Abstract
Baroreflex sensitivity (BRS) using spontaneous sequence analysis in the time domain is not fully applicable in infancy, as the time delay for heart period to change (heart period delay, HPD) after an arterial pressure change is unknown. We estimated and compared HPD and BRS in the frequency (BRSsp, HPDsp) and time domains (BRSseq, HPDseq) from systolic blood pressure (SBP) and heart period fluctuations. Continuous SBP, using photoplethysmography, and heart period measurements were performed on 30 term infants at 2–4 weeks, 2–3 months and 5–6 months postnatal age. Cross-spectral analysis between SBP and heart period fluctuations was used to estimate BRSsp and HPDsp. Spontaneous sequence analysis was used to estimate BRS using a fixed beat delay of 1–12 beats (BRSseq) or a variable delay identified by a novel method accounting for epoch–epoch variability in HPD (BRSseqvar). HPDsp averaged 3.4 s (∼7 beats); BRSsp averaged 11.4 ms mmHg−1. BRSseq and BRSseqvar were consistently lower than BRSsp (P < 0.05), but the three BRS estimates were strongly correlated using a HPD of ∼5–6 beats. BRSseqvar resulted in the average estimate (8.9 ms mmHg−1) closest to BRSsp and overall had the strongest correlation with BRSsp (R2= 0.61; P < 0.001). All three BRS estimates increased progressively with postnatal age, with BRSsp averaging 6.4, 10.5 and 16.0 ms mmHg−1 at 2–4 weeks, 2–3 months and 5–6 months, respectively (P < 0.05). Accounting for the HPD of infancy provides estimates of BRS in the time domain that closely parallel spectral estimates, and provides a novel analytical tool to assess normal development and dysfunction of the baroreflex in infants.
Introduction
The baroreflex, the autonomic mechanism primarily responsible for the short term control of blood pressure, is present and functional from early fetal life (Shinebourne et al. 1972; Yardley et al. 1983) and undergoes significant maturation in utero (Shinebourne et al. 1972). After birth, control of blood pressure undergoes further significant maturation as blood pressure declines over the first 3 months of infancy, then increases at 6 months of age (Yiallourou et al. 2008a). Preterm birth appears to alter the normal maturational pattern, as hypotension is common in extremely preterm infants (Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians, 1992; Munro et al. 2004). Moreover, prematurity may impart long-lasting effects on blood pressure control of preterm infants, as blood pressure is lower and the blood pressure response to body tilting is impaired compared to full-term born infants matched for gestational age (Witcombe et al. 2008, 2010). Speculatively, age-related and preterm birth-related impairment of blood pressure regulation may allow uncompensated hypotension leading to the sudden infant death syndrome (SIDS) (Ledwidge et al. 1998; Harper, 2000; Matthews, 2002). This is supported by the presence of a nadir in infant blood pressure corresponding to the age of greatest risk for SIDS, and by the finding that blood pressure control is also impaired at this age when infants sleep prone, the major risk factor for SIDS (Witcombe et al. 2008; Yiallourou et al. 2008a).
Presently, there is limited information on the maturation of baroreflex function during infancy. Past assessment of baroreflex sensitivity (BRS) has been limited in infants due to the inability to non-invasively measure beat–beat blood pressure, but new methods validated by ourselves and others have recently made this possible (Drouin et al. 1997b; Harrington et al. 2001; Andriessen et al. 2004; Yiallourou et al. 2006). Using continuous blood pressure recording, BRS in infants has been assessed by examining spontaneous sequences of beat–beat fluctuations of blood pressure and heart period in the time domain (Drouin et al. 1997a; Yiallourou et al. 2006) and cross-spectral analysis of blood pressure and heart period in the frequency domain (Andriessen et al. 2003; Andriessen et al. 2005). Both time and frequency domain methods of spontaneous BRS assess baroreflex function only at the operating point of the sigmoidal baroreflex function curve. Spontaneous sequence analysis of BRS enables the separation of centrally driven changes on the heart and vessels (non-baroreflex changes) from purely baroreflex related influences on heart period control, thus providing a quantification of BRS unaffected by higher neural influences, such as those arising from behavioural changes (Zoccoli et al. 2001). However, this form of analysis requires the assumption of a heart period delay (HPD), the delay time of reflex-affected heart period changes after an arterial pressure change, which as yet, is undefined for infants. Cross-spectral analysis of BRS provides frequency-dependent estimates of sensitivity and gain (Robbe et al. 1987), but is limited in that it does not discriminate between central or baroreflex mediated changes (Zoccoli et al. 2001). Nevertheless, the cross-spectral technique does allow estimation of HPD (deBoer et al. 1987), though as yet, the approach to defining HPD has not been fully applied in infancy as it has in adults (deBoer et al. 1987; Jo et al. 2003). Preliminary evidence suggests that HPD may be much longer in neonates than adults (Andriessen et al. 2003, 2005) but whether subsequent HPD changes occur across development during infancy are unknown. Should HPD changes occur, significant errors in BRS estimates might arise from uncorrected spontaneous sequence analysis, making developmental descriptions inaccurate (Laude et al. 2009).
Given the importance and uncertainty of blood pressure regulatory mechanisms in early human life, we aimed to define BRS changes over the first 6 months after birth. As the combination of spontaneous sequence analysis and cross-spectral analysis has proven a powerful approach to BRS analysis in the adult (Tank et al. 2001; Laude et al. 2004), we employed both these approaches in our study.
Methods
Ethical approval
This study was approved by the Southern Health and Monash University Human Research Ethics Committees (021464B) and conformed with the principles outlined in the Declaration of Helsinki. Prior to commencement of the study, written parental consent was obtained for all subjects. No monetary incentive was provided for participation.
Subjects
The study group consisted of 30 healthy term infants (15F/15M) born at 38–42 weeks of gestation with birth weights of 3.6 ± 0.1 kg (mean ±s.e.m.). Each infant was studied on three occasions at 2–4 weeks, 2–3 months and 5–6 months postnatal age with daytime polysomnography.
Recording methods
Electrodes and measuring devices for polysomnography were attached during the infant's morning feed. Following their normal sleep routine, infants were placed in a pram and allowed to sleep naturally in the supine position in a darkened room at constant temperature (22–23°C), as previously described (Yiallourou et al. 2008a,b;). Studies were performed in quiet sleep identified using electroencephalograph, behavioural, HR and breathing pattern criteria (Anders et al. 1971; Curzi-Dascalova & Challamel, 2000).
