Abstract
Protein hepatocyte nuclear factor 4α (HNF-4α) is atypically activated in the liver of diabetic rodents and contributes to hepatic glucose production. HNF-4α and Foxo1 can physically interact with each other and represent an important signal transduction pathway that regulates the synthesis of glucose in the liver. Foxo1 and HNF-4α interact with their own binding sites in the phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) promoters, and this binding is required for their effects on those promoters. However, the effect of physical activity on the HNF-4α/Foxo1 pathway is currently unknown. Here, we investigate the protein levels of HNF-4α and the HNF-4α/Foxo1 pathway in the liver of leptin-deficient (ob/ob) and diet-induced obese Swiss (DIO) mice after acute exercise. The ob/ob and DIO mice swam for four 30 min periods, with 5 min rest intervals for a total swimming time of 2 h. Eight hours after the acute exercise protocol, the mice were submitted to an insulin tolerance test (ITT) and determination of biochemical and molecular parameters. Acute exercise improved insulin signalling, increasing insulin-stimulated Akt and Foxo1 phosphorylation and decreasing HNF-4α protein levels in the liver of DIO and ob/ob mice under fasting conditions. These phenomena were accompanied by a reduction in the expression of gluconeogenesis genes, such as PEPCK and G6Pase. Importantly, the PI3K inhibitor LY292004 reversed the acute effect of exercise on fasting hyperglycaemia, confirming the involvement of the PI3K pathway. The present study shows that exercise acutely improves the action of insulin in the liver of animal models of obesity and diabetes, resulting in increased phosphorylation and nuclear exclusion of Foxo1, and a reduction in the Foxo1/HNF-4α pathway. Since nuclear localization and the association of these proteins is involved in the activation of PEPCK and G6Pase, we believe that the regulation of Foxo1 and HNF-4α activities are important mechanisms involved in exercise-induced improvement of glucose homeostasis in insulin resistant states.
Introduction
Type 2 diabetes (T2DM) constitutes a burgeoning epidemic that is expected to afflict over 300 million individuals worldwide by 2015 (Zimmet et al. 2001). The main clinical features of T2DM include glucose intolerance, insulin resistance, hyperglycaemia, and hyperinsulinaemia (Biddinger & Kahn, 2006). One of the diagnostic criteria for diabetes is fasting hyperglycaemia, which is mainly due to increased hepatic glucose production (Hwang et al. 1995; Hundal et al. 2000).
Several studies have shown that fasting hyperglycaemia occurs when there is defective insulin signal transduction in the liver (Anai et al. 1998; Buettner et al. 2005; Wan et al. 2009). Insulin's molecular action is initiated upon ligand binding to the insulin receptor, which autophosphorylates and activates phosphatidylinositol 3-kinase (PI3K), mediating the activation of Akt. Importantly, Akt activation leads to the phosphorylation of a member of the forkhead transcription factor family (Foxo1), which is expressed abundantly in the liver, and acts as a major target for insulin signalling (Biggs et al. 1999; Altomonte et al. 2003). Foxo1 belongs to a nuclear protein subfamily (Kops et al. 1999; Nakae et al. 1999, 2001; Kaestner et al. 2000) and its phosphorylation blocks the expression of a number of genes involved in gluconeogenesis (Kops et al. 1999; Nakae et al. 1999; Puigserver et al. 2003). In contrast, in its non-phosphorylated state, Foxo1 leads to augmented expression of key gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (Schmoll et al. 2000).
In addition to Foxo1, studies show that the transcription factor protein hepatocyte nuclear factor 4α (HNF-4α) has an important role in regulating glucokinase and glucose-6-phosphatase (G6Pase) genes in response to fasting and feeding (Hirota et al. 2008). HNF-4α belongs to the steroid/thyroid hormone receptor superfamily of transcription factors and was first identified by its interaction with a cis-regulatory sequence of liver-specific gene promoters (Sladek et al. 1990). Once active, HNF-4α binds to a specific DNA element as a homodimer and regulates the expression of a number of genes involved in glucose metabolism (Diaz et al. 1993; Drewes et al. 1996; Stoffel & Duncan, 1997).
An elegant study showed that one of the mechanisms involved in the suppressive effects of insulin on gluconeogenesis depends on its ability to reduce the expressions of HNF-4α and Foxo1 in the liver (Hirota et al. 2003). In fact, in models of insulin resistance, both during steady-state and after insulin stimulation, HNF-4α and Foxo1 protein levels are increased (Hirota et al. 2003; Ganjam et al. 2009). It has been suggested that the HNF-4α mediates glucose metabolism in the liver by controlling transcription of both the glucokinase (GK) and G6Pase genes (Hirota et al. 2008), which catalyse the first and last rate-limiting steps in glycolysis and gluconeogenesis, respectively. Thus, the specific interaction of Foxo1 and HNF-4α can simultaneously generate an activation of gluconeogenic and repression of glycolytic genes. To elucidate this, Hirota and colleagues focused on the functional interplay between HNF-4α and Foxo1. They found that Foxo1 represses HNF-4α potentiated transcription of the GK gene and that it synergizes with HNF-4α in G6Pase gene transcription (Hirota et al. 2008). These reciprocal reactions, exerted by Foxo1, took place simultaneously, and, importantly, insulin treatment of the cells abrogated both reactions (Hirota et al. 2008).
Exercise is generally recommended for the treatment of type 2 diabetes, because of its beneficial effects on blood glucose control (Diabetes Prevention Program Research Group, 2002). Increased physical exercise has been linked to improved glucose homeostasis and enhanced insulin sensitivity. After acute exercise, the sensitivity to insulin is enhanced in tissues such as the skeletal muscle, adipose, liver and hypothalamus (Luciano et al. 2002; Aoi et al. 2004; Peres et al. 2005; Flores et al. 2006; Ropelle et al. 2006; Pauli et al. 2008). In addition, we have shown that exercise greatly improves glucose homeostasis in animal models of obesity (Peres et al. 2005; Ropelle et al. 2006, 2009). However, the effects of exercise on the HNF-4α/Foxo1 pathway have not been investigated. Based on these principles, it is possible that acute exercise can alter the Foxo1/HNF-4α pathway, and reduce hepatic glucose production, improving the body's glucose homeostasis. Thus, in the present study, we investigated the effect of acute exercise on the Foxo1/HNF-4α pathway in the liver of diabetic mice.
