Abstract
The spinal substantia gelatinosa (SG; lamina II) is a major synaptic zone for unmyelinated (C) primary afferents. Whereas a substantial proportion of intrinsic SG neurones are GABAergic inhibitory, their relationship to afferent activity is unknown. In spinal cord slices from a transgenic mouse in which certain GABAergic lamina II neurones are labelled with green fluorescent protein (GFP), we compared primary afferent input with local efferent connections made by inhibitory SG neurones. Simultaneous whole-cell recordings from characterized neurones establish that inhibitory SG neurones receive monosynaptic input from a subset of unmyelinated primary afferents and connect to other lamina II cells that have input from a different set of afferents, permitting interactions between distinctive afferent messages. Certain lamina II inhibitory cells were found to connect to one another by reciprocal links. Inhibitory lamina II connections appear arranged to modulate activity from different sets of peripheral unmyelinated fibres through neural circuitry that includes disinhibition.
Introduction
Neural messages responsible for mammalian somatic sensation are transmitted to the central nervous system by a variety of primary afferents. Unmyelinated primary afferent fibres are the most numerous and include categories that signal tissue damage, skin temperature, particular forms of mechanical disturbance, and chemical changes (Perl, 1992).
The spinal cord has transfer stations for several modes of somatic sensory information. Its dorsal horn is a laminated arrangement of neurones, an organization respected by the central projections of functional categories of primary afferents (Rexed, 1952; Light, 1992). The substantia gelatinosa (SG), lamina II of the spinal dorsal horn, is a narrow cellular band near the dorsal limit of the grey matter (Pearson, 1952; Szentágothai, 1964; Réthelyi & Szentágothai, 1969; Ralston, 1979; Ralston & Ralston, 1979). The SG receives input from several categories of thin afferent fibres and appears to be involved in manipulating primary sensory information. Inhibitory neurones form a prominent proportion of the SG's complement of cells (Todd & McKenzie, 1989), but how such cells participate in transfer of first-order afferent signals is unknown. We report here observations that bear upon this.
The variety of neurones in the spinal cord SG complicates study. In part to circumvent this problem, we took advantage of a transgenic mouse line in which a set of lamina II GABAergic inhibitory neurones, with homogeneous functional and morphological features, express green fluorescent protein (GFP; Hantman et al. 2004; Hantman & Perl, 2005). Morphological and physiological features document that GFP neurones in the SG have characteristics of tonic central cells (Hantman et al. 2004; Hantman & Perl, 2005). The selective labelling of a subset of SG cells in this transgenic mouse provides an advantage for circuit studies over other mouse lines (e.g. the GAD-GFP mouse) in which GFP-expressing neurones display considerable heterogeneity (Heinke et al. 2004; Labrakakis et al. 2009). We used recordings from spinal cord slices of the prion promoter (PrP) transgenic mouse to analyse local connections of the GFP-expressing inhibitory neurones. Our observations indicate that SG-GFP neurones facilitate cross-modal interactions at first synaptic transfers for unmyelinated primary afferents. We also found mutual reciprocal inhibitory connections between specific SG neurones, suggestive of arrangements conducive for dynamic processing. A preliminary report of this work was presented at the 2007 Society for Neuroscience meeting (Zheng et al. 2007).
Methods
Ethical approval
The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill reviewed and approved all procedures on living animals. Use of animals was conducted in accordance with the Society for Neuroscience's Policies on the Use of Animals and Humans in Neuroscience Research and the American Physiological Society's Guiding Principles for the Care and Use of Animals in Research.
Spinal slice preparation
Tissue was obtained from 198 white Swiss-Webster transgenic mice (4–6 weeks postpartum) expressing enhanced green fluorescent protein (GFP) under control of the mouse prion promoter (PrP-GFP; van den Pol et al. 2002). Under deep urethane anaesthesia (1.5 g kg−1, i.p.), mice were perfused through the left ventricle with ice-cold, low Na+ and Ca2+ artificial cerebrospinal fluid (ACSF; in mm: 75 sucrose, 80 NaCl, 2.5 KCl, 0.5 CaCl2, 3.5 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 0.4 ascorbic acid, 2.0 pyruvate). After a laminectomy, the lumbar spinal cord was quickly removed and the animals were killed by decapitation (perfusion and tissue removal do not lead to immediate death). Parasagittal slices with an attached segmental dorsal root were prepared with a vibrating microtome (Vibratome; St Louis, MO, USA). The slices were incubated at room temperature for 1 h in ACSF equilibrated with 95% O2 and 5% CO2 (ACSF, in mm: 125 NaCl, 2.5 KCl, 2.0 CaCl2, 1.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 25 d-glucose, 0.4 ascorbic acid, 2.0 pyruvate).
Electrophysiology
The electrophysiological recording procedures were similar to those previously described (Grudt & Perl, 2002; Lu & Perl, 2003; Lu & Perl, 2005). Recordings were made from spinal cord slices suprafused with ACSF at 23–25°C (6–10 ml min−1) in a recording chamber mounted on a compound upright microscope. Infrared and fluorescence images of neurones in the slice were captured by a CCD video camera and displayed on a CCD monitor. Pipette microelectrodes of 5–8 MΩ impedance were filled with a solution containing (in mm) 130 potassium gluconate, 5 KCl, 4 Mg-ATP, 10 phosphocreatinine, 0.3 Li-GTP, 10 Hepes (pH 7.3, 310 mosmol l−1), and backfilled with 0.5% biocytin. Tight-seal, whole-cell recordings were guided by visible-light and infrared images. Careful note was made of the locus of each recorded cell relative to landmarks on the slice. Electrophysiological analyses were usually initiated by a visually guided whole-cell recording from a GFP fluorescent neurone. Confirmation of recording from the GFP cell was provided by visualization of green fluorescent fluid in the tip of the whole cell electrode. Another electrode introduced in the vicinity (within 200 μm) of the targeted GFP neurone was manipulated to make a second whole-cell recording, usually from a neurone without GFP. Action potentials were generated in one cell by suprathreshold current (10 ms, 0.1 Hz, unless otherwise stated) while the other neurone of the pair was monitored in current-clamp for evoked synaptic potentials. The membrane potential was held at −60 mV for presynaptic and −50 mV for postsynaptic cells. The firing pattern to 1 s depolarizing pulses from a holding potential of −60 mV was used to help characterize each recorded neurone.
