Abstract
Hebbian long-term potentiation (LTP) develops at specific synapses onto hippocampal CA1 oriens/alveus interneurons (OA-INs), suggesting selective regulation of distinct input pathways. Afferent-specific properties at interneuron synapses have been characterized extensively in CA3 stratum lucidum cells, but given interneuron diversity these rules of transmission and plasticity may not hold in other interneuron types. Here, we used paired recordings and demonstrate that CA2/3 pyramidal cell (PC) feedforward and CA1 PC feedback synapses onto OA-INs show distinct AMPA receptor rectification and Ca2+ permeability, short-term plasticity and mGluR2/3-mediated inhibition. Only feedback synapses undergo Hebbian LTP. OA-IN firing during repeated synaptic stimulation displays onset-transient or late-persistent responses consistent with activation of feedforward and feedback inputs, respectively. Input–output functions are preserved after theta-burst stimulation, but late-persistent responses selectively show mGluR1-dependent long-term increases. Thus, cell type- and afferent-specific rules of transmission and plasticity underlie distinct OA-IN input–output functions, providing selective long-term regulation in feedback inhibitory networks.
Introduction
Inhibitory interneurons participate in setting and maintaining hippocampal network oscillations and may thus contribute to memory formation. Leading hypotheses about memory stipulate that synaptic strength between principal cells increases, making associations between stimuli permanent (Bliss & Collingridge, 1993). However, facilitated transmission between excitatory neurons comes at a price: dominant oscillation frequencies may change over very large areas (Grunze et al. 1996; Bibbig et al. 2002). Fortunately, hippocampal principal cells are controlled by interneuron networks that selectively target specific cellular compartments (Toth & McBain, 1998; Poncer et al. 2000). Thus, interneurons could restrain changes in excitatory transmission if inhibitory output were adjusted in response to variations in principal cell networks (Grunze et al. 1996; Bibbig et al. 2002). Moreover, hippocampal interneurons are not mere passive relays of inhibitory information but perform complex computations like input selection and dendritic integration. They receive multiple synaptic inputs with afferent-specific mechanisms. For instance, CA3 stratum lucidum interneurons (SL-INs) receive mossy fibre (feedforward) and CA3 Schaffer collateral (feedback) inputs, distinguished by specific postsynaptic targeting of Ca2+-permeable (CP) and Ca2+-impermeable (CI) AMPA receptors, respectively (Toth & McBain, 1998; Salin et al. 1996). Additionally, mossy fibre synapses are presynaptically modulated by group II metabotropic glutamate receptors (mGluR2/3) whereas Schaffer collateral synapses are not (Toth & McBain, 1998).
Afferent specific synaptic mechanisms result in dynamically regulated inhibition of pyramidal cells (PCs). Stimulation of mossy fibre inputs to CA3 produces massive feedforward inhibition that readily prevents pyramidal cell firing. During repeated stimulation, feedforward excitatory afferents on interneurons depress, leading to increased pyramidal cell firing (Mori et al. 2004). Synaptic mechanisms, however, are heterogeneous between interneuron subtypes. For example, in CA1 feedback inhibitory networks, repeated activation of interneuron excitatory inputs leads pyramidal cells to shift from transient somatic inhibition by basket cells to persistent dendritic inhibition by oriens/alveus interneurons (OA-INs) (Pouille & Scanziani, 2004). Finally, synapses onto interneurons also undergo heterogeneous forms of use-dependent long-term plasticity (McBain & Fisahn, 2001; Kullmann & Lamsa, 2007). Interestingly, long-term plasticity is highly afferent specific in interneurons (Maccaferri et al. 1998), as established for CA3 pyramidal cells (Salin et al. 1996). Thus, feedforward mossy fibre synapses on CA3 SL-INs undergo mGluR7-dependent long-term depression (LTD) after tetanization whereas feedback Schaffer collateral inputs do not show plasticity (Pelkey et al. 2005). LTD in CA3 feedforward inhibitory circuits is in contrast to NMDAR-dependent long-term potentiation (LTP) in CA1 stratum radiatum interneurons, which has been proposed to maintain spike timing precision during LTP at pyramidal cell synapses through an increase in feedforward inhibition (Lamsa et al. 2005).
Clearly, considering the diversity of hippocampal interneurons (McBain & Fisahn, 2001; Freund & Buzsaki, 1996; Somogyi & Klausberger, 2005), the functional implications of the vast repertoire of synaptic mechanisms and plasticity remain largely unknown. Pathway specific mechanisms have been addressed only in CA3 SL-INs (Toth & McBain, 1998), which show predominantly LTD of feedforward inputs (but see Pelkey et al. 2005). In contrast, CA1 OA-INs are preferential targets of CA1 pyramidal cell recurrent collaterals (Maccaferri & McBain, 1995; Maccaferri & McBain, 1996) and play an important role in feedback inhibition (Lacaille et al. 1987). They may also be innervated by Schaffer collaterals (Ishizuka et al. 1990) and can mediate feedforward inhibition. OA-INs represent a heterogeneous subgroup of interneurons with particular properties of synaptic plasticity. Specifically, OA-IN synapses show prominent mGluR1-dependent Hebbian LTP (Perez et al. 2001; Lapointe et al. 2004; Le Vasseur et al. 2008) as well as an anti-Hebbian form of LTP (Lamsa et al. 2007). Interestingly, previous work indicated that Hebbian LTP does not develop at a subset (about 20%) of OA-IN synapses (Perez et al. 2001; Lapointe et al. 2004). Therefore, we used whole-cell and cell-attached recordings, as well as pharmacological approaches, to examine afferent specific synaptic mechanisms and plasticity at OA-IN synapses and determine their role in regulation of OA-IN input–output function. Our findings uncover interneuron subtype-specific rules of transmission and plasticity in feedback and feedforward pathways that result in precise long-term regulation of hippocampal feedback inhibitory networks.
Methods
Acute hippocampal slices
Sprague–Dawley rats (18– 25 days old) were anaesthetized with isoflurane and killed by decapitation, in compliance with ethical guidelines in place at Université de Montréal and The Journal of Physiology (Drummond, 2009). Transverse hippocampal slices (300 μm thick) were prepared as previously described (Lapointe et al. 2004; Chapman & Lacaille, 1999). Individual slices were submerged in a chamber mounted on an upright microscope (Nikon Canada Inc) equipped with a long-range water immersion objective (×40, Nomarski optics) and infrared video camera. Slices were perfused with ACSF containing (in mm): 124 NaCl, 5 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3 and 10 dextrose, saturated with 95% O2–5% CO2 (pH 7.3–7.4, 300 mosmol l−1).
Electrophysiological recordings
Recordings were made at room temperature (20–22°C) from visually identified CA1 interneurons located in stratum oriens/alveus (Lapointe et al. 2004) using a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA, USA). Data were low pass filtered at 1 kHz, digitized at 10 or 20 kHz, and acquired using pCLAMP (Molecular Devices). For whole-cell voltage-clamp recordings, pipettes were filled with a solution containing (in mm): 130 CsMeSO3, 5 NaCl, 1 MgCl2, 10 Hepes, 10 phosphocreatine, 0.1 spermine, 2 ATP-Tris, 0.4 GTP-Tris and 0.1% biocytin (pH 7.2–7.3, 290 mosmol l−1). During voltage-clamp recording, interneurons were held at −60 mV, and series resistance was regularly monitored. Recordings were analysed only if the holding current and series resistance were stable. The latter had average values of 18.3 ± 0.6 MΩ for interneuron (n= 43) and 19.3 ± 1.2 MΩ for pyramidal cell (n= 11) recordings. For current-clamp recordings, CsMeSO3 was replaced with potassium gluconate and CsOH was omitted. Cell-attached recordings were performed with pipettes containing ACSF and 0.1% biocytin.
