wildlife rehabilitators were song sparrows (34, 0.059), red-winged blackbirds (22, 0.091), plain pigeons (22, 0.273), common ravens (5, 0.200), barn owls (5, 0.200), red-tailed hawks (4, 0.500), and red-shouldered hawks (1,1.000). Species bled, but never found to be positive for WNV or flavivirus, were American crows (20 tested), black-headed grosbeaks (9), lesser goldfinches (32), chipping sparrows (8), gray-headed juncos (6), tricolored blackbirds (2), Lincoln's sparrows (2), golden-crowned sparrows (2), European starlings (2), blue grosbeaks (2), western screech owl (1), rufous-crowned sparrow (1), ringed turtle dove (1), pine siskin (1), bullock's oriole (1), northern mockingbird (1), merlin (1), hermit thrush (1), great horned owl (1), dark eyed junco (1), Cooper's hawk (1), common poorwill (1), California quail (1), and black phoebe (1).
Seroprevalence for house finches and house sparrows calculated biweekly ranged between 0 and 0.46, with the overall maximum in December 2004 (Figure 7). Three important temporal trends were observed in our data. The first trend was the impact of herd immunity. After seroprevalence exceeded 0.25 during 2004, tangential transmission and therefore positive dead birds and human cases decreased. Dead birds and human cases were detected during subsequent years whenever seroprevalence decreased below 0.10. The second trend was hatching year birds. Fledging of nestlings was associated with reduced seroprevalence during each summer and was the primary factor associated with progressive seasonal decreases in seroprevalence. The third trend was winter seroprevalence. Low seroprevalence during later winter and spring most likely facilitated early season amplification transmission and was associated with increased human cases during the following summer. During 2008, seroprevalence was 0.03, 0.10, and 0.11 during January, February and March, respectively, and was followed by an outbreak of human cases.
Figure 7.
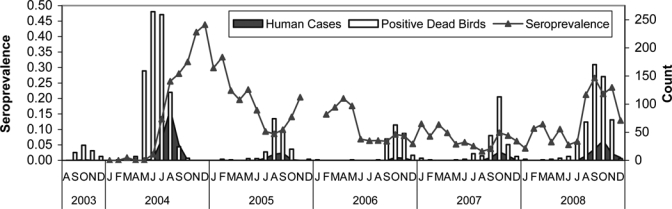
Summary of house finch and house sparrow seroprevalence in Los Angeles County during 2004–2008 (number positive/total collected/month) compared with the counts of human cases and positive dead birds by monthly interval. Positive dead birds indicates virus detection in bird carcasses by reverse transcription–polymerase chain reaction and seroprevalence indicates detection of antibody to West Nile virus by enzyme immunoassay in live-captured house finches and house sparrows.
Discussion
Similar to published reports describing WNV invasion into new geographic areas, WNV enzootic and epidemic activity in Los Angeles followed a three-year pattern of introduction, amplification, and subsidence,38,39 with subsequent resurgence during 2008. Resurgence in 2008 followed similar spatial and temporal patterns as in the 2004 epidemic year, with increases in proportion of positive dead birds, mosquito infection incidence, sentinel chicken seroconversions, and number of human cases. Interestingly, the 2008 outbreak followed the progressive decrease in avian herd immunity, perhaps indicating the importance of early season increased seroprevalence and depopulation in dampening viral amplification events.
Case detection in Greater Los Angeles appeared to be skewed towards severe disease, because the ratio of WNND to West Nile fever cases was consistently greater than the 1:256 and 1:140 ratios estimated in North Dakota and New York City.40,41 This finding, combined with the increase in the numbers of ASDs detected by routine blood donor screening, suggested that the 2008 epidemic may have been greater in magnitude than that in 2004, but was not captured by passive detection of human infection, which failed to adequately reflect febrile illness. Human cases were primarily in the Los Angeles Basin in 2004 (n = 85) and 2008 (n = 45), and lower epidemic peaks consistently occurred in the San Fernando Valley. There were only seven human cases reported in the Santa Clarita Valley over the entire study period. Mosquito infection incidence in Cx. p. quinquefasciatus consistently preceded human case incidence and was similar in magnitude in 2004 (mean = 4.73/1,000 mosquitoes) and 2008 (mean = 3.50/1,000 mosquitoes) (F = 0.32, df = 1, 45, P = 0.57). Sentinel chicken seroconversions peaked concurrently with human cases and the proportion observed was not significantly different between 2004 and 2008 (χ2 = 1.42, df = 1, P = 0.23).