Methods
Experimental animals
Male leptin-deficient (ob/ob) and lean control mice, originally imported from the Jackson Laboratories (Bar Harbor, ME, USA), were used in the experiments. All studies were conducted in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA), and the Ethics Committee at the State University of Campinas approved the experiments. Ten-week-old mice were divided into three groups: control mice (lean); obese mice (ob/ob), and an obese group submitted to acute exercise (Exe). Room temperature was maintained stable (28 ± 1°C) and mice were housed in individual cages, under a standard light–dark cycle (06.00 h to 18.00 h/18.00 h to 06.00 h), with food and water ad libitum.
Importantly, DIO may be different from hyperphagic induced obesity on hepatic metabolism, and this was the rationale for using a second type of obese mice. For this, male Swiss mice from the University of Campinas Central Animal Breeding Center were used in the experiments. The 4-week-old Swiss mice were divided into three groups: control mice (lean) fed standard rodent chow; obese mice, fed on an high fat diet (Table 1) for 2 months (DIO); and a third group which also received the high fat diet, but were submitted to acute exercise (DIO+Exe).
Table 1.
Composition of standard chow and high-fat diet
| Standard chow |
High fat diet |
|||
|---|---|---|---|---|
| Ingredients | g kg−1 | kcal kg−1 | g kg−1 | kcal kg−1 |
| Cornstarch (Q.S.P) | 398 | 1590 | 116 | 462 |
| Casein | 200 | 800 | 200 | 800 |
| Sucrose | 100 | 400 | 100 | 400 |
| Dextrinated starch | 132 | 528 | 132 | 528 |
| Lard | — | — | 312 | 2808 |
| Soybean Oil | 70 | 630 | 40 | 360 |
| Cellulose | 50 | — | 50 | — |
| Mineral mix | 35 | — | 35 | — |
| Vitamin mix | 10 | — | 10 | — |
| l-Cystine | 3 | — | 3 | — |
| Choline | 2.5 | — | 2.5 | — |
| Total | 1000 | 3948 | 1000 | 5358 |
Exercise protocol
The swimming protocol employed has been previously described (Ryder et al. 1999). Adult mice were acclimatized to swimming for 10 min on each of two days. Eight animals swam together in plastic containers measuring 45 cm in diameter. Water temperature was maintained at 32–33°C. Mice swam for four 30 min periods, with 5 min rest periods for a total swimming time of 2 h. After the acute exercise protocol (Exe group for ob/ob or DIO+Exe for Swiss), animals were fed ad libitum for 2 h and food was withdrawn 6 h before the analysis. Eight hours after the exercise protocol, the mice were anaesthetized with i.p. injection of sodium thiopental (40 mg (kg body weight)−1). Following the experimental procedures, the mice were killed under anaesthesia (thiopental 200 mg kg−1). A different set of mice were used for each experimental procedure.
Fasting glucose, insulin tolerance test (ITT), serum insulin determination and glycogen content
After the exercise protocol, mice (n= 8) were submitted to an insulin tolerance test (ITT; 1.5 U (kg body weight)−1 of insulin) after 6 h of fasting. Briefly, 1.5 IU kg−1 of human recombinant insulin (Humulin R) from Eli Lilly (Indianapolis, IN, USA) was injected i.p. into anaesthetized mice, and blood samples were then collected at 0, 5, 10, 15, 20, 25 and 30 min from the tail for serum glucose determination. The rate constant for plasma glucose disappearance (kITT) was calculated using the formula, 0.693/biological half-life (t1/2). The plasma glucose t1/2 was calculated from the slope of the least squares analysis of the plasma glucose concentration during the linear phase of decline (Bonora et al. 1989). Plasma glucose was determined using a glucose meter (Advantage – Boehringer Mannheim, Irvine, CA, USA). Plasma was separated by centrifugation (1500 g) for 15 min at 4°C and stored at −80°C until assay. Radioimmunoassay (RIA) was employed to measure serum insulin, according to a previous description (Scott et al. 1981). Glycogen content in liver fragments was measured according to a previously described method (Pimenta et al. 1989).
Clamp studies
After a 6 h fast, a 2 h euglycaemic–hyperinsulinaemic clamp study was performed (n= 6) (De Souza et al. 2005). Under sodium thiopental anaesthesia and aseptic conditions, a mono-occlusive polyethylene catheter was inserted into the carotid artery for infusion of insulin and glucose. A second polyvinyl catheter was inserted into the jugular vein, for blood sampling, and the animal was kept in a heated box (37°C) throughout the study. During the first phase of the study (30 min), a priming dose of insulin was infused at a rate of 3.6 mU kg−1 min−1 to achieve steady state concentrations of plasma insulin. After glucose equilibration, insulin infusion was maintained for 2 h with constant rate, and a variable infusion of glucose (5% solution) was adjusted to maintain the serum glucose concentration at approximately 6.6 mm. Blood samples were collected from the jugular vein every 5 min for serum glucose measurement and every 30 min for measurement of plasma insulin.
Protein analysis by immunoblotting
As soon as anaesthesia was assured by the loss of pedal and corneal reflexes the abdominal cavity was opened, the cava vein exposed, and 0.2 ml of normal saline (−) or 0.2 ml of insulin (10−6m) (+) was injected. After 30 s (proximal molecules) and 90 s (distal molecules) of insulin injection, hepatic tissue was excised. Seven hours after the acute exercise, a group of animals was subjected to a single i.p. injection (400 μl, 5 μm) of a PI3-K inhibitor (LY294002 – Calbiochem, La Jolla, CA, USA) and animals were killed after 1 h for extraction of hepatic tissue (n= 6). To evaluate the efficiency of the treatment, the phosphorylation of Akt was determined 1 h after LY294002 injection (n= 6) (see experimental design). The tissues were pooled, minced coarsely and homogenized immediately in extraction buffer (mm) (1% Triton X-100, 100 mm Tris, pH 7.4, containing 100 mm sodium pyrophosphate, 100 mm sodium fluoride, 10 mm EDTA, 10 mm sodium vanadate, 2 mm PMSF and 0.1 mg of aprotinin ml−1) at 4°C with a Polytron PTA 20S generator (Brinkmann Instruments model PT 10/35, Westbury, NY, USA) operated at maximum speed for 30 s. The extracts were centrifuged at 9000 g and 4°C in a Beckman 70.1 Ti rotor (Palo Alto, CA, USA) for 40 min to remove insoluble material, and the supernatants of these tissues were used for protein quantification, using the Bradford method (Bradford, 1976). Proteins were denaturated by boiling in Laemmli sample buffer (Laemmli, 1970) containing 100 mm dithiothreitol (DTT), run on SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were blocked, probed and developed. Insulin receptor (IR), insulin receptor substrate 2 (IRS-2), HNF-4α and Foxo1 were immunoprecipitated from samples of mouse liver protein extracts, with or without a previous insulin injection in the cava vein. Antibodies used for immunoblotting were anti-phosphotyrosine (PY), anti-IR, anti-phospho-Akt, anti-Akt, anti-pFoxo1, anti-Foxo1, anti-HNF4α, anti-histone (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-IRS-2 (Upstate Biotechnology, Lake Placid, NY, USA), anti-G6Pase, anti-PEPCK and β-actin (Cell Signaling Techonology, Beverly, MA, USA). Chemiluminescence detection was performed with horseradish peroxidase-conjugated secondary antibodies. Visualization of protein bands was performed by exposure of membranes to RX-films. The original membrane was stripped and reblotted with β-actin for loading protein. After transfer, the membrane was stained with Ponceau, and the bands were visualized, photographed and quantified of the primary antibody, to control of the transfer.