The segmental dorsal root was stimulated by a suction electrode. Graded stimulation (less than 0.1 Hz with 0.1 or 0.5 ms pulse width) was applied to evoke EPSPs in SG cells. The threshold of the dorsal root (DR)-stimulation-evoked EPSPs was defined as the intensity used to evoke 50% of the maximal EPSP amplitude. Identification of monosynaptic EPSPs was based upon a constant latency (minimal jitter; variations <2%; Yoshimura & Jessell, 1989; Li & Perl, 1995) and absence of failures when repeated, suprathreshold stimulation was used (1 Hz for C-fibre input and 10 Hz for Aδ-fibre input). Direct primary afferent input to each recorded neurone was evaluated by the response to graded volleys of impulses in the attached dorsal root. Dorsal-root conduction velocity (CV) was estimated from the latency of monosynaptic EPSPs and the conduction distance (synaptic delay was estimated as 1 ms). For mEPSC recordings the membrane potential of cells was held at −70 mV. In the presence of tetrodotoxin (TTX, 0.5–1 μm), mEPSCs were recorded for 3 min as control and transient receptor potential (TRP) receptor agonists (capsaicin, icilin or menthol) were applied for 1 min. Changes in mEPSC frequency were analysed offline by the MiniAnalysis program (Synaptosoft, Decatur, GA, USA). In all electrophysiological experiments the series resistance was monitored during recording; data were not used if series resistance changed by more than 20%. Data were collected using AxoPatch 1D and AxoPatch 200B amplifiers, a Digidata 1200A analog-to-digital interface, and pCLAMP 6 software (Molecular Devices, Sunnyvale, CA, USA). Data are presented as the mean ±s.e.m. Student's one sample t test and paired t test were used to estimate the differences in threshold of evoked EPSPs and conduction velocities of primary afferents to each of a pair of synaptically connected cells. The differences in threshold of evoked EPSPs and conduction velocity of primary afferents projecting to a given type of neurone (e.g. GFP cell and islet cell) in different connection patterns was estimated by one-way ANOVA (P < 0.05 was taken as significant).
Chemical compounds and pharmaceutical agents were obtained from Sigma-Aldrich Chemical Company (St Louis, MO, USA).
Histological and morphological procedures
After a minimum of 20 min of whole-cell recording, the slice was fixed in 4% paraformaldehyde for 2–7 days and then immersed in 30% sucrose in phosphate buffer for 24–48 h. Intracellular biocytin was visualized using a Streptavidin–Texas Red conjugate (Vector Laboratories, Burlingame, CA, USA). GFP and the Texas Red fluorescence signals were viewed and analysed in a confocal microscope. In a continuous scanning mode, the confocal focus was carefully measured from the surface to the deepest extent of the neural image for determination of the Z-axis scanning range. The distance between top and bottom scanning positions for most cells ranged between 30 and 60 μm, adjusted for the medial–lateral dimension and the relevant location of the neuronal soma along the Z-axis. Serial scanning was started at a step of 1 μm; the averaged image for eight scans at each step was projected to the XY plane. The rostral–caudal and dorsal–ventral extensions of each neurone's dendritic arbours were measured on stacked images created from the plane scans to help establish the cell's morphological category.
Classification of neurones
Recorded neurones were categorized according to criteria established by Grudt & Perl (2002) and by Lu & Perl (2003, 2005) in hamster and rat, respectively. The classification scheme considers both physiological and morphological features. PrP-GFP neurones consistently are of the tonic central cell category, discharging more or less regularly for the full duration of a depolarizing current; their dendritic tree extends approximately 200 μm rostrocaudally and less than 60 μm in the dorsal–ventral plane. Islet cells also tonically discharge during maintained depolarization. On confocal reconstruction islet neurones have dendritic trees extending in the rostral–caudal direction over 400 μm with particularly extensive dendritic branching. Vertical cells display a delayed discharge of action potentials to step-depolarization and on visualization of the biocytin label have major dendritic distributions ventral to the cell soma. Transient central (TrC) neurones are recognized by a promptly appearing, but transient discharge to step depolarization. Their dendritic distribution is oriented rostrocaudally, extending overall less than 300 μm in a sagittal plane. Whereas the overall appearance of transient central morphology is similar to that of PrP-GFP neurones, their electrophysiological features are distinctive.
Results
In over 500 simultaneous recordings from two SG neurones, we obtained sufficient data to morphologically and physiologically categorize both cells of 53 connected pairs. Action potentials in one neurone evoked monosynaptic postsynaptic potentials (PSPs; 0.5 to 8 mV) in the simultaneously recorded cell. An example of a monosynaptic inhibitory connection appears in Fig. 1. Connection from one spinal cord neurone to another is presumed to be monosynaptic when (1) the peak of action potentials in the presynaptic (sending) neurone preceded the initial phase of PSPs in the receiving cell by short (<2 ms), near constant, delays (coefficient of variation <2%; Li & Perl, 1994), and (2) the IPSPs persist in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm), a non-N-methyl-d-aspartic acid (NMDA) receptor antagonist, and dl-2-amino-5-phosphonovaleric acid (APV; 50 μm), an NMDA receptor antagonist. Functional monosynaptic inhibitory connections between 10% of the paired recordings were noted in our sample of randomly selected, closely located cells. As detailed in Table 1, the observations included multiple instances of particular direct inhibitory linkages (i.e. between particular combinations of SG neurones). Inhibitory connections were repeatedly observed between pairs comprised of two of four categories of SG cells: PrP-GFP, islet, TrC, and vertical. Some other SG cell categories (e.g. radial and unidentified cells) did not manifest inhibitory connections and are not considered in the present report.
