Abstract
Background
Plant-pathogenic begomoviruses have a complex association with their insect vectors. The interactions of begomoviruses and reproduction of their vectors are poorly understood. Bemisia tabaci is known to transmit many begomoviruses, and the spread of B. tabaci, especially the B and Q ‘biotypes’, has been accompanied by the epidemics of begomoviruses. One of these identified disease-causing agents was Tomato yellow leaf curl China virus (TYLCCNV).
Methodology/Principal Findings
In this study, we compared the egg production and realized fecundity of two ‘biotypes’ or putative species of the whitefly B. tabaci, including the alien invasive B and the indigenous ZHJ1 from Zhejiang, China, feeding on either healthy or TYLCCNV-infected tobacco plants. The ovary of the whitefly was composed of 12–22 telotrophic ovarioles. According to the morphology of the oocytes and level of yolk content, oocytes in ovarioles were divided into four developmental phases (I-IV). Significantly higher proportion of immature oocytes (phase II, III) and mature oocytes (phase IV) was observed in ovary of females that fed on TYLCCNV-infected tobacco compared to that on healthy plants. Moreover, there was significant increase of eggs laid of B whitefly that fed on TYLCCNV-infected tobacco plants during the early developmental stages. In contrast, the proportion of oocytes of different developmental phases and eggs laid had no significant differences between ZHJ1 whiteflies feeding on TYLCCNV-infected and non-infected host plants.
Conclusions/Significance
The invasive B whitefly benefits from feeding on a begomovirus-infected plant through increased egg production and realized fecundity.
Introduction
Plant pathogen-vector systems are characterized by complex interactions. Some plant- pathogenic geminiviruses have caused serious problems to crops [1]–[3]. Most of these plant viruses are vectored by insects and the interactions between plant viruses and their insect vectors have been shown to be beneficial, neutral, or antagonistic, depending on the species involved [4], [5]. Generally, some of the plant viruses were observed to have intimate molecular interactions with their vectors, including movement of the viruses from the gut back to the mouthparts through salivary gland, and the transcription and replication of virus within the vector [6]. Moreover, it was reported that the longevity and fecundity of insect vectors were affected when fed on virus-infected plants [7]–[9]. However, the mechanisms underlying the interactions between the plant viruses and the reproduction of its vector insect are poorly understood.
The whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) is genetically diverse and a vector of many begomoviruses [10]–[13]. Recent phylogenetic analysis combined with a pattern of reproductive isolation among genetic groups within B. tabaci indicates that the complex contains at least 24 cryptic species, some of which have been referred to as ‘biotypes’ in the last 20 years [14], [15]. As the separation at the species level within the B. tabaci complex is yet to be fully resolved, the commonly used term ‘biotypes’ to refer cryptic species has been sometimes retained [16].
Two cryptic species of the B. tabaci complex, i.e., the Mediterranean and the Middle East-Minor Asia 1 [14], which have commonly referred to as the Q biotype (Q hereafter) and the B biotype (hereafter B), have spread into much of the rest of the world from its presumed origin in the past 20 years, and have displaced some indigenous cryptic species of this species complex in the regions of invasion [2], [17], [18]. However, the mechanisms underlying the displacement of indigenous whiteflies by B are not thoroughly clear. Previously, it has been found that mating behavioral interactions between different cryptic species of the whitefly are important in the invasion by B [2], [19]. In another study, Jiu et al. [8] demonstrated that the invasive B feeding on begomovirus-infected plants substantially increased its longevity and fecundity compared to that on uninfected plants while the indigenous ZHJ1 whitefly (hereafter ZHJ1, which belongs to the Asia II 3 cryptic species as named by Dinsdale et al. [14]) was unable to do so, and speculated that this indirect vector-virus mutualism through their shared host plant may accelerate the population increase of the invasive B in the field [8]. However, the physiological mechanisms of this indirect mutualism are not known.
Moreover, compared to its performance on uninfected plants, B feeding on begomovirus-infected plants performed better [20], [21], similarly [3], [22], [23], or less well [24]. Virus-infected plants undergo changes that could affect the biology of insect vectors [25]. These variations of relationships may affect the process of biological invasion and the displacement of indigenous species by invaders when the invasive and indigenous organisms occur with niche overlap but differ in the interactions [8]. Therefore, a more thorough understanding on how the begomoviruses (as well as others) affect the reproduction of their vectors seems necessary.
The virus vectors of this study include the B and ZHJ1 whiteflies, which are known to belong to different putative species of the B. tabaci species complex [14], [15]. The notorious invasive B entered China in the late 1990s and now is the predominant or only biotype in many regions of the country [2], [26], [27]. The virus, Tomato yellow leaf curl China virus (TYLCCNV) is a whitefly-transmitted begomovirus that has become widespread in south China for years [28], [29]. Here, the anatomy of the reproductive organ of the whitefly was observed, the percentage of oocytes in ovaries at different developmental phases and the fecundity between the invasive B and the indigenous ZHJ1 whiteflies feeding on healthy and TYLCCNV-infected tobacco plants were compared.
