Abstract
Purinergic regulation of airway innate defence activities is in part achieved by the release of nucleotides from epithelial cells. However, the mechanisms of airway epithelial nucleotide release are poorly understood. We have previously demonstrated that ATP is released from ionomycin-stimulated airway epithelial goblet cells coordinately with mucin exocytosis, suggesting that ATP is released as a co-cargo molecule from mucin-containing granules. We now demonstrate that protease-activated-receptor (PAR) agonists also stimulate the simultaneous release of mucins and ATP from airway epithelial cells. PAR-mediated mucin and ATP release were dependent on intracellular Ca2+ and actin cytoskeleton reorganization since BAPTA AM, cytochalasin D, and inhibitors of Rho and myosin light chain kinases blocked both responses. To test the hypothesis that ATP is co-released with mucin from mucin granules, we measured the nucleotide composition of isolated mucin granules purified based on their MUC5AC and VAMP-8 content by density gradients. Mucin granules contained ATP, but the levels of ADP and AMP within granules exceeded by nearly 10-fold that of ATP. Consistent with this finding, apical secretions from PAR-stimulated cells contained relatively high levels of ADP/AMP, which could not be accounted for solely based on ATP release and hydrolysis. Thus, mucin granules contribute to ATP release and also are a source of extracellular ADP and AMP. Direct release of ADP/AMP from mucin granules is likely to provide a major source of airway surface adenosine to signal in a paracrine faction ciliated cell A2b receptors to activate ion/water secretion and appropriately hydrate goblet cell-released mucins.
Introduction
The airway mucociliary clearance (MCC) system is crucial for innate lung defence. The major components of MCC are ciliary beating and airway surface liquid (ASL) generated by mucin secretion and electrolyte/fluid transport. The balance among ASL volume, mucin secretion and ciliary beating ensures the rapid removal of inhaled foreign materials. Failure in this balance may lead to airway obstructive and inflammatory diseases. For example, cystic fibrosis results from aberrant ion transport activities and impaired hydration of the ASL, mucus accumulation and ultimately chronic obstructive pulmonary disease (Boucher, 2007). Thus, a key physiological component of lung defence involves mucin hydration and clearance.
However, mucin secretion and ASL hydration/clearance functions reside in distinct cell types, i.e. goblet and ciliated cells, respectively, and thus coordination of these activities is required for effective mucus clearance. A body of evidence suggests that ATP release from airway epithelial cells provides a mechanism for the control of MCC functions (reviewed in Lazarowski & Boucher, 2009). Extracellular ATP and its metabolite adenosine activate subsets of purinergic receptors expressed on the mucosal surface of airway epithelial cells. The predominant nucleotide-sensing receptor in the airways is the Gq-coupled P2Y2 receptor, which is activated by ATP and UTP. P2Y2 receptor activation promotes mucin secretion and ciliary beating, inhibition of the epithelial Na+ channel ENaC, and activation of cystic fibrosis transmembrane conductance regulator (CFTR) and the Ca2+-activated Cl− channel. Extracellular ATP hydrolysis results in formation of adenosine, which activates the Gs-coupled A2b receptor, promoting cyclic AMP-regulated CFTR Cl− channel activity and thus fluid secretion (Lazarowski & Boucher, 2009). Functional and biochemical evidence suggest that adenosine/A2b receptor is the major regulator of CFTR activity in airway epithelium (Lazarowski et al. 2004). ATP and adenosine are naturally occurring components of ASL (Lazarowski et al. 2004), but the cellular pathways that yield these extracellular molecules are poorly defined.
Given the complex cellular composition of airway epithelia, multiple mechanisms and pathways are likely to participate in the release of nucleotides into ASL. Nucleotide release from airway epithelial cells has been proposed to occur via different mechanisms: (i) tonic release from vesicles via a constitutive pathway in non-mucous cells (Sesma et al. 2009); (ii) a conductive mechanism likely to involve pannexin hemichannels, potentially localized within ciliated cells (Ransford et al. 2009; Seminario-Vidal et al. 2009); and (iii) ATP release associated with Ca2+-regulated exocytosis (Boudreault & Grygorczyk, 2004).