Figure 1.
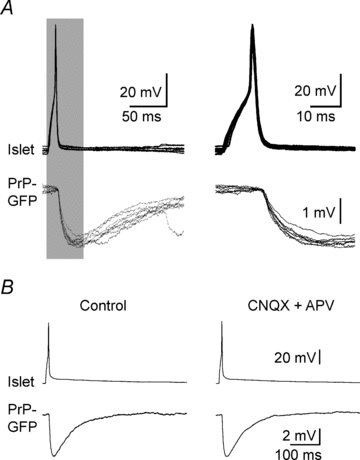
A monosynaptic inhibitory connection between an islet and a PrP-GFP neurone A, eight successive pulses (5 Hz, 10 ms duration) initiated action potentials in the presynaptic islet cell (upper traces) evoking IPSPs in the postsynaptic PrP-GFP cell (lower traces). Shaded portions of the records on the left are on the right expanded in time to show the IPSP latencies. Traces are aligned relative to the peak of the presynaptic action potential. Note the one-to-one generation of fixed latency IPSPs relative to presynaptic action potentials. B, left, averaged traces from 10 successive recordings (0.2 Hz, 10 ms duration). Right, the same protocol was repeated in the presence of glutamate receptor antagonists CNQX (20 μm) and APV (50 μm) to test for multisynaptic linkages.
Table 1.
Summary of inhibitory connection patterns
| GFP and islet cell |
|||||||
|---|---|---|---|---|---|---|---|
| GFP and GFP cell | GFP to islet cell | Islet to GFP cell | Reciprocal | GFP and TrC cell | GFP and vertical cell | Totals | |
| Connected | 0 | 4 | 15 | 8 | 0 | 20 | 47 |
| Unconnected | 8 | 127 | 43* | 129 | 307 | ||
| Totals | 8 | 154 | 43 | 149 | 354 | ||
| Islet and islet cell | Islet and TrC cell | Islet and vertical cell | TrC and TrC cell | TrC and vertical cell | Vertical and vertical cell | Totals | |
| Connected | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| Unconnected | 14 | 19 | 19 | 12 | 16* | 17 | 97 |
| Totals | 14 | 25 | 19 | 12 | 16 | 17 | 103 |
Most data are expressed as number of inhibitory connections/total observations for each combination of a pair of cells.
In two instances, excitatory connections are noted. TrC cell, transient central cell.
Synaptic connections of SG inhibitory neurones
The SG PrP-GFP neurones present a coherent population. PrP-GFP cells are distributed over the full length of the SG (Supplemental Fig. 1). Their afferent and efferent connections form repeated patterns. Twenty-seven PrP-GFP cells exhibited monosynaptic inhibitory linkages to or from a nearby islet cell, another inhibitory neurone. An example is shown in Fig. 1. Many islet neurones show immunocytochemical features of GABAergic cells and are presumed to be inhibitory (Todd & McKenzie, 1989). Three connection patterns are evident in the links between these two kinds of inhibitory neurones (Table 1). In four cases, an action potential in the PrP-GFP neurone evoked a bicuculline-sensitive IPSP in the islet cell. In another 15 instances, islet cell action potentials evoked bicuculline-sensitive IPSPs in the PrP-GFP neurone, suggesting that both of the IPSPs were mediated by GABAA receptors.
Eight pairs of PrP-GFP-islet recordings document mutual reciprocal, monosynaptic linkages. Action potentials in either neurone of this group evoked bicuculline-sensitive IPSPs in the other cell. Fig. 2 presents whole-cell recordings and cell labelling for a reciprocally connected PrP-GFP–islet pair. In all reciprocally linked pairs, the islet to PrP-GFP projection was stronger (a larger IPSP) than the converse PrP-GFP to islet cell connection (e.g. Fig. 2).
Figure 2.
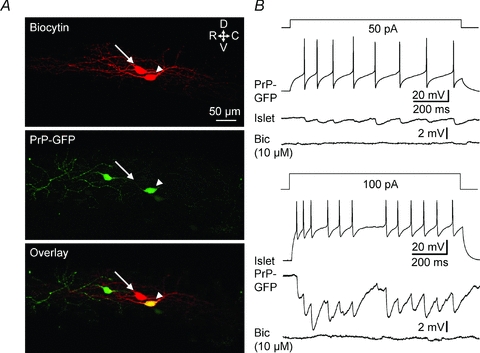
Reciprocal inhibitory connections between PrP-GFP and islet neurones A, maximum projection confocal images. Upper, biocytin-labelled cells (arrowhead, PrPGFP; arrow, islet). Middle: PrP-GFP (arrowhead). Lower, overlay (arrowhead, PrP-GFP; arrow, islet). Part of the dendritic processes of the islet cell are out of the field of view. B, depolarization by current injection (1 s) in PrP-GFP and islet cells induces tonic firing that generates monosynaptic IPSPs in the other neurone. The IPSPs evoked in each neurone were blocked by 10 μm bicuculline. Resting membrane potential: −60 mV for the presynaptic cell; −50 mV for the postsynaptic cell. Bic: bicuculline.
