Abstract
Vascular changes associated with brain functions are thought to be tightly coupled with neuronal activity through neuronal glucose consumption or the local release of vasoactive agents. In contrast, another view suggests that cortical blood flow is strongly regulated by the nucleus basalis of Meynert (NBM), independently of regional metabolism. Thus, although cortical regional cerebral blood flow (rCBF) variations induced by somatosensory stimulation are strongly linked to neuronal activity, they may also be partly controlled by the NBM. In the present study, cortical rCBF alterations in response to innocuous brushing of the hindlimb (HL) were investigated by laser speckle contrast imaging. The contribution of NBM to these changes was examined after injection of the GABAergic agonist muscimol into the right NBM, allowing comparison of somatosensory-evoked cortical rCBF modifications before and after NBM inactivation. As expected, HL brushing elicited a robust rCBF increase in the contralateral parietal cortex (PC), over the representation of the HL. However, these alterations were decreased, by approximately 40%, in the hemisphere ipsilateral to muscimol inactivation of NBM, whereas vehicle injection did not produce any significant variation. The results demonstrate that cortical rCBF changes induced by somatosensory stimulation are partly regulated by NBM.
Introduction
The link between neuronal activity and cerebral blood flow was discovered by the Italian physiologist Angelo Mosso and was studied for the first time by Roy and Sherrington in the late 19th century (Roy & Sherrington, 1890). Since then, the mechanisms of neurovascular coupling have been widely investigated but are still not completely understood.
Vascular changes associated with brain functions are thought to be connected to neuronal activity through neuronal glucose consumption, which, in turn, increases regional cerebral blood flow (rCBF; Magistretti, 2006; Raichle & Mintun, 2006). Neuronal activity can also elicit blood flow variations directly, through the local release of vasoactive agents, such as nitric oxide (Cauli et al. 2004; Rancillac et al. 2006). In either case, rCBF changes are strongly coupled with local neuronal activity.
In contrast, another view suggests that cortical blood flow is strongly regulated by the nucleus basalis of Meynert (NBM; Biesold et al. 1989; Adachi et al. 1992; Lacombe et al. 1997; Vaucher et al. 1997). The NBM sends diffuse cholinergic projections to the cortex (Kurosawa et al. 1989; Sato & Sato, 1990; for review see Sato & Sato, 1995), enabling cortical blood flow control independently of regional metabolism (Kimura et al. 1990; Hallström et al. 1990). Thus, although cortical rCBF changes induced by somatosensory stimulation are robustly associated with neuronal activity, they may also be partly regulated by the NBM. Consistent with this idea, most NBM neurons projecting to the ipsilateral parietal cortex (PC) are recruited by noxious mechanical stimulation of the fore- or hindlimb (HL) skin in rats (Akaishi et al. 1990), and such stimulation also evokes rCBF alterations that are partly independent of variability in blood pressure (Adachi et al. 1990). In both these studies, NBM neuronal activity and associated rCBF changes were elicited by noxious stimulation, whereas innocuous mechanical stimulation of the skin, such as 15–20 s of brushing at a frequency of 1 Hz, was ineffective in modulating NBM responses (Akaishi et al. 1990) or cortical rCBF (Adachi et al. 1990). In contrast, subsequent experiments showed that the same innocuous skin stimulation for 10 min produced a significant increase in cortical acetylcholine (ACh) release (Kurosawa et al. 1992) that would evoke changes in cortical blood flow. These results indicate that stimulus duration and temporal summation may be critical factors in augmenting rCBF by innocuous stimulation.
Altogether, such studies suggest that cortical rCBF alterations induced by somatosensory stimulation are coupled with local neuronal activity but that NBM activation also underlies part of these changes. To explore this possibility, the present investigation aimed to determine whether somatosensory-evoked cortical rCBF variations could be modulated by pharmacological inactivation of the NBM. To that end, rats were anaesthetized with urethane, and changes in cortical rCBF were measured by laser speckle contrast imaging, in response to innocuous HL brushing. Alterations in rCBF were then compared in three sets of conditions: (1) innocuous brushing; (2) innocuous brushing with control (vehicle) injection into the NBM unilaterally; and (3) innocuous brushing with injection of the GABAA agonist muscimol into the NBM unilaterally. We hypothesized that GABAergic inhibition of the NBM by muscimol would partly decrease somatosensory-evoked rCBF changes in the cortex, according to the previously described role of NBM in rCBF regulation.
Methods
General experimental conditions
Experiments were performed on nine adult male Wistar rats (body weight 330–430 g) bred at the Tokyo Metropolitan Institute of Gerontology. The study was conducted with the approval of and in accordance with the guidelines for animal experimentation prepared by the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Gerontology.