A photoplethysmographic cuff (Finapres Medical Systems, Amsterdam, The Netherlands) placed around the infant's wrist measured blood pressure non-invasively and continuously according to methods described previously (Yiallourou et al. 2006; Witcombe et al. 2008; Yiallourou et al. 2008a,b;). Blood pressure was recorded in 2 min epochs, allowing a 2 min rest period prior to each measurement. A maximum of 2 min was selected to avoid blood pooling in the hand.
All data were recorded at a frequency of 400 Hz and amplified via a polygraph (Grass Instrument Co., Quincy, MA, USA) and digitized via a 16-channel Powerlab system (ADInstruments, Sydney, Australia). All signals were recorded onto a computer for data storage, analysis and visualization (Chart 5.2, ADInstruments).
Data analysis
Beat–beat blood pressure and heart period data were obtained from 2 min epochs recorded during each episode of quiet sleep at each postnatal age. Epochs were selected to be free of artifacts arising from movement, apnoeas, sighs or periodic breathing. In some cases an artifact was observed late in the epoch; the data preceding the artifact were retained provided >1 min of artifact-free measurement was recorded. Analysis was performed using MATLAB (The Mathworks, Natick, MA, USA). Systolic blood pressure was determined from blood pressure recordings and R-waves were determined from ECG recordings via peak detection, with the sampling error minimised using a quadratic fit method for optimal localisation of the peak (Andriessen et al. 2003). Heart period interval was taken as the time between two consecutive heart beats and assigned the time of the latter beat. Beat–beat data for systolic blood pressure and heart period were resampled (200 Hz, cubic interpolation) to achieve a continuous equidistantly spaced time series for subsequent analysis.
Cross spectral analysis
Spectral analysis procedures were adapted from methods described previously (deBoer et al. 1987; Robbe et al. 1987; Andriessen et al. 2005). To determine the gain, phase shift, and coherence of the coupling between systolic blood pressure and heart period, both the transfer function and coherence were estimated. The transfer function provides a representation of gain and phase characteristics of baroreflex function as a function of frequency, assuming a linear time-invariant system with a single input (systolic blood pressure fluctuations) and single output (heart period fluctuations). Coherence, also a function of frequency, provides values between 0 and 1 that indicate the strength of the correlation between the input and output at each frequency. For both analyses, systolic blood pressure and heart period were de-trended, divided into four equal segments with 75% overlap, and each windowed using a Hamming window. After determining the transfer function and coherence, the value of gain (BRS) was chosen at the frequency of maximum coherence between systolic blood pressure and heart period, within 0.04–0.15 Hz. Likewise, the phase shift ϕ and associated transfer delay (HPDsp), where HPDsp=ϕ/(2πf), were taken from the transfer function at f= the frequency of maximum coherence. Gain (BRS) values with a corresponding negative phase shift (HPDsp) were excluded, under the assumption that a negative phase shift infers that R–R interval changes lead systolic blood pressure changes, and therefore are not consistent with baroreflex activity.
Spontaneous sequence analysis
BRS was calculated using methods adopted from Bertinieri et al. (1988) and Zoccoli et al. (2001). To minimise the influence of respiration on systolic blood pressure and heart period via respiratory sinus and intrathoracic pressure variations, the continuous heart period and systolic blood pressure traces were filtered using a second-order 2 Hz low-pass filter (Zoccoli et al. 2001). Individual beats were subsequently extracted from the filtered records for analysis. Each epoch was scanned to identify increasing/decreasing blood pressure ramps characterised by a systolic blood pressure change of >0.5 mmHg (Tank et al. 2001; Gross et al. 2002) during each of three or more blood pressure waves. Baroreflex sequences were defined by changes in systolic blood pressure and heart period proceeding in the same direction, i.e. sequences having positive slopes. Based on the range of the HPDsp, spontaneous sequence analysis was performed using two methods. BRSseq was assessed using fixed HPD of 1 through 12 beats for each blood pressure rise/fall identified. In addition, we assessed BRSseq using a variable HPD for each blood pressure rise/fall detected. Details are outlined below. A mean value of BRS was computed for each blood pressure and heart period epoch, and then pooled and averaged in each infant.
BRSseq calculated with a fixed heart period delay
Once sequences were identified, linear regression was performed between systolic blood pressure values and heart period changes using a fixed HPD of 1 through to 12 beats, producing 12 different values of BRS for each sequence. For each sequence the systolic blood pressure-heart period regression coefficient was computed and used as a measure of BRS. Only sequences with a coefficient of determination (R2) > 0.8 (Bertinieri et al. 1988) were included in the BRS analysis.
BRSseq calculated with a variable heart period delay
Previously, a cross-correlation method has been employed to assess BRS in a manner which accounts for a variable HPD (Westerhof et al. 2004). Based on a similar concept, we also computed BRS using a novel ‘variable HPD’ that we refer to as BRSseqvar. For each blood pressure ramp, linear regression was performed between systolic blood pressure and heart period with a beat delay of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13 beats, and the regression coefficients and R2 values calculated. For each sequence and each beat delay, BRS was calculated from the slope of the systolic blood pressure–heart period regression equation. The optimal BRS and HPD were chosen from the regression with the highest R2 value. To ensure only baroreflex mediated changes on HR were selected, the sequence was excluded if the correlation was negative, if the R2 was <0.8, or if the highest R2 value occurred with a beat delay of 0 or 13; the latter criterion ensured that the best beat delay selected was truly a local minimum value within 1–12 beats. Along with the BRS value, the mean beat delay chosen was computed for each epoch in each infant.
Determination of optimal heart period delay for spontaneous sequence analysis
In overall analysis, we identified the optimal HPD for spontaneous sequence analysis by considering: (1) the correlation between BRSseq with BRSsp; (2) the precision, estimated by the coefficient of variation, which is the standard deviation/mean normalised for mean BRS at each postnatal age; and (3) the sequence yield, determined by the number of positive systolic blood pressure and heart period ramps for each beat delay criterion.