On the basis of infection incidence, the Culex species most frequently involved in enzootic and epidemic transmission in urban Los Angeles appeared to be Cx. p. quinquefasciatus, Cx. stigmatosoma, and Cx. tarsalis. Using serologic methods, we determined that 58% (n = 98), 95% (n = 19), and 100% (n = 3) of these species collected resting at residences in the Los Angeles Basin previously were found to blood feed on avian hosts, respectively.42 Frequent mammal feeding by Cx. p. quinquefasciatus also was reported recently in Houston, Texas,43 supporting the role of this species in epidemic transmission. Culex pipiens complex populations in Los Angeles were presumed to be homogenously Cx. p. quinquefasciatus on the basis of genetic and morphologic studies of mosquito collections south of the Tehachapi Mountains.44–46 Vector competence studies indicated that the Los Angeles populations of Cx. p. quinquefasciatus were moderately susceptible to WNV infection by pledget feedings,47 as were Cx. tarsalis, whereas Cx. stigmatosoma were highly susceptible. Culex tarsalis from the San Fernando Valley were more capable to transmit than other species collected in the Los Angeles area, and Cx. stigmatosoma were more susceptible to infection. Repeated vector competence studies of Los Angeles mosquito populations have consistently shown that Cx. stigmatosoma was the most competent species in California, followed by Cx. tarsalis and Cx. p. quinquefasciatus.48 Our current study demonstrated concordant high infection rates in Cx. stigmatosoma. However, this species was not abundant in collections and not an early indicator of viral activity when compared with other Culex species and avian indicators. This finding may be confounded by low and intermittent collections, perhaps caused by a lack of a suitable and competitive trap attractant.
Collections of Cx. tarsalis were consistently lower than collections of Cx. p. quinquefasciatus, but higher than collections of Cx. stigmatosoma, making them a suitable indicator in time series. Unfortunately the collections of Cx. tarsalis were made almost exclusively with EVS traps in the Los Angeles area, and given the trap bias noted with Cx. p. quinquefasciatus in urban settings, our findings may represent underestimates of abundance and infection. This underestimation is apparent in the comparisons made in Tables 1 and 2, where in 2004 the infection rates in Cx. tarsalis were similar to that in Cx. stigmatosoma in magnitude, and no infected mosquitoes were detected in 2003 or 2006, whereas infected Cx. p. quinquefasciatus mosquitoes were detected annually.
Culex p. quinquefasciatus were the most abundant species in Los Angeles and provided the most consistent indication of WNV activity as measured by females per trap night and infection. Collections of this species in urban settings were biased towards gravid individuals because of the comparative sensitivity of gravid and EVS traps. However, in the Santa Clarita Valley, where the human population density is the lowest of the three regions, no significant difference was noted in abundance estimated by the two traps. Although this finding may have been an effect of low sample size, our results also may be explained by the higher density of potential hosts in an urban environment competing with the CO2 emitted by the dry ice bait. Conversely, medium for gravid traps may represent not only a suitable but perhaps a limiting resource for oviposition.
Dead bird reports were not a useful surveillance tool because they were consistently reported long before the mosquito season began and were confounded by bird death unrelated to WNV. The proportion of dead birds tested during epidemic years 2004 and 2008 were significantly different (χ2 = 1376.6, df = 1, P < 0.001), as were the proportion of dead birds testing positive (χ2 = 162.879, df = 1, P < 0.001). This lack of association was likely confounded by reduced media attention, public concern, and funding, resulting in zip code closures earlier in the 2008 mosquito season despite a similar magnitude epidemic. Dead bird species in Table 6 that had high percentages of WNV-positive individuals were species that were dying above their background death rate because of WNV infection. In experimental infections, for example, 63% of house finches and all western scrub-jays, but only 16% of house sparrows, died of infection.47 This trend was reflected in the percent reported positive in Table 6 and in the increased seroprevalence in trapped birds.