Surgical procedures were initiated after the animals had been deeply anaesthetized with urethane (1.1–1.2 g kg−1i.p.). The right jugular vein was catheterized, and additional urethane doses were administered by bolus i.v. injection (approximately 10% of the initial dose) to maintain the depth of anaesthesia. In addition to stable systemic arterial blood pressure, the depth of anaesthesia was routinely confirmed by the absence of withdrawal (paw and tail pinching) and corneal reflexes. Systemic mean arterial pressure (MAP) was recorded continuously from a canula (AT601-G, Nihon Kohden, Tokyo, Japan) inserted into the right axillary artery. The animals were also artificially ventilated via a tracheal canula (model SN-480-7, Shinano, Tokyo, Japan). End-tidal CO2 concentration was maintained at 3.0% during surgical preparation and was increased to 4% for experimentation (Microcap, Oridion Medical, Jerusalem, Israel) by controlling respiratory rate and tidal volume. Body temperature was monitored with a rectal probe and maintained at approximately 37.0°C with a body temperature control system (ATB-1100, Nihon Kohden) during surgical preparation, and elevated to 37.5°C for experimentation.
Laser speckle contrast imaging
The animals were placed in a prone position, and their head was fixed with ear bars in a stereotaxic frame. The skull was made transparent with a dental drill, allowing the visualization of rCBF changes through the bone and leaving the meninges and cerebrospinal fluid intact. The laser speckle contrast imaging device was then fixed, and the zoom was adjusted to cover the dorsal surface of the brain from the most anterior part of the olfactory bulbs to the most posterior aspect of the occipital cortex with polarizer lens carefully adjusted to minimize speckle reflection.
Laser speckle contrast imaging was performed with a Moor full-field perfusion imaging device consisting of an infrared laser diode (785 nm wavelength) and a CCD camera (Moor Instruments, Axminster, UK). The viewing field covered about 300 mm2 (20 mm × 15 mm) with a matrix of 760 × 568 pixels, giving an approximate resolution of 26.4 μm per pixel. Images were sampled at 1 Hz with an exposure time of 4 ms. Online averaging generated one mean image every 3 s. These images were acquired continuously during the whole trial, providing 100 images over a period of 5 min.
Stimulation protocol
Innocuous stimulation, with a soft-hair brush at a frequency of 3 Hz on the posterior aspect of the HL covering the area from the buttock to the sole of the paw, was delivered for 3 min preceded by 1 min of baseline recording and followed by 1 min of recovery. Brushing was performed manually and was paced with an auditory cue. This protocol was undertaken three times for each HL and for each set of conditions: control, vehicle injection and muscimol injection. For vehicle and muscimol injections, the trials were performed 30 min postinjection to allow diffusion of the solution and to minimize mechanical distension effects.
In a preliminary experiment, brushing was continuous for 15 min at a frequency of 1 Hz in three rats. Although the rCBF and blood pressure changes were similar to those induced by 3 Hz stimulation, rCBF alterations occurred with longer latency, usually after 2 min or more (see Results). Therefore, 3 Hz stimulation was chosen for the following experiments to minimize the length of the experimental session.
Pharmacological NBM inactivation
A 1 μl Hamilton microsyringe (7001-N, Hamilton, Reno, NV, USA) was oriented caudally over the frontal cortex at a 30 deg angle relative to the coronal plane. A small hole was made in the skull by dental drill to allow penetration of the needle and the injection of solutions into the right NBM, located 1.4 mm posterior, 2.5 mm lateral and 7.6 mm ventral to bregma (Paxinos & Watson, 1986). This approach was chosen to avoid potential lesions of the thalamocortical afferents localized more posteriorly. The experimental injection delivered 60 ng muscimol (Alexis Biochemicals, San Diego, CA, USA) in 300 nl phosphate-buffered saline solution. The control (vehicle) injection consisted of 300 nl phosphate-buffered saline solution. Injections were given at a rate of 60 nl min−1 to minimize the distension/compression of brain tissue. In addition, 300 nl of Evans Blue dye was administered at the end of the experiment to localize the injection site. Rats were killed by injecting an overdose of pentobarbital. The brains were removed, fixed by formalin, and then placed in a 30% sucrose solution. The position of the needle was verified histologically on frozen coronal brain sections, cut to a thickness of 100 μm.
Data analyses
For the analysis of spatial changes in rCBF, mean images (see ‘Laser speckle contrast imaging’) were further averaged over 30 s time bins (10 images per time bin), leading to 10 averaged images. The first image of the series (baseline) was then subtracted from the nine subsequent images to assess relative rCBF changes.
To quantify temporal rCBF alterations (in arbitrary units, a.u.), time courses were extracted from the 10 averaged images (see above) in two regions of interest (ROI), including the PC over the representation of the HLs bilaterally. These time courses were the averaged signal over 30 s time bins. The values were then averaged across three trials (see ‘Stimulation protocol’) separately for each HL and each set of conditions (control, vehicle and muscimol). The averages were compared statistically.