Connections of SG inhibitory neurones to SG excitatory neurones
Vertical neurones are SG cells with dendrites mainly oriented in the dorsal–ventral plane. Although there are immunohistochemical studies showing that some vertical cells contain an enzyme important for the synthesis of inhibitory neurotransmitter (Maxwell et al. 2007), in our paired recordings from both rat and mouse spinal slices, vertical cells have only excitatory synaptic projections to postsynaptic neurones, most of which are lamina I cells (Lu & Perl, 2005, and our unpublished observations). Therefore, in the present analysis vertical cells are classified as excitatory interneurones. In 20 of 149 simultaneous recordings of a PrP-GFP cell and a vertical neurone, action potentials in the PrP-GFP cell initiated bicuculline-sensitive monosynaptic IPSPs in the vertical neurone (see Supplemental Fig. 2A). Reciprocal vertical to PrP-GFP neurone connections were not seen (n= 20).
In six of 25 paired recordings, GABAergic islet neurones were connected to SG transient central cells, as is reported for rat (Lu & Perl, 2003; see Table 1). Action potentials in these islet cell evoked monosynaptic IPSPs, suppressed by bicuculline, in the transient central neurone (see Supplemental Fig. 2C). Reciprocal, transient central to islet linkages were not noted (n= 6).
Primary afferent inputs to connected SG cells
Insight into modulation or transformation of primary afferent messages is dependent upon knowledge about afferent signals at each stage of the circuit. The present observations confirm for mouse that volleys of impulses in DR fibres evoke monosynaptic EPSPs in a large proportion of lamina II neurones (Yoshimura & Jessell, 1989; Grudt & Perl, 2002). This includes the lamina II inhibitory cells involved in the synaptic connections described above. The DR-initiated monosynaptic EPSPs in SG neurones have latencies consistent with conduction by either unmyelinated or thinly myelinated fibres (Lawson et al. 1997; Woodbury & Koerber, 2003).
As a start to addressing the nature of primary afferent inputs to SG neurones in the present experiments, we used the DR-conduction velocity or latency of the DR-evoked EPSP. An approximate inverse relationship is known to exist between cross-sectional diameter of fibres in a nerve bundle and the threshold amplitude of an extracellular electrical stimulating pulse (Lloyd, 1943; Tasaki, 1953). Thus, systematically grading a DR stimulating pulse from weak to strong will produce afferent volleys containing progressively thinner fibres. The electrical stimulus thresholds of DR-EPSPs in neurones of linked pairs proved consistently lower for the EPSP with the shorter latency. Examples are shown in Fig. 3. Considerable evidence is consistent with functionally distinct types of primary sensory neurones often having DR fibres clustered in a narrow range of fibre diameters, related CVs, and electrical DR thresholds (Bradley & Eccles, 1953; Douglas & Ritchie, 1957; Burgess & Perl, 1973). Monosynaptic DR-EPSPs recorded from two synaptically connected lamina II neurones consistently differ in their thresholds and latencies. Such differences in the DR-EPSP thresholds and latencies proved closely comparable for different examples of a connected category. This is illustrated by recordings from islet–PrP-GFP pairs in Fig. 3A–C, and for PrP-GFP–vertical and islet–transient central cell pairs (Supplemental Fig. 2B and D). Thresholds of DR-EPSPs are similar for PrP-GFP or islet neurones participating in different connection patterns (P= 0.97 for GFP cells and 0.99 for islet cells, one-way ANOVA). Thus, in a given spinal slice, the electrical threshold of the DR pulse evoking an EPSP will have a defining value.
Figure 3.
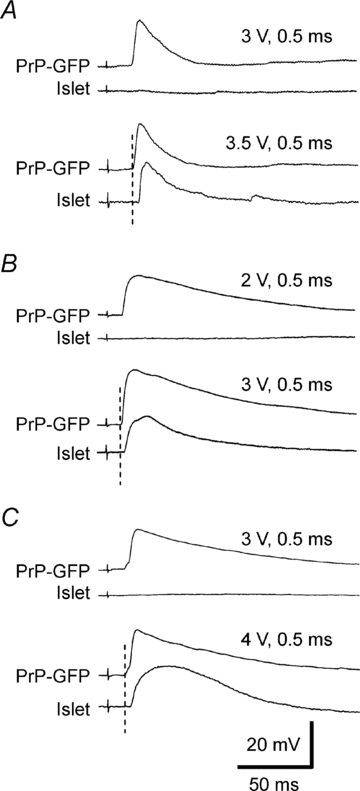
Differences in threshold and latency of EPSPs evoked by graded DR stimulation in pairs of synaptically connected PrP-GFP and islet neurones A, reciprocally connected PrP-GFP and islet cells. B, unidirectional connection from PrP-GFP to an islet cell. C, unidirectional connection from islet to PrP-GFP cell. Note that the threshold and latency show a consistent difference for the two cells in each connection pattern. All resting membrane potentials were −60 mV.
Simultaneous recordings allowed comparison of two unconnected neurones of the same type. In these unconnected cases, the thresholds and latencies for the DR-EPSPs in the two cells proved essentially the same (data not shown). Thresholds of evoked DR-EPSPs and estimated CVs of primary afferents suggest that a particular class of SG neurone receives input from a limited range of primary afferent neurones (Table 2 and Supplemental Table 1). The distinctions in threshold and latency of DR-evoked EPSPs from connected lamina II neurones are taken as indicative of systematic differences in the primary afferent distribution to lamina II neurones and to their synaptic partners. Although the differences in thresholds and CVs are consistent for each pair of connected cells, the threshold differences are considered the more reliable in documenting differences in projecting primary afferents. (The precision of a CV calculation will be affected by factors such as the length of dorsal root in slice preparation and unknown differences in trajectory of primary afferent fibres to each neurone within the spinal cord.)