Nuclear extraction
To evaluate the subcellular localization of proteins, a fractionation protocol was employed as described previously (Prada et al. 2006). Liver fragments from mice of all groups (except LY290402 treatment) were minced and homogenized in 2 volumes of STE buffer (0.32 m sucrose, 20 mm Tris-HCl (pH 7.4), 2 mm EDTA, 1 mm DTT, 100 mm sodium fluoride, 100 mm sodium pyrophosphate, 10 mm sodium orthovanadate, 1 mm PMSF, and 0.1 mg aprotinin ml−1) at 4°C with a Polytron homogenizer. The homogenates were centrifuged (1000 g, 25 min, 4°C) and the pellets were washed once with STE buffer (1000 g, 10 min, 4°C) and suspended in Triton buffer (1% Triton X-100, 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 200 mm EDTA, 10 mm sodium orthovanadate, 1 mm PMSF, 100 mm NaF, 100 mm sodium pyrophosphate, and 0.1 mg aprotinin ml−1), kept on ice for 30 min, and centrifuged (15,000 g, 30 min, 4°C) to obtain the nuclear fraction. The samples (1 mg) were used for immunoprecipitation with Foxo1 antibody and Protein A-Sepharose 6MB (GE Healthcare, Little Chalfont, UK). After addition of Laemmli buffer with 100 mm DTT, samples were heated in a boiling water bath for 5 min, and aliquots (100 μg of protein) were subjected to SDS-PAGE and Western blotting with anti-IRS-2; alternatively, anti-histone antibodies were used to demonstrate the efficiency of the method.
Statistical analysis
Results were expressed as means ±s.e.m. Differences between the lean groups and mice at rest (ob/ob or Swiss DIO) and after the exercise protocol were evaluated using one-way analysis of variance (ANOVA). When the ANOVA indicated significance, Tukey's post hoc test was performed.
Results
Experimental design
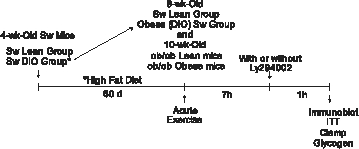 |
A single physical exercise bout improves fasting glucose and insulin action
Both ob/ob and DIO mice presented a significant increase in body mass, blood insulin and glucose concentrations, accompanied by impaired insulin action as compared with respective lean controls (Table 2). Following an acute bout of exercise, no changes were detected in body mass and blood insulin concentration. However, the exercise significantly reduced blood glucose levels in both ob/ob and DIO mice (Table 2). This was, at least in part, due to improved insulin action, as demonstrated by an increased kITT (Table 2).
Table 2.
Metabolic parameters of ob/ob and DIO mice at rest and after an acute bout of exercise
| Groups (n= 5) | Body weight (g) | Fasting insulin (ng ml−1) | Fasting glucose (mg dl−1) | kITT (% min−1) |
|---|---|---|---|---|
| lean | 26.8 ± 1.32 | 1.23 ± 0.49 | 86.3 ± 9.16 | 4.3 ± 0.76 |
| ob/ob | 45.7 ± 4.81* | 8.9 ± 3.04* | 239.5 ± 15.97* | 2.1 ± 0.37* |
| Exe | 49.4 ± 2.61* | 9.7 ± 2.11* | 109.5 ± 5.26*,# | 3.5 ± 0.96*,# |
| (C) control | 29.8 ± 2.26 | 1.68 ± 0.54 | 100.9 ± 9.62 | 4.2 ± 0.56 |
| DIO | 53.9 ± 5.88* | 13.9 ± 2.72* | 275.2 ± 22.83* | 2.0 ± 0.27* |
| DIO+Exe | 50.7 ± 4.32* | 15.6 ± 3.33* | 141.1 ± 15.79*,# | 3.3 ± 0.71*,# |
P < 0.05 vs. respective lean control mice;
P < 0.05 vs. respective obese sedentary mice; n= 8.
Acute exercise improves hepatic insulin signal transduction
The effects of in vivo insulin injection were examined in the livers of lean and obese mice submitted, or not, to an acute bout of exercise. Insulin-induced tyrosine phosphorylation of the IR was reduced by 2.0- and 2.5-fold in the liver of ob/ob and DIO mice, respectively, as compared with respective lean mice (Fig. 1A and B). Following exercise, increases of 1.6- and 1.7-fold insulin-induced IR tyrosine phosphorylation were detected in ob/ob and DIO mice, respectively, as compared to respective obese mice at rest (Fig. 1A and B). There were no differences in basal (non-insulin stimulated) levels of IR tyrosine phosphorylation between the groups (data not shown). The IR protein levels were not different between the groups (Fig. 1A and B, lower panels).
Figure 1. Insulin signalling in the hepatic tissue of controls, ob/ob and swiss DIO mice at rest or after acute exercise.