Statistical analyses
All data, expressed as means ±s.e.m., followed a normal distribution and were therefore analysed by parametric tests. The rCBF changes evoked by brushing (3 min at 3 Hz and 15 min at 1 Hz) were assessed separately for each ROI by one-way repeated-measures ANOVA, followed by Dunnett's multiple comparison test. Two-way repeated-measures ANOVA followed by planned contrasts was also performed on peak rCBF changes in the control conditions for left and right HL stimulation, to gauge differences in rCBF alterations between hemispheres (left vs. right ROI).
Next, peak rCBF changes evoked by brushing of the left and right HLs in the different conditions (control, vehicle injection and muscimol injection) were appraised separately for each ROI by one-way repeated-measures ANOVA, followed by Dunnett's multiple comparison test. Two-way repeated-measures ANOVA, with planned contrasts, was also performed on peak rCBF changes elicited by brushing of the left and right HLs across conditions (control, vehicle injection and muscimol injection) to calculate differences between hemispheres (left vs. right ROI). For all analyses, homogeneity of variance and sphericity was assessed by Levene and Mauchley's test, respectively, and lack of sphericity was corrected with Greenhouse–Geisser and Huynh–Feldt procedures when appropriate. Values of P < 0.05 (two-tailed) were considered to be statistically significant for all analyses.
Results
Spatial and temporal rCBF changes with innocuous stimulation
Variations in rCBF and MAP induced by 3 min innocuous brushing of the HL at a frequency of 3 Hz were first measured in the control conditions (see individual example in Fig. 1). In the neocortex, rCBF was increased during brushing and declined slowly after the end of stimulation (Fig. 1A–D). In contrast, MAP was unchanged, indicating that rCBF modifications were not evoked by systemic blood pressure elevation (Fig. 1E). Blood flow changes were most obvious in the PC, contralateral to the stimulated HL (Fig. 1A–B). Nevertheless, blood flow variations were also detected ipsilaterally and contralaterally in the frontal, parietal and occipital areas (Fig. 1B). Importantly, rCBF alterations in the contralateral PC occurred within 10 s from the onset of stimulation (Fig. 1D). These changes were robust and reproducible in successive trials and across animals (Fig. 2).
Figure 1. Spatiotemporal changes in rCBF evoked by innocuous 3 Hz brushing for 3 min.
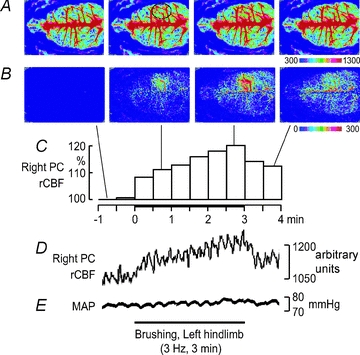
Individual example showing rCBF variations in the right PC induced by innocuous brushing of the contralateral left HL. A, averaged signal over selected periods of 30 s. B, differential signal change taken from A when subtracting the baseline signal from subsequent images. C, percentage signal change in the right PC averaged every 30 s during the 5 min of the trial (data extracted from the ROI indicated by the black circle in A). D, rCBF signal from the same ROI sampled at 1 Hz and temporally smoothed with a time constant of 1 s. E, MAP during the 5 min trial.
Figure 2. Group analyses of rCBF changes induced by innocuous 3 Hz brushing of left HL (A, C and E) and right HL (B, D and F) for 3 min.
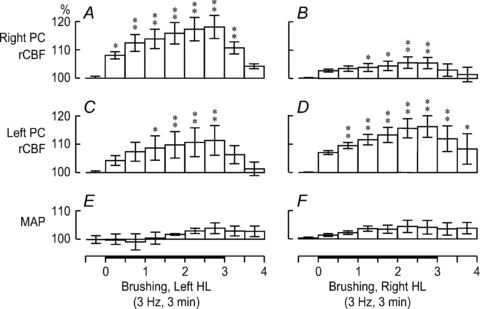
The bars show the rCBF variations during the 5 min trial averaged every 30 s in the right PC (A and B) and left PC (C and D). In E and F, slight changes in MAP were not significant. *P < 0.05, **P < 0.01.
Mean changes in MAP and rCBF in the right and left PCs elicited by 3 min brushing of the left and right HLs, respectively, at a frequency of 3 Hz were assessed by one-way repeated-measures ANOVA and Dunnett's multiple comparison test (see summary in Fig. 2). As illustrated in Fig. 2A (changes in right PC rCBF due to left HL stimulation) and D (changes in left PC rCBF due to right HL stimulation), brushing produced a robust rCBF increase in the contralateral PC from the first to the third minute (peak change: 118.1 ± 4.1%, P < 0.01, for the left HL and right PC, and 116.2 ± 3.8%, P < 0.01, for the right HL and left PC). In contrast, as shown in Fig. 2B (changes in right PC rCBF due to right HL stimulation) and C (changes in left PC rCBF due to left HL stimulation), the ipsilateral PC revealed significant but subtle rCBF alterations with right HL stimulation (peak change: 105.5 ± 2.0%, P < 0.01) and modest rCBF variations with left HL stimulation (peak change: 111.4 ± 5.2%, P < 0.01). Furthermore, two-way repeated-measures ANOVA of peak changes disclosed significant interaction between the hemisphere and the stimulated side (P < 0.001), indicating that rCBF modifications in the contralateral PC were significantly greater than those in the ipsilateral PC. Conversely, MAP was not significantly affected by brushing of either the right or left HL (P > 0.05), again indicating that rCBF changes cannot be explained by variations in systemic blood pressure (Fig. 2E and F).