Synaptic responses were evoked in interneurons using constant current pulses (50 μs) via an ACSF-filled bipolar theta-glass electrode positioned in oriens/alveus within 100 μm lateral to the recorded cell soma. Putative single-fibre EPSCs were evoked at 0.5 Hz using minimal stimulation (Stevens & Wang, 1994; Raastad, 1995), as previously described (Perez et al. 2001; Lapointe et al. 2004). In some experiments, the location of the stimulation electrode was moved toward the CA3 or subiculum in attempts to recruit the different afferent pathways, but no systematic relationship was found. To determine current–voltage (I–V) relations, EPSCs were evoked at voltages from −60 mV to +40 mV in 20 mV increments. Short-term plasticity was examined during repetitive stimulation (5 pulses at 20 Hz; repeated 10 times at 30 s intervals) and was quantified as the ratio EPSC5/EPSC1. Long-term potentiation (LTP) was induced by three episodes (given at 30 s intervals) of theta-burst stimulation (TBS) of afferents (five bursts each consisting of four pulses at 100 Hz with 250 ms interburst interval) paired with postsynaptic depolarization (five depolarizing steps to −20 mV, 60 ms long). EPSCs were usually characterized in one cell per slice. In cell-attached recording experiments, series of stimulation (10 pulses at 20 Hz) and TBS were given using a whole cell patch pipette filled with ACSF.
Organotypic slice cultures
Cultured hippocampal slices were prepared from 6- to 10-day-old rats as previously described (Stoppini et al. 1991). Cultured hippocampal slices were chosen because the synaptic network is preserved and the frequency of obtaining monosynaptic connections between cell pairs is improved (Scanziani et al. 1998; Debanne et al. 1995). After 1–2 weeks in vitro, individual slice cultures were placed in a perfusion chamber mounted on an upright microscope (Eclipse E600FN, Nikon Canada Inc.). Paired whole cell recordings were obtained from pyramidal cells and interneurons as described by Scanziani et al. (1998). CA1 OA-INs and pyramidal cells in CA1 or CA2/3 were visually identified and selected for whole cell recordings. Pyramidal cells were recorded in the whole-cell current-clamp configuration. Single presynaptic action potentials were evoked by injecting depolarizing currents (200–700 pA; at 3 s intervals) in pyramidal cells. EPSCs were recorded in the whole-cell voltage-clamp configuration in synaptically coupled interneurons at a holding potential of −60 mV.
Pharmacology
EPSCs were recorded in ACSF containing bicuculline (10 μm), (±)-2-amino-5-phosphonovalerate (AP-5, 50 μm) and elevated concentrations of Ca2+ and Mg2+ (4 mm each; Perez et al. 2001). AP-5 was added to the bath to avoid long-term plasticity in polysynaptic pathways (Maccaferri & McBain, 1995). Note that Hebbian LTP which is mGluR1-dependent has been shown to be unaffected by the presence or absence of AP-5 (Perez et al. 2001). When indicated, philanthotoxin-433 tris-trifluoracetate (PhTX, 1 μm), GYKI-52466 hydrochloride (30 μm), (2S,2′R,3′R)-2-(2′,3-dicarboxycylopropyl)glycine (DCG-IV, 1 μm), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were added to the superfusion medium. All drugs were obtained from Sigma (Ontario, Canada) except DCG-IV which was purchased from Tocris Bioscience (Ellisville, MO, USA).
Measurements and statistical analysis
Responses were analysed off-line using Clampfit (v. 9; Molecular Devices) and Igor Pro (4.06 Carbon, WaveMetrics, Lake Oswego, OR, USA). EPSC amplitude was measured as peak amplitude minus average baseline points preceding stimulation. The rectification index (RI) of EPSCs was calculated from the amplitude of average EPSCs (20 traces) at different holding potentials. The RI was taken as the ratio of EPSC conductances at −60 mV and +40 mV (Itazawa et al. 1997). A RI > 1.0 was defined as a linear I–V relation; RI < 0.25 as an I–V relation with inward rectification; and 0.25 ≤ RI ≤ 1.0 as an I–V relation with intermediate rectification (Itazawa et al. 1997). Mean amplitude of average EPSCs including failures, mean amplitude of average EPSCs excluding failures (EPSC potency) and failure rate (number of failures as percentage of total number of events) were calculated for 2 min bins. Long-term changes were taken as a significant change (Student's t test) in EPSC amplitude at 30 min post-pairing (Perez et al. 2001; Lapointe et al. 2004). Group comparisons were made using Student's t test (paired or independent, as appropriate). In cell attached experiments, repeated measures ANOVA was performed on the raw data to determine changes in firing probability. These results were then normalized to baseline responses for display. The level of significance was set at P < 0.05 and data were expressed as means ±s.e.m.
Biocytin labelling
To verify the morphology of recorded cells, slices were fixed overnight in 4% paraformaldehyde in 0.1 m phosphate buffer (PB, pH 7.4). Slices were washed in PB, treated with 0.3% H2O2 (30 min) and incubated in avidin–biotin complex (Elite ABC kit; 1:200, 24 h; Vector Laboratories, Burlingame, CA, USA). The reaction product was visualized using 0.05% diaminobenzidine (DAB, Sigma), 0.2% nickel sulphate (Sigma), 0.1 m imidazole and 0.0015% H2O2 in Tris buffer (TB, 0.05 m, pH 7.6).
Results
Two types of excitatory synapses based on Ca2+ permeability of AMPARs and modulation by mGluR2/3
First we used whole cell recordings from CA1 OA-INs and evoked EPSCs with minimal stimulation at the oriens–alveus border (Perez et al. 2001; Lapointe et al. 2004) to distinguish two distinct types of excitatory inputs based on their sensitivity to the polyamine toxin philanthotoxin (PhTX). This drug selectively blocks Ca2+-permeable AMPA receptors (CP-AMPARs) that lack the GluR2 subunit (Toth & McBain, 1998; Washburn & Dingledine, 1996). OA-IN EPSCs demonstrated significant reductions in EPSC amplitude after adding PhTX (1 μm) in the bath in 61% of cells tested (to 37.6 ± 4.9% of control; 11/18 cells; Fig. 1A and B), indicating the presence of CP-AMPARs at these synapses. The remaining 39% of OA-IN EPSCs were unaffected by PhTX (94.0 ± 3.1% of control; Fig. 1C and D), suggesting that these EPSCs were due to Ca2+-impermeable AMPAR (CI-AMPAR) activation.
Figure 1. Two types of excitatory synapses onto OA-INs.