Materials and Methods
Whiteflies
Two cryptic species of the whitefly Bemisia tabaci (Gennadius) species complex were used. The B whitefly (GenBank accession no. AJ867555) population was first collected from cabbage, Brassica oleracea var. capitata L. (Cruciferae), and the indigenous ZHJ1 whitefly (GenBank accession no. AJ867556) population from cotton, Gossypium hirsutum L., in 2003 in Hangzhou, Zhejiang, China (32.2 °N, 120.1 °E, with an elevation of 6 m a.s.l.) [3], [8]. Stock cultures of two cryptic species were maintained on cotton G. hirsutum L. cv. Zhemian 1973 in separate climate chambers at 28±1 °C, 14 h light: 10 h darkness and 70±10% r. h. The purity of the cultures was monitored every 3-5 generations using the random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) technique [30], and measures were taken to use only pure sub-cultures of the two cryptic species for experiments. Viruliferous whitefly colonies were reared separately at the same condition as described above.
Viruses
Infectious clones of TYLCCNV and their satellite DNA molecules (named DNA β) constructed previously [28], [29] were used as inocula, and the viruses were maintained on plants of tobacco Nicotiana tabacum L. cv. NC89.
Plants
Tobacco Nicotiana tabacum L. cv. NC89, a host plant of TYLCCNV, and cotton (cv. Zhemian 1973), a non-host plant of TYLCCNV, were used. Uninfected tobacco and cotton plants were grown in insect-proof cages under natural lighting and ambient temperature in screen houses as described in Jiu et al. [8]. The virus-infected tobacco plants were acquired by inoculating with TYLCCNV and its DNA β at the 4–5 true-leaf stage by agro-inoculated as previously described [1], [28], [29]. Healthy and TYLCCNV-infected tobacco plants were grown to the 6–7 true-leaf stage for experiments. The virus infection of test plants was judged by the characteristic symptoms caused by the virus and further confirmed by molecular marks as previously described [8]. All plants were watered every 3–4 days as necessary and fertilized once a week. All experiments were conducted at 26±1 °C, 40–60% relative humidity, and a photoperiod of 14 h light: 10 h darkness.
Light microscopy
The whitefly adults of different developmental stages reared on cotton plant were insufflated into centrifuge tube on ice. The ovaries of each female were then dissected in phosphate buffered saline (PBS) (pH 7.4) and the epidermis was removed. Different types of ovarioles were separated using a stretched microcapillary. The morphology of ovary samples and ovarioles were photographed with a phase contrast microscope (Leica 216, Germany) fitted with a light source. Digital images were captured using a digital camera and modified using Adobe Photoshop®.
Development of ovaries in initially non-viruliferous adults feeding on healthy or virus-infected tobacco
These experiments were conducted to examine the realized fecundity of adult whiteflies which were reared on cotton from egg to pupa, and then transferred upon emergence onto healthy or virus-infected tobacco plants. The experiment was carried out concurrently using the procedure as described earlier by Jiu et al. [8] with a few modifications. Approximately 600 pairs of newly emerged (0–12 h) adult whiteflies were collected from the culture on cotton, and divided randomly into two groups, 300 pairs of adults each. Each group was used for inoculation onto either healthy or TYLCCNV-infected tobacco plants. On each type of plants, 10 females were dissected everyday for counting the number of oocytes at different developmental phases in ovaries until 15 d after eclosion.
Fecundity of initially non-viruliferous adults feeding on healthy or virus-infected tobacco
The number of eggs laid on healthy or virus-infected tobacco by the whiteflies was examined using the clip-cages described by Zang et al. [30]. Each cage contained one male and one female. The total number of eggs laid was examined daily for the first 8 d after eclosion using the microscope (Leica, Germany) as leaves covered by the clip-cages started to wilt after 8 d.
Statistical analysis
All statistical analysis were conducted using the DPS© package (Version 8.01 for Windows) [31]. To analyze the effects of TYLCCNV infection on the reproduction of the same cryptic species of whiteflies feeding on healthy and virus-infected plants, data were subjected to Student t-test. The effects of virus infection and age on the reproduction of B or ZHJ1 were analyzed using two-factors ANOVA and Tukey's multiple range test (MRT). The total number of oocytes and eggs laid of different cryptic species of whiteflies feeding on healthy and virus-infected plants in the first 2 d were analyzed using two-factors ANOVA and Tukey's MRT. All proportion data were transformed by arcsine root before analysis, but untransformed means are presented.