Next, rCBF modifications induced by continuous 1 Hz brushing for 15 min were assessed by one-way repeated-measures ANOVA. As expected, this stimulation also produced significant rCBF alterations in the contralateral PC (peak change: 160.4 ± 14.1%, P < 0.01; Fig. 3A–C) and in the ipsilateral PC (peak change: 132.8 ± 4.7%, P < 0.01; Fig. 3A, B and D) without significant MAP variations (all P > 0.05; Fig. 3E). However, the onset of rCBF changes occurred with longer latency, usually after 2 min or more (Fig. 3), indicating that stimulus frequency can influence the temporal characteristics of rCBF modifications.
Figure 3. Spatiotemporal changes in rCBF evoked by innocuous 1 Hz brushing for 15 min.
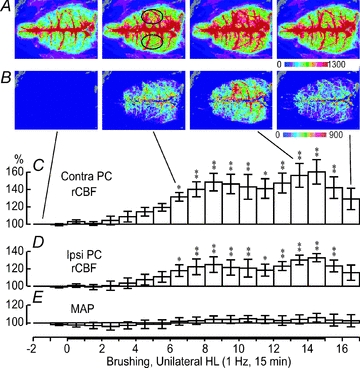
A, averaged signal over selected periods of 1 min during the whole trial. B, differential signal change taken from A when subtracting the baseline signal from subsequent images. C and D, percentage change in signal in the right and left PC averaged every 30 s when the left or right HL, respectively, was stimulated (data extracted from the ROI indicated by black circles in A). *P < 0.05, **P < 0.01. E, MAP changes averaged every 1 min. No significant variation was observed.
Effects of pharmacological NBM inactivation by muscimol
Mean variations in rCBF elicited by 3 min brushing, with vehicle or muscimol injections in the right NBM (Fig. 4A), were assessed by one-way repeated-measures ANOVA and Dunnett's multiple comparison test. In the right PC (ipsilateral to injections in the NBM), stimulation of the left contralateral HL after vehicle injection induced rCBF alterations comparable to those in the control conditions (peak change: 115.8 ± 3.8%, P < 0.01; Fig. 4B). In contrast, stimulation of the left contralateral HL after muscimol injection produced significant but attenuated rCBF modifications (peak change: 109.3 ± 4.5%, P < 0.01; Fig. 4C). With stimulation of the right ipsilateral HL, rCBF modifications were still significant after vehicle injection (peak change: 106.2 ± 1.9%, P < 0.001; Fig. 4D) but not after muscimol injection (non-significant main effect, P= 0.58; peak change: 103.8 ± 1.8%; Fig. 4E).
Figure 4. Effect of vehicle or muscimol injection into the right NBM on rCBF changes evoked by innocuous 3 Hz brushing.
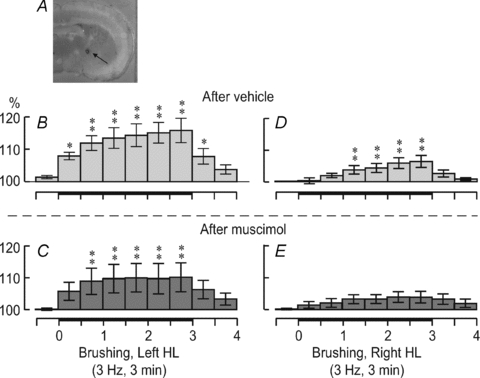
The NBM injection site is indicated by Evans Blue dye injected at the end of the experiment (A). The scale bar represents 2 mm. The histograms show rCBF changes in the right PC after vehicle injection (B and D) and after muscimol injection (C and E) with left HL stimulation (B and C) and right HL stimulation (D and E). *P < 0.05, **P < 0.01.
In the left PC, rCBF variations were comparable to those in the control conditions (peak values are shown in Fig. 5C and D). Indeed, stimulation of the right contralateral HL elicited significant rCBF alterations after vehicle injection (peak change: 115.0 ± 4.4%, P < 0.001) and after muscimol injection (peak change: 117.2 ± 6.2%, P < 0.001). Moreover, stimulation of the left ipsilateral HL also induced significant rCBF variations after vehicle injection (peak change: 118.3 ± 6.1%, P < 0.001) and after muscimol injection (peak change: 116.2 ± 7.4%, P < 0.001).