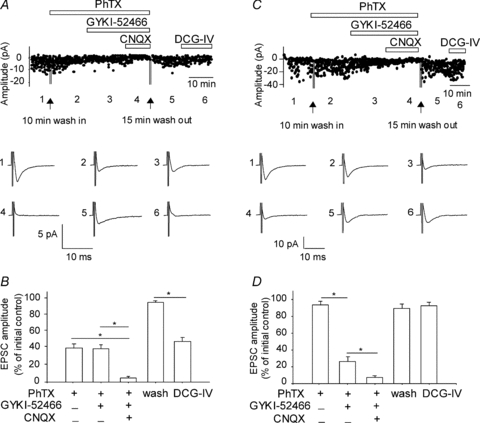
A, example from a representative interneuron of EPSCs partly blocked by the Ca2+-permeable AMPA receptor (CP-AMPAR) antagonist PhTX (1 μm). Graph of amplitude of individual EPSCs (top) evoked by minimal stimulation throughout the different drug applications. Traces (below) represent averages of 100 responses taken in different conditions at time points indicated by the numbers (1–6). Residual EPSCs in PhTX were insensitive to the AMPAR antagonist GYKI-52466 (30 μm) but sensitive to the AMPAR/KAR antagonist CNQX (20 μm). After washout of AMPAR/KAR antagonists, application of the mGluR2/3 agonist DCG-IV (1 μm) depressed these EPSCs. B, bar graph illustrating the mean drug effects for EPSCs for all cells with a CP-AMPAR component (n= 11), and indicating the implication of CP-AMPARs and KARs, as well as a negative modulation via mGluR2/3, at these synapses. C, representative example of an interneuron with EPSCs not antagonized by PhTX (1 μm). These EPSCs were sensitive to the AMPAR antagonist GYKI-52466. Residual EPSCs in the presence of AMPAR antagonists were inhibited by CNQX. After washout of AMPAR/KAR antagonists, application of DCG-IV did not depress EPSCs. D, bar graph for all cells tested that did not have a CP-AMPAR component (n= 7), showing the contribution of calcium-impermeable AMPARs (CI-AMPARs) and KARs, as well as absence of modulation by mGluR2/3, at these synapses.
To tease apart the relative contribution of AMPARs and kainate receptors (KARs) to these synapses, the selective AMPAR antagonist GYKI-52466 (Goldin et al. 2007) and the non-selective AMPAR and KAR antagonist CNQX were sequentially added to the slice. The residual EPSC of PhTX-sensitive responses was unaffected by additional application of GYKI-52466 (30 μm; 36.8 ± 4.8% of control; Fig. 1A and B), indicating that these EPSCs did not implicate CI-AMPARs. However, subsequent perfusion of the non-selective AMPAR/KAR antagonist CNQX (20 μm) abolished residual EPSCs (4.2 ± 1.8% of control), implicating KARs. On the other hand, PhTX-resistant EPSCs were significantly inhibited by GYKI-52466 (to 26.2 ± 5.8% of control; Fig. 1C and D), indicating that these synaptic responses were mediated by CI-AMPARs. Further addition of CNQX essentially blocked transmission at these synapses (6.8 ± 2.4% of control), also implicating KARs. These pharmacological data indicate the existence of two distinct excitatory inputs to OA-INs, the first consisting of CP-AMPARs and KARs, the second consisting of CI-AMPARs and KARs.
Previous work identified two types of OA-IN excitatory synapses based on their modulation by presynaptic group II metabotropic glutamate receptors (mGluR2/3) (Lapointe et al. 2004). We hypothesized that this presynaptic mGluR2/3 distinction may also correlate with the distinct postsynaptic AMPAR subpopulations. To test this, the mGluR2/3 agonist DCG-IV (1 μm) was added after AMPAR/KAR pharmacological identification (Fig. 1). Following a 20 min washout of AMPAR/KAR antagonists, PhTX-sensitive EPSCs were depressed by DCG-IV (45.5 ± 4.6% of control; Fig. 1A and B). In contrast, PhTX-insensitive EPSCs were unaffected by DCG-IV (92.0 ± 4.6% of control; Fig. 1C and D). Our results indicate that OA-INs are innervated by two pharmacologically distinct types of excitatory synapses. The first is composed of Ca2+-permeable AMPARs and KARs which are negatively modulated by presynaptic mGluR2/3. The second consists of Ca2+-impermeable AMPARs and KARs which are not subject to presynaptic modulation by mGluR2/3.
Synapse specific I–V relations
To further characterize the two types of OA-IN synapses, the I–V relationships of EPSCs were examined. CP-AMPARs are known to display a voltage-dependent block by intracellular polyamines that results in inward rectification at depolarized voltages (Koh et al. 1995; Rozov & Burnashev, 1999). On the other hand, CI-AMPARs have been shown to possess linear I–V relationships (Toth & McBain, 1998). The rectification index (RI) of OA-IN EPSCs was therefore computed, and cells were separated into (1) inwardly/intermediately rectifying (RI ≤ 1.0) and (2) non-rectifying (1.0 < RI; (Itazawa et al. 1997). Accordingly, 50% of OA-IN EPSCs sampled (n= 9) showed inward rectification (RI = 0.11 ± 0.01; Fig. 2A,C). Furthermore, 23% of EPSCs tested (n= 4) displayed intermediate rectification (RI = 0.53 ± 0.05). Importantly, EPSCs with inward/intermediate rectification were antagonized by PhTX (22.1 ± 4.4% of control; n= 10) confirming that these EPSCs were mediated by CP-AMPARs. In contrast, the remaining 27% of OA-IN EPSCs (n= 5) showed linear I–V relationships (RI = 1.35 ± 0.05) and were insensitive to PhTX (96.0 ± 0.9% of control; n= 4). There was a significant negative correlation between the percentage reduction in EPSC amplitude by PhTX application and the rectification index (r=−0.81; Fig. 2E). Thus, these results are consistent with the presence of two populations of OA-IN excitatory afferents which are distinguishable by their I–V relationship and sensitivity to PhTX.
Figure 2. Different I–V relation of EPSCs with CP- vs. CI-AMPARs.

A, representative example of EPSCs evoked at different membrane potentials (−60 to +40 mV) which showed inward rectification (RI = 0.08) (left) and were antagonized by PhTX (right). B, EPSCs from another OA-IN with linear I–V relationship (RI = 1.0) which were insensitive to PhTX (right). C and D, graph showing I–V relationships for all OA-INs with inward rectification (C; n= 9) or linear I–V (D; n= 5). E, scatter plot showing the significant correlation between EPSC sensitivity to PhTX (expressed as percentage reduction) and rectification index (n= 17).
Synapse-specific plasticity
The existence of synapse-specific CP- and CI-AMPARs in OA-INs has important implications for plasticity. Indeed, it is known that synapses with CP- versus CI-AMPARs display fundamentally different forms of short-term plasticity in SL-INs (Toth & McBain, 1998; Toth et al. 2000). Thus, next we examined short-term plasticity of the two types of OA-IN EPSCs. After characterization of their I–V curve, five pulses were administered at 20 Hz. EPSCs with inward/intermediate rectification (RI = 0.12 ± 0.04; n= 11) generally exhibited short-term facilitation, which was incremental during the 20 Hz stimulation and reached 409.6 ± 122.3% (EPSC5/EPSC1 for all cells; Fig. 3A and B). Although a majority of EPSCs with inward/intermediate rectification showed facilitation (490.5 ± 132.6%; n= 10 OA-INs), a minority showed short-term depression (52.7 ± 2.8%; n= 2 OA-INs; Fig. 3C). In contrast, EPSCs with linear I–V relations (RI = 1.37 ± 0.08; n= 6) showed no change during repetitive stimulation when considered as a group (98.7 ± 19.2% EPSC5/EPSC1 for all cells; Fig. 3D,E). However, a majority of EPSCs showed short-term depression (69.5 ± 5.7%; n= 4 OA-INs), and a minority displayed facilitation (157.1 ± 14.3%; n= 2 OA-INs; Fig. 3F). Overall, these results indicate that EPSCs with inward/intermediate rectification, characteristic of CP-AMPAR containing synapses, generally exhibit short-term facilitation, whereas EPSCs with linear I–V relations, associated with CI-AMPAR containing synapses, usually show short-term depression during short trains.