We recently demonstrated that ATP release from goblet cell-like Calu-3 cells was associated with Ca2+-promoted mucin secretion (Kreda et al. 2007). An attractive hypothesis derived from this observation is that mucin granules store ATP, and probably other purine nucleotides. Relevant to this hypothesis, a recent mathematical model of nucleotide regulation in the ASL predicts the release of AMP and ADP accompanying ATP from a vesicular pool (Zuo et al. 2008). Thus, a mix of nucleotides released upon mucin granule exocytosis could provide paracrine signalling to ciliated cells to increase ion and water secretion to support mucin hydration and ultimately mucus clearance. However, the contribution of mucin granule exocytosis to ASL nucleotides has awaited quantification of nucleotide concentration within mucin granules.
Recently, we discovered that the serine protease thrombin elicited robust ATP release from well-differentiated primary cultures of normal human bronchial epithelial (WD-HBE) cells (Seminario-Vidal et al. 2009). Thrombin-promoted ATP release was mediated via activation of cognate G-protein coupled protease-activated receptors (PARs). The family of PARs includes four members (PAR1, PAR2, PAR3 and PAR4). Thrombin is the physiological activator of PAR1, PAR3 and PAR4; however, other proteases can cleave these receptors and may contribute to their function in vivo (Russo et al. 2009). PAR2 is activated by multiple serine proteases including trypsin and tryptase, but not by thrombin (Russo et al. 2009). Relevant to our studies, activation of PARs has been described to induce mucin secretion from gastrointestinal and airway epithelial cells (Kawabata et al. 2001; Lin et al. 2008). Thus, PAR activation of airway goblet cells may provide a useful model to investigate the potential coordination between ATP release and mucin secretion.
In the present study, we used WD-HBE and airway goblet-like Calu-3 cells to investigate (i) whether PAR agonist-elicited mucin exocytotic secretion is associated with enhanced nucleotide release, and (ii) whether mucin granules purified from airway goblet cells contain ATP and possibly other adenyl nucleotide species.
Methods
Cell culture
Airway epithelial Calu-3 cells are derived from pleural effusion associated with human lung adenocarcinoma (Shen et al. 1994). Unless otherwise indicated, Calu-3 cells were grown on 12 mm Transwell supports and maintained at air–liquid interface for at least 2 weeks, as described previously (Kreda et al. 2007). Well-differentiated human bronchial epithelial cells were grown on collagen-coated 12 mm Transwell supports and maintained at the air–liquid interface for at least 4 weeks, as described previously (Kreda et al. 2005). Human tissue specimens for cell culture production and mRNA expression analyses were collected according to the guidelines of the Institutional Review Board for Protection of Human Rights at the University of North Carolina at Chapel Hill.
RT-PCR analysis
Total RNA was prepared using the RNeasy Mini Kit (Qiagen) and reverse-transcribed using Super-Script III reverse transcriptase (RT, Invitrogen). RT-PCR analyses were performed at the University of North Carolina at Chapel Hill (UNC-CH) Cystic Fibrosis Center Molecular Biology Core Lab using standardized protocols. Amplified PCR products were identified by sequence analysis at the UNC-CH DNA sequencing facility. Primers used to amplify human PAR1, PAR2, PAR3 and PAR4 were prepared according to Seminario-Vidal et al. (2009). A fragment between bp 71 and 339 of human VAMP-8 was amplified with forward primer (F) 5′-AGGTGGAGGAAATGATCGTG and reverse primer (R) 5′-TGGCAAAGAGCACAATGAAG.