Statistical analysis
Statistical analysis was performed using SigmaStat (Systat Software Inc., San Jose, CA, USA). Data were first tested for normality and equal variance in order to utilize appropriate tests. The effects of postnatal age on BRS, HPD and the frequency of maximum coherence were compared using one way repeated measures ANOVA. For regression analysis between BRSsp and BRSseq, data were log10 transformed to achieve normality. Correlation between BRSsp and BRSseq, the frequency of maximum coherence and transfer function variables (HPDsp and phase), BRSseqvar and sequence variables (heart rate, blood pressure and HPD) was tested using Pearson correlation. Differences between BRSsp and BRSseq, BRSseqvar and BRSseq calculated using a fixed HPD, and positive and negative blood pressure ramps for BRSseqvar were tested using Mann–Whitney rank sum tests. Results are presented as means ±s.e.m., with significance taken at the P < 0.05 level.
Results
A total of 180 blood pressure and heart period epochs across the three age groups met the selection criteria and produced a mean BRS estimate using both frequency and time domain techniques.
Cross-spectral analysis
A typical example of the cross spectral analysis performed between systolic blood pressure and heart period series in an infant during quiet sleep at 2–3 months postnatal age is shown in Fig. 1A–E. In this epoch, both BRS (6.6 ms mmHg−1) and HPD (4.8 s) were calculated from the values of transfer gain (Fig. 1D) and phase delay (Fig. 1E) at the frequency of maximum coherence (0.07 Hz) (Fig. 1E). Mean values of the frequency of maximum coherence in the low frequency band were 0.10 ± 0.01 Hz at 2–4 weeks, 0.10 ± 0.01 Hz at 2–3 months and 0.09 ± 0.00 Hz at 5–6 months postnatal age. There was no effect of postnatal age on the frequency of maximum coherence.
Figure 1. Spectral power curves, transfer function and coherence parameters of blood pressure and heart period recordings measured in an infant at 2–3 months postnatal age.
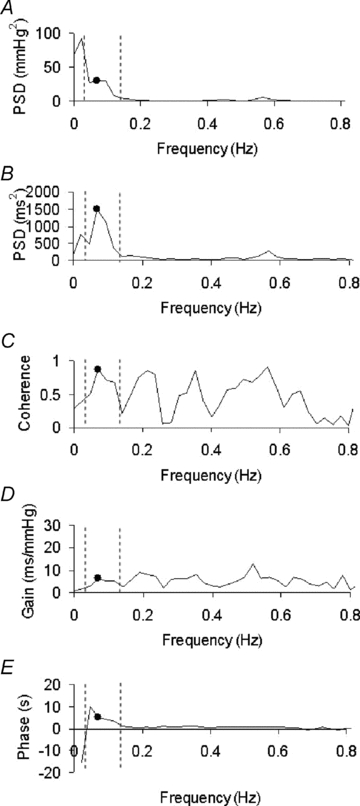
This figure shows the results of spectral analysis from a 1–2 min epoch of continuous blood pressure and heart period recording. The dashed lines represent the low-frequency range used to assess baroreflex changes (0.04–0.15 Hz.). A, the power spectral density of fluctuations in systolic blood pressure. Note the significant power in the low frequency band and the decline above 0.09 Hz. B, the power spectral density for heart period fluctuations. Note that peaks occur at 0.07 Hz, within the range of baroreflex mediated changes (Robbe et al. 1987; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996) and at 0.57 Hz corresponding to respiratory modulation at the infant's respiratory frequency ∼40 breaths min−1.C–E, the coherence, gain and phase delay of the relationship. Dots indicate the values of each spectral parameter at the frequency of maximum coherence (0.07 Hz, C). Values of gain at this frequency were used to determine baroreflex sensitivity (BRS), and values of phase to determine heart period time delay of the baroreflex loop (HPD).
Cross-spectral heart period delay (HPDsp)
Mean values for HPDsp were 3.2 ± 0.4 s at 2–4 weeks, 3.1 ± 0.2 s at 2–3 months and 3.8 ± 0.4 s at 5–6 months postnatal age. Based on individual heart rate values calculated for each epoch, the cross spectral HPDsp equated to 7 ± 1 beats at 2–4 weeks, 7 ± 0 beats at 2–3 months and 7 ± 1 beats at 5–6 months postnatal age. Postnatal age had no effect on HPDsp or when values were converted to heart beats. Marked variability of HPDsp was observed within infants with ranges being as great as 8 s in some infants (Fig. 2). Variability was also large between infants with a mean HPDsp range of 1.5 to 8.3 s.
Figure 2. Infant heart period delay (HPD) values measured from cross-spectral analysis.
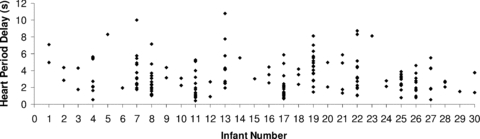
Values of HPD calculated from the gain of the transfer function between spontaneous fluctuations in blood pressure and heart period in a low frequency band of 0.04–0.15 Hz. Blood pressure and heart period were recorded continuously in 1–2 min epochs (n= 180) in 30 infants aged 2–4 weeks, 2–3 months and 5–6 postnatal age. Numbers on the x-axis represent the individual infants studied numbered 1 to 30. Note the variability both within and between infants.
To investigate the source of the variability of HPDsp we analysed the relationship between the frequency of the oscillation at which BRS was determined (the frequency of maximum coherence) with both HPDsp and phase. There was a significant negative correlation between the frequency of maximum coherence and HPDsp at 2–4 weeks (P < 0.001), 2–3 months (P < 0.001) and 5–6 months postnatal age (P < 0.001) (Fig. 3). There was no correlation between the frequency of maximum coherence and phase at 2–4 weeks and 2–3 months, but a weak negative correlation was found at 5–6 months postnatal age (P < 0.01).
Figure 3. Relationship between frequency at maximum coherence and the heart period delay (HPD).