High death rates in nature were associated with significant population decreases in California American crow and house finch populations since the arrival of WNV.49 In marked contrast, house sparrow abundance has increased significantly, perhaps because of reduced competition for peridomestic nesting sites and predation by corvids. In 2004, the proportion tested decreased during the summer months because of zip code closures after many positive birds had been detected. Increases in the proportion tested occurred during winter and early spring months when fewer birds were reported dead by the public, more were in testable condition, and zip code closures had not been initiated. The clustering of WNV-positive dead birds near large crow roosts suggests that this species contributed significantly to local virus amplification.50,51
Sentinel chicken seroconversions were detected each year, and occurred closest in time with the first Cx. stigmatosoma infections. This association is intuitive given the ornithophilic nature of Cx. stigmatosoma. However, more data will be required for a more robust time series analysis of this coincidence. Further examination of the enzootic circumstances relating to sentinel chicken seroconversion will be forthcoming in a separate report.
In summary, the two WNV epidemics in Los Angeles County provided a unique opportunity to track patterns in the epizootic progression of WNV through time and space and evaluate the utility of different epizootic surveillance measures during invasion and resurgence. From this experience, it was clear that gravid Cx. p. quinquefasciatus infection provided the primary indication of urban WNV activity. Improved sampling methods are needed to effectively sample urban populations of the other more competent Culex species. Dead birds positive for WNV were a more relevant measure of WNV activity than just dead bird reports, especially during inter-epidemic periods when most birds died of other causes. Wild bird seroprevalence provided an estimate of herd immunity or portions of the free-ranging reservoir population that were no longer contributing to enzootic transmission. These factors plus variation in temperature and mosquito abundance appeared to give rise to different seasonal patterns of virus amplification and human disease.
Acknowledgments
We thank the Scientific-Technical Department of the Greater Los Angeles County Vector Control District for braving the Los Angeles freeway system to provide field data used in the study; Ying Fang for managing laboratory work completed at the University of California, Davis, Center for Vectorborne Disease Research; the California Department of Public Health Dead Bird Hotline staff for collecting all dead bird records and testing blood samples from sentinel chickens; and Drs. Christopher Barker, Danh Nguyen, and Tim Carpenter (University of California, Davis) for providing instruction in spatial statistics and critically reviewing the manuscript.
Footnotes
Financial support: This study supported, in part, by grant R01-AI055607 from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, and grant RM08-6044 from the National Aeronautics and Space Administration Applied Sciences Program Decision Support through Earth Science Research Results. Additional funding and resources were provided by the Greater Los Angeles County Vector Control District and by the Research and Policy for Infectious Disease Dynamics Program of the Science and Technology Directorate, the U.S. Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Authors' addresses: Jennifer L. Kwan and William K. Reisen, Center for Vectorborne Diseases, School of Veterinary Medicine, University of California, Davis, CA, E-mails: jnwilson@ucdavis.edu and wkreisen@ucdavis.edu. Susanne Kluh and Minoo B. Madon, Greater Los Angeles County Vector Control District, Santa Fe Springs, CA, E-mails: skluh@glacvcd.org and minoovecterminator@yahoo.com.
Reprint requests: William K. Reisen, Center for Vectorborne Diseases, School of Veterinary Medicine, University of California, Old Davis Road, Davis, CA 95616, E-mail: wkreisen@ucdavis.edu.