Table 2.
Threshold differences of DR-evoked EPSPs
| GFP–islet cell pairs |
GFP–vertical cell pairs |
|||||
|---|---|---|---|---|---|---|
| GFP to islet (n= 3) | Islet to GFP (n= 15) | Reciprocal (n= 6) | Aδ (n= 7) | C (n= 9) | Islet–TrC cell pairs (n= 5) | |
| Ratio | 0.84 ± 0.1 | 0.80 ± 0.04*** | 0.77 ± 0.06** | 3.01 ± 0.50** | 0.80 ± 0.06** | 0.86 ± 0.05* |
Data were obtained from connected cell pairs in which both cells received monosynaptic dorsal root (DR) inputs. Graded DR stimulation was applied to estimate the thresholds of DR-evoked EPSPs. Pulse width was 0.5 ms for C fibres and 0.1 ms for Aδ fibres. Ratio was computed by threshold of GFP cell/threshold of non-GFP cell, or threshold of islet cell/threshold of transient central (TrC) cell. One sample t test was adopted for statistical analysis with the null hypothesis of mean = 1.
P < 0.05;
P < 0.01;
P < 0.001.
The connected pairs in our experiments were obtained from combinations of four types of neurones: PrP-GFP (tonic central), islet, vertical and transient central. Assuming that the CVs of fibres projecting to an SG neurone have the same relationships in different individuals of a mouse strain, information on CVs, and therefore fibre diameters, can be used to estimate relative sizes of the primary afferent fibres evoking the EPSPs. In pairs consisting of a PrP-GFP neurone connected to an islet cell, the DR-EPSP of the PrP-GFP cell was generated by fibres with a lower electrical threshold and higher CV than the input to the islet cell (see Yasaka et al. 2007). In connected pairs of islet and transient central neurones, the threshold of fibres initiating the islet DR-EPSP was lower than that evoking the DR-EPSP in the transient central cell (Supplemental Fig. 2D). Details of the thresholds and CV differences are summarized for each connection pattern in Table 2 and Supplemental Table 1.
Signalling function of the primary afferents
Unmyelinated (C) primary afferents are a diverse population that send activity to the CNS more or less selectively related to body tissue injury (nociceptors), skin temperature, mechanical events affecting various organs, and tissue fluid or molecular concentration. Differences in threshold and conduction velocity of the DR-evoked EPSPs in synaptically connected neurones may reflect modality-related segregation in afferent signalling. The basis of certain stimulus preferences by primary afferents can be attributed to activation of particular receptive molecules. The ‘capsaicin receptor’, transient receptor potential vanilloid 1 (TRPV1), is proposed to mediate excitation of particular unmyelinated dorsal root ganglion (DRG) nociceptors by capsaicin, noxious heat and acid (Caterina et al. 1997). Similarly, related molecules, transient receptor potential channel of melastatin type 8 (TRPM8) and transient receptor potential ankyrin 1 (TRPA1), are argued to provide subsets of unmyelinated fibre DRG afferent responsiveness to low tissue temperatures and cooling (Peier et al. 2002; Reid et al. 2002; Dhaka et al. 2008). TRPM8 is manifest in a relatively small number of DRG neurones (Peier et al. 2002; Story et al. 2003). Cells expressing TRPM8 and/or TRPA1 are potently excited by exposure to menthol and icilin, chemicals evoking sensory experiences of cooling and cold (Peier et al. 2002; Story et al. 2003).
To provide additional information on the nature of DR afferent input to lamina II neurones, we examined the effects of capsaicin and icilin/menthol suprafusion on whole-cell recordings from identified cells. The logic of this experiment argues that the background generation of mEPSCs at presynaptic terminals bearing TRPV1 or TRPM8 would increase in frequency with agonist activation of receptive molecules. In the presence of TTX (0.5–1 μm), capsaicin (1 μm) evoked sharp increases in mEPSC frequency in both PrP-GFP (n= 12; 275 ± 35%) and islet cells (n= 5; 263 ± 73%), whereas icilin (20 μm, n= 3) or menthol (500 μm, n= 7) increased mEPSC occurrence only in PrP-GFP neurones (n= 10; 66 ± 18%, Fig. 4). Increase of mEPSC frequency is taken to result from increased release of transmitter vesicles from presynaptic terminals expressing TRPV1 and/or TRPM8 (Larkman et al. 1991; Li & Perl, 1995; Bao et al. 1998). This logic implies that DR C-fibres excited by activation of TRPM8/TRPA1 are monosynaptically connected to lamina II GFP neurones, but not to lamina II islet cells.
Figure 4.
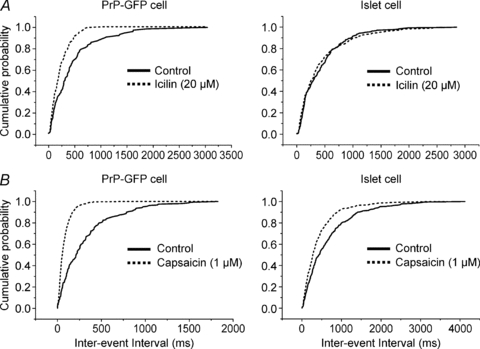
The effects of TRPM8 and TRPV1 receptor agonists on the frequency of mEPSCs recorded from reciprocally connected PrP-GFP and islet cells A, TRPM8 receptor agonist, icilin (20 μm), increased the frequency of mEPSCs recorded from the PrP-GFP neurone but not from the islet cell. B, TRPV1 receptor agonist, capsaicin (1 μm), increased the frequency of mEPSCs recorded from both cells. TTX (1 μm) was present during all mEPSC recordings. Membrane potential: −70 mV for voltage-clamp recordings.
mEPSC frequency in vertical neurones is not changed by either bath-applied capsaicin or icilin (n= 7). Vertical cells in our preparations exhibit high mEPSC frequencies (data not shown) and some have DR Aδ monosynaptic input (Supplemental Fig. 2B). On the other hand, capsaicin (6 out of 8) but not icilin (n= 6) evokes increase in mEPSC frequency in transient central neurones without postsynaptic membrane effects. The latter suggests that some DR afferents to transient central neurones are from capsaicin-sensitive elements.