Figure 5. Effect of vehicle or muscimol injection into the right NBM on peak rCBF changes evoked by innocuous 3 Hz brushing.
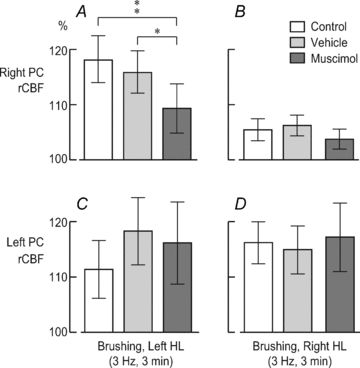
Muscimol significantly decreased rCBF in the right PC when the left HL was stimulated (A). *P < 0.05, **P < 0.01. No other significant effect was observed in the right PC (A and B). In the left PC, rCBF changes were not significantly influenced by the vehicle or muscimol after either left or right HL stimulation (C and D).
These results were subsequently corroborated by a two-way repeated-measures ANOVA of rCBF peak alterations (Fig. 5). Muscimol significantly inhibited stimulus-evoked rCBF changes in the right ipsilateral PC when the left HL was stimulated (P= 0.008; Fig. 5A). Moreover, this effect was specific to muscimol, since the vehicle did not significantly modulate rCBF variations (P= 0.45), and the rCBF changes elicited after muscimol were significantly smaller, by approximately 40%, than those induced after vehicle injection (P= 0.017; Fig. 5A). With stimulation of the right HL (Fig. 5B), rCBF modifications in the right PC were not affected by the vehicle but were slightly inhibited by muscimol, although this effect did not reach statistical significance (P > 0.05). In the left PC, somatosensory-evoked rCBF changes were not significantly affected by either vehicle or muscimol injection (all P > 0.05; Fig. 5C and D). All in all, these results clearly demonstrate GABAergic inhibition of stimulus-evoked rCBF alterations by muscimol with a specific effect on the PC ipsilateral to the injection.
In contrast, basal rCBF was not significantly affected by vehicle or muscimol injections in either hemisphere (P > 0.1; see Fig. 6). For the right PC, basal rCBF slightly declined following vehicle and muscimol injections, but these effects were not significant (Fig. 6A). For the left PC, basal rCBF was comparable between control conditions, vehicle injection and muscimol injection (Fig. 6B).
Figure 6. Effect of vehicle or muscimol injections into the right NBM on basal rCBF in the right PC (A) and left PC (B).
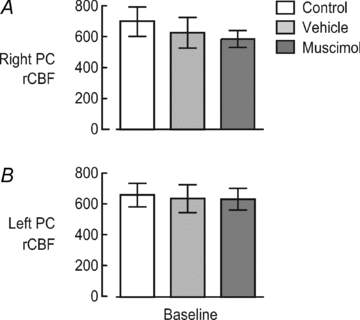
For the right PC, basal rCBF slightly but not significantly declined following vehicle and muscimol injections. Compared with basal rCBF after the vehicle injection, rCBF after the muscimol injection was decreased by only 6%. For the left PC, basal rCBF was comparable across conditions and showed no significant change.
Discussion
In the present study, somatosensory-evoked rCBF changes were investigated by high spatiotemporal resolution with laser speckle contrast imaging. The contribution of NBM to these variations was examined after injection of the GABAergic agonist muscimol into the right NBM. This allowed comparison of somatosensory-evoked rCBF changes over most of the neocortex before and after NBM inactivation. Innocuous brushing of the HLs induced widespread alterations in rCBF, including a robust increase in the contralateral PC, over the representation of the HL. The results further demonstrated that these changes were greatly decreased by GABAergic modulation of the ipsilateral NBM by muscimol. This is the first study to show that stimulus-evoked rCBF changes can be affected by NBM inactivation. We propose that stimulus-evoked rCBF changes are not only related to focal metabolic variations or to the local release of vasoactive agents as proposed in previous studies, but are also partly regulated by NBM as shown by pharmacological inactivation of NBM in the present study.