Quantification of ATP with the luciferin–luciferase assay
To measure ATP release, WD-HBE and Calu-3 cell cultures were rinsed and pre-incubated in 0.4 ml basolateral, 0.25 ml mucosal Hanks’ balanced salt solution (HBSS) with 20 mm Hepes and 1.6 mm Ca2+ and 1.8 mm Mg2+. ATP hydrolysis inhibitors (30 μm ebselen and 300 μmβ, γ-methylene-ATP) were added for 5 min prior to stimuli. At the end of the incubation, aliquots of the extracellular baths were removed, and heated to 95°C to inactivate nucleotidases, as described (Seminario-Vidal et al. 2009). The luciferin–luciferase reaction mix was added to tubes and luminescence recorded in an Auto-Lumat LB953 luminometer (Lazarowski et al. 2000). An ATP standard curve was performed in parallel. None of the reagents used during ATP release measurements interfered with the luciferase reaction.
Mucin secretion
MUC5AC release by WD-HBE and Calu-3 cells was determined by immuno-slot blot analysis of the extracellular media, as previously described (Kreda et al. 2007). Slot blots were scanned and quantified in a LI-COR Odyssey system (Lincoln, NE, USA).
Intracellular calcium measurements
Calu-3 cells grown on glass coverslips were loaded with Fura-2 AM for 15–30 min. Cells were washed, mounted on a platform of a fluorometer-coupled Nikon microscope, and fluorescence from 30–40 cells was acquired alternately at 340 and 380 nm. Other details were as previously described (Seminario-Vidal et al. 2009).
Immunofluorescence and confocal microscopy
Cell cultures were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100 for 10 min, and subjected to immunofluorescence staining and confocal microscopic analysis, as previously described (Kreda et al. 2005, 2007).
Quinacrine associated granule fluorescence
Calu-3 cell secretory granules were pre-labelled with 10 μm quinacrine, as previously described (Kreda et al. 2007). Cells were mounted on the stage of the confocal microscope and real-time recording was performed every 10 and 30 s in the xy axes. The fluorescence intensity of all the pixels contained within granules of 1–2 μm diameter were measured at each time point, normalized to basal values (time = 0), and averaged using Leica software. To confirm the cellular location of quinacrine-loaded granules, y-stacks were generated at the end of each experiment.
Mucin granule isolation
Calu-3 cells were grown in four 75 cm2 culture flasks for at least 10 days. Cells were detached using Varsene solution. In some experiments, cells were loaded with quinacrine for 15 min prior to detaching from the flask. Cells were pelleted at 500 g and resuspended in 4 ml of ice-cold lysis buffer (Pipes 20 mm pH 6.8, 130 mm potassium glutamate, 3 mm MgCl2, 0.1 mm CaCl2, and 3 mm EGTA). Cells were disrupted by cavitation (800–1000 p.s.i., 30 min on ice). Lysate was centrifuged for 3 min at 500 g and the supernatant pelleted at 3000 g for 3 min. The resulting pellet was resuspended in isotonic 50% Percoll suspension prepared in lysis buffer and centrifuged at 4°C for 20 min at 60,000 g using a TLS 55 rotor (TL100 ultracentrifuge, Beckman, USA). The first 0.5 ml of each gradient was collected, mixed with 1.5 ml isotonic 50% Percoll suspension, and submitted to a second ultra-centrifugation for 30 min at the same speed. Twenty fractions (100 μl each) were collected and stored at −80°C for further analyses. In experiments using quinacrine-loaded cells, fraction aliquots were examined under the fluorescence microscope to visualize granule-associated fluorescence. The fractions of the second gradient were analysed for nucleotide concentration and by immuno-slot blot (Kreda et al. 2007), using antibodies against cellular markers MUC5AC and VAMP-8 (mucin granules), MUC1 (plasma membrane), GM 130 and p230 (Golgi), protein disulfide isomerase (PDI, endoplasmic reticulum), LAMP-1 (lysosome), and mitochondrial cytochrome oxidase subunits III/IV and ATP-synthase α-subunit.