Discussion
Our purpose in undertaking the present study was to establish some understanding of the part played by the numerous inhibitory neurones in the SG given the latter's receipt of a large projection from thin primary afferents. Evidence is provided that inhibitory neurones are part of an organization that imposes opportunities for preset interactions between different afferent inputs. The observations document specific connections from SG neurones that are related to the cells’ DR input. Thus, there appears to be definitive circuitry associated with the afferent and efferent connections of particular populations of SG neurones. The fact that similar connection patterns appear in different animals strongly suggests that this connectivity is a product of programmed development of the circuitry. In other words, the extant circuitry of inhibitory neurones appears to represent modules that are part of the system's functional organization.
Evidence for differences in unmyelinated afferent projection to individual SG neurones comes principally from in vitro observations on DR-evoked EPSPs. In pairs of lamina II neurones, directly linked by a synapse, the DR-EPSP latency is consistently shorter and the electrical threshold lower for one neurone than for the other. That finding suggests that the PSPs are generated by different populations of afferents. The data provide a relative order of latencies and thresholds for afferent DR volleys evoking EPSPs in different types of lamina II neurones. The most rapidly conducting afferent fibres and lowest DR threshold evoking DR-EPSPs in our sample of lamina II cells are Aδ fibres projecting to vertical cells. Next in threshold is the C-fibre connection to PrP-GFP neurones. The DR projection to islet cells is a lower threshold category of afferents than those evoking DR-EPSPs in transient central cells. These conclusions are consonant with the concept that the population of afferent fibres forming C deflections in the DR compound action potential consists of distinct subsets. It seems reasonable to postulate that the afferent C-fibre subsets originate from different receptive elements and may serve particular sensory modalities.
Clues to the afferent signals represented in the different DR projection patterns also come from the selective actions of TRP agents on synaptic transmission from primary afferents. TRP channels are established on DR fibres. The TRPV1 receptor, for example, has been shown to undergo a bidirectional axoplasmic transport toward both peripheral and central terminals (Guo et al. 1999). The superficial dorsal horn is densely innervated by TRPV1 terminals (Tominaga et al. 1998), and ultrastructural studies show TRPV1 receptors are associated with plasma membrane of presynaptic terminals in the dorsal horn (Guo et al. 1999). This location enables TRPV1 channels to mediate Ca2+ entry and to enhance glutamate release (see Medvedeva et al. 2008) and to directly interact with other presynaptic membrane receptors such as mGluRs (Kim et al. 2009). Immunocytochemical study of TRPM8 receptors is difficult; however, a recent genetic study showed that TRPM8 receptor-expressing sensory neurons terminate in the superficial dorsal horn (Dhaka et al. 2008). Similar to TRPV1's action, activation of TRPM8 receptors increases the frequency of glutamatergic mEPSCs recorded from a particular subset of superficial dorsal horn neurons (Wrigley et al. 2009). Overall, these studies suggest that TRPV1 and TRPM8 channels are largely located on plasma membranes of DR presynaptic terminals.
The selective responses of SG neurones to TRP agonists suggest focused projection of particular primary afferents to a set of SG neurones. Capsaicin, an agonist of the TRPV1 channel, increases mEPSC frequency in islet and PrP-GFP neurones, two categories of inhibitory SG neurones, but not in vertical cells, a category of SG excitatory neurones. The mEPSC frequency increase is attributable to enhancement of transmitter release at presynaptic terminals (Larkman et al. 1991; Nicholls et al. 1992). In our experiments, multisynaptic effects were blocked by inhibition of action potentials by TTX. It has been reported that TRPV1 is expressed by intrinsic dorsal horn neurones; however, expression of TRPV1 markers is markedly reduced by suppression of primary afferent terminations in the spinal dorsal horn. Thus, most TRPV1 receptors in the dorsal horn are evident on central terminals of DRG neurones (Szallasi et al. 1994; Guo et al. 1999; Valtschanoff et al. 2001).
In contrast to capsaicin's actions, exposure to icilin and menthol increases mEPSC frequency in PrP-GFP cells, but not in SG islet neurones. Icilin and menthol are agonists of TRPM8, a receptor that has been related to cooling sense (McKemy, 2005); however, both icilin and menthol are reported to activate TRPA1 receptors as well (Karashima et al. 2007; Bandell et al. 2007). Our findings can be attributed to activation of TRPM8 rather than TRPA1 (Garcia-Añoveros & Nagata, 2007; Wrigley et al. 2009). The EC50 for increased mEPSC frequency in a study of SG neurones (Wrigley et al. 2009) is 1.5 μm for icilin and 263 μm for menthol; the TRPA1 EC50 for increased mEPSC frequency for icilin is reported to be 79 μm. We employed a concentration of icilin (20 μm) that is well below the EC50 for TRPA1 activation. Moreover, the concentration of menthol we used (500 μm) is associated with TRPA1 channel block rather than its activation (Karashima et al. 2007).