Figure 3. Short-term plasticity at synapses with different I–V properties.
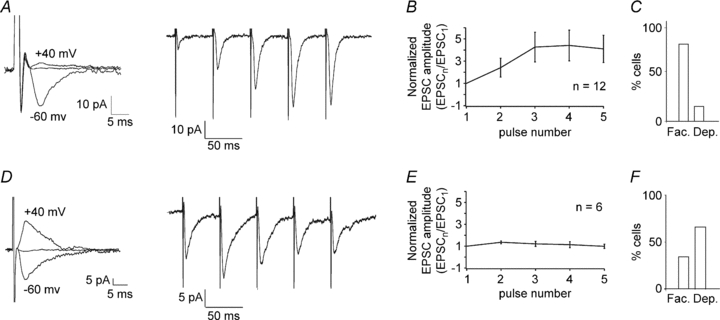
A, EPSCs from a representative OA-IN showing inward rectification (RI = 0.18; left) and facilitation during repetitive stimulation (5 pulses at 20 Hz) (average of 15 traces; right). B, graph of EPSC amplitude during the train (normalized to EPSC1) for all cells with inward/intermediate rectification (n= 12), showing facilitation at these synapses. C, summary histogram of synapses with inward/intermediate rectification, showing most often facilitation (Fac.) versus depression (Dep.). D, example of EPSCs with linear I–V relation (RI = 1.4; left) which showed initial facilitation followed by depression during repetitive stimulation (right). E, graph of EPSC amplitude during the train for all OA-INs with linear I–V relation (n= 6), illustrating the initial facilitation and subsequent depression at these synapses. F, summary histogram of synapses with linear I–V relation, showing usually depression over facilitation at the end of the train.
Likewise, induction of long-term plasticity is also dependent on the AMPA receptor subtype found at SL-IN synapses (Toth et al. 2000). Using a paired theta-burst stimulation and postsynaptic depolarization induction protocol (Perez et al. 2001; Lapointe et al. 2004), we found that EPSCs with inward/intermediate rectification (RI = 0.29 ± 0.06; n= 8; Fig. 4A and B), characteristic of CP-AMPAR containing synapses, showed LTP. For all cells tested, the amplitude of average EPSCs (including failures) was significantly increased at 30 min (263 ± 64% of control) following the induction protocol (Fig. 4E). The EPSC potency was significantly increased (141 ± 22% of control; Fig. 4F), and the failure rate was significantly decreased (Fig. 4G). In contrast, EPSCs with linear I–V relations (RI = 1.19 ± 0.04; n= 6; Fig. 4C and D), associated with CI-AMPAR containing synapses, did not develop LTP. In these cells, the amplitude of average EPSCs (including failures) was not significantly affected at 30 min (90 ± 13% of control) after pairing (Fig. 4E), and neither were EPSC potency (92 ± 11% of control; Fig. 4F) and failure rate (Fig. 4G). Therefore, these data indicate that the two types of OA-IN synapses show a differential capacity for TBS pairing-induced long-term potentiation. Interestingly, the direction of plastic changes at CP-AMPAR-specific synapses in OA-INs is different from the long-term depression found at CP-AMPAR-containing synapses in SL-INs (Toth and McBain, 1998), indicating that synapse-specific rules of plasticity are interneuron subtype-dependent.
Figure 4. Different capacity for Hebbian long-term potentiation (LTP) at synapses with distinct I–V properties.

A, representative example of EPSCs with inward rectification (RI = 0.25). B, graph of EPSC amplitude (left) and EPSC traces (right) from the same neuron, showing LTP after pairing theta-burst stimulation with postsynaptic depolarization (TBS+depo) delivered at time = 0 (vertical line). Ten consecutive traces are shown to the right for the control period (top) and 30 min post-pairing (middle). Superimposed average EPSCs (n= 100, including failures) illustrating the increase in response at 30 min post-pairing (bottom). C, example of EPSCs with linear I–V relation (RI = 1.3). D, graph of EPSC amplitude and EPSC traces from the same cell, indicating that TBS+depo did not induce LTP. E–G, bar graphs for all synapses tested with inward/intermediate rectification (n= 8, filled bars) and for all synapses recorded with linear I–V relations (n= 6; open bars). E, average EPSCs including failures before and 30 min after TBS+depol. F, average EPSCs excluding failures (potency) before and 30 min after TBS+depol. G, percentage failure rate of evoked EPSCs before and 30 min after TBS+depol.
Afferent-specific properties of excitatory synapses
Overall, the previous series of results indicate that OA-INs receive two different types of excitatory synapses. Could these synapses represent two distinct sources of afferents onto single OA-INs? It is well known that CA1 OA-INs receive excitatory inputs from both feedback axon collaterals of CA1 pyramidal cells and feedforward afferents from the Schaffer collaterals of CA3 pyramidal neurons (Lacaille et al. 1987; Scanziani et al. 1998). In initial attempts to answer this question, biocytin labelling of OA-INs revealed that the two types of EPSCs (with inward/intermediate rectification or linear I–V relations) were observed in the same morphological subtypes of OA-INs, cells with horizontally oriented dendrites and axon in stratum oriens (horizontal interneurons) (Fig. 5A and D) and cells with an axon projecting to stratum lacunosum-moleculare (O-LM interneurons) (Fig. 5B and E). Likewise, while recording from a single OA-IN, it was possible to induce the two types of EPSCs by changing the location of the stimulation electrode (Fig. 5C and F). In cells that showed changes in responses (n= 6 of 12 cells), the RI of EPSCs was significantly different for the first and second stimulation sites (RI = 1.21 ± 0.05 vs. 0.36 ± 0.03, respectively; P < 0.05) whereas in the remaining cells the RI of responses was not different (RI = 0.30 ± 0.10 vs. 0.22 ± 0.09, respectively; P= 0.58). Thus, the two types of EPSCs appear to converge on single OA-INs.
Figure 5. Two types of EPSCs in single interneurons of multiple subtypes.

A and B, camera lucida drawings of biocytin-labelled interneurons from which EPSCs with inward/intermediate rectification were recorded (top traces; RI = 0.2 and 0.25, respectively for A and B), showing different interneuron subtypes with horizontally oriented soma and dendrites in stratum oriens, and axons either arborizing in stratum oriens, characteristic of horizontal subtypes of interneurons (A), or projecting to stratum lacunosum-moleculare, characteristic of O-LM interneurons (B). C, representative example of responses evoked at different membrane potentials recorded from a single cell. Minimal stimulation at a first site (top) evoked EPSCs (left) that showed an inwardly rectifying I–V relation (RI = 0.2; right). Stimulation at a second site (bottom) elicited EPSCs that also displayed an inwardly rectifying I–V relationship (RI = 0.1). D and E, examples of two other biocytin-filled interneurons with dendritic and axonal arborization patterns of horizontal (D) and O-LM (E) cells, from which EPSCs with linear I–V relation (RI = 1.2; RI = 1.5, respectively for D and E) were recorded. F, example of EPSCs recorded in a single interneuron evoked from two different stimulation sites which demonstrated inward rectification (top) and linear I–V relations (bottom), respectively (RI = 0.2 at first stimulation site; RI = 1,38 at second stimulation site). alv., alveus; ori., oriens; pyr., pyramidale; rad., radiatum; l.-m., lacunosum-moleculare.