Quantification of adenyl purines via etheno-derivatization and HPLC analysis
Cell cultures were rinsed and incubated as above, except that ATP hydrolysis inhibitors were omitted due to interference with the detection of etheno-derivatives. To quantify adenyl species within isolated mucin granules, purified granules were disrupted with ice-cold 5% trichloroacetic acid followed by ethyl ether extraction, as previously described (Lazarowski et al. 2000). Samples were derivatized with chloroacetaldehyde and the resulting fluorescent etheno-species analysed by HLPC, as previously described (Lazarowski et al. 2004).
ATP hydrolysis
Isolated mucin granules (2 μg protein) were resuspended in ice-cold 100 μl Hepes-buffered HBSS (pH 7.4) containing 0.1 μCi [3H]ATP (100 μm). Reactions were initiated by transferring the tubes to a 37°C water bath followed by the immediate addition of either vehicle or 0.1% Triton X-100. At the end of the incubation, samples were heated (2 min at 95°C) to inactivate ATPase activities. The resulting [3H]-species were separated by HPLC, as previously described (Lazarowski et al. 2000).
Reagents
Bafilomycin A1, cytochalasin D, ionomycin, H-1152, ML7 and Y27632 were purchased from Calbiochem (San Diego, CA, USA). 2-Phenyl-1,2-benzisoselenazol-3(2H)-one (ebselen), β, γ-methylene-ATP, luciferase from Photinus pyralis, Percoll and quinacrine were purchased from Sigma (St Louis, MO, USA). BAPTA AM, fluorescently-labelled phalloidin, Fura-2 AM, and antibodies against cytochrome oxidase subunit III and IV and ATP-synthase α-subunit were purchased from Molecular Probes (Eugene, OR, USA). [3H]ATP (20 Ci mmol−1) was purchased from GE Healthcare (formerly Amersham; Piscataway, NJ, USA). MUC5AC and MUC1 antibodies (Kreda et al. 2007) were purchased from LabVision (Fremont, CA, USA). VAMP-8 antibodies were from Abcam (Cambridge, MA, USA) and Synaptic Systems (Goettingen, Germany). Luciferin and antibodies against GM 130, p230 antibodies, protein disulfide isomerase, and LAMP-1 were from BD Biosciences Pharmingen (San Jose, CA, USA). Secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA) and LI-COR (Lincoln, NE, USA). Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN, USA). The PAR1-activating peptide TFLLRNPNDK and the PAR2-activating peptide SLIGKV were synthesized at Tufts University Peptide Synthesis Core Facility. Other chemicals were of the highest purity available and from sources previously reported.
Statistics
Student's paired t test was performed using Microsoft Excel 2003; P < 0.01 was accepted as indicating statistical significance.
Results
PAR agonists elicit release of ATP associated with mucins in WD-HBE cells
Recently, we reported that basolateral, but not mucosal, addition of the serine protease thrombin resulted in enhanced mucosal ATP release from WD-HBE cultures (Seminario-Vidal et al. 2009). Since cellular responses to thrombin and other serine proteases are mediated by members of the family of PARs, we investigated whether PAR stimulation in WD-HBE cells elicited mucin secretion coordinate with ATP release. Expression of PARs was verified in WD-HBE cells by RT-PCR analysis. PAR1, PAR2 and PAR3, but not PAR4, transcripts were amplified in WD-HBE cultures (Fig. 1A). Thrombin (50 nm, 5 min), a physiological agonist for PAR1 and PAR3 (Russo et al. 2009), promoted both mucin secretion (Fig. 1B) and ATP release (Fig. 1C) into the apical bath of WD-HBE cultures. Activation of PARs by their cognate proteases involves proteolytic cleavage of the amino-terminal exodomain of the receptor, generating a new amino terminus that functions as a tethered ligand (Russo et al. 2009). Synthetic peptides, mimicking the tethered ligand, can selectively activate PAR1 and PAR2 independently of receptor cleavage. Human PAR3 is not activated by PAR3 mimicking peptides (Russo et al. 2009). Basolateral incubation of WD-HBE cells for 5 min with PAR1 and PAR2 activating peptides (PAR1P and PAR2P, respectively) resulted in enhanced mucosal mucin secretion and ATP release (Fig. 1B and C). PAR activation did not elicit mucin or ATP release into the basolateral bath (not shown).