Results
Anatomy of ovary in the whiteflies
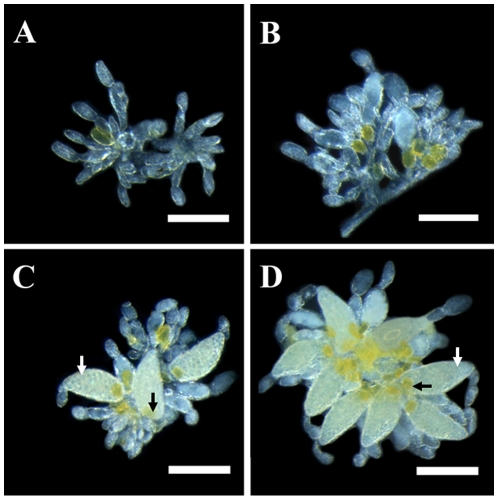
The ovaries of the whiteflies are formed by a mass of ovarioles, of which the number for a pair of ovary is ranged from 12–22 in various developmental stages. Every ovariole contains two or three oocytes in various stages of maturity (Fig. 1). The ovary can be divided into four stages during its development: (1) stage 1, within 2 h after eclosion, wherein one or more oocytes were transparent (Fig. 1A); (2) stage 2, 1–2 d after eclosion, wherein one or more oocytes were opaque and with yolk contents (Fig. 1B). The bacteriocytes formed around the immature oocytes and some of them were transferred into the immature eggs in the region where the pedicel was ultimately formed; (3) stage 3, 3–10 d after eclosion, wherein one or more oocytes were filled with yolk contents and an increasing number of ovarioles and mature oocytes were observed (Fig. 1C); (4) stage 4, 11–14 d after eclosion, wherein most oocytes were full with yolk content. Mature oocytes were always observed at the third stage (Fig. 1C) and the number of mature oocytes increased to the maximum from 7 to 11 d after eclosion (Fig. 1D).
Figure 1. Ovary of B whitefly at different developmental stages after eclosion.
A: the ovary of freshly emerged whitefly; B: 1–2 d after eclosion; C: 3–10 d after eclosion; D: 11–14 d after eclosion. Mature oocytes were showed with white arrows. Bacteriocyte sphere was showed with blank arrows. Scale bar: 0.10 mm.
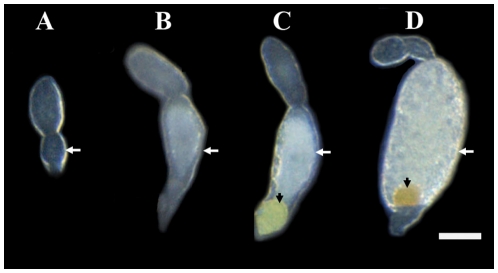
According to the main morphological characteristics of oocytes and the level of yolk content, the oocytes were classified into four developmental phases, separately named phase I, II, III and IV (Fig. 2). No yolk protein was observed in phase I oocyte. The phase II and III were identified by the presence of yolk content. Oocytes with<50% yolk content were named phase II and those with >50% yolk content phase III. The phase ІV were mature eggs. A rounded bacteriocyte sphere was transferred into oocyte at the terminal of ovariole in phase III and embedded in mature oocyte (Fig. 2).
Figure 2. Oocytes of different developmental phases in the ovarioles of whitefly.
A, B, C and D are termed as phase I, phase II, phase III and phase IV, respectively (showed with white arrows). Bacteriocyte sphere was showed with blank arrows. Scale bar: 0.05 mm.
Effects of TYLCCNV on proportions of oocytes in ovaries of whiteflies
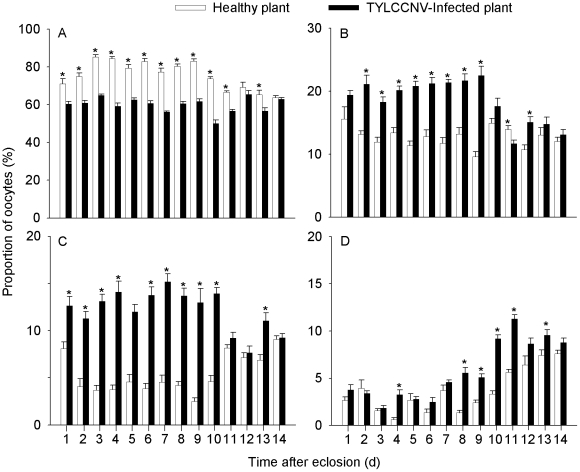
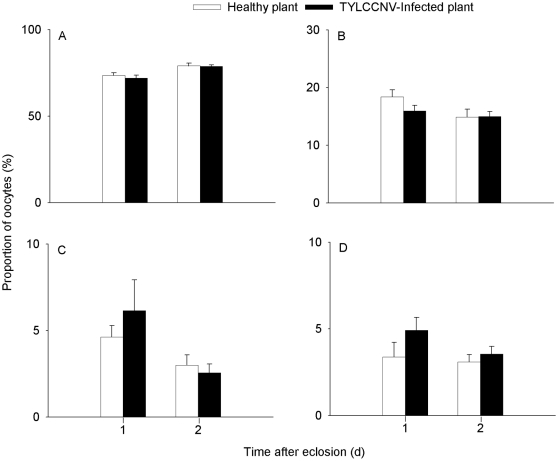
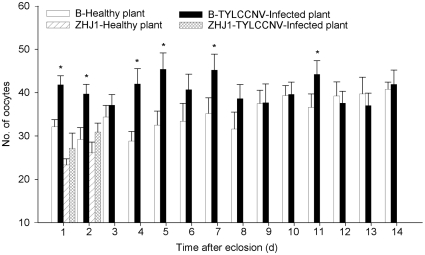
B adults feeding on virus-infected plants had a significantly lower proportion of phase I oocytes but significant higher proportions of phase II, III, and IV oocytes, compared to the B adults feeding on healthy plants (Table 1; Figure 3). In contrast, the proportions of oocytes at different phases in ZHJ1 were similar between adults feeding on healthy plants and those feeding on virus-infected plants (Table 1; Figure 4). In the B whiteflies, compared with adults feeding on healthy plants, those feeding on virus-infected plants had a significantly higher total number of oocytes. However, in ZHJ1 no significant difference was observed between adults feeding on virus-infected and healthy plants (Fig. 5).