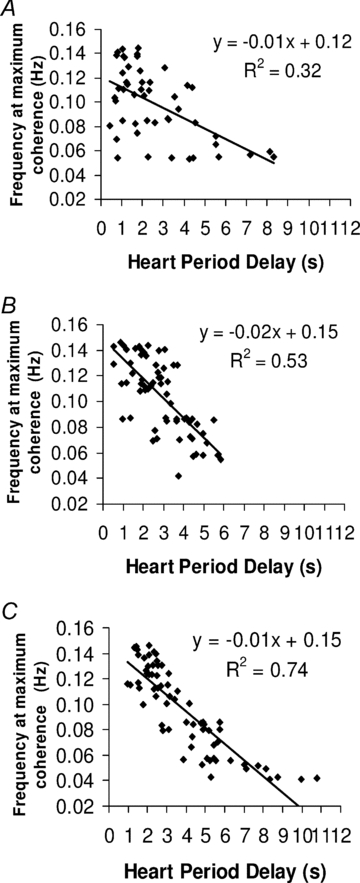
Correlation between the frequency at maximum coherence and the HPD estimated from cross-spectral analysis performed between systolic blood pressure and heart period changes in infants at 2–4 weeks (A), 2–3 months (B), and 5–6 months (C) postnatal age. Note the negative correlation between the frequency at maximum coherence and HPD at all ages.
Spontaneous sequence analysis
BRSseqvar
HPD calculated from BRSseqvar was longer at 5–6 months (6 ± 1 beats) than at 2–4 weeks (5 ± 1 beats) and 2–3 months postnatal age (5 ± 0 beats) (P < 0.01). The average number of beats for each sequence was 4.
There was no difference between BRSseqvar values with increasing and decreasing ramps of blood pressure at any of the ages studied. Correlation between the initial and mean systolic blood pressure and heart period of the sequence with BRSseqvar identified a positive correlation between BRSseqvar and both the initial heart period and mean heart period of the sequence at all ages studied (P < 0.01). Initial and mean systolic blood pressure of the sequence were negatively correlated with BRSseqvar at 2–4 weeks postnatal age (P < 0.001). BRSseqvar was negatively correlated with HPD at both 2–4 weeks (P < 0.05) and 2–3 months postnatal age (P < 0.05).
BRSseqvarvs. BRS fixed heart period delay
Differences between BRSseqvar and BRS calculated with a fixed HPD are presented in Fig. 4. All BRS values calculated using a fixed HPD were significantly lower than BRSseqvar (P < 0.05; Fig. 4A). We found that BRSseq decreased with an increase in fixed HPD values (R2= 0.98), with the steepest decline in mean BRS occurring between 6 and 12 beats (Fig. 4A). Overall, BRSseq estimated with a fixed HPD (1–12 beats) yielded less sequences (n= 210–385) than BRSseqvar (n= 507) (Fig. 4B). The number of sequences yielded for each fixed HPD was lowest in beat delays ranging from 8 to 12 beats. The coefficient of variation for BRSseqvar was 0.6, which was within the range of fixed HPD of 5–8 beats which displayed the lowest coefficient of variation values of 0.5–0.6 (Fig. 4C).
Figure 4. Effect of different heart period delays (HPDs) on BRS estimates in the time domain.
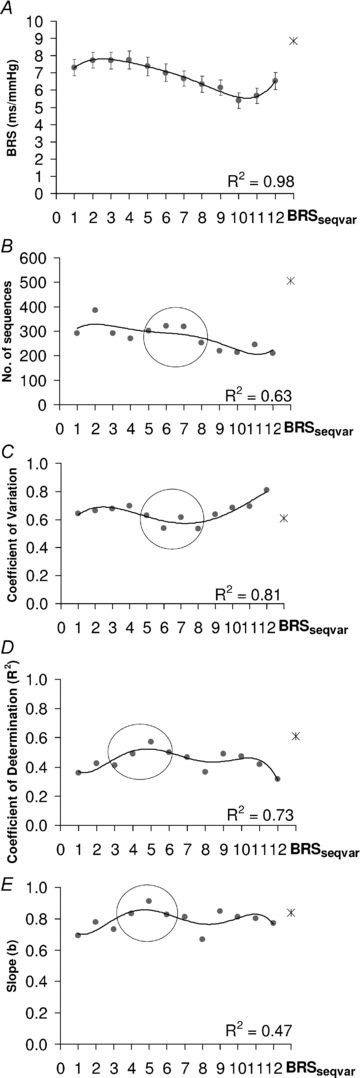
Effect of different heart period delays (HPDs) on mean baroreflex sensitivity (BRS) (A), number of sequences yielding heart period–systolic blood pressure regression of R2 > 0.8 (B), coefficient of variation for BRS (C), the coefficient of determination (R2) for the regression between BRSsp and spontaneous BRSseq (D), and the slope for the regression between BRSsp and spontaneous BRSseq (E). BRS was estimated from 1–2 min epochs of blood pressure and heart period epochs (n= 180) during quiet sleep in infants aged 2–4 weeks, 2–3 months and 5–6 months postnatal age (n= 30). A total of 13 separate sequence analyses were performed using different HPD: one sequence with a variable HPD (BRSseqvar, represented by the star) and 12 using a fixed HPD of 1–12 beats, represented by filled circles. Regression equation: Y=aXb. R= correlation coefficient between logY and logX. To characterise any trends for fixed beat delay criteria, 5th order polynomial regression was fitted. BRSseqvar yielded the highest value of BRS (A), number of sequences (B), and R2 (D) and slope (E) for the regression between BRSsp and BRSseq. For fixed beat delays, the number of sequences was highest and coefficient of variation lowest with fixed HPD of mid range (circle). Note (1) the steep decline in BRS for fixed beat delays of >6 beats, (2) the high yield of sequences of R2 > 0.8 (see text) for delays of 5–8 beats, (3) the low coefficient of variation of BRS estimates for delays of 5–8 beats, and (4) the peak values for R2 and slope for fixed HPD at 5 beats (circle).
Cross spectral analysis vs. spontaneous sequence analysis
The mean value for BRSsp (pooled for postnatal age) was 11.4 ± 0.6 ms mmHg−1. Comparison of regression analyses between BRSsp and BRSseq are presented in Fig. 4D and E for each HPD method. There was a significant and positive correlation between BRSsp and BRSseq for both variable and all fixed HPD (P < 0.001; for all beat delays). However, for each HPD method, BRSseq consistently produced lower estimates compared to BRSsp. BRSseqvar (8.9 ± 0.5 ms mmHg−1) produced the closest pooled value to BRSsp and the strongest correlation with BRSsp (R2= 0.61) (Fig. 4A and D). Of the fixed HPD values, a HPD equal to 5 beats correlated the best with BRSsp (Fig. 4D). Notably, the average HPD calculated from BRSseqvar of 5 beats yielded the BRSseq with the closest correlation with BRSsp. The coefficient of variation for BRSsp was equal to 0.61, which was close to the coefficient of variation determined for BRSseqvar (0.60) and fixed HPD values ranging from 5 to 8 beats (0.53 to 0.61) (Fig. 4C).