To directly test the distinct origins of the two types of synapses, dual whole cell recordings between presynaptic CA1 or CA2/3 pyramidal neurons and postsynaptic CA1 OA-INs were performed in hippocampal slice cultures (Scanziani et al. 1998). Experiments with paired recordings were conducted in organotypic slice cultures to improve the yield of successful synaptic connections, especially between CA2/3 pyramidal neurons and CA1 OA-INs (Scanziani et al. 1998). Latencies between the peak of presynaptic action potentials and the peak of the first derivative of EPSCs varied by less than ±1 ms, suggesting that connections between cell pairs were monosynaptic (Scanziani et al. 1998). We characterized the EPSCs originating from the two pathways in terms of sensitivity to the CP-AMPAR antagonist PhTX, modulation by mGluR2/3, rectification properties and short-term plasticity. In all CA1 pyramidal cell–OA-IN synaptic connections tested, PhTX (1 μm) decreased EPSC amplitude (to 35.4 ± 5.6% of control; n= 5 pairs; Fig. 6A and G). In contrast, in all CA2/3 pyramidal cell–OA-IN synapses tested, PhTX did not affect EPSCs (amplitude 94.9 ± 0.8% of control; n= 4 pairs; Fig. 6D and G). After washout of PhTX, we determined the sensitivity of EPSCs to the mGluR2/3 agonist DCG-IV. In CA1 pyramidal cell–OA-IN pairs, EPSCs were depressed by DCG-IV (1 μm; to 15.4 ± 3.3% of control; Fig. 6A and G), whereas they were unaffected (90.7 ± 0.7% of control; Fig. 6D and G) in CA2/3 pyramidal cell–OA-IN pairs. These results indicate that CA1 pyramidal cell feedback excitatory synapses involve CP-AMPARs and are modulated by presynaptic mGluR2/3, whereas CA2/3 pyramidal cell feedforward connections implicate CI-AMPARs and are not modulated by presynaptic mGluR2/3.
Figure 6. Afferent-specific properties of excitatory synapses from CA2/3 and CA1 pyramidal cells.

A and D, schematic representation of recording arrangements (top) and EPSCs from CA1 OA-INs in paired whole cell recordings with presynaptic CA1 (A–C; blue traces) or CA2/3 (D–F; red traces) pyramidal cells. Average responses (n= 100 traces) of presynaptic action potentials and OA-IN EPSCs taken at time points indicated by numbers (1–4) in the graph (below) of EPSC amplitude over time. In CA1 pyramidal cell–OA-IN pairs (A), EPSCs were reversibly antagonized by PhTX (1 μm); after washout of PhTX, DCG-IV (1 μm) caused a depression of EPSCs. B and C, in the same cell pair, EPSCs displayed an I–V relationship with inward rectification (B; RI = 0.2; blue) and showed facilitation (C; average of 15 traces; blue) during repeated stimulation (5 pulses at 20 Hz). In CA2/3 pyramidal cell–OA-IN pairs (D), EPSCs were not affected by PhTX (1 μm) nor by DCG-IV (1 μm). E and F, in the same cell pair, EPSCs exhibited a linear I–V relation (E; RI = 1.5; red) and underwent depression (F; red) during 20 Hz stimulation. G–I, summary bar graphs for all cell pairs tested, illustrating the different properties of EPSCs originating from CA1 (blue) and CA2/3 (red) pyramidal cells with respect to contributions of CP-AMPARs and modulation by mGluR2/3 (G; n= 5 CA1 PYR/INT and 4 CA2/3 PYR/INT pairs), I–V relationships (H; n= 5 CA1 PYR/INT and 5 CA2/3 PYR/INT pairs), and plasticity during 20 Hz stimulation (I; n= 5 CA1 PYR/INT and 4 CA2/3 PYR/INT pairs).
We also assessed the I–V relation and short-term plasticity of excitatory connections in the two afferents. Consistent with their sensitivity to PhTX, EPSCs of CA1 pyramidal cell–OA-IN pairs were inwardly rectifying (RI = 0.15 ± 0.02; n= 5; Fig. 6B and H). In contrast, EPSCs of CA2/3 pyramidal cell–OA-IN pairs displayed a linear I–V relationship (RI = 1.5 ± 0.13, n= 5; Fig. 6E–H) consistent with their insensitivity to PhTX. Moreover, at a holding potential of −60 mV, repetitive presynaptic stimulation (five action potentials evoked in current-clamp by current pulses at 20 Hz) evoked EPSCs that exhibited facilitation in CA1 pyramidal cell–OA-IN pairs (Fig. 6C). For all pairs, facilitation reached 254.4 ± 25.7% at the end of the train (EPSC5/EPSC1; n= 5; Fig. 6I). In contrast, repetitive stimulation of CA2/3 pyramidal cell–OA-IN pairs produced depression of EPSCs (Fig. 6F), attaining 30.2 ± 6.7% of initial responses (EPSC5/EPSC1; n= 4; Fig. 6I). Biocytin labelling confirmed the identity of pre- and postsynaptic cells (Fig. 7). Biocytin labelled axons originating from CA2/3 neurons coursed in strata oriens and radiatum of CA1, as well as in CA3, thus confirming the feedforward nature of these connections to CA1 OA-INs. In contrast, CA1 pyramidal cell axons projected to CA1 stratum oriens and alveus. Examples in Fig. 7 illustrate biocytin-filled cell pairs and the inwardly rectifying I–V relation for EPSCs of a CA1 pyramidal cell–OA-IN pair (RI = 0.16; Fig. 7A) and the linear I–V relation for EPSCs of a CA2/3 pyramidal cell–OA-IN pair (RI = 1.4; Fig. 7B). Overall, this series of results directly demonstrate the afferent-specific properties of excitatory synapses onto OA-INs: feedback synapses from CA1 pyramidal cells are composed of CP-AMPARs and show inward rectification, short-term facilitation and presynaptic modulation by mGluR2/3; feedforward synapses from CA2/3 pyramidal cells are composed of CI-AMPARs and exhibit a linear I–V relationship, short-term depression and no modulation by mGluR2/3. These findings indicate that the afferent-specific properties of CA1 and CA2/3 pyramidal cell synapses onto OA-INs are likely to underlie the two distinct types of OA-IN EPSCs found in acute slices.
Figure 7. Biocytin labelling of synaptically connected cell pairs.
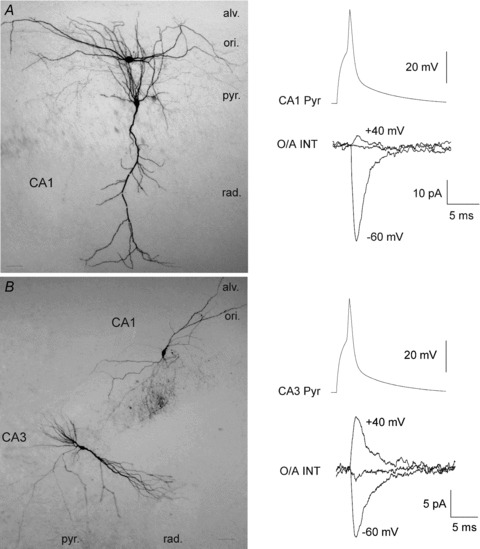
A, image of a biocytin-labelled CA1 pyramidal cell–CA1 OA-IN pair (left), and EPSCs evoked in the postsynaptic interneuron showing inward rectification (RI = 0.1; right). B, example of a biocytin-labelled CA2/3 pyramidal cell–CA1 OA-IN pair and EPSCs exhibiting a linear I–V relation (RI = 1.4). al., alveus; ori., oriens; pyr., pyramidale; rad., radiatum. (Scale bars = 50 μm).