Table 1. Two-factors ANOVA on different phase of oocytes in the ovary of B and ZHJ1 whiteflies.
| Parameters measured | Percentage (%) of oocytes in pairs of ovary | Two-factors ANOVA | ||
| TYLCCNV- Infected plants | Healthy plants | |||
| B | Phase I | 59.89±6.50 b | 75.51±9.66 a | F A = 57.6880, df = 1; P<0.0001 |
| F B = 1.3930, df = 13; P = 0.2793 | ||||
| F A×B = 7.7570, df = 13; P<0.0001 | ||||
| Phase II | 18.48±5.01 a | 12.67±4.14 b | F A = 151.3340, df = 1; P<0.0001 | |
| F B = 4.1150, df = 13; P<0.0001 | ||||
| F A×B = 5.3880, df = 13; P<0.0001 | ||||
| Phase III | 12.12±3.90 a | 4.71±0.353 b | F A = 426.4280, df = 1; P<0.0001 | |
| F B = 1.9950, df = 13; P = 0.0216 | ||||
| F A×B = 11.4570, df = 13; P<0.0001 | ||||
| Phase IV | 9.51±0.717 a | 7.11±0.603 b | F A = 4.3230, df = 1; P<0.0480 | |
| F B = 5.7900, df = 13; P = 0.0017 | ||||
| F A×B = 5.9440, df = 13; P<0.0001 | ||||
| ZHJ1 | Phase I | 75.42±6.45 a | 76.32±6.66 a | F A = 1.5310, df = 1; P = 0.4328 |
| F B = 71.8390, df = 1; P = 0.0748 | ||||
| F A×B = 0.1530, df = 1; P = 0.6979 | ||||
| Phase II | 15.49±3.42 a | 16.64±5.31 a | F A = 0.7850, df = 1; P = 0.5384 | |
| F B = 2.9790, df = 1; P = 0.3343 | ||||
| F A×B = 0.8680, df = 1; P = 0.3577 | ||||
| Phase III | 4.86±0.343 a | 3.81±0.254 a | F A = 0.4910, df = 1; P = 0.6108 | |
| F B = 4.3780, df = 1; P = 0.2838 | ||||
| F A×B = 3.5060, df = 1; P = 0.0693 | ||||
| Phase IV | 4.24±0.246 a | 3.23±0.258 a | F A = 3.5570, df = 1; P = 0.3104 | |
| F B = 2.4300, df = 1; P = 0.3631 | ||||
| F A×B = 0.4400, df = 1; P = 0.5112 | ||||
Note: the value for each parameter measured is from the analysis of all data during the whole experiment period, and is expressed as mean ± standard error (n = 140). In the same row, the data followed by the same lowercase letter do not differ significantly according to two-factors ANOVA. Factor A: types of tobacco plants for feeding the Bemisia tabaci (TYLCCNV-infected plants versus healthy plants); and Factor B: time after eclosion.
Figure 3. Proportion of different phase oocytes in the ovary of B whitefly.
A, B, C, D: phase I, II, III, IV. The data are expressed as means ± SE (n = 10). At the same day, values followed by the asterisk (*) represent significant differences (P<0.05, Student t-test).
Figure 4. Proportion of different phase oocytes in the ovary of ZHJ1 whitefly.
A, B, C, D: phase I, II, III, IV. The data are expressed as means ± SE (n = 10).
Figure 5. Total numbers of oocytes in the ovaries of two cryptic B. tabaci.
The data are expressed as means ± SE (n = 10). At the same day, values followed by the asterisk (*) represent significant differences (P<0.05, Student t-test).
Due to the short lifespan of ZHJ1 on healthy and virus-infected plants, these parameters were compared statistically at the first two days after emergence with B. The total number of oocytes in B was significantly higher than that of ZHJ1 (Table 2).
Table 2. Two-factors ANOVA on fecundity of B and ZHJ1 whiteflies feeding on healthy or TYLCCNV-infected plants in the first two days after eclosion.
| Parameters | TYLCCNV-Infected plants | Healthy plants | ||
| B | ZHJ1 | B | ZHJ1 | |
| No. of oocytes per ovary a | 40.75±2.33 a | 29.05±0.89 bc | 30.65±1.32 b | 24.7±0.82 c |
| No. of eggs per cage b | 7.9±1.43 a | 4.4±0.62 c | 5.75±1.18 b | 3.8±0.51 c |
Two-factors ANOVA: F biotype = 35.8, df = 1; P<0.05; F plant = 24.7, df = 1; P<0.05; F biotype* plant = 3.8, df = 1; P<0.05.