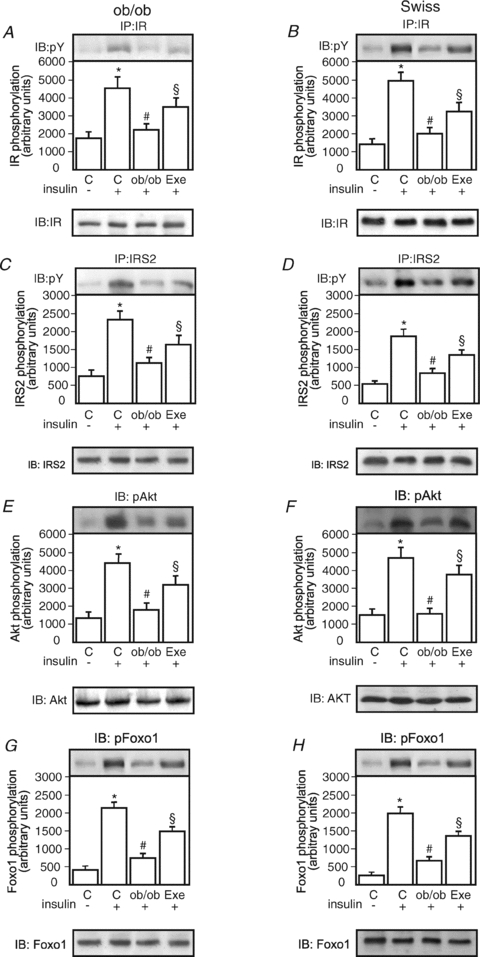
Liver extracts from mice injected with saline or insulin were prepared as described in Methods. A and B, tissue extracts were immunoprecipitated (IP) with anti-IR antibody and blotted (IB) with anti-PY antibody (upper panels) or anti-IR antibody (lower panels). C and D, tissue extracts were IP with anti-IRS-2 antibody and IB with anti-PY (upper panels) and anti-IRS-2 antibody (lower panels). E and F, liver extracts were IB with anti-phospho Akt or anti-Akt antibody (upper and lower panels, respectively). G and H, liver extracts were IB with anti-phospho Foxo1 or anti-Foxo1 antibody (upper and lower panels, respectively). The results of scanning densitometry are expressed as arbitrary units. Bars represent means ±s.e.m. of n= 6 mice. *P < 0.05, control stimulated-insulin (+) versus no insulin (−); #P < 0.05 ob/ob or DIO mice versus respective control; and §P < 0.05, exercised group versus ob/ob or DIO mice at rest.
Insulin-induced tyrosine phosphorylation of the IRS-2 was reduced by 2.0- and 2.1-fold in the liver of ob/ob and DIO mice, respectively, as compared with respective lean mice (Fig. 1C and D). Following exercise, increases of 1.4- and 1.5-fold insulin-induced IRS-2 tyrosine phosphorylation were detected in ob/ob and DIO mice, respectively, as compared to respective obese mice at rest (Figs 1C and 2D). There were no differences in basal (non-insulin stimulated) levels of IRS-2 tyrosine phosphorylation between the groups (data not shown). The IRS-2 protein levels were not different between the groups (Figs 1C and 2D, lower panels).
Figure 2. Isolation, immunoprecipitation, and nuclear extract expression of Foxo1 and HNF-4α in control, ob/ob and DIO mice at rest or after acute exercise.

Liver extracts from mice injected with saline or insulin were prepared as described in Methods. A and B, evaluation of the efficiency of isolation. IRS-2 expression in cytoplasm and nuclear extracts in ob/ob and DIO mice (upper panel) and histone expression in cytoplasm and nuclear extracts in ob/ob and DIO mice (lower panel). C and D, liver extracts were IB with anti-HNF-4α antibody. E and F, liver extracts were IB with anti-Foxo1 antibody. G and H, nuclear extracts were immunoprecipitated (IP) with anti-HNF-4α antibody and blotted (IB) with anti-Foxo1 antibody. Bars represent means ±s.e.m. of n= 6 mice. *P < 0.05, control stimulated-insulin (+) versus no insulin (−); #P < 0.05 ob/ob or DIO mice versus respective control; and §P < 0.05, exercised group versus ob/ob or DIO mice at rest.
Insulin-induced serine phosphorylation of Akt was reduced by 2.4- and 3.2-fold in the liver of ob/ob and DIO mice, respectively, as compared with respective lean mice (Fig. 1E and F). Following exercise, increases of 1.7- and 2.6-fold insulin-induced Akt serine phosphorylation were detected in ob/ob and DIO mice, respectively, as compared to respective obese mice at rest (Figs 1E and 2F). There were no differences in basal (non-insulin stimulated) levels of Akt serine phosphorylation between the groups (data not shown). The Akt protein levels were not different between the groups (Figs 1E and 2F, lower panels).
Insulin induced an increase in Foxo1 phosphorylation in the livers of both groups’ lean controls (Fig. 1G and H). Insulin-induced phosphorylation of Foxo1 was reduced by 3.0- and 3.0-fold in the liver of ob/ob and DIO mice, respectively, as compared with respective lean mice (Fig. 1G and H). Following exercise, increases of 2.9- and 2.5-fold insulin-induced Foxo1 phosphorylation were detected in ob/ob and DIO mice, respectively, as compared to respective obese mice at rest (Fig. 1G and H). There were no differences in basal (non-insulin stimulated) levels of Foxo1 phosphorylation between the groups (data not shown). The Foxo1 protein levels were not different between the groups (Fig. 1G and H, lower panels).
Acute exercise reduces HNF-4α/Foxo1 pathway in the liver
The efficiency of the method used for subcellular fractionation was evaluated. Figure 2A and B shows the protein levels of IRS-2, restricted to the cytosol (Fig. 2A), and the presence of histones, confined to the nucleus (Fig. 2B). Following insulin injection, the nuclear localization of HNF-4α was reduced by 1.6- and 2.3-fold in liver extracts of both groups’ controls (Fig. 2C and D). In resting obese ob/ob and DIO mice, nuclear protein levels of HNF-4α was increased by 2.9- and 3.2-fold, respectively, as compared to respective lean mice (Fig. 2C and D). In acutely exercised ob/ob and DIO mice, the nuclear protein level of HNF-4α was reduced by 2.3- and 1.8-fold, respectively, as compared to respective obese mice at rest (Fig. 2C and D).