References
- 1.Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisen WK, Lothrop HD, Chiles RE, Madon MB, Cossen C, Woods L, Husted S, Kramer VL, Edman JD. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson J, Hazelrigg JE, Reisen WK, Madon MB. Invasion of Greater Los Angeles by West Nile virus - 2003. Proc Mosq Vector Control Assoc Calif. 2004;72:6–11. [Google Scholar]
- 4.Reeves WC, Asman SM, Hardy JL, Milby MM, Reisen WK. Epidemiology and Control of Mosquito-borne Arboviruses in California, 1943–1987. Proc Mosq Vector Control Assoc Calif. 1990;71:17–27. [Google Scholar]
- 5.Reisen WK, Milby MM, Presser SB, Hardy JL. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987–1990. J Med Entomol. 1992;29:582–598. doi: 10.1093/jmedent/29.4.582. [DOI] [PubMed] [Google Scholar]
- 6.Murray RA, Habel LA, Mackey KJ, Wallace HG, Peck BA, Mora SJ, Ginsberg MM, Emmons RW. Epidemiological aspects of the 1984 St. Louis encephalitis epidemic in southern California. Proc Calif Mosq Vector Control Assoc. 1985;53:5–9. [Google Scholar]
- 7.Emmons RW, Milby MM, Walsh JD, Reeves WC, Bayer EV, White K, Woodie JD, Murray RA. Surveillance for arthropod-borne viral activity and disease in California during 1984. Proc Calif Mosq Vector Control Assoc. 1985;53:1–4. [Google Scholar]
- 8.Los Angeles County 2009. http://portal.lacounty.gov/wps/portal/lac/residents/ Available at.
- 9.U.S. Census Bureau . United States Census Bureau QuickFacts. Bureau USC, ed. State and County QuickFacts. Washington, DC: U.S. Census Bureau; 2009. http://quickfacts.census.gov/qfd/states/06/06037.html Available at. [Google Scholar]
- 10.Reveal JL. Biomes of North America. College Park, MD: Norton-Brown Herbarium, University of Maryland; 2000. [Google Scholar]
- 11.State of California Department of Water Resources . California Irrigation Management Information System. Sacramento, CA: State of California Department of Water Resources; 2009. http://www.water.ca.gov/ Available at. [Google Scholar]
- 12.Los Angles County Department of Public Works 2009. http://dpw.lacounty.gov Available at.
- 13.Greater Los Angels County Vector Control District 2009. http://glacvcd.org Available at.
- 14.Melton F, Nemani RR, Michaelis A, Barker CM, Park B, Reisen WK. Monitoring and modeling environmental conditions related to mosquito abundance and virus transmission risk with the NASA Terrestrial Observation and Prediction System. Proc Mosq Vector Control Assoc Calif. 2008;76:4. [Google Scholar]
- 15.PostgreSQL Global Development Group 2009. http://www.postgresql.org/ Available at.
- 16.Centers for Disease Control and Prevention . West Nile Virus: Clinical Description. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 17.Newhouse VF, Chamberlain RW, Johnston JG, Jr, Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News. 1966;26:30–35. [Google Scholar]
- 18.Rohe DL, Fall RP. A miniature battery powered CO 2 baited trap for mosquito borne encephalitis surveillance. Bull Soc Vector Ecol. 1979;4:24–27. [Google Scholar]
- 19.Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 1992;60:170–176. [Google Scholar]
- 20.Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506–4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham RR, Hardy JL, Presser SB. Use of the in situ enzyme immunoassay for the rapid detection of arbovirus infections in mosquitoes in California. Proc Calif Mosq Vector Control Assoc. 1986;54:10. [Google Scholar]
- 22.Chiles RE, Green EN, Fang Y, Reisen WK, Edman JD, Brault AC. Surveillance for arboviruses in California mosquito pools: current and future protocols. Proc Mosq Vector Control Assoc Calif. 2004;72:15–17. [Google Scholar]
- 23.Biggerstaff BJ. Pooled Infection Rate. Fort Collins, CO: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 24.McCaughey K, Miles SQ, Woods L, Chiles RE, Hom A, Kramer VL, Jay-Russel M, Sun B, Reisen WK, Scott TW, Hui LT, Steinlein DB, Castro M, Houchin A, Husted S. The California West Nile virus dead bird surveillance program. Proc Mosq Vector Control Assoc Calif. 2003;71:38–43. [Google Scholar]