Two-factors ANOVA: F biotype = 77.9, df = 1; P<0.05; F plant = 40.5, df = 1; P<0.05; F biotype* plant = 60.8, df = 1; P<0.05.
In the same row, means followed by the same lowercase letters do not differ significantly according to two-factors ANOVA and Tukey's multiple-range test. Data are represented as means ± standard error (n = 20).
Effects of TYLCCNV on fecundity of whiteflies
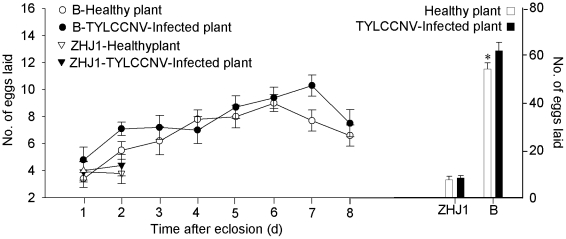
Compared with the ZHJ1, B whiteflies laid 5–8 times more eggs on either healthy or virus-infected plants (Fig. 6, Table 2). The B adults feeding on TYLCCNV-infected plants laid significantly more eggs than those feeding on healthy plants; in contrast, the ZHJ1 adults feeding on healthy and virus-infected plants laid similar numbers of eggs (Figure 6; Table 2)
Figure 6. Fecundity of non-viruliferous adults fed on healthy or virus-infected tobacco of two cryptic whiteflies in the first 8 d after eclosion.
The data are expressed as means ± SE (n = 10). Values followed by the asterisk (*) represent significant differences (P<0.05, Student t-test).
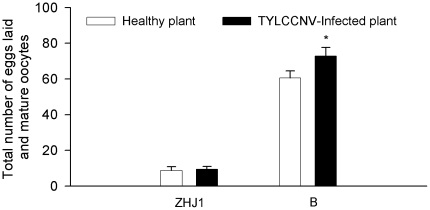
When the number of mature oocytes in ovaries and that of eggs laid was added, the total number in B adults was 4–6 time higher than that in ZHJ1 adults feeding on either healthy or virus-infected plants (Fig. 7).
Figure 7. Total number of eggs plus mature oocytes of non-viruliferous adult fed on healthy or virus-infected tobacco of two cryptic whiteflies in the first 2 d after eclosion.
The data are expressed as means ± SE (n = 80). Values followed by the asterisk (*) represent significant differences (P<0.05, Student t-test).
Discussion
The ovariole of insects is usually divided into three types: the polytrophic type, telotrophic type and the panoistic type [32]. In the present study, it was assumed that the ovarioles of B. tabaci belongs to the telotrophic type. This type ovariole had been reported in three species of aphid, Stomaphis quercus, Phylloxera coccinea and Phylloxera glabra [33], [34]. In B. tabaci, the ovaries were characterized by an increasing number of ovarioles and mature oocytes after eclosion and 12–22 ovarioles were observed in its adult stage. Our results are consistent with Gameel et al. [35] who depicted 15 ovarioles in B. tabaci. Previous reports had provided only a brief description of the ovarian structure and oogenesis in green house whitefly Trialeurodes vaporariorum [36]. In another report, the paired ovaries of whitefly Aleurochiton aceris are composed of 5 short telotrophic ovarioles [37]. In B. tabaci, each ovariole contained two or three oocytes and a germarium. Once a mature oocyte was present in ovariole, three oocytes would be observed in the same ovariole. This ovarian system, based on morphological examination of oocyte of the four developmental phases, was used to evaluate the effects of virus infection of the plants on the whitefly reproduction.
A rounded bacteriocyte sphere was observed to be transferred into the oocyte at the terminal of ovariole in oocyte III and embedded in mature oocyte. The bacteriocytes housing symbiotic bacteria had been examined in whiteflies [37]–[39]. However, the interactions of this bacteriocyte with the oogenesis and reproduction of its host were poorly known. Gottlieb et al. [38] demonstrated the presence of five types of secondary symbionts, including Hamiltonella, Arsenophonus, Cardinium, Wolbachia, and Rickettsia, in a bacteriocyte of whitefly. This phenomenon may be a result of the direct enclosure of the bacteriocyte in the egg during oogenesis, providing a useful mechanism for efficient vertical transmission of endosymbiotic microorganisms. These endosymbiotic microorganisms had been reported not only in whitefly, but also in aphids and other insects [40]. In the aphid S. quercus, trophocyte cytoplasm is filled with endosymbiotic microorganisms [35].