Figure 1. PAR agonists stimulate mucin and ATP release from WD-HBE cells.
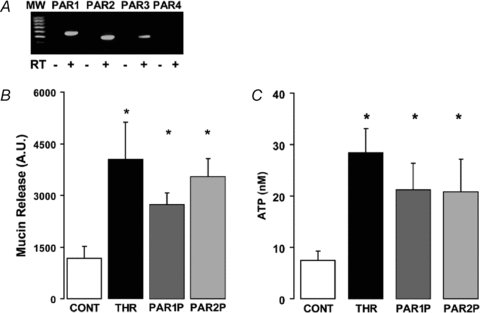
A, RT-PCR analysis indicating that PAR1, PAR2 and PAR3 (but not PAR4) transcripts were amplified in WD-HBE cells; RT, reverse transcriptase. B and C, WD-HBE cultures were incubated basolaterally with vehicle, 50 nm thrombin, 100 μm PAR1P, or 100 μm PAR2P for 5 min at 37°C. The apical bath was analysed for mucin content by immuno-slot blot (B) and ATP content by the luciferin–luciferase assay (C). Experiments were performed in quadruplicate with cultures from three different donors. The results are expressed as the mean ±s.e.m. (*P < 0.01).
PARs promote Ca2+-dependent mucin secretion from Calu-3 cells
Goblet cells are sparsely expressed in WD-HBE cell cultures, making purification of goblet cell granules difficult. Therefore, airway goblet-like Calu-3 cells (Kreda et al. 2007) were utilized as a cell model to investigate the contribution of mucin granule exocytosis to ATP release. Based on a previous study suggesting the presence of Ca2+-mobilizing PARs in Calu-3 cells (Palmer et al. 2006), the expression of PAR transcripts in these cells was examined by RT-PCR. As illustrated in Fig. 2A, transcripts for PAR1, PAR2 and PAR3, but not for PAR4, could be amplified in these cells.
Figure 2. Calu-3 express PARs.
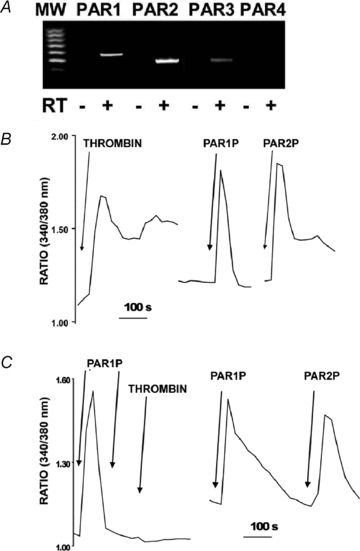
A, RT-PCR analysis indicating that PAR1, PAR2 and PAR3 (but not PAR4) transcripts were expressed in Calu-3 cells. B, intracellular calcium mobilization was assessed in independent cultures of Fura-2-loaded Calu-3 cells exposed to 50 nm thrombin, 100 μm PAR1P, or 100 μm PAR2P. C, left, fura-2 loaded cells were challenged with 100 μm PAR1AP and, after the Ca2+ signal relaxed, a second dose of 100 μm PAR1AP was added followed by 50 nm thrombin (left tracing); right, Calu-3 cells were exposed to 100 μm PAR1AP and then to 100 μm PARA2P. The tracings are representative of three independent experiments performed in duplicate.
Consistent with the concept that all PARs couple to Gq and phospholipase C activation (Russo et al. 2009; Seminario-Vidal et al. 2009), Calu-3 cells loaded with Fura-2 AM displayed increased intracellular Ca2+ mobilization in response to thrombin (50 nm), PAR1P (100 μm), or PAR2P (100 μm) (Fig. 2B). As shown in Fig. 2C, thrombin-evoked Ca2+ mobilization was negligible in PAR1P-pretreated cells, suggesting that desensitization of PAR1 prevented Calu-3 cells from responding to thrombin. Control experiments indicated that PAR2P-evoked responses were not affected by PAR1P pre-treatment (Fig. 2C). Although the data cannot rule out a contribution of PAR3 to thrombin-evoked responses, the nearly identical efficacies of thrombin and PAR1P and the PAR1P desensitization effect on thrombin in eliciting Ca2+ mobilization suggest that PAR1 is the major thrombin receptor expressed in Calu-3 cells. Our results suggest that PAR2 is also robustly expressed in these cells.