Stimulus-evoked rCBF changes
In the present study, 3 min brushing of the HLs at a frequency of 3 Hz was highly effective in increasing cortical rCBF, especially in the contralateral PC, over the representation of the HL. This is consistent with previous laser speckle contrast imaging experiments on rCBF changes associated with somatosensory stimulation in rats (Durduran et al. 2004; Dunn et al. 2005; Royl et al. 2006). In those investigations, a restricted area of the somatosensory cortex was imaged at very high spatial and temporal resolution to determine the characteristics of haemodynamic modifications and of neurovascular coupling during somatosensory stimulation. In the first of those studies, the magnitude of changes in somatosensory cortex rCBF was shown to scale with stimulus amplitude (Durduran et al. 2004). The experiments by Dunn et al. (2005) established that the metabolic oxygen consumption rate was spatially coupled to increased rCBF. The subsequent investigation by Royl et al. (2006) disclosed that rCBF changes were non-linearly dependent on neural activity, as measured by somatosensory-evoked potentials. The present work adds to these findings and shows that somatosensory stimulation induces widespread cortical rCBF modifications, including the PC, occipital and frontal cortex. Interestingly, rCBF variations in the PC peaked with shorter latency at higher stimulus frequency and were of greater amplitude with prolonged stimulation at lower stimulus frequency, reaching up to 60% of increase after 15 min. These characteristics are consistent with the temporal summation of haemodynamic responses associated with each brush stroke. Accordingly, a previous study, using electrical HL stimulation, demonstrated that the blood flow response in the PC, as measured by laser Doppler flowmetry, was influenced by stimulus duration and frequency (Ureshi et al. 2004). The present findings may, therefore, explain the results of a previous investigation, with innocuous brushing at a frequency of 1 Hz, showing no significant rCBF change in the cortex after 15 s (Adachi et al. 1990). In that study, stimulus frequency and duration may not have allowed sufficient summation of the haemodynamic responses to produce a significant modification of rCBF, as measured by laser Doppler flowmetry. The present results corroborate previous findings and emphasize that stimulus parameters greatly influence the characteristics of haemodynamic changes. From these data, the modulation of brushing-induced rCBF changes was assessed after GABAergic treatment of the NBM, to test its involvement in stimulus-evoked rCBF variations. The results of this experiment are discussed in the following section.
Regulation of stimulus-evoked rCBF changes by the NBM
The main objective of this study was to determine whether NBM plays a significant role in somatosensory-evoked rCBF changes. Considering the important GABAergic innervations of NBM (Gritti et al. 1993), its activity was modulated by muscimol injection before the brushing stimulus was applied. We demonstrated that the pharmacological inactivation of the right NBM by muscimol injection produced a large decrease of brushing-induced rCBF changes, indicating that it partly regulates somatosensory-evoked variations in cortical blood flow. Interestingly, the effect was specific to the hemisphere ipsilateral to the NBM that received the muscimol injection. These novel findings may be explained by at least two mechanisms.
First, one likely mechanism is the inactivation of the basalocortical vasodilative system consisting of diffuse, ipsilateral cholinergic projections (Biesold et al. 1989; Kurosawa et al. 1989; Adachi et al. 1992; Lacombe et al. 1997; Vaucher et al. 1997; Sato & Sato, 1990; for review see Sato & Sato, 1995). Accordingly, basalocortical projections enable strong rCBF modulation in the cortex independently of regional metabolism (Hallström et al. 1990; Kimura et al. 1990). Thus, GABAergic inactivation of NBM activity may have altered ACh release in the cortex, decreasing, in turn, the blood flow response to brushing, independently of cortical neural activity. Previous studies have shown that the diameter of pial arteries is not affected when rCBF is increased by focal electrical stimulation of NBM (Adachi et al. 1992), while the diameter of parenchymal microvessels is increased (Hotta et al. 2004). Nucleus basalis of Meynert activity-induced parenchymal vasodilatation is thought to be effective in recruiting the regional haemodynamic response around the somatosensory focus located in the parenchyma with an increased neuronal activity.
Another possibility that may explain part of the present results is the modulation of somatosensory-evoked cortical activity by GABAergic inactivation of NBM. Tetanic stimulation of NBM can increase cortical activity and fast oscillations through cholinergic neurotransmission, as measured by EEG (Metherate et al. 1992). Moreover, the pairing of cutaneous electrical stimulation with NBM stimulation enhances evoked potentials in the rat somatosensory cortex through cholinergic neurotransmission (Verdier & Dykes, 2001). Accordingly, cortical activation is decreased and slow oscillations are increased when NBM is lesioned with ibotenic acid (Buzsáki et al. 1988). Another study also showed that muscimol can enhance slow oscillations and diminish wakefulness when injected in NBM (Manfridi et al. 2001). However, we suggest that alteration of stimulus-evoked rCBF changes is less likely to be explained by a global alteration of cortical activity, because the effect of muscimol was specific to the hemisphere ipsilateral to NBM injection, just like the cholinergic basalocortical projections described above. Furthermore, basal rCBF was not affected after the muscimol injection in the present study, as reported after electrical lesion of bilateral NBM or after i.v. injection of cholinergic receptor blockers (Uchida et al. 2000). Such a restricted outcome could hardly be explained by decreased cortical activity, as reported when NBM was lesioned or inactivated pharmacologically (Buzsáki et al. 1988; Manfridi et al. 2001).
The fact that rCBF changes were not completely blunted by muscimol may support the role of regional cortical activity and local release of vasoactive agents triggered by brushing in producing rCBF variations independently of NBM. However, considering that the injection was very strictly localized (as shown in Fig. 4A), the residual response may also be due to partial blocking of NBM activity, resulting in residual cholinergic output from the NBM still inducing some rCBF changes in the cortex. Nevertheless, the present data indicate that the relative contribution of NBM to the somatosensory-evoked rCBF changes is at least 40%.