Both B and ZHJ1 whiteflies are effective vectors of TYLCCNV [41]. In the present study, compared with its fecundity on healthy tobacco, B whitefly experienced a significant increase in its mean number of oocytes in the ovary through feeding on plants infected with TYLCCNV. In contrast, the potential fecundity of ZHJ1 whitefly feeding on virus-infected plants was similar to that of adults feeding on healthy plants. Previous studies have demonstrated that B and ZHJ1 are reproductively isolated [30], and B has a wider host range than ZHJ1 and generally performs better on their shared host plants [42]. Furthermore, the tobacco cultivar used in this study is a relatively poor host plant for both whitefly cryptic species [8]. However, the invasive B has a significant advantage via its mutualism with the viruses to increase the suitability of host plants and to have a higher number of immature (phase II, III) and mature oocytes in the ovaries, while the indigenous ZHJ1 is unable to do so (Fig. 3, 4). It was assumed that the vector-virus mutualism increased the differentiation of oocytes in B during oogenesis. This was verified by the higher total number of oocytes in ovaries of B whiteflies feeding on virus-infected plants (Fig. 5). Moreover, previous studies had shown that tomato yellow leaf curl virus (TYLCV) appeared to reduce whitefly fitness, whereas tomato mottle virus (ToMoV) did not [43], [44]. It had been speculated that this may be the result of the ability of TYLCV to replicate in the whitefly. A subsequent study indicated that TYLCV transcripts were stimulated to accumulate in the whitefly [45], whereas ToMoV transcripts were not. Whether TYLCCNV could replicate and accumulate transcripts in the whitefly or not warrants investigations in the future.
In plant-pathogen-vector systems such as the one examined in this study, the pathogen depends on the arthropod herbivore vector for transmission and dispersal. Thus higher proportion of mature oocytes in the ovaries and consequently higher numbers of eggs will help increase vector population and this will in turn facilitate the spread of virus disease pandemic in the field. This kind of vector-virus mutualism in assisting in the spread of virus disease pandemic had been seen in other vector-virus-plant systems, such as some aphid-transmitted viruses in wheat, barley and oats [46], . Laboratory and field evidence indicates that this kind of vector-virus mutualism had played a significant role in driving the spread of a cassava mosaic disease pandemic in Uganda [4], [48]. Since the effect of plant viruses on the vector can vary from positive to negative, effort has been made to reveal patterns of the effects according to the type of plant-virus-vector relationships [3], [8]. However, it is not yet clear how much role the differential effects of virus-infection of plants on the two cryptic species of the whitefly have played in their interactions and competitions in the field. Our results showed that TYLCCNV infection of a host plant would help to induce different appearance of ovarian development between two cryptic species of B. tabaci. The yolk protein is the main composition of egg content in many insects during oogenesis. In the ovaries of the two cryptic species of the whitefly, whether the different proportions of mature oocytes were due to the synthesis and uptake of yolk protein needs to be elucidated. Further, whether feeding on the virus-infected plants could lead to structural changes of the ovaries or changes in hormone synthesis in the vectors remain to be investigated.
Acknowledgments
We thank Mr. Gen-hong Yan for help in culturing the host plants of cotton and tobacco.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support from the National Nature Science Foundation of China (30730061) (http://www.nsfc.gov.cn/Portal0/default124.htm) and the National Basic Research Programme of China (2009CB119203) (http://www.most.gov.cn/) is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhou XP, Xie Y, Tao XR, Zhang ZK, Li ZH. Characterization of DNA beta associated with begomoviruses in China and evidence for co-evolution with their cognate viral DNA-A. J Gen Virol. 2003;84:237–247. doi: 10.1099/vir.0.18608-0. [DOI] [PubMed] [Google Scholar]
- 2.Liu SS, De Barro PJ, Xu J, Luan JB, Zang LS, et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 2007;318:1769–1172. doi: 10.1126/science.1149887. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Zhao H, Jiang K, Zhou XP, Liu SS. Differential indirect effects of two plant viruses on an invasive and an indigenous whitefly vector: implications for competitive displacement. Annals Appl Biol. 2009;155:439–448. [Google Scholar]