Similarly, following acute insulin injection, the nuclear localization of Foxo1 was reduced by 3.9- and 4.6-fold in the livers of ob/ob and DIO, respectively (Fig. 2E and F). In resting ob/ob and DIO mice, the nuclear protein levels of Foxo1 was increased by 3.9- and 3.8-fold, respectively, as compared to respective lean mice (Fig. 2E and F). After an acute bout of exercise, the nuclear localization of Foxo1 was reduced by 1.9- and 1.9-fold, respectively, as compared to respective obese mice (Fig. 2E and F). Coimmunoprecipitation assays were used to determine the association of Foxo1 and HNF-4α. In the hepatic tissue, insulin injection led to reduced association HNF-4α/Foxo1 of 2.5- and 1.6-fold in groups controls (Fig. 2G and H). In resting obese ob/ob and DIO mice, the HNF-4α/Foxo1 association was increased by 2.8- and 2.7-fold, respectively, as compared to respective lean mice (Fig. 2C and D). In acutely exercised ob/ob and DIO mice the HNF-4α/Foxo1 association was reduced by 1.1- and 1.8-fold, respectively, as compared to respective obese mice at rest (Fig. 2C and D). There was no difference in basal levels of HNF-4α/Foxo1 association between the groups (data not shown).
Acute exercise reduces G6Pase and PEPCK protein levels and increases glycogen content in the hepatic tissue
We next determined the expression of PEPCK in the liver of lean controls and obese mice at rest or submitted to an acute bout of exercise. In ob/ob and DIO mice at rest, the expression of PEPCK was increased by 2.4-fold and 2.3-fold, respectively. Interestingly, at 8 h after acute exercise, the PEPCK protein level was decreased by 1.8- and 1.6-fold in ob/ob and DIO, respectively, as compared with respective obese mice at rest (Fig. 3A and B). We next measured the expression of G6Pase in the liver of lean controls and obese mice at rest or submitted to acute exercise. In the hepatic tissue of ob/ob and DIO mice at rest, the expression of G6Pase was increased by 4.0- and 2.6-fold in ob/ob and DIO mice, respectively, as compared to the respective lean control mice. After acute exercise, the protein levels of G6Pase was decreased by 1.6- and 1.7-fold in ob/ob and DIO groups, respectively, as compared to obese diabetic mice at rest (Fig. 3C and D).
Figure 3. PEPCK and G6Pase expression, hepatic glycogen contents and clamp under fasting conditions in control, ob/ob and DIO mice at rest or after acute exercise.
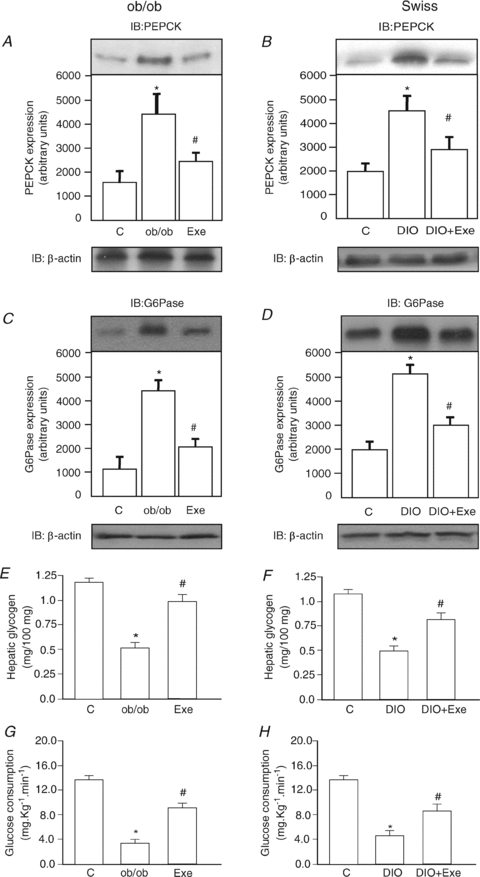
Liver extracts from mice were prepared as described in Methods. A and B, tissue extracts were blotted (IB) with anti-PEPCK antibody in ob/ob and DIO mice, respectively. C and D, tissue extracts were blotted (IB) with anti-G6Pase antibody in ob/ob and DIO mice, respectively. The results of scanning densitometry are expressed as arbitrary units (n= 6). E and F, glycogen contents (mg per 100 mg of tissue) (n= 8). G and H, the rate of glucose consumption (mg kg−1 min−1) during a euglycaemic–hyperinsulinaemic clamp is presented (n= 6). Bars represent means ±s.e.m.*P < 0.05, ob/ob or DIO mice versus respective control and #P < 0.05, exercised group versus ob/ob or DIO mice at rest.
Hepatic gluconeogenesis is tightly controlled by insulin. Since acute exercise leads to high glucose turnover in spite of low basal and stimulated insulin levels, we decided to evaluate glycogen contents in the livers of our experimental groups. In resting ob/ob mice, glycogen content was reduced by 2.0-fold, when compared with the lean controls (Fig. 3E). In resting DIO mice, glycogen content was reduced by 2.2-fold, when compared with lean controls (Fig. 3F). Following an acute bout of exercise, glycogen content increased by 1.5- and 1.6-fold, in ob/ob and DIO mice, respectively as compared to the respective obese groups at rest (Fig. 3E and F). Finally, during the euglycaemic–hyperinsulinaemic clamp, we observed an increase in the glucose consumption rate of 2.3- and 2.0-fold in acutely exercised ob/ob and DIO mice, respectively, as compared to respective obese mice at rest (Fig. 3G and H). Interestingly, after acute exercise, the obese groups showed the same values as the non-exercising lean groups, demonstrating that exercise in obese models restored levels to those seen in non-exercising controls.
A PI3K inhibitor reverses the protective effect of acute exercise on the Foxo1/HNF-4α association
In order to determine the role played by the PI3K/Akt signalling pathway in the protective effect of acute exercise on hepatic insulin resistance, we used the PI3K inhibitor, LY294002, and evaluated signal transduction and the HNF-4α/Foxo1 pathway. LY294002 treatment led to 16- and 15-fold reduction of insulin-induced Akt serine phosphorylation in acutely exercised ob/ob and DIO respectively, as compared to acutely exercised, non-LY294002 treated mice (Fig. 4A and B). In addition, LY294002 produced a 14- and 32-fold reduction in insulin-induced Foxo1 phosphorylation in acutely exercised ob/ob and DIO, respectively, as compared to acutely exercised, non-LY294002 treated mice (Fig. 4C and D).