Input–output relations of OA-INs
Thus far, our results have uncovered that CA1 and CA2/3 inputs to OA-INs have different synaptic mechanisms and long-term plasticity. To determine if these synaptic differences are reflected in interneuron integration and output firing, we performed cell-attached recordings in acute slices and examined the temporal pattern of OA-IN discharge in response to series of synaptic stimulation. Consistent with previous reports (Pouille & Scanziani, 2004), stimulation of the oriens-alveus region with series of 10 stimuli at 20 Hz revealed two distinct response patterns in OA-INs: (a) onset-transient responses characterized by a high firing probability evoked by the first two stimuli followed by a reduction in firing probability during the train (n= 4; Fig. 8B); and (b) late-persistent responses showing no initial firing and a gradual increase in firing probability that reached asymptote by the third stimulus (n= 14; Fig. 8C). Such responses patterns were previously reported to arise from target cell-dependent synaptic mechanisms in CA1 feedback pathways to somatic versus dendritic projecting cells, respectively (Pouille & Scanziani, 2004). Our results suggest that, in addition, these distinct discharge patterns can also arise from activation of different afferents with distinct synaptic mechanisms onto OA-INs.
Figure 8. Input–output function of interneurons and theta-burst stimulation (TBS)-induced increases in firing probability.

A, diagram of stimulation protocol before, during and after TBS. B, 10 consecutive onset-transient responses recorded in cell-attached mode before (a) and 30 min after (b) TBS. Plot of average response probability (c) as a function of stimulus number for the population of onset-transient responses (black trace), showing the characteristic transient high firing probability at onset followed by decrease in firing during subsequent stimulations. Firing probability during onset-transient responses was not affected by TBS (grey trace). C, examples of OA-IN late-persistent responses before (a) and 30 min after (b) TBS. Plot of average response probability to each stimulus for the population of late-persistent responses (c) revealing the initial low firing and progressive increase in response probability that reaches a plateau by the 3rd to 4th stimulus (black line). TBS produced an upward shift in the response probability curve (grey line), indicating a long-term potentiation of firing probability. D, summary bar graph of average firing probability for late-persistent responses at 30 min (normalized to pre-TBS firing) for all cells that received no TBS (n= 6), TBS in ACSF (TBS; n= 14), or TBS in the presence of LY367385 (100 μm; n= 3). The mGluR2/3 agonist DCG-IV reduced average response probability of late-persistent responses (n= 6). E and F, in a different group of cells (n= 25), stimulation location was changed 3–5 times to test whether the response pattern was dependent on the stimulation location. In 5 of 25 cells (E), varying the stimulation location transformed an initial late-persistent (grey trace) into an onset-transient (black trace) response pattern. At right are shown 10 overlaid traces illustrating the two types of responses in the same cell evoked by stimulus trains (black arrows) delivered at the first (above) and second (below) locations. In 20 of 25 cells (F), changing the stimulation location produced no observable change in the response pattern. In E and F, ∼50% of cells were recorded in ACSF with or without AP-5 and both types of responses were observed in the two conditions, so data were pooled.
Moreover we found that both types of discharge patterns could be evoked in the same OA-IN by stimulation of different sites (Fig. 8E and F). In these experiments, 13 cells were tested in ACSF containing AP-5 and 12 in normal ACSF. Since the two different response patterns were observed in both conditions this indicated that onset-transient and late-persistent responses are independent of NMDA receptor blockade and present with intact NMDAR-mediated synaptic transmission (see also Pouille & Scanziani, 2004); thus, these data were pooled. In 5 of the 25 cells (2 in ACSF with AP-5 and 3 in ACSF without AP-5), onset-transient and late-persistent responses were evoked in the same OA-IN from two distinct stimulation sites (firing probability significantly different at the beginning and at the end of the stimulation trains; Fig. 8E; repeated measures ANOVA, P < 0.05, n= 5 of 25). In the other 20 cells, late-persistent response patterns were similarly evoked from both stimulation sites (Fig. 8F). Thus, both response patterns were elicited in the same OA-IN and late-persistent responses were evoked most often, suggesting that this synaptic input and response pattern may be predominant. That onset-transient and late-persistent responses may be due to activation of distinct afferents onto OA-INs is also consistent with their pattern of short term plasticity: CA2/3 feedforward inputs show short term depression underlying onset-transient responses and CA1 feedback inputs undergo short term facilitation generating late-persistent responses.
We next examined if application of a TBS protocol to the alveus, known to produce lasting enhancement of pyramidal cell GABAA inhibition via mGluR1-dependent LTP of OA-INs synapses (Lapointe et al. 2004), would preferentially regulate late-persistent responses of OA-INs. After determining the basal response pattern, TBS was applied (see Fig. 8A). Analysis of firing probability during the TBS revealed that interneurons fired on average to 74.9 ± 7.9% of the stimuli, suggesting that the stimulation protocol induced Hebbian rather than anti-Hebbian plasticity. A comparison of responses before and 30 min after the TBS revealed no change in onset-transient responses (Fig. 8B; repeated measures ANOVA; n= 4). In contrast, late-persistent responses showed a significant increase in discharge probability (Fig. 8C; repeated measures ANOVA; n= 14). Interestingly, the overall pattern of response was not changed by the increase in firing probability, demonstrating that the late-persistent input–output function is maintained following LTP. The average latency of responses was also significantly reduced to 76.56 ± 8.27% of control at 30 min post-TBS. Omission of TBS resulted in stable response probabilities over the 30 min recording period (Fig. 8D; n= 6). Bath application of the mGluR1 antagonist LY367385 (100 μm) during the TBS protocol blocked the increase in late-persistent responses (Fig. 8D; n= 3), suggesting that the TBS-induced increase in late-persistent firing necessitated the activation of mGluR1. These results are consistent with the involvement of mGluR1-dependent LTP in CA1 feedback pathways onto OA-INs. Finally, we verified that synaptic pathways generating late-persistent responses were sensitive to mGluR2/3. Bath application of DCG-IV (1 μm) significantly reduced response probability during late-persistent responses (n= 6; Fig. 8D), confirming that synaptic pathways for late-persistent responses are modulated by presynaptic mGluR2/3, and thus likely to involve feedback inputs onto OA-INs. This series of results suggest that afferent-specific synaptic properties underlie different input–output functions in feedforward and feedback excitatory pathways onto OA-INs. Moreover, they indicate that afferent-specific plasticity leads to selective long-term regulation of feedback inhibitory networks via an mGluR1-dependent long-term increase in level without affecting spike train dynamics of OA-IN input–output function.