Postnatal maturation of baroreflex sensitivity
Figure 5 presents the effect of postnatal age on BRSsp, BRSseqvar and BRS calculated with a 5 beat delay. A maturational effect was apparent across the first 6 months of life, with BRS being higher at 5–6 months postnatal age compared to both 2–3 months (BRSsp, P < 0.05; BRSseqvar, P < 0.05; BRS 5 beat delay, P < 0.05) and 2–4 weeks postnatal age (BRSsp, P < 0.05; BRSseqvar, P < 0.05; BRS 5 beat delay, P < 0.05).
Figure 5. Effects of postnatal age on baroreflex sensitivity.
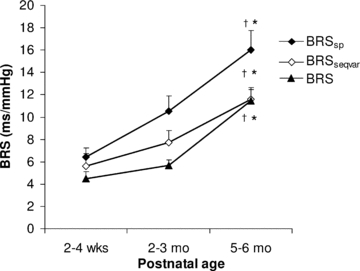
Postnatal age effects on BRS calculated using frequency and time domain methods during quiet sleep in the first 6 months of life (n= 30). BRS was estimated in the frequency domain (BRSsp) from the gain of the transfer function between heart period and systolic blood pressure changes within a LF band of 0.04–0.15 Hz. In the time domain, BRS was estimated using spontaneous sequence analysis with a variable heart period delay (BRSseqvar) and a 5 beat fixed delay (BRS). †P < 0.05, 2–3 months vs. 5–6 months; *P < 0.05, 5–6 months vs. 2–4 weeks. Note that baroreflex sensitivity increases with postnatal age using all three modes of analysis.
Discussion
Using non-invasive techniques to assess beat–beat changes in arterial blood pressure, this study used cross-spectral analysis to calculate HPD for the baroreceptor loop in infants across the first 6 months of life. We found that HPD was significantly longer than that calculated for adults and commonly used in infant studies. We also identified that there was intrinsic variability in HPDsp and applied this information to spontaneous sequence analysis methods to optimise assessment of BRS in the time domain. In this first comparison of the two methods in infants, both spectral and time domain methods have shown that BRS increases progressively across the first 6 months of life.
Heart period delay
The HPD identified from cross-spectral analysis in infants of 3.1–3.8 s (7 beats) was longer than that described in adults (deBoer et al. 1987; Badra et al. 2001; Jo et al. 2003). Using a model analysis to assess BRS in sleeping adults, Jo et al. (2003) found that the ‘time to peak response’, akin to our HPD, was 2.2 and 2.3 s (∼2 beats) in REM and stage 2 sleep, respectively (Jo et al. 2003). In agreement with our findings, Andriessen et al. (2005) also described a greater time delay (∼3 s) between fluctuations of blood pressure and heart period in preterm neonates (Andriessen et al. 2003, 2005). Differences between infant and adult HPD could be associated with maturational changes within a number of segments of the baroreceptor loop. Previous studies have quantified the individual components of ‘baroreflex latency’, which comprise: arterial transduction (Katona et al. 1968), central transmission (Kunze, 1972), efferent vagus conduction (Brown & Eccles, 1934) and the effector response (Fagius et al. 1987). It has been estimated that the delay between acetylcholine release and depolarisation of the sinoatrial node depolarisation represents approximately two-thirds of the baroreflex latency (Fagius et al. 1987; Eckberg & Sleight, 1992). Thus, given that the effector response constitutes the majority of the latency, we speculate that developmental changes in BRS are likely to reflect maturational changes within this component of the reflex arc, in keeping with the substantial growth in vagal influence on the heart during infancy (Schechtman et al. 1989; Lipsitz et al. 1997).
Interestingly, we also identified that HPDsp was characterised by marked variability both between and within the term infants studied. This finding is in contrast to latencies measured in the adult, which have been found to be remarkably constant (Badra et al. 2001). Variability in infant HPD may also arise at the level of the sinoatrial node given its dominant influence on HPD. Previous studies have shown that the timing of pacemaker discharge, depends on the timing of the vagal input within the cardiac cycle: thus, if the vagal input arrives late in the cycle the vagally mediated change in pacemaker periodicity is delayed to the next cycle (Slenter et al. 1984). Therefore, variability such as that we have seen may arise in part from complex frequency-dependent patterns of vagus–SA node interactions (Slenter et al. 1984). In addition, variability may be amplified as responses to abrupt alterations of afferent baroreceptor traffic that have been shown to be mediated by fluctuations of efferent cholinergic activity are quite non-linear (Eckberg, 1980). Alternatively, we found that the frequency of the oscillation at which BRS was calculated was negatively correlated with HPDsp, which indicates that the source of the variability is in part due to the period of the blood pressure oscillation. Consistent with this suggestion, early studies of the timing of the baroreflex loop in adults suggest that the HPD may be dependent on the rate of blood pressure rise, where slowly rising pressures produced a longer delay (Koepchen et al. 1961). Thus, heterogeneous populations of neurons may discharge in response to different pressures and rates of blood pressure changes, which may in turn alter conduction time of the reflex loop. Similarly, the rate of pressure change during each pulse is known to influence the rate of baroreceptor nerve firing in infants (Blanco et al. 1985).
We have also considered that the variability in HPD may be due to respiratory-mediated changes which can affect the low frequency component of heart-period and blood pressure variability. However, it has been previously demonstrated that removal of respiratory mediated changes, using partial coherence analysis, did not influence the phase angles and coherence between systolic pressures and R–R intervals at low frequencies (Badra et al. 2001). To minimize respiratory-related variations we only included data that were recorded in quiet sleep, which is characterised by a strikingly monotonous regularity and lack of breath–breath variability in breathing (Sheldon, 2005). In addition, we excluded respiratory events such as sighs, apnoeas, periodic breathing and arousal, in an effort to exclude low frequency respiratory related changes. Furthermore, as HPD was averaged over 1–2 min of data with no bias toward inspiration or expiration, respiratory-mediated effects on baroreflex function such as respiratory gating (Eckberg & Orshan, 1977; Eckberg et al. 1980; Eckberg, 2003) should be effectively cancelled out.