Figure 4. Analysis of insulin-induced phosphorylation of the Akt and Foxo1, expression of HNF-4α and immunoprecipitation of Foxo1 in the liver of ob/ob and DIO mice submitted to the exercise protocol plus single injection of LY294002.

Liver extracts from mice were prepared as described in Methods. A and B, liver extracts were IB with anti-phospho Akt or anti-Akt antibody (upper and lower panels, respectively). C and D, liver extracts were IB with anti-phospho Foxo1 or anti-Foxo1 antibody (upper and lower panels, respectively). E and F, liver extracts were IB with anti-phospho HNF-4α or anti-β-actin antibodies (upper and lower panels, respectively). G and H tissue extracts were IP with anti-Foxo1 and IB anti-HNF-4α (upper panels) or IB anti-HNF-4α antibodies (lower panels). Bars represent means ±s.e.m. of n= 6 mice. *P < 0.05, control stimulated-insulin (+) versus no insulin (−); #P < 0.05 ob/ob or DIO mice versus respective control; §P < 0.05, exercised group versus ob/ob or DIO mice at rest, and $P < 0.05, exercised plus LY290402 groups versus ob/ob or DIO exercised mice.
In hepatic tissue of acutely exercised ob/ob and DIO mice, the treatment with LY294002 produced increases in HNF-4α protein levels of 2.3- and 2.3-fold, respectively, as compared with respective exercised, non-LY290402 treated obese mice (Fig. 4E and F). Finally, the association of HNF-4α/Foxo1 was increased by 2.2- and 2.3-fold in the livers of acutely exercised, LY294002-treated ob/ob and DIO mice, respectively (Fig. 4G and H). There was no difference in the basal levels of the HNF-4α/Foxo1 association in the groups (data not shown).
Inhibition of PI3K reverses the exercise-induced reduction of PEPCK and G6Pase protein levels
To determine the role of the PI3K/Akt pathway in mediating the effects of acute exercise on the expression of enzymes involved in gluconeogenesis, obese mice were treated with LY292004, and PEPCK and G6Pase expressions were determined by immunoblot. As shown in Fig. 5A and B, inhibition of PI3K resulted in 1.9- and 2.8-fold increases of PEPCK protein levels in the liver of acutely exercised ob/ob and DIO mice, respectively, as compared to obese mice non-treated with LY292004. In addition, LY292004 treatment resulted in 2.5- and 2.0-fold increases in G6Pase protein levels in the liver of exercised ob/ob and DIO mice, respectively, as compared to obese mice not treated with LY292004 (Fig. 5C and D).
Figure 5. PEPCK and G6Pase expression in control, ob/ob and DIO mice after acute exercise plus a single injection of LY294002.

Liver extracts from mice were prepared as described in Methods. A and B, tissue extracts were blotted (IB) with anti-PEPCK or anti-β-actin antibodies (upper and lower panels, respectively). C and D, tissue extracts were blotted (IB) with anti-G6Pase or anti-β-actin antibodies (upper and lower panels, respectively). The results of scanning densitometry are expressed as arbitrary units. Bars represent means ±s.e.m. of n= 6 mice. *P < 0.05, ob/ob or DIO mice versus respective control; #P < 0.05, exercised group versus ob/ob or DIO mice at rest; and §P < 0.05, exercised plus LY290402 groups versus ob/ob or DIO exercised mice.
Discussion
In type 2 diabetes, excessive hepatic glucose production is a major mechanism involved in fasting hyperglycaemia. It results from many causes, including increased glycogenolysis, decreased glycogen synthesis, decreased glycolytic flux and gluconeogenisis. Insulin resistance plays an important role in this process, including the inability of insulin to inhibit PEPCK and G6Pase (for review, see Barthel & Schmoll, 2003). Many factors are likely to contribute to the effects of insulin on PEPCK and G6Pase expression, including effects on CBP, TORC proteins, PGC-1α, SREBP-1c, Foxo1 and HNF-4α (Yoon et al. 2001; Boustead et al. 2003; Hirota et al. 2003; Hirota et al. 2008). Since physical activity is known to favour glucose homeostasis through a number of mechanisms, including the reduction of hepatic glucose production (Hoene et al. 2009; Lima et al. 2009; Ropelle et al. 2009), we decided to evaluate the role of acute exercise in the control of Foxo1 and HNF-4α and in the regulation of PEPCK and G6Pase protein levels.
In the first part of the study, we employed two distinct methods, ITT and clamp, to demonstrate the beneficial effect of acute exercise on whole body insulin action in two distinct models of obesity and diabetes. Also, by immunoblot, we showed that insulin signal transduction through the IR/IRS-2/Akt signalling pathway was improved in the livers of acutely exercised animals. Moreover, glycogen levels were also increased in the liver of these animals.
In the present study, we observed that, in both ob/ob and DIO mice at rest, there was an increase in the protein levels of Foxo1 and HNF-4α in the hepatic tissue. Following acute exercise, significant reductions in the protein levels of both Foxo1 and HNF-4α were observed. Previous studies have shown that the regulation of transcriptional activity of the HNF-4α/Foxo1 pathway depends upon insulin signal transduction through the insulin/Foxo1 pathway (Hirota et al. 2008). To test whether this signalling system may mediate the effects of exercise on Foxo1 and HNF-4α, we employed an inhibitor of PI3K. Our results show that a single dose of LY292004 suppressed the phosphorylation of Akt and Foxo1 and inhibited the increased sensitivity to insulin in acutely exercised mice. This effect was accompanied by increased Foxo1 and HNF-4α protein levels and hepatic protein levels of the enzymes PEPCK and G6Pase in both of the strains of mice examined.
Foxo1 plays a key role in switching between glycolysis and gluconeogenesis, and HNF-4α is an indispensable component of this switching mechanism. In the absence of insulin, Foxo1 localizes to the nucleus; simultaneously, Foxo1 and HNF-4α synergistically activate the G6Pase promoter, presumably via PGC-1α, to favour gluconeogenesis. In the presence of insulin, Foxo1 is phosphorylated and excluded from the nucleus, resulting in its dissociation from HNF-4α; thus, HNF-4α can activate the GK gene promoter. In this way, insulin signalling shifts the metabolic balance in favour of glycolysis by suppressing G6Pase gene expression and activating GK gene expression (Hirota et al. 2008).