- 4.Colvin J, Omongo CA, Govindappa MR, Stevenson PC, Maruthi MN. Host plant viral infection effects on arthropod-vector population growth, development and behavior: management and epidemiological implications. Adv Virol Res. 2006;67:419–452. doi: 10.1016/S0065-3527(06)67011-5. [DOI] [PubMed] [Google Scholar]
- 5.Stout MJ, Thaler JS, Thomma BPHJ. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol. 2006;51:663–689. doi: 10.1146/annurev.ento.51.110104.151117. [DOI] [PubMed] [Google Scholar]
- 6.Nault LR. Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am. 1997;90:521–541. [Google Scholar]
- 7.Belliure B, Janssen A, Maris PC, Peters D, Sabelis MW. Herbivore arthropods benefit from vectoring plant viruses. Ecol Lett. 2005;8:70–79. [Google Scholar]
- 8.Jiu M, Zhou XP, Tong L, Xu J, Yang X, et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE. 2007;2:e182. doi: 10.1371/journal.pone.0000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubinstein G, Czosnek H. Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J Gen Virol. 1997;78:2683–2689. doi: 10.1099/0022-1317-78-10-2683. [DOI] [PubMed] [Google Scholar]
- 10.Boykin LM, Shatters RG, Rosell RC, McKenzie CL, Bagnall RA, et al. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COΙ DNA sequences. Mol Phylogenet Evol. 2007;44:1306–1319. doi: 10.1016/j.ympev.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Varma A, Malathi VG. Emerging geminivirus problems: a serious threat to crop production. Ann Appl Biol. 2003;142:145–464. [Google Scholar]
- 12.Seal SE, van den Bosch F, Jeger MJ. Factors influencing Begomovirus evolution and their increasing global significance: implications for sustainable control. Crit Rev Plant Sciences. 2006;25:23–46. [Google Scholar]
- 13.Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol. 2008;46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 14.Dinsdale A, Cook L, Riginos C, Buckley Y, De Barro PJ. Refined global analysis of Bemisia tabaci (Gennadius) (Hemiptera: Sternorrhyncha: Aleyrodoidea) mitochondrial CO1 to identify species level genetic boundaries. Ann Entomol Soc America, 2010;103:196–208. [Google Scholar]
- 15.Xu J, De Barro PJ, Liu SS. Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res. 2010;100:1–8. doi: 10.1017/S0007485310000015. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Hu J, Xu FC, Liu SS. Transmission of Tomato yellow leaf curl virus by two invasive biotypes and a Chinese indigenous biotype of the whitefly Bemisia tabaci. Inter J Pest Manag. 2010;56 in press, DOI:10.1111/j.1744-7917.2010.01354.x. [Google Scholar]
- 17.Perring TM, Cooper AD, Rodriguez RJ, Farrar CA, Bellows TS. Identification of a whitefly species by genomic and behavioral studies. Science. 1993;259:74–77. doi: 10.1126/science.8418497. [DOI] [PubMed] [Google Scholar]
- 18.Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies, biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol. 1995;40:511–534. [Google Scholar]
- 19.Crowder DW, Horowitz AR, De Barro PJ, Liu SS, Showalter AM, et al. Mating behaviour, life history and adaptation to insecticides determine species exclusion between whiteflies. J Animal Ecol. 2010;79:563–570. doi: 10.1111/j.1365-2656.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie CL. Effect of tomato mottle virus (Tomov) on Bemisia tabaci biotype B (Homoptera: Aleyrodidae) oviposition and adult survivorship on healthy tomato. Florida Entomol. 2002;85:367–368. [Google Scholar]
- 21.McKenzie CL, Shatters RG, Jr, Doostdar H, Lee SD, Inbar M, et al. Effect of geminivirus infection and Bemisia infestation on accumulation of pathogenesis-related proteins in tomato. Arch Insect Biochem Physiol. 2002;49:203–214. doi: 10.1002/arch.10020. [DOI] [PubMed] [Google Scholar]
- 22.Lapidot M, Friedmann M, Pilowsky M, Ben-Joseph R, Cohen S. Effect of host plant resistance to Tomato yellow leaf curl virus(TYLCV) on virus acquisition and transmission by its whitefly vector. Virol. 2001;91:1209–1213. doi: 10.1094/PHYTO.2001.91.12.1209. [DOI] [PubMed] [Google Scholar]
- 23.Mann RS, Sidhu JS, Butter NS, Sohi AS, Sexhon PS. Performance of Bemisia tabaci (Hemiptera: Aleyrodidae) on healthy and cotton leaf curl virus infected cotton. Florida Entomo l. 2008;91:249–255. [Google Scholar]
- 24.Polston JE, Toapanta M. The effect of begomoviruses on whitefly fitness. J Insect Science. 2008;8:39. [Google Scholar]
- 25.Maris PC, Joosten NN, Goldbach RW, Peters D. Tomato spotted wilt virus infection improves host suitability for its vector, Frankliniella occidentalis. Phytopathol. 2004;94:706–711. doi: 10.1094/PHYTO.2004.94.7.706. [DOI] [PubMed] [Google Scholar]
- 26.Luo C, Yao Y, Wang RJ, Yan FM, Hu DX, et al. The use of mitochondrial cytochrome oxidase I (mt COI) gene sequences for the identification of biotype of Bemisia tabaci (Gennadius) in China. Acta Entomol Sin. 2002;45:759–763. [Google Scholar]