The secreted mucin MUC5AC is highly expressed in Calu-3 cells as revealed by fluorescence microscopy analyses that identified ∼1 μm diameter MUC5AC-immunoreactive granules in 30–40% of Calu-3 cell cultures (Kreda et al. 2007). Having verified that Calu-3 cells express Ca2+-mobilizing PARs, we asked whether activation of these receptors resulted in enhanced mucin secretion. Incubation of the cells with thrombin, PAR1P, or PAR2P (5 min) resulted in marked (∼70%) loss of MUC5AC intracellular immunoreactive granules (Fig. 3A and B), suggesting that PAR agonists elicited mucin granule secretion from these cells. To further verify the effect of PARs on mucin secretion, polarized monolayers of Calu-3 cells were stimulated basolaterally with PAR agonists and the MUC5AC content in the extracellular solution assessed by immuno-slot blot analysis. (Consistent with previous observations that PARs are selectively expressed on the basolateral surface of airway epithelial cells (Seminario-Vidal et al. 2009), PAR agonists promoted no responses when added to the apical surface of polarized Calu-3 cells; Kreda & Seminario-Vidal, unpublished observation.) Incubation of cells with thrombin, PAR1P, or PAR2P resulted in enhanced secretion of MUC5AC into the apical extracellular solution (Fig. 3C). Similarly to previous observations with ionomycin-stimulated Calu-3 cells (Kreda et al. 2007), negligible mucin secretion to the basolateral solution was observed in PAR-stimulated Calu-3 cells (not shown). Thus, PAR-elicited mucin secretion reflects an exocytotic process associated with the apical plasma membrane of Calu-3 cells.
Figure 3. PAR agonists stimulate mucin release from Calu-3 cells.
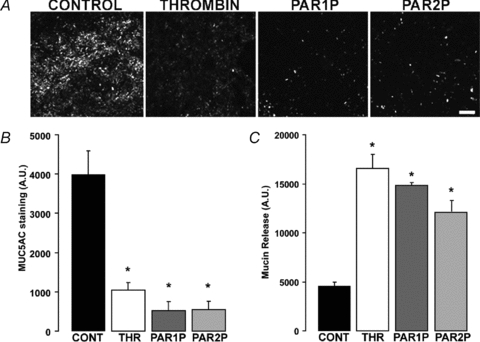
Calu-3 cells were challenged with vehicle, 50 nm thrombin, 100 μm PAR1P, or 100 μm PAR2P for 5 min at 37°C. A, mucin granule content was determined by immunostaining with a MUC5AC antibody followed by confocal microscopy analysis (bar = 100 μm). B, quantification of MUC5AC immunostaining in Calu-3 cultures. C, mucin release in the lumenal bath was assessed by slot blot as in Fig. 1. The results of a representative experiment are illustrated, and the data are expressed in arbitrary units and are the mean ±s.e.m. (n= 4; *P < 0.01). Similar results were obtained in three independent experiments.