The lack of mEPSC frequency increase in islet cells to icilin and menthol implies that TRPM8 afferents do not synapse on them. Furthermore, Mrgprd nociceptors, which represent many of the DRG neurones that do not express TRPV1 and TRPM8 (Dong et al. 2001; Dussor et al. 2008), are reported to establish synaptic links to numerous SG cells, except to islet neurones (Wang & Zylka, 2009). Taken together, it appears that TRPV1 positive afferents are prominent DR inputs to islet cells. In a functional context, the presence of TRPV1 channels defines heat-sensitive C primary afferents with particularly slow conduction (Lawson et al. 2008). We suggest that at a first CNS synapse islet cells are activated by heat but not by cooling. In distinction, PrP-GFP neurones exhibit increased synaptic effects by both TRPV1 and TRPM8 agonists. Current evidence suggests that these two TRP receptors characterize distinct populations of primary sensory neurones (Peier et al. 2002; Dhaka et al. 2008), agreeing with conclusions drawn by Wrigley et al. (2009) that the majority of superficial dorsal horn neurons which respond to cooling, icilin and menthol are also excited by capsaicin. Despite uncertainty of the detailed composition of primary afferent populations mediating responses in vitro, the action of TRP channel agonists supports the idea of distinction in primary afferent input to different categories of inhibitory neurones. The latter is supported by the consistent differences in threshold and latency of DR-evoked EPSCs from PrP-GFP and from islet cells. Therefore, these observations support the view that SG inhibitory circuits uncovered in the present study represent programmed cross-modal interactions between primary afferent messages signalling noxious cutaneous stimulation and skin cooling. Human experience indicates that these two sensory modalities often interact, with cooling stimulation usually suppressing nociception (or pain) (Gammon & Starr, 1941; Bini et al. 1984).
Our findings provide insights on components of circuitry facilitating interactions between afferents contributing to different sensory modalities. The diagram in Fig. 5 summarizes synaptic connections between SG neurones deduced from the present observations. That circuitry includes reciprocal interaction between two inhibitory neurones, PrP-GFP and islet cells. Many vertical cells have input from Aδ primary afferent fibres while transient central cells receive slowly conducting C-fibre projections; both vertical and transient central categories of cells have upstream targets of lamina-I projection cells (Lu & Perl, 2005; our unpublished observations). Menthol and/or icilin's action in increasing mEPSC frequency suggests that PrP-GFP cells receive excitatory input from afferents expressing TRPM8 receptors. A cooling or cold input would activate inhibitory PrP-GFP projections to lamina II vertical cells and thereby inhibit output to lamina I projection neurones (Lu & Perl, 2005). Activation of PrP-GFP neurones would also inhibit at least some islet cells; islet cell activity inhibits transient central cells. Thus, cooling-induced outcome in this network could be the activation of transient central cells by disinhibition. Similarly, a sensory input that selectively activates islet cells would shift the dynamic balance of the network activity to the vertical cell excitatory pathway, with suppression of the transient central cell path. One can conclude that inhibitory neurones of the SG are arranged to permit dynamic cross-modal interactions between inputs from different sets of somatic unmyelinated afferents.
Figure 5.
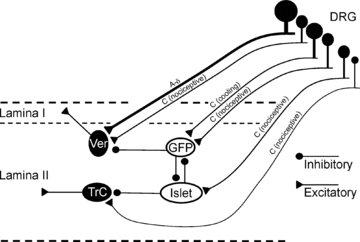
Schematic illustration of inhibitory neuronal networks in the spinal SG The relative conduction velocities of direct primary afferent input are estimated from comparisons of latencies of DR evoked EPSPs in pairs of linked neurones. Vertical and transient central neurones receive inputs from different populations of primary afferents and represent two distinct excitatory pathways in the SG. DRG: dorsal root ganglia; GFP: PrP-GFP neurone; Ver: vertical neurone; TrC: transient central neurone.
Partially stereotyped arrangements for modulation of afferent signals in one class of receptive neurones by activity generated by another class appears common in mammalian sensory systems. In our postulate, activation of TRPM8 receptors (cooling and cold) would lead to inhibition of myelinated-fibre input to vertical cells. Tentatively, it can be surmised that such programmed interactions based upon inhibition of activity generated by one sense organ or modality of sensation by that initiated by another afferent input is arranged to give priority to situations compelling for survival (e.g. feeding, reproduction). In any case, the interconnections uncovered by the present study are not a rigid system. They are structured as part of dynamic networks operating in part by inhibitory linkages.
In summary, the present analysis illuminates features of the functional organization of the murine spinal SG. (1) Demonstration of selective projection of subsets of C afferent fibres to particular kinds of lamina II neurones supports the concept that unmyelinated primary afferents differentially terminate in the SG in a specifically organized fashion. (2) Inherent SG circuitry implements interactions between inputs arriving from different classes of C-primary afferents. Ultimately, these arrangements provide for either a modal (functionally alike) or a ‘cross-modal’ (functionally dissimilar) afferent modulation of other C-fibre inputs. (3) SG inhibitory neurones are part of circuit modules arranged to permit dynamic interactions. (4) Inhibition of inhibitory connections (disinhibition) represents one mechanism for functional interaction in the spinal SG.
Acknowledgments
This work was supported by Research Grant NS-10321 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health. We thank Carolyn Suitt and Kirk McNaughton for histological assistance and Adam Hantman, Thomas Jessell and Mark Zylka for manuscript suggestions. The help provided by Bonnie Taylor-Blake in preparation of the manuscript was substantial and invaluable.
Glossary
Abbreviations
- APV
dl-2-amino-5-phosphonovaleric acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CV
conduction velocity
- DR
dorsal root
- DRG
dorsal root ganglion
- GFP
green fluorescent protein
- NMDA
N-methyl-d-aspartic acid
- PrP
prion promoter
- TrC
transient central
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankrin 1
- TRPM8
transient receptor potential channel of melastatin type 8
- TRPV1
transient receptor potential vanilloid 1
Author contributions
All experiments were conducted in the Department of Cell and Molecular Physiology at the University of North Carolina, Chapel Hill. All authors approved the final version of the manuscript for publication. E.R.P. conceived and designed the experiments; J.Z. and Y.L. collected, analysed and interpreted the data; E.R.P. and J.Z. drafted the article.