- 25.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2003;40:968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- 27.Reisen WK, Lin J, Presser SB, Enge B, Hardy JL. Evaluation of new methods for sampling sentinel chickens for antibodies to WEE and SLE viruses. Proc Calif Mosq Vector Control Assoc. 1993;61:33–36. [Google Scholar]
- 28.Patiris PJ, Peck GW, Chiles RE, Reisen WK, Hanson CV. Serological diagnosis of West Nile and St. Louis encephalitis virus infections in domestic chickens. Am J Trop Med Hyg. 2008;78:7. [PubMed] [Google Scholar]
- 29.McClure HE. Bird Banding. Pacific Grove, CA: Boxwood Press; 1984. [Google Scholar]
- 30.Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- 31.Kulldorff M., and Information Management Services Inc. SaTScantm v8.0: Software for the Spatial and Space-Time Scan Statistics. Rockville, MD: Information Management Services, Inc; 2009. [Google Scholar]
- 32.Kulldorff M, Athas WF, Feuer EJ, Miller BA, Key CR. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am J Public Health. 1998;88:1377–1380. doi: 10.2105/ajph.88.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- 34.Reisen WK, Lothrop HD. Effects of sampling design on the estimation of adult mosquito abundance. J Am Mosq Control Assoc. 1999;15:104–114. [PubMed] [Google Scholar]
- 35.Dawson SW, Jr, Ebel GD, Young DS, Galinski DS, Pensabene JP, Franke MA, Eidson M, Kramer LD. Crow deaths caused by West Nile virus during winter. Emerg Infect Dis. 2007;12:1912–1914. doi: 10.3201/eid1312.070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oke TR. The energetic basis of the urban heat-island. Q J R Meteorol Soc. 1982;108:1–24. [Google Scholar]
- 37.Acute Communicable Disease Control Los Angeles County, Department of Health Services, Public Health West Nile virus. Wkly Epidemiol Rep. 2004;42:2. [Google Scholar]
- 38.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- 40.Busch MP, Wright DJ, Custer B, Tobler LH, Stramer SL, Kleinman SH, Prince HE, Bianco C, Foster G, Petersen LR, Nemo G, Glynn SA. West Nile virus infections projected from blood donor screening data, United States, 2003. Emerg Infect Dis. 2006;12:395–402. doi: 10.3201/eid1203.051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 42.Reisen WK, Meyer RP, Tempelis CH, Spoehel JJ. Mosquito abundance and bionomics in residential communities in Orange and Los Angeles Counties, California. J Med Entomol. 1990;27:356–367. doi: 10.1093/jmedent/27.3.356. [DOI] [PubMed] [Google Scholar]
- 43.Molaei G, Andreadis TG, Armstrong PM, Bueno R, Dennett JA, Real SV, Sargent C, Bala A, Randle Y, Guzman H, da Rosa AT, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- 44.Urbanelli S, Silvestrini F, Reisen WK, deVito E, Bullini L. California hybrid zone between Culex pipiens pipiens and Cx. p. quinquefasciatus revisited (Diptera: Culicidae) J Med Entomol. 1997;34:116–127. doi: 10.1093/jmedent/34.2.116. [DOI] [PubMed] [Google Scholar]
- 45.Barr AR. The distribution of Culex p. pipiens and Culex p. quinquefasciatus in North America. Am J Trop Med Hyg. 1957;6:153–165. doi: 10.4269/ajtmh.1957.6.153. [DOI] [PubMed] [Google Scholar]
- 46.Tabachnick WJ, Powell JR. Genetic analysis of Culex pipiens populations in the central valley of California. Ann Entomol Soc Am. 1983;76:715–720. [Google Scholar]
- 47.Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of west nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 48.Reisen WK, Barker CM, Fang Y, Martinez VM. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler SS, Barker CM, Fang Y, Armijos MV, Carroll BD, Husted S, Johnson WO, Reisen WK. Differential impacts of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen CF, Reisen WK. West Nile virus-infected dead corvids increase the risk of infection in Culex mosquitoes (Diptera: Culicidae) in domestic landscapes. J Med Entomol. 2007;44:1067–1073. doi: 10.1603/0022-2585(2007)44[1067:wnvdci]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, Fang Y, Shafii M, Kahl N, Ashtari S, Kramer V, Glaser C, Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]