In the time domain, based on the characteristics of HPDsp, we assessed a novel sequence analysis method using a variable HPD that we refer to as BRSseqvar. The average HPD calculated from BRSseqvar, of 5–6 beats, was shorter than HPDsp (7 beats). This discrepancy between time and frequency domain HPD may be explained by the properties of the HPDsp computed from the phase lag of the transfer function between systolic blood pressure and heart period changes. The phase lag calculated from the transfer function is composed of a contribution from the ‘pure delay’ of the system in addition to a response lag (ascribed to time constants) of the system. Thus, the HPDsp may slightly overestimate the ‘pure delay’ seen in the time domain. Nonetheless, HPD averaged for both BRSsp and BRSseqvar were similar, and were still longer than that described for the adult.
Spontaneous sequence analysis
In addition to BRSseqvar, using the range of HPDsp, we also assessed spontaneous sequence analysis using a fixed HPD ranging from 1 to 12 beats. Of the different HPD methods evaluated, BRSseqvar yielded the highest number of sequences (more than 500 in total), had a low coefficient of variation, and provided the closest pooled estimate to BRSsp. Bertinieri et al. (1988), suggest that evaluation of BRS is optimised by the collection of a large number of sequences, as the sensitivity of the baroreflex is not stable, and is characterised by a pronounced short-term variability. Using a fixed HPD, the number of sequences obtained was substantially lower than BRSseqvar (less than 400). Therefore, using a fixed HPD may limit the number of sequences used and consequently the confidence limits for the estimate.
Interestingly, we identified that the use of a greater HPD decreased estimates of BRSseq (Fig. 4A). This decrease in BRSseq, suggests that overestimation of HPD leads to underestimation of BRS in the time domain. Therefore, the accuracy of the sequence method may critically depend on the ability to determine the timing of the peak slope in a spontaneous change in systolic blood pressure and the peak slope in the corresponding spontaneous change in heart period. Such underestimation of BRS using the sequence method with any fixed HPD is likely to be due to difficulties in capturing the peak of the change in heart period; over- or under-estimation of the timing may therefore both lead to underestimation of the peak heart period slope and therefore of BRS.
Correlation of cross spectral analysis and spontaneous sequence analysis
A good correlation between both cross-spectral and spontaneous sequence analysis is important as assessment of BRS using both non-invasive methods can allow different characteristics of the baroreflex response to be evaluated. As in adults (Laude et al. 2004), we identified that BRSseq and BRSsp were significantly correlated, regardless of the HPD method used, though BRSseq consistently produced a lower estimate than BRSsp in infants. In agreement with our findings, another study in preterm infants has reported a lower mean BRS estimate in the time domain (based on the standard deviation of heart period and systolic blood pressure) and a strong correlation with BRSsp (Andriessen et al. 2003). Our analysis of the correlation between BRSsp and BRSseq showed that BRSseqvar produced the closest results to BRSsp and identified an average HPD of 5–6 beats. Similarly, of the fixed HPDs compared, we found that a fixed HPD of 5 beats also produced the best correlation, and was close to the HPDsp. Thus, accounting for the variability and length of HPD improved the correlation between sequence and spectral methods.
Optimal HPD for spontaneous sequence analysis
Previously in infants, spontaneous sequence analysis has been assessed using a constant HPD equal to 1 beat (Drouin et al. 1997a; Gournay et al. 2002). However, we have now shown that infant HPD is generally greater than one heart beat and is not constant but quite variable from minute to minute (Fig. 2). Under- or over- estimation of HPD may lead to errors in capturing the peak heart period slope and therefore to inaccuracy of BRS assessed in the time domain. Our BRSseqvar represents an improved estimate of BRS in the time domain as it is adjusted for variability in HPD.
To adequately account for the timing of the baroreflex loop during infancy we suggest using a variable HPD, or if a fixed HPD is required, a delay of 5 beats. Without an established gold standard method for determination of BRS in infancy we are unable to assess whether, by altering HPD, the accuracy of BRS assessment is improved in the time domain. However, as we have produced a BRS estimate which (1) accounts for the timing properties of the baroreflex-cardiac response, (2) optimises the amount valid BRS sequences, and (3) improves the correlation between cross-spectral and sequence techniques, we are confident that we have produced criteria for sequence analysis that are more appropriate for infant studies.
Postnatal development of BRS
Importantly, both frequency and time domain methods of assessing BRS, have identified that maturation of baroreflex gain occurs within the first 6 months of postnatal life. Our estimates of BRS at 2–4 weeks (5–6 ms mmHg−1) were somewhat lower than those previously reported in the first week of life (∼8 to 10 ms mmHg−1) (Drouin et al. 1997a; Gournay et al. 2002). Discrepancies between studies may be due to critical differences in the sequence analysis methods used, as both Drouin et al. and Gournay et al. used the adult HPD of a one beat delay and limited blood pressure ramps to a 10–20% rise/fall in baseline blood pressure (Drouin et al. 1997a; Gournay et al. 2002). Across infancy we identified a progressive increase of BRS to a value of ∼11 to 16 ms mmHg−1 at 5–6 months of age, which is equivalent to BRS measured in normal adult subjects (La Rovere et al. 2008). As there are few data, it is unclear which neural mechanisms might be improving baroreflex maturation. Previous studies in animal models have shown that postnatal maturation of central neural regulation of cardiovascular function occurs (Gootman et al. 1983). Maturation of baroreflex function may be closely coupled to maturational changes of parasympathetic control, as it is thought that the infant baroreflex depends primarily on parasympathetic modulation of heart rate rather than sympathetic modulation of vascular tone (Andriessen et al. 2005). Similarly, it has been previously suggested that cardiovagal activity may serve as an indicator of the efficiency of central parasympathetic signal processing in early life (Lenard et al. 2004). Moreover, studies of postnatal maturation of autonomic heart rate control have shown that parasympathetic drive increases with increasing postnatal age (Andriessen et al. 2005). In addition, previous demonstrations of age-related increases in BRS are directly proportional to cardiovagal activity (Lenard et al. 2004). Taken together, these observations suggest that central parasympathetic signal processing may dominate the maturation of baroreflex autonomic function.