Authors’ present addresses
J. Zheng: Urogenix, Inc., Durham, NC 27713, USA.
Y. Lu: Department of Anesthesiology, Xijing Hospital, The Fourth Military Medical University, 15 West Changle Road, Xi’an, 710032 China.
Supplemental material
Supplemental Figure 1
Supplemental Figure 2
Supplemental Table 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bandell M, Macpherson L, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci. 1998;18:8740–8750. doi: 10.1523/JNEUROSCI.18-21-08740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjörk E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- Bradley K, Eccles JC. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953;122:462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Cutaneous mechanoreceptors and nociceptors. In: Iggo A, editor. Handbook of Sensory Physiology, vol II, Somatosensory System. Berlin: Springer-Verlag; 1973. pp. 29–78. [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ritchie JM. A technique for recording functional activity in specific groups of medullated and non-medullated fibres in whole nerve trunks. J Physiol. 1957;138:19–30. doi: 10.1113/jphysiol.1957.sp005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor G, Zylka MJ, Anderson DJ, McCleskey EW. Cutaneous sensory neurons expressing the Mrgprd receptor sense extracellular ATP and are putative nociceptors. J Neurophysiol. 2008;99:1581–1589. doi: 10.1152/jn.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon GD, Starr I. Studies on the relief of pain by counterirritation. J Clin Invest. 1941;20:13–20. doi: 10.1172/JCI101190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Añoveros J, Nagata K. Trpa1. Handb Exp Pharmacol. 2007:347–362. doi: 10.1007/978-3-540-34891-7_21. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Perl ER. Molecular and genetic features of a labelled class of spinal substantia gelatinosa neurons in a transgenic mouse. J Comp Neurol. 2005;492:90–100. doi: 10.1002/cne.20709. [DOI] [PubMed] [Google Scholar]
- Hantman AW, Van Den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkühler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci. 2009;29:10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrakakis C, Lorenzo LE, Bories C, Ribeiro-da-Silva A, De Koninck Y. Inhibitory coupling between inhibitory interneurons in the spinal cord dorsal horn. Mol Pain. 2009;5:24. doi: 10.1186/1744-8069-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A, Stratford K, Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991;350:344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72:1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Li J, Perl ER. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR. The Initial Processing of Pain and its Descending Control: Spinal and Trigeminal Systems. Vol. 12. Basel: S. Karger AG; 1992. [Google Scholar]
- Lloyd DPC. Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J Neurophysiol. 1943;6:293–314. [Google Scholar]
- Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fibre input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory neurons in superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva YV, Kim MS, Usachev YM. Mechanisms of prolonged presynaptic Ca2+ signalling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci. 2008;28:5295–5311. doi: 10.1523/JNEUROSCI.4810-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JG, Martin AR, Wallace BG. From Neuron to Brain: A Cellular and Molecular Approach to the Function of the Nervous System. Sunderland, MA: Sinauer Associates; 1992. [Google Scholar]
- Pearson AA. Role of gelatinous substance of spinal cord in conduction of pain. Arch Neurol Psychiatry. 1952;68:515–529. doi: 10.1001/archneurpsyc.1952.02320220092011. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Perl ER. Function of dorsal root ganglion neurons: An overview. In: Scott SA, editor. Sensory Neurons: Diversity, Development & Plasticity. New York: Oxford University Press; 1992. pp. 3–23. [Google Scholar]
- Ralston HJ, III, Ralston DD. The distribution of dorsal root axons in laminae I, II and III of the macaque spinal cord: a quantitative electron microscope study. J Comp Neurol. 1979;184:643–683. doi: 10.1002/cne.901840404. [DOI] [PubMed] [Google Scholar]
- Ralston HJ., III The fine structure of laminae I, II and III of the macaque spinal cord. J Comp Neurol. 1979;184:619–641. doi: 10.1002/cne.901840403. [DOI] [PubMed] [Google Scholar]
- Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J Physiol. 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réthelyi M, Szentágothai J. The large synaptic complexes of the substantia gelatinosa. Exp Brain Res. 1969;7:258–274. doi: 10.1007/BF00239033. [DOI] [PubMed] [Google Scholar]
- Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952;96:415–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM, Nilsson S, Hokfelt T, Lundberg JM. Visualization by [3H]resiniferatoxin autoradiography of capsaicin-sensitive neurons in the rat, pig and man. Eur J Pharmacol. 1994;264:217–221. doi: 10.1016/0014-2999(94)00526-5. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. Neuronal and synaptic arrangement in the substantia gelatinosa rolandi. J Comp Neurol. 1964;122:219–239. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- Tasaki I. Nervous Transmission. Springfield, IL: Charles C. Thomas; 1953. [Google Scholar]
- Todd AJ, McKenzie J. GABA-immunoreactive neurons in the dorsal horn of the rat spinal cord. Neuroscience. 1989;31:799–806. doi: 10.1016/0306-4522(89)90442-9. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates mutliple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol. 2001;436:225–235. [PubMed] [Google Scholar]
- Van Den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci. 2003;23:601–610. doi: 10.1523/JNEUROSCI.23-02-00601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley PJ, Jeong HJ, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009;157:371–380. doi: 10.1111/j.1476-5381.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lu Y, Perl ER. Washington, DC: Society for Neuroscience; 2007. Inhibitory interactions including mutual inhibition in the spinal substantia gelatinosa (lamina II) 2007 Abstract Viewer/Itinerary Planner, Programme No. 714.18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