Hirota and coworkers showed that Foxo1 confers either positive or negative transcriptional influence on HNF-4α-potentiated gene activity in a promoter context-dependent manner. It has been reported that G6Pase contains binding sites for both HNF-4α and Foxo1; in this case, these molecules play synergistic effects (Hirota et al. 2008). Our results suggest a possible inhibition of the synergistic effect induced by acute exercise mediated by the improvement in hepatic insulin signalling and Foxo1 phosphorylation and extrusion from the nucleus. Finally, the present study clearly demonstrates the effects of exercise on Foxo1 and HNF-4α levels and activities, which appear to be relevant for the control of hepatic gluconeogenic gene expression. Unfortunately, the present study has limitations, especially regarding measurement of the GK protein level.
One point to be explored is the participation of PGC-1α in this process. According to the reports described above, Foxo1 and HNF-4α synergistically activate the G6Pase promoter, presumably via PGC-1α. Additional studies have shown the participation of the co-activator of transcription, PGC-1α, in the control of hepatic gluconeogenesis (Yoon et al. 2001; Iordanidou et al. 2005). PGC-1α can increase the HNF-4α-mediated transactivation of PEPCK and G6Pase genes and, therefore, play an important role in the process described previously (Lacorte et al. 1997; Lavrentiadou et al. 1999; Yoon et al. 2001). PGC-1α is a versatile coactivator of transcriptional factors such as HNF-4α (Yoon et al. 2001). Coactivation of HNF-4α activity by PGC-1α has also been implicated in the nutritional regulation of hepatic gluconeogenic genes (Yoon et al. 2001; Rhee et al. 2003). As a part of the current study, we evaluated the expression of PGC-1α, by immunoblotting, and observed a reduction following exercise. A single dose of LY292004 significantly increased PGC-1α expression (data not shown). These findings are in accordance with a recent study by our group (Ropelle et al. 2009) demonstrating that acute exercise reduces the Foxo1/PGC-1α pathway in the liver of diet-induced obesity rodents. It seems clear that acute exercise acts to control liver production of glucose by the integration of a number of mechanisms, such as that involving sterol responsive element binding protein 1c (SREBP-1c). SREBP-1c has been implicated as a physiological inhibitor of hepatic glucose production (Bécard et al. 2001; Chakravarty et al. 2004). Consistent with these earlier studies, SREBP-1c inhibited transcription of PEPCK by interfering in the action of HNF-4α (Yamamoto et al. 2004). In addition, in DIO-induced obese mice submitted to 8 weeks of endurance (treadmill running), we observed a reduction in SREBP-1c protein levels (manuscript submitted).
In summary, the present study shows that exercise acutely improves the action of insulin in the liver of animal models of obesity and diabetes, resulting in increased phosphorylation of Foxo1, and a reduction in the Foxo1/HNF4α pathway. Foxo1 and HNF-4 are important for stimulating expression of PEPCK and G6Pase, and exercise appears to promote the phosphorylation of Foxo1 and suppress levels of both Foxo1 and HNF-4; this is likely to be important for suppressing PEPCK and G6Pase, and possibly reduces hepatic glucose output/gluconeogenesis. We believe that the regulation of Foxo1 and HNF4α protein levels is an important mechanism involved in exercise-induced improvement of hepatic production of glucose in insulin-resistant states.
Acknowledgments
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We thank Dr Nicola Conran for English grammar editing.
Glossary
Abbreviations
- G6Pase
glucose-6-phosphatase
- GK
glucokinase
- HNF-4α
protein hepatocyte nuclear factor 4α
- IR
insulin receptor
- IRS-2
insulin receptor substrate 2
- ITT
insulin tolerance test
- kITT
rate constant for plasma glucose disappearance
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PI3K
phosphatidylinositol 3-kinase
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
Author contributions
C.T.D.S.: conception, design, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content; M.J.S.F.: conception and design; G.L.: conception and design; D.E.C.: conception, design, analysis and interpretation of data; E.R.R.: conception, design, analysis and interpretation of data; J.R.P.: conception, design, analysis and interpretation of data; L.A.V.: conception, design, analysis and interpretation, drafting the article and revising it critically for important intellectual content.
References
- Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab. 2003;285:E718–E728. doi: 10.1152/ajpendo.00156.2003. [DOI] [PubMed] [Google Scholar]
- Anai M, Funaki M, Ogihara T, Terasaki J, Inukai K, Katagiri H, Fukushima Y, Yazaki Y, Kikuchi M, Oka Y, Asano T. Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes. 1998;47:13–23. doi: 10.2337/diab.47.1.13. [DOI] [PubMed] [Google Scholar]
- Aoi W, Ichiishi E, Sakamoto N, Tsujimoto A, Tokuda H, Yoshikawa T. Effect of exercise on hepatic gene expression in rats: a micro array analysis. Life Sci. 2004;75:3117–3128. doi: 10.1016/j.lfs.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285:E685–692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- Bécard D, Hainault I, Azzout-Marniche D, Bertry-Coussot L, Ferré P, Foufelle F. Adenovirus-mediated overexpression of sterol regulatory element binding protein-1c mimics insulin effects on hepatic gene expression and glucose homeostasis in diabetic mice. Diabetes. 2001;50:2425–2430. doi: 10.2337/diabetes.50.11.2425. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–378. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- Boustead JN, Stadelmaier BT, Eeds AM, Wiebe PO, Svitek CA, Oeser JK, O’Brien RM. Hepatocyte nuclear factor-4α mediates the stimulatory effect of peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) on glucose-6-phosphatase catalytic subunit gene transcription in H4IIE cells. Biochem J. 2003;369:17–22. doi: 10.1042/BJ20021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buettner C, Patel R, Muse ED, Bhanot S, Monia BP, McKay R, Obici S, Rossetti L. Severe impairment in liver insulin signalling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty K, Wu SY, Chiang CM, Samols D, Hanson RW. SREBP-1c and Sp1 interact to regulate transcription of the gene for phosphoenolpyruvate carboxykinase (GTP) in the liver. J Biol Chem. 2004;279:15385–15395. doi: 10.1074/jbc.M309905200. [DOI] [PubMed] [Google Scholar]