Importantly, we have identified that during the time of increased SIDS risk, baroreflex function is immature in healthy term infants. Post-mortem studies have shown that some SIDS victims had a pre-existing abnormality in brainstem areas responsible for the autonomic control of both heart rate and blood pressure (Kinney, 2005). Immature baroreflex function, in combination with low blood pressure and a pre-existing impaired autonomic control may dampen the response to a life threatening hypotension in infants that is thought to play a role in SIDS (Ledwidge et al. 1998; Harper, 2000; Matthews, 2002).
Conclusions
This study has identified characteristics of infant HPD and assessed a novel method to measure BRS in the time domain. Assessment of BRS in infancy is important as altered cardiovascular regulation has been implicated in SIDS and also manifests as a legacy of preterm birth. This is the first study to provide normative data on the maturation of baroreflex function in healthy term infants over the first 6 months of postnatal life. Further research using frequency and time domain techniques to assess BRS is required to shed more light into infant conditions and pathologies where impaired baroreflex function has been implicated, such as SIDS and prematurity.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (Project 284357) and by The Kaarene Fitzgerald Fellowship awarded by SIDS and KIDS Victoria. The authors thank the parents and infants who participated in this study and the staff of the maternity wards at Monash Medical Centre and Jessie McPherson Private Hospital and at the Melbourne Children's Sleep Unit.
Glossary
Abbreviations
- BRS
baroreflex sensitivity
- BRSseq
baroreflex sensitivity (spontaneous sequence analysis)
- BRSseqvar
baroreflex sensitivity (spontaneous sequence analysis using variable beat delay)
- BRSsp
baroreflex sensitivity (spectral analysis)
- HPD
heart period delay
- ϕ
heart period phase lag
Author contributions
All research studies were performed at the Ritchie Centre for Baby Health Research. R.S.C.H., S.R.Y. and A.M.W. were involved in the conception and design of the studies. S.R.Y. and R.S.C.H. performed the studies. S.R.Y. and S.A.S. performed data analysis and were involved in the interpretation of data together with R.S.C.H. and A.M.W., S.R.Y. and S.A.S. had equal contributions in writing the paper with critical input from R.S.C.H. and A.M.W. All authors approved the final version of the manuscript for publication.
References
- Anders T, Emde R, Parmelle A. A Manual of Standardized Terminology, Techniques and Criteria for Scoring States of Sleep and Wakefuleness in Newborn Infants. Los Angeles: UCLA Brain Information Service/BRI; 1971. [Google Scholar]
- Andriessen P, Koolen AM, Berendsen RC, Wijn PF, ten Broeke ED, Oei SG, Blanco CE. Cardiovascular fluctuations and transfer function analysis in stable preterm infants. Pediatr Res. 2003;53:89–97. doi: 10.1203/00006450-200301000-00016. [DOI] [PubMed] [Google Scholar]
- Andriessen P, Oetomo SB, Peters C, Vermeulen B, Wijn PF, Blanco CE. Baroreceptor reflex sensitivity in human neonates: the effect of postmenstrual age. J Physiol. 2005;568:333–341. doi: 10.1113/jphysiol.2005.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriessen P, Schoffelen RL, Berendsen RC, de Beer NA, Oei SG, Wijn PF, Blanco CE. Noninvasive assessment of blood pressure variability in preterm infants. Pediatr Res. 2004;55:220–223. doi: 10.1203/01.PDR.0000104152.85296.4F. [DOI] [PubMed] [Google Scholar]
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–2688. doi: 10.1152/ajpheart.2001.280.6.H2674. [DOI] [PubMed] [Google Scholar]
- Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol Heart Circ Physiol. 1988;254:H377–383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- Blanco C, Dawes G, Hanson M, McCooke H. Studies of carotid baroreceptor afferents in fetal and newborn lambs. In: Jones C, Nathanielsz P, editors. The Physiological Development of the Fetus and Newborn. London: Academic Press; 1985. pp. 596–598. [Google Scholar]
- Brown GL, Eccles JC. The action of a single vagal volley on the rhythm of the heart beat. J Physiol. 1934;82:211–241. doi: 10.1113/jphysiol.1934.sp003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzi-Dascalova L, Challamel M. Neurophysiological basis of sleep development. In: Loughlin G, Carroll J, Marcus C, editors. Sleep and Breathing in Children. New York: Marcal Dekker; 2000. pp. 3–37. [Google Scholar]
- deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol Heart Circ Physiol. 1987;253:H680–689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Drouin E, Gournay V, Calamel J, Mouzard A, Roze JC. Assessment of spontaneous baroreflex sensitivity in neonates. Arch Dis Child Fetal Neonatal Ed. 1997a;76:F108–112. doi: 10.1136/fn.76.2.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin E, Gournay V, Calamel J, Mouzard A, Roze JC. Feasibility of using finger arterial pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 1997b;77:F139–140. doi: 10.1136/fn.77.2.f139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg D, Sleight P. Human Baroreflexes in Health and Disease. Oxford: Clarendon Press; 1992. [Google Scholar]
- Eckberg DL. Nonlinearities of the human carotid baroreceptor-cardiac reflex. Circ Res. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol. 1980;304:489–502. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. J Clin Invest. 1977;59:780–785. doi: 10.1172/JCI108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Sundlof G, Wallin BG. Variation of sympathetic reflex latency in man. J Auton Nerv Syst. 1987;21:157–165. doi: 10.1016/0165-1838(87)90018-x. [DOI] [PubMed] [Google Scholar]
- Gootman PM, Gootman N, Buckley BJ. Maturation of central autonomic control of the circulation. Fed Proc. 1983;42:1648–1655. [PubMed] [Google Scholar]
- Gournay V, Drouin E, Roze JC. Development of baroreflex control of heart rate in preterm and full term infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F151–154. doi: 10.1136/fn.86.3.F151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V, Plehm R, Tank J, Jordan J, Diedrich A, Obst M, Luft FC. Heart rate variability and baroreflex function in AT2 receptor-disrupted mice. Hypertension. 2002;40:207–213. doi: 10.1161/01.hyp.0000027279.69240.75. [DOI] [PubMed] [Google Scholar]