Discussion
Our main findings are that CA1 and CA2/3 PC afferents onto CA1 OA-INs have distinct synaptic properties, resulting in differential use-dependent plasticity and distinct integrative functions. Afferent-specific synaptic properties have important functional implications because many interneuron subtypes receive mixed feedforward and feedback inputs (Freund & Buzsaki, 1996). The consequences of afferent-specific mechanisms in inhibitory interneurons have been extensively studied in CA3 SL-INs. In these interneurons, synapses from CA3 PC feedback and dentate granule cell feedforward afferents are discriminated by their CI- and CP-AMPAR composition, respectively (Maccaferri et al. 1998; Toth et al. 2000). The selective targeting of different postsynaptic receptors to distinct afferent synapses confers pathway-specific computational properties. The short-term depression occurring at CI-AMPAR-containing synapses contributes to depression of pyramidal cell feedback inhibition, whereas the short-term facilitation at CP-AMPAR-containing synapses promotes facilitation of feedforward inhibition by SL-INs during repeated activation (Maccaferri et al. 1998; Toth et al. 2000). We show that OA-INs also possess afferent-specific mechanisms of transmission. However, the underlying rules are different, resulting in opposite dynamic properties for CA1 PC inhibition during repeated activation of OA-INs: facilitation of feedback inhibition and depression of feedforward inhibition. Thus afferent-specific synaptic properties in hippocampal interneurons are cell type-specific, which increases computational capacities in hippocampal inhibitory networks.
Interestingly, we observed that KARs also partake in transmission at identified synapses onto OA-INs. In fact, feedback pathways activate a major CP-AMPAR and a minor KAR component. At feedforward synapses, PhTX did not affect responses but GYKI-52466 did, and the residual response was reduced by CNQX, indicating that they activate a major CI-AMPAR and a minor KAR component. Our results confirm previous reports (Goldin et al. 2007; Cossart et al. 2002) of a role for KARs at stratum oriens interneuron synapses, but indicate a contribution of KARs at both feedforward and feedback synapses. Given that KARs play a role in tuning OA-INs to operate at theta frequency (Goldin et al. 2007), their participation in OA-IN synaptic plasticity relative to that of CP-AMPARs will be interesting to explore.
In addition to the differences in afferent-specific properties of interneuron synapses, the type of afferent-specific long term synaptic plasticity differs between interneurons. Following high frequency stimulation, feedforward synapses onto SL-INs show long-term depression and previously depressed feedforward synapses display LTP (Pelkey et al. 2005). Whereas, feedback synapses from CA3 pyramids onto SL-INs are unaffected by high-frequency stimulation (Maccaferri et al. 1998). The afferent-specific long term plasticity uncovered in OA-INs differs in two ways. First, feedback synapses onto OA-INs develop long-term plasticity, whereas feedforward synapses are unaffected. So, the type of afferent showing plasticity differs. Second, the direction of change is opposite. OA-IN synapses develop long-term potentiation after pairing TBS and depolarization. Since both LTD (McMahon & Kauer, 1997) and LTP (Lamsa et al. 2005) have been observed in CA1 interneurons of stratum radiatum following tetanization, it will be interesting to examine how afferent-specific (Schaffer collateral vs. temporo-ammonic pathway) and cell-specific properties contribute to the different synaptic plasticity in these interneurons.
Our data suggest that multiple subtypes of OA-INs receive inputs from two distinct afferent sources. We did not find a consistent relation between the location of the stimulation electrode and the type of synaptic response elicited, indicating a lack of location dependency of the effect in our conditions. This may be due to the horizontal arrangement of feedforward and feedback afferent pathways in stratum oriens-alveus with both pathways potentially recruited by local electrical stimulation. Morphological identification using biocytin labelling confirmed that OA-INs included in our study corresponded to O-LM and other interneuron types with horizontally oriented dendrites and axons in stratum oriens (see Fig. 5). Thus the rules of afferent-specific mechanisms of transmission and plasticity uncovered here are not unique to a single interneuron type but hold for multiple OA-IN subtypes with terminal fields covering distal (O-LM interneurons) and more proximal (horizontal interneurons) dendrites of CA1 pyramidal cells (Freund & Buzsaki, 1996). It should be emphasized that both afferent specificity and target cell identity may be involved in the properties of the observed responses since we did not examine the detailed anatomical identification of each recorded interneuron (e.g. axonal targeting, immunohistochemistry). However, our results clearly reveal that two distinct input pathways with afferent-specific properties converge on individual OA-IN subtypes (Figs 5 and 8). Consistent with previous reports that axons of CA3 and CA1 pyramidal neurons course in stratum oriens (Ishizuka et al. 1990; Blasco-Ibanez & Freund, 1995; Wittner et al. 2007), we have identified two distinct inputs to OA-INs; that is, both CA1 and CA2/3 PCs excite OA-INs. Overall, our observations of the relative incidence of each type of synaptic inputs suggest that feedback pathways may be more predominant than feedforward inputs to these cells, as previously reported (Blasco-Ibanez & Freund, 1995). Nevertheless, our findings indicate that, despite a less-pronounced incidence, feedforward CA2/3 synaptic inputs are present onto OA-INs and are capable of influencing their activity in a pathway-specific manner.
It should be noted that our dual whole-cell recording experiments were performed in organotypic slice cultures. Although the local connectivity between CA1 principal cells and interneurons is well preserved in acute slices (Ali et al. 1998), the probability of obtaining long-range CA2/3 to CA1 connectivity is improved in organotypic slice cultures (Debanne et al. 1995; Scanziani et al. 1998). Thus, it would be important to verify our findings with dual recordings in acute slices. Despite this caveat, our results with dual recordings in slice cultures are consistent with those in acute slices showing two afferent inputs with distinct properties converging onto CA1 OA-INs.
Our results indicate that OA-INs perform a dual function in the hippocampal network. First, sustained activity in CA1 pyramidal cell afferents enhances the inhibitory output of OA-INs (Lacaille et al. 1987). This is unlikely to be due to changes in overall excitability before and after TBS because the increased spiking reported here is afferent specific and because the presence of AP-5 in our recording conditions rules out possible NMDA-dependent EPSP-spike potentiation (Marder & Buonomano, 2004). Thus, the inhibitory impact on distal dendrites increases as pyramidal cell activity levels rise, an effect that is in part due to the late-persistent mode of OA-IN firing (Pouille & Scanziani, 2004). We found that late-persistent firing of OA-INs develops an mGluR1-dependent long-term increase following theta burst activity. The overall firing pattern remains unchanged, suggesting that the incremental dendritic feedback inhibition during sustained activity is preserved. Therefore, LTP at CA1 PC feedback synapses onto OA-INs increases their input–output function, resulting in increased level of recurrent inhibition. The fact that response delays are reduced suggests that LTP at these synapses may also enhance temporal fidelity in feedback inhibitory circuits.
Feedforward inhibition of CA1 pyramidal cells is another major function of interneurons. It is more pronounced at the soma than in distal dendrites and restricts temporal summation of excitatory inputs in pyramidal neurons to a narrow time window (<2 ms; Pouille & Scanziani, 2001). LTP of excitatory inputs to CA1 feedforward interneurons maintains this synchronous activation of pyramidal inputs in the event of LTP at pyramidal cell synapses (Lamsa et al. 2005). Since our results suggest that feedforward afferents to OA-INs do not develop LTP, temporal fidelity is mostly maintained by synaptic plasticity in other types of feedforward interneurons such as those in stratum radiatum (Lamsa et al. 2005). These findings further highlight that afferent-specific synaptic plasticity in interneurons is dependent on cell type.