Basal cortical rCBF following GABAergic inactivation of NBM
As opposed to the very clear decrease in stimulus-evoked rCBF changes in the parietal cortex ipsilateral to the inactivated NBM, basal rCBF was unaffected by muscimol injection. This result indicates that acute unilateral GABAergic inactivation of NBM is not sufficient to alter basal rCBF, consistent with previous studies showing unaltered basal rCBF after unilateral ibotenic acid lesion of NBM (Namba et al. 1991; Scremin et al. 1991). In contrast, another study showed a significant decrease of basal rCBF following unilateral ibotenate injections in NBM in rats (Gomi et al. 1991). These studies underscore that the role of NBM in regulating basal rCBF is still controversial. Nevertheless, the present results clearly indicate that the decrease in stimulus-evoked rCBF changes in the parietal cortex ipsilateral to the inactivated NBM was not related to decreased basal rCBF.
Limitations and future directions
The foremost limitation of this study is the lack of measurement to monitor neuronal activity in parallel with the rCBF changes. Since NBM can influence cortical neuronal activity, the present results warrant further investigation to clarify neurovascular coupling properties during the modulation of somatosensory-evoked rCBF variations by NBM inactivation. In addition, more studies are needed to determine the precise mechanisms underlying NBM effects on stimulus-evoked rCBF changes, such as the role of cholinergic projections and the receptors involved. Lastly, it would be interesting to assess whether the present results could be generalized to other stimulus-evoked rCBF alterations, such as those elicited by auditory and visual stimulation.
One implication of the present data concerns neuroimaging studies. In investigations with haemodynamic-dependent methods, it is assumed that local neuronal activity underlies the observed signal changes. However, the present findings show that additional processes involving NBM also contribute to these changes in the cortex, which potentially allow flow–metabolism uncoupling in some conditions. This suggests that individual differences in cortical activation patterns, especially for somatosensory tasks, may be explained, at least in part, by the level of activity in NBM. Future neuroimaging studies may benefit from controlling for this potential effect. It remains to be determined, however, whether this also applies to subcortical structures, in which NBM stimulation is reported to induce an increase in regional blood flow, associated with flow–metabolism coupling (Vaucher et al. 1997).
Conclusion
The present work shows that somatosensory-evoked rCBF changes induced by innocuous HL brushing are influenced by stimulus frequency and duration. Importantly, the present results suggest that at least 40% of these changes depend on parenchymal vasodilatation involving the ipsilateral NBM.
Acknowledgments
The experiments were done in Tokyo Metropolitan Institute of Gerontology. Mathieu Piché thanks ‘La Fondation de recherche en chiropratique du Quebec’ for financial support.
Glossary
Abbreviations
- ACh
acetylcholine
- HL
hindlimb
- MAP
mean arterial pressure
- NBM
nucleus basalis of Meynert
- PC
parietal cortex
- rCBF
regional cerebral blood flow
- ROI
region of interest
Author contributions
M.P., S.U., S.H. and H.H. contributed to the experimental design, experiment performance, data analyses, data interpretation and manuscript writing. Y.A. contributed to manuscript writing.
Author's present address
M. Piché is a visiting scientist at the Department of Autonomic Neuroscience, Tokyo Metropolitan Institute of Gerontology.
References
- Adachi T, Baramidze DG, Sato A. Stimulation of the nucleus basalis of Meynert increases cortical cerebral blood flow without influencing diameter of the pial artery in rats. Neurosci Lett. 1992;143:173–176. doi: 10.1016/0304-3940(92)90259-a. [DOI] [PubMed] [Google Scholar]
- Adachi T, Meguro K, Sato A, Sato Y. Cutaneous stimulation regulates blood flow in cerebral cortex in anaesthetized rats. Neuroreport. 1990;1:41–44. doi: 10.1097/00001756-199009000-00012. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Kimura A, Sato A, Suzuki A. Responses of neurons in the nucleus basalis of Meynert to various afferent stimuli in rats. Neuroreport. 1990;1:37–39. doi: 10.1097/00001756-199009000-00011. [DOI] [PubMed] [Google Scholar]
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Durduran T, Burnett MG, Yu G, Zhou C, Furuya D, Yodh AG, Detre JA, Greenberg JH. Spatiotemporal quantification of cerebral blood flow during functional activation in rat somatosensory cortex using laser-speckle flowmetry. J Cereb Blood Flow Metab. 2004;24:518–525. doi: 10.1097/00004647-200405000-00005. [DOI] [PubMed] [Google Scholar]