- Harper RM. Sudden infant death syndrome: a failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–142. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Harrington C, Kirjavainen T, Teng A, Sullivan CE. Cardiovascular responses to three simple, provocative tests of autonomic activity in sleeping infants. J Appl Physiol. 2001;91:561–568. doi: 10.1152/jappl.2001.91.2.561. [DOI] [PubMed] [Google Scholar]
- Jo JA, Blasi A, Valladares E, Juarez R, Baydur A, Khoo MC. Model-based assessment of autonomic control in obstructive sleep apnea syndrome during sleep. Am J Respir Crit Care Med. 2003;167:128–136. doi: 10.1164/rccm.200202-096OC. [DOI] [PubMed] [Google Scholar]
- Joint Working Group of the British Association of Perinatal Medicine and the Research Unit of the Royal College of Physicians. Development of audit measures and guidelines for good practice in the management of neonatal respiratory distress syndrome. Arch Dis Child. 1992;67:1221–1227. doi: 10.1136/adc.67.10_spec_no.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona PG, Poitras JW, Pantelakis N, Jensen EW, Barnett GO. Deterministic nature of baroreceptor firing. Am J Physiol. 1968;215:1–7. doi: 10.1152/ajplegacy.1968.215.1.1. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Koepchen HP, Lux HD, Wagner PH. [Studies on time requirement and central development of the pressor receptor heart reflex.] Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;273:413–430. [PubMed] [Google Scholar]
- Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol. 1972;222:1–15. doi: 10.1113/jphysiol.1972.sp009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude D, Baudrie V, Elghozi JL. Tuning of the sequence technique. IEEE Eng Med Biol Mag. 2009;28:30–34. doi: 10.1109/MEMB.2009.934630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude D, Elghozi JL, Girard A, Bellard E, Bouhaddi M, Castiglioni P, Cerutti C, Cividjian A, Di Rienzo M, Fortrat JO, Janssen B, Karemaker JM, Leftheriotis G, Parati G, Persson PB, Porta A, Quintin L, Regnard J, Rudiger H, Stauss HM. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study) Am J Physiol Regul Integr Comp Physiol. 2004;286:R226–231. doi: 10.1152/ajpregu.00709.2002. [DOI] [PubMed] [Google Scholar]
- Ledwidge M, Fox G, Matthews T. Neurocardiogenic syncope: a model for SIDS. Arch Dis Child. 1998;78:481–483. doi: 10.1136/adc.78.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–2312. doi: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Pincus SM, Morin RJ, Tong S, Eberle LP, Gootman PM. Preliminary evidence for the evolution in complexity of heart rate dynamics during autonomic maturation in neonatal swine. J Auton Nerv Syst. 1997;65:1–9. doi: 10.1016/s0165-1838(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Matthews T. Sudden infant death syndrome: a defect in circulatory control? Child Care Health Dev. 2002;28(Suppl 1):41–43. doi: 10.1046/j.1365-2214.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114:1591–1596. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- Robbe HW, Mulder LJ, Ruddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;10:538–543. doi: 10.1161/01.hyp.10.5.538. [DOI] [PubMed] [Google Scholar]
- Schechtman VL, Harper RM, Kluge KA. Development of heart rate variation over the first 6 months of life in normal infants. Pediatr Res. 1989;26:343–346. doi: 10.1203/00006450-198910000-00011. [DOI] [PubMed] [Google Scholar]
- Sheldon S. Physiological variations during sleep in children. In: Sheldon S, Ferber R, Kryger M, editors. Principles and Practice of Pediatric Sleep Meicine. Philadelphia: Elsevier Saunders; 2005. pp. 73–84. [Google Scholar]
- Shinebourne EA, Vapaavuori EK, Williams RL, Heymann MA, Rudolph AM. Development of baroreflex activity in unanesthetized fetal and neonatal lambs. Circ Res. 1972;31:710–718. doi: 10.1161/01.res.31.5.710. [DOI] [PubMed] [Google Scholar]
- Slenter VA, Salata JJ, Jalife J. Vagal control of pacemaker periodicity and intranodal conduction in the rabbit sinoatrial node. Circ Res. 1984;54:436–446. doi: 10.1161/01.res.54.4.436. [DOI] [PubMed] [Google Scholar]
- Tank J, Jordan J, Diedrich A, Stoffels M, Franke G, Faulhaber HD, Luft FC, Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Westerhof BE, Gisolf J, Stok WJ, Wesseling KH, Karemaker JM. Time domain cross-correlation baroreflex sensitivity: performance on the EUROBAR data set. J Hypertension. 2004;22:1371–1380. doi: 10.1097/01.hjh.0000125439.28861.ed. [DOI] [PubMed] [Google Scholar]
- Witcombe NB, Yiallourou SR, Walker AM, Horne RS. Blood pressure and heart rate patterns during sleep are altered in preterm-born infants: implications for sudden infant death syndrome. Pediatrics. 2008;122:e1242–1248. doi: 10.1542/peds.2008-1400. [DOI] [PubMed] [Google Scholar]
- Witcombe NB, Yiallourou SR, Walker AM, Horne RS. Delayed blood pressure recovery after head-up tilting during sleep in preterm infants. J Sleep Res. 2010 doi: 10.1111/j.1365-2869.2009.00793.x. (in press) [DOI] [PubMed] [Google Scholar]
- Yardley RW, Bowes G, Wilkinson M, Cannata JP, Maloney JE, Ritchie BC, Walker AM. Increased arterial pressure variability after arterial baroreceptor denervation in fetal lambs. Circ Res. 1983;52:580–588. doi: 10.1161/01.res.52.5.580. [DOI] [PubMed] [Google Scholar]
- Yiallourou S, Walker A, Horne R. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep. 2006;29:1083–1088. doi: 10.1093/sleep/29.8.1083. [DOI] [PubMed] [Google Scholar]
- Yiallourou SR, Walker AM, Horne RS. Effects of sleeping position on development of infant cardiovascular control. Arch Dis Child. 2008a;93:868–872. doi: 10.1136/adc.2007.132860. [DOI] [PubMed] [Google Scholar]
- Yiallourou SR, Walker AM, Horne RS. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep. 2008b;31:1139–1146. [PMC free article] [PubMed] [Google Scholar]
- Zoccoli G, Andreoli E, Bojic T, Cianci T, Franzini C, Predieri S, Lenzi P. Central and baroreflex control of heart rate during the wake-sleep cycle in rat. Sleep. 2001;24:753–758. [PubMed] [Google Scholar]