Congruent with previous reports using whole-cell recordings (Perez et al. 2001; Lapointe et al. 2004), the loose cell-attached recordings performed here show that OA-INs readily develop Hebbian LTP. Loose cell-attached recordings preserve the intracellular milieu, thus ruling out possible artefactual effects of cytoplasmic washout. Moreover, recordings during the theta-burst stimulation confirmed that a majority of pulses in each theta-burst produce supra-threshold depolarization and associated spikes. Thus, under these conditions, OA-INs demonstrate contiguous pre- and postsynaptic activity, coherent with Hebbian rather than anti-Hebbian (Lamsa et al. 2007) LTP. Firing during TBS is also consistent with the relatively depolarized resting membrane potential of OA-INs (Lacaille & Williams, 1990) and single spikes in individual CA1 PCs producing unitary EPSPs in OA-INs that summate and trigger action potentials (Lacaille et al. 1987). These factors possibly account for predominant Hebbian LTP observed in these interneurons. However, at more hyperpolarized membrane potentials, anti-Hebbian LTP is uncovered (Lamsa et al. 2007). We did not attempt to induce anti-Hebbian plasticity in OA-INs, but both Hebbian and anti-Hebbian are likely to occur at OA-IN synapses, albeit under different conditions. Given the afferent specific properties we uncovered in the present study, including in morphologically identified O-LM interneurons which were previously reported to undergo anti-Hebbian LTP (Lamsa et al. 2007), it will be interesting to examine the afferent specificity of this type of long-term plasticity in these cells. At present, it is still unclear how and why these two forms of LTP co-exist. One possibility is that large network oscillations, such as slow wave sleep, generate instances where the membrane potential of hippocampal interneurons hyperpolarizes during neocortical down states (Hahn et al. 2006), and thus may create windows permitting anti-Hebbian LTP.
We found that OA-INs perform distinct functions that depend on afferent origin. Previous findings showed that distinct interneuron types generated different dynamic profiles of feedback inhibition: basket cells with perisomatic projections generated onset-transient responses and O-LM cells with dendritic projections produced late-persistent responses (Pouille & Scanziani, 2004). Our results indicate that distinct afferents to single OA-INs can perform such dual dynamic inhibitory functions: feedforward afferents generating onset-transient responses and feedback afferents producing late-persistent responses. Prolonged CA2/3 pyramidal cell firing produces an initially strong OA-IN activity that rapidly decreases. This suggests that CA2/3 spikes will likely produce monosynaptic excitation of CA1 pyramids, followed by powerful disynaptic inhibition. The consequences of CA1 pyramidal cell activity will be different. Sustained local CA1 pyramidal cell activity leads to a gradual increase in inhibition (Pouille & Scanziani, 2004) that may favour low frequency activity. Following global increases in CA1 activity, LTP at OA-IN synapses may prevent bursting and may maintain temporal fidelity of transmission between pyramidal cells (see Fig. 9). Recent evidence suggests that OA-IN firing relative to CA1 principal neurons is modulated as a function of hippocampal theta and ripple oscillations (Klausberger and Somogyi, 2008). Inasmuch as firing patterns are preserved, perhaps OA-IN LTP can enhance the level of inhibition without modifying the phase-locking of hippocampal neurons to oscillations.
Figure 9. Afferent-specific long-term regulation of OA-IN output function.
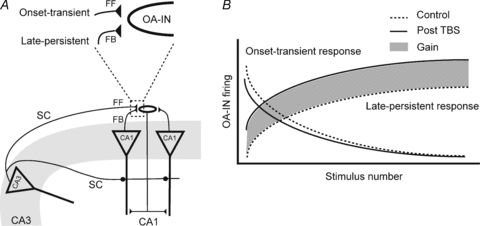
A, diagram showing two CA1 pyramidal neurons with feedback projections (FB) to a single OA-IN, and a CA2/3 pyramidal cell with Schaffer collateral projections to the CA1 pyramidal cells and OA-IN (feedforward; FF). Above, enlargement of indicated region illustrating OA-IN dual afferents: CA2/3 feedforward inputs (generating onset-transient responses), and feedback inputs (producing late-persistent responses). B, graph illustrating onset-transient and late-persistent OA-IN firing as a function of repetitive synaptic activation. Under normal conditions of repetitive activation of selective afferents (control, dotted lines) OA-IN output will consist of onset-transient responses (FF afferents) or late-persistent responses (FB afferents). After theta burst stimulation (continuous lines), LTP is induced at FB afferent synapses and there is an increase in late-persistent responses. The area shaded in grey represents the total gain of function in feedback inhibition due to LTP at OA-IN synapses.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (to J.-C.L; MOP-10848) and the Fonds de la Recherche en Santé du Québec (GRSNC). J.-C.L. is the recipient of the Canada Research Chair in Cellular and Molecular Neurophysiology. A.C. and M.T. were supported by postdoctoral fellowships of the Groupe de Recherche sur le Système Nerveux Central (GRSNC) and J.G.P. from the FRSQ. We thank France Morin for comments on a previous version of the manuscript.
Glossary
Abbreviations
- CI
Ca2+ impermeable
- CP
Ca2+ permeable
- LTD
long-term depression
- LTP
long-term potentiation
- OA-IN
oriens/alveus interneuron
- PC
pyramidal cell
- SL-IN
stratum lucidum interneuron
- TBS
theta-burst stimulation
Author contributions
All experiments were carried out in the Department of Physiology at Université de Montréal. J.C.L. conceived and supervised the project. J.C.L., A.C. and J.G.P. designed the research. A.C., J.G.P. and M.T. performed the experiments, analyzed the data and prepared illustrations. All authors participated in writing the manuscript and approved the final version of the manuscript for publication.
References
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbig A, Traub RD, Whittington MA. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88:1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: structural basis of feed-back activation. Eur J Neurosci. 1995;7:2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Lacaille JC. Intrinsic theta-frequency membrane potential oscillations in hippocampal CA1 interneurons of stratum lacunosum-moleculare. J Neurophysiol. 1999;81:1296–1307. doi: 10.1152/jn.1999.81.3.1296. [DOI] [PubMed] [Google Scholar]
- Cossart R, Epsztein J, Tyzio R, Becq H, Hirsch J, Ben-Ari Y, Crepel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crepel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci. 2007;27:9560–9572. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat Neurosci. 2006;9:1359–1361. doi: 10.1038/nn1788. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Itazawa SI, Isa T, Ozawa S. Inwardly rectifying and Ca2+-permeable AMPA-type glutamate receptor channels in rat neocortical neurons. J Neurophysiol. 1997;78:2592–2601. doi: 10.1152/jn.1997.78.5.2592. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Williams S. Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience. 1990;36:349–359. doi: 10.1016/0306-4522(90)90431-3. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vasseur M, Ran I, Lacaille JC. Selective induction of metabotropic glutamate receptor 1- and metabotropic glutamate receptor 5-dependent chemical long-term potentiation at oriens/alveus interneuron synapses of mouse hippocampus. Neuroscience. 2008;151:28–42. doi: 10.1016/j.neuroscience.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fibre plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Marder CP, Buonomano DV. Timing and balance of inhibition enhance the effect of long-term potentiation on cell firing. J Neurosci. 2004;24:8873–8884. doi: 10.1523/JNEUROSCI.2661-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A Hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol. 2000;528:123–130. doi: 10.1111/j.1469-7793.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Raastad M. Extracellular activation of unitary excitatory synapses between hippocampal CA3 and CA1 pyramidal cells. Eur J Neurosci. 1995;7:1882–1888. doi: 10.1111/j.1460-9568.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proc Natl Acad Sci U S A. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Toth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fibre synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther. 1996;278:669–678. [PubMed] [Google Scholar]
- Wittner L, Henze DA, Zaborszky L, Buzsaki G. Three-dimensional reconstruction of the axon arbor of a CA3 pyramidal cell recorded and filled in vivo. Brain Struct Funct. 2007;212:75–83. doi: 10.1007/s00429-007-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