- Gomi S, Gotoh F, Ishihara N, Tanaka K, Ishikawa Y, Takashima S, Mihara B. Effects of lesioning of the substantia innominata on autoregulation of local cerebral blood flow in rats. J Cereb Blood Flow Metab. 1991;11:66–71. doi: 10.1038/jcbfm.1991.7. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Hallström A, Sato A, Sato Y, Ungerstedt U. Effect of stimulation of the nucleus basalis of Meynert on blood flow and extracellular lactate in the cerebral cortex with special reference to the effect of noxious stimulation of skin and hypoxia. Neurosci Lett. 1990;116:227–232. doi: 10.1016/0304-3940(90)90415-6. [DOI] [PubMed] [Google Scholar]
- Hotta H, Kanai C, Uchida S, Kanda K. Stimulation of the nucleus basalis of Meynert increases diameter of the parenchymal blood vessels in the rat cerebral cortex. Neurosci Lett. 2004;358:103–106. doi: 10.1016/j.neulet.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Kimura A, Sato A, Takano Y. Stimulation of the nucleus basalis of Meynert does not influence glucose utilization of the cerebral cortex in anaesthetized rats. Neurosci Lett. 1990;119:101–104. doi: 10.1016/0304-3940(90)90766-3. [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Sato A, Sato Y. Cutaneous mechanical sensory stimulation increases extracellular acetylcholine release in cerebral cortex in anaesthetized rats. Neurochem Int. 1992;21:423–427. doi: 10.1016/0197-0186(92)90194-v. [DOI] [PubMed] [Google Scholar]
- Lacombe P, Sercombe R, Vaucher E, Seylaz J. Reduced cortical vasodilatory response to stimulation of the nucleus basalis of Meynert in the aged rat and evidence for a control of the cerebral circulation. Ann N Y Acad Sci. 1997;826:410–415. doi: 10.1111/j.1749-6632.1997.tb48494.x. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron–glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Manfridi A, Brambilla D, Mancia M. Sleep is differently modulated by basal forebrain GABAA and GABAB receptors. Am J Physiol Regul Integr Comp Physiol. 2001;281:R170–R175. doi: 10.1152/ajpregu.2001.281.1.R170. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba H, Irie T, Fukushi K, Yamasaki T, Tateno Y, Hasegawa S. Lesion of the nucleus basalis magnocellularis does not affect cerebral cortical blood flow in rats. Neurosci Res. 1991;12:463–467. doi: 10.1016/0168-0102(91)90079-e. [DOI] [PubMed] [Google Scholar]
- Paxinos GE, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rancillac A, Rossier J, Guille M, Tong XK, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B. Glutamatergic control of microvascular tone by distinct GABA neurons in the cerebellum. J Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royl G, Leithner C, Sellien H, Müller JP, Megow D, Offenhauser N, Steinbrink J, Kohl-Bareis M, Dirnagl U, Lindauer U. Functional imaging with laser speckle contrast analysis: vascular compartment analysis and correlation with laser Doppler flowmetry and somatosensory evoked potentials. Brain Res. 2006;1121:95–103. doi: 10.1016/j.brainres.2006.08.125. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y. Cerebral cortical vasodilatation in response to stimulation of cholinergic fibres originating in the nucleus basalis of Meynert. J Auton Nerv Syst. 1990;30 Suppl:S137–S140. doi: 10.1016/0165-1838(90)90118-3. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y. Cholinergic neural regulation of regional cerebral blood flow. Alzheimer Dis Assoc Disord. 1995;9:28–38. doi: 10.1097/00002093-199505000-00007. [DOI] [PubMed] [Google Scholar]
- Scremin OU, Torres C, Scremin AME, O’Neal M, Heuser D, Blisard KS. Role of nucleus basalis in cholinergic control of cortical blood flow. J Neurosci Res. 1991;28:382–390. doi: 10.1002/jnr.490280310. [DOI] [PubMed] [Google Scholar]
- Uchida S, Kagitani F, Suzuki A, Aikawa Y. Effect of acupuncture-like stimulation on cortical cerebral blood flow in anaesthetized rats. Jpn J Physiol. 2000;50:495–507. doi: 10.2170/jjphysiol.50.495. [DOI] [PubMed] [Google Scholar]
- Ureshi M, Matsuura T, Kanno I. Stimulus frequency dependence of the linear relationship between local cerebral blood flow and field potential evoked by activation of rat somatosensory cortex. Neurosci Res. 2004;48:147–153. doi: 10.1016/j.neures.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Borredon J, Bonvento G, Seylaz J, Lacombe P. Autoradiographic evidence for flow-metabolism uncoupling during stimulation of the nucleus basalis of Meynert in the conscious rat. J Cereb Blood Flow Metab. 1997;17:686–694. doi: 10.1097/00004647-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Verdier D, Dykes RW. Long-term cholinergic enhancement of evoked potentials in rat hindlimb somatosensory cortex displays characteristics of long-term potentiation. Exp Brain Res. 2001;137:71–82. doi: 10.1007/s002210000646. [DOI] [PubMed] [Google Scholar]