- 27.Li ZX, Hu DX. Rapid identification of B biotype of Bemisia tabaci (Homoptera: Aleyrodidae) based on analysis of internally transcribed spacer 1 sequence. J Insect Science. 2005;12:421–427. [Google Scholar]
- 28.Cui XF, Tao XR, Xie Y, Fauquet CM, Zhou XP. A DNAβ associated with tomato yellow leaf curl China virus is required for symptom induction. J Virol. 2004;78:13966–13974. doi: 10.1128/JVI.78.24.13966-13974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li ZH, Xie Y, Zhou XP. Tobacco curly shoot virus DNAβ is not necessary for infection but intensifies symptoms in a host-dependent manner. Phytopathol. 2005;95:902–908. doi: 10.1094/PHYTO-95-0902. [DOI] [PubMed] [Google Scholar]
- 30.Zang LS, Liu SS, Liu YQ, Chen WQ. A comparative study on the morphological and biological characteristics of the B biotype and a non-B biotype (China-ZHJ-1) of Bemisia tabaci (Homoptera: Aleyrodidae) from Zhejiang, China. Acta Entomol Sin. 2005;48:742–748. [Google Scholar]
- 31.Tang, QY, Feng, MG DPS© Data Processing System: Experimental Design, Statistical Analysis and Data Mining Science Press, Beijing, China. 2007. [DOI] [PubMed]
- 32.Klowden MJ. Elsevier Academic Press, London, UK; 2007. Physiological systems in insects. [Google Scholar]
- 33.Pyka-fosciak G, Szklarewicz T. Germ cell cluster formation and ovariole structure in viviparous and oviparous generations of the aphid Stomaphis quercus. Int J Dev Biol. 2008;52:259–265. doi: 10.1387/ijdb.072338gp. [DOI] [PubMed] [Google Scholar]
- 34.Szklarzewicz T, Jankowska W, Wieczorek K, Wegierek P. Structure of the ovaries of the primitive aphids Phylloxera coccinea and Phylloxera glabra (Hemiptera, Aphidinea: Phylloxeridae). Acta Zool. 2008;90:123–131. [Google Scholar]
- 35.Gameel OI. Some aspects of the mating and oviposition behavior of the cotton whitefly Bemisia tabaci (Germ). Rev Zool Afr. 1974;88:784–88. [Google Scholar]
- 36.Buning J. Ultrastructure, previteltogenic growth and evolution. Chapman and hall, New York; 1994. The insect ovary. [Google Scholar]
- 37.Szklarzewicz T, Moskal A. Ultrastructure, distribution, and transmission of endosymbionts in the whitefly Aleurochiton aceris Modeer (Insecta, Hemiptera, Aleyrodinea) Protoplasma. 2001;218:45–53. doi: 10.1007/BF01288359. [DOI] [PubMed] [Google Scholar]
- 38.Lambiase S, Grigolo A, Laudani U, Sacchi L, Baccetti B. Pattern of bacteriocyte formation in Periplaneta americana (L.) (Blattaria: Blattidae). Int J Insect Morphol Embryol. 1997;26:9–19. [Google Scholar]
- 39.Costa HS, Toscano NC, Henneberry TJ. Mycetocyte inclusion in the oocytes of Bemisia argentifolii (Homoptera: Aleyrodidae). Ann Entomol Soc Am. 1996;89:694–699. [Google Scholar]
- 40.Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F, et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 2008;22:2591–2599. doi: 10.1096/fj.07-101162. [DOI] [PubMed] [Google Scholar]
- 41.Jiu M, Zhou XP, Liu SS. Acquisition and transmission of two begomoviruses by the B and a non-B biotype of Bemisia tabaci from Zhejiang, China. J Phytopath. 2006;154:587–591. [Google Scholar]
- 42.Zang LS, Chen WQ, Liu SS. Comparison of performance on different host plants between the B biotype and a non-B biotype of Bemisia tabaci from Zhejiang, China. Entomol Exp Appl. 2006;121:221–227. [Google Scholar]
- 43.Mehta P, Wyman JA, Nakhla MK, Maxwell DP. Transmission of tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J Econ Entomol. 1994;87:1291–1297. doi: 10.1093/jee/87.5.1285. [DOI] [PubMed] [Google Scholar]
- 44.Zeidan M, Czosnek H. Acquisition of tomato yellow leaf curl virus by the whitefly Bemisia tabaci. J Gen Virol. 1991;72:2607–2614. doi: 10.1099/0022-1317-72-11-2607. [DOI] [PubMed] [Google Scholar]
- 45.Sinisterra XH, Mckenzie CL, Hunter WB, Powell CA, Shatters RG. Differential transcriptional activity of plant-pathogenic begomoviruses in their whitefly vector (Bemisia tabaci, Gennadius: Hemiptera Aleyrodidae). J Gen Virol. 2005;86:1525–1532. doi: 10.1099/vir.0.80665-0. [DOI] [PubMed] [Google Scholar]
- 46.Fereres A, Lister RM, Araya JE, Foster JE. Development and reproduction of the English grain aphid (Homoptera: Aphididae) on wheat cultivars infected with barley yellow dwarf virus. Environ Entomol. 1989;18:388–393. [Google Scholar]
- 47.Quiroz C, Lister RM, Araya JE, Foster JE. Effect of symptom variants derived from the NY-MAV isolate of barley yellow dwarf virus on the life cycle of the English grain aphid (Homoptera: Aphididae) and on yield components in wheat and oats. J Econ Entomol. 1991;84:1920–1925. [Google Scholar]
- 48.Colvin J, Omongo CA, Maruthi MN, Otim-Nape GW, Thresh JM. Dual begomovirus infections and high Bemisia tabaci populations: two factors driving the spread of a cassava mosaic disease pandemic. Plant Pathol. 2004;53:577–584. [Google Scholar]