- De Souza CT, Araújo EP, Prada PO, Saad MJ, Boschero AC, Velloso LA. Short-term inhibition of peroxisome proliferator-activated receptor-γ coactivator-1α expression reverses diet-induced diabetes mellitus and hepatic steatosis in mice. Diabetologia. 2005;9:1860–1871. doi: 10.1007/s00125-005-1866-4. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction of the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Guerra MJ, Bergot MO, Martinez A, Cuif MH, Kahn A, Raymondjean M. Functional characterization of the L-type pyruvate kinase gene glucose response complex. Mol Cell Biol. 1993;13:7725–7733. doi: 10.1128/mcb.13.12.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes T, Senkel S, Holewa B, Ryffel GU. Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol. 1996;16:925–931. doi: 10.1128/mcb.16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–2561. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- Ganjam GK, Dimova EY, Unterman TG, Kietzmann T. FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. J Biol Chem. 2009;284:30783–30797. doi: 10.1074/jbc.M109.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Daitoku H, Matsuzaki H, Araya N, Yamagata K, Asada S, Sugaya T, Fukamizu A. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. J Biol Chem. 2003;278:13056–13060. doi: 10.1074/jbc.C200553200. [DOI] [PubMed] [Google Scholar]
- Hirota K, Sakamaki J, Ishida J, Shimamoto Y, Nishihara S, Kodama N, Ohta K, Yamamoto M, Tanimoto K, Fukamizu A. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J Biol Chem. 2008;47:32432–32441. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- Hoene M, Lehmann R, Hennige AM, Pohl AK, Häring HU. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol. 2009;587:241–252. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin dependent diabetic subjects during mixed meal ingestion: a 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95:783–787. doi: 10.1172/JCI117727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanidou P, Aggelidou E, Demetriades C, Hadzopoulou-Cladaras M. Distinct amino acid residues may be involved in coactivator and ligand interactions in hepatocyte nuclear factor-4α. J Biol Chem. 2005;23:21810–21819. doi: 10.1074/jbc.M501221200. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Gene Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Lacorte JM, Ktistaki E, Beigneux A, Zannis VI, Chambaz J, Talianidis I. Activation of CAAT enhancer-binding protein δ (C/EBPδ) by interleukin-1 negatively influences apolipoprotein C-III expression. J Biol Chem. 1997;38:23578–23584. doi: 10.1074/jbc.272.38.23578. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavrentiadou SN, Hadzopoulou-Cladaras M, Kardassis D, Zannis VI. Binding specificity and modulation of the human ApoCIII promoter activity by heterodimers of ligand-dependent nuclear receptors. Biochemistry. 1999;38:964–975. doi: 10.1021/bi981068i. [DOI] [PubMed] [Google Scholar]
- Lima AF, Ropelle ER, Pauli JR, Cintra DE, Frederico MJ, Pinho RA, Velloso LA, De Souza CT. Acute exercise reduces insulin resistance-induced TRB3 expression and amelioration of the hepatic production of glucose in the liver of diabetic mice. J Cell Physiol. 2009;221:92–97. doi: 10.1002/jcp.21833. [DOI] [PubMed] [Google Scholar]
- Luciano E, Carneiro EM, Carvalho CR, Carvalheira JB, Peres SB, Reis MA, Saad MJ, Boschero AC, Velloso LA. Endurance training improves responsiveness to insulin and modulates insulin signal transduction through the phosphatidylinositol 3-kinase/Akt-1 pathway. Eur J Endocrinol. 2002;147:149–157. doi: 10.1530/eje.0.1470149. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park B-C, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Pauli JR, Ropelle ER, Cintra DE, Carvalho-Filho MA, Moraes JC, De Souza CT, Velloso LA, Carvalheira JB, Saad MJ. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. J Physiol. 2008;586:659–671. doi: 10.1113/jphysiol.2007.142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres SB, de Moraes SM, Costa CE, Brito LC, Takada J, Andreotti S, Machado MA, Alonso-Vale MI, Borges-Silva CN, Lima FB. Endurance exercise training increases insulin responsiveness in isolated adipocytes through IRS/PI3-kinase/Akt pathway. J Appl Physiol. 2005;98:1037–1043. doi: 10.1152/japplphysiol.00536.2004. [DOI] [PubMed] [Google Scholar]
- Pimenta WP, Saad MJ, Paccola GM, Piccinato CE, Foss MC. Effect of oral glucose on peripheral muscle fuel metabolism in fasted men. Braz J Med Biol Res. 1989;4:465–476. [PubMed] [Google Scholar]
- Prada PO, Pauli JR, Ropelle ER, Zecchin HG, Carvalheira JB, Velloso LA, Saad MJ. Selective modulation of the CAP/Cbl pathway in the adipose tissue of high fat diet treated rats. FEBS Lett. 2006;20:4889–4894. doi: 10.1016/j.febslet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through Foxo1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci U S A. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Cintra DE, Frederico MJ, de Pinho RA, Velloso LA, De Souza CT. Acute exercise modulates the Foxo1/PGC-1α pathway in the liver of diet-induced obesity rats. J Physiol. 2009;587:2069–2076. doi: 10.1113/jphysiol.2008.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol. 2006;577:997–1007. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Kawano Y, Galuska D, Fahlman R, Wallberg-Henriksson H, Charron MJ, Zierath JR. Postexercise glucose uptake and glycogen synthesis in skeletal muscle from GLUT4-deficient mice. FASEB J. 1999;13:2246–2256. doi: 10.1096/fasebj.13.15.2246. [DOI] [PubMed] [Google Scholar]
- Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHREvidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- Scott AM, Atwater I, Rojas E. A method for the simultaneous measurement of insulin release and B cell membrane potential in single mouse islets of Langerhans. Diabetologia. 1981;21:470–475. doi: 10.1007/BF00257788. [DOI] [PubMed] [Google Scholar]
- Sladek FM, Zhong WM, Lai E, Darnell JE., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4α regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;24:13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan XD, Yang WB, Xia YZ, Wang JF, Lu T, Wang XM. Disruption of glucose homeostasis and induction of insulin resistance by elevated free fatty acids in human L02 hepatocytes. J Endocrinol Invest. 2009;32:454–459. doi: 10.1007/BF03346485. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimano H, Nakagawa Y, Ide T, Yahagi N, Matsuzaka T, Nakakuki M, Takahashi A, Suzuki H, Sone H, Toyoshima H, Sato R, Yamada N. SREBP-1 interacts with hepatocyte nuclear factor-4α and interferes with PGC-1 recruitment to suppress hepatic gluconeogenic genes. J Biol Chem. 2004;279:12027–12035. doi: 10.1074/jbc.M310333200. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]