It has been well established that receptor-mediated mucin secretion reflects a Ca2+-dependent process (Davis & Dickey, 2008). Incubation of Calu-3 cells with BAPTA AM to chelate intracellular Ca2+ impaired thrombin-induced mucin secretion, as assessed by the fluorescence microscopy observation of MUC5AC-immunostained cells (Fig. 4A) and immuno-slot blot analysis of Calu-3 cell apical secretions (Fig. 4B). Consistent with the notion that actin cytoskeleton remodelling is required for mucin granule exocytosis (Kreda et al. 2007; Davis & Dickey, 2008), thrombin-promoted mucin secretion was inhibited by cytochalasin D (which disrupts the actin cytoskeleton), ML7 (a myosin light chain kinase inhibitor), and H1152 and Y27632 (inhibitors of Rho kinase, a known upstream effector of myosin light change kinase and actin cytoskeleton remodelling during exocytosis; Davis & Dickey, 2008) (Fig. 4A and B). PAR1P- and PAR2P-stimulated cells also displayed reduced MUC5AC secretion when pre-incubated with the Rho kinase inhibitor H1152 (Fig. 4A and B). Moreover, changes in the organization of actin cytoskeleton were observed in PAR-stimulated Calu-3 cells labelled with fluorescent phalloidin as previously described in thrombin-stimulated non-epithelial cells (Kreda et al. 2008) (not shown).
Figure 4. PAR-stimulated mucin release is Ca2+ and cytoskeleton dependent.
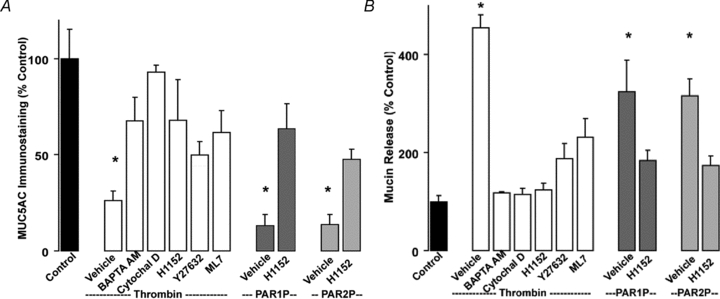
Calu-3 cells were pre-incubated for 30 min at 37°C with vehicle, 10 μm BAPTA AM, 5 μm cytochalasin D, 100 nm H1152, 10 μm Y27632, or 1 μm ML7. Cells were challenged with vehicle, 50 nm thrombin, 100 μm PAR1P, or 100 μm PAR2P for 5 min at 37°C. A, mucin granule content was quantified by immunostaining as in Fig. 3. B, mucin release in the apical bath was assessed by slot blot as in Fig. 1. Experiments were performed three times, each condition in quadruplicate. The results of a representative experiment are illustrated and data are expressed as percentage of control (mean ±s.e.m.; *P < 0.01 vs. control).
PAR-promoted mucin secretion is associated with enhanced release of ATP
Incubation of Calu-3 cells with PAR agonists resulted in enhanced release of ATP into the mucosal (but not basolateral) compartment (Fig. 5). Manoeuvres that affected mucin granule exocytosis in Calu-3 cells, such as chelating intracellular Ca2+ (BAPTA AM), inhibiting Rho kinase (HH152) and myosin light chain kinase (ML7), or disruption of the actin cytoskeleton (cytochalasin D), reduced, but did not abolish, ATP release in thrombin-stimulated Calu-3 cells (Fig. 5). Bafilomycin A1, an inhibitor of the vesicular H+-ATPase that loads ATP into specialized granules in secretory cells (Bankston & Guidotti, 1996), also partially inhibited (∼30% inhibition) ATP release from thrombin-stimulated Calu-3 cells (Fig. 5). Altogether, our results are consistent with the hypothesis that a vesicular/granular component, e.g. mucin granules, contributed at least in part to ATP release from PAR-stimulated Calu-3 cells.
Figure 5. PAR-stimulated ATP release involves a vesicular, Ca2+- and cytoskeleton- dependent mechanism.
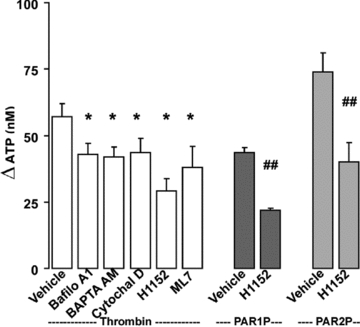
Calu-3 cells were pre-incubated with inhibitors as in Fig. 4, or with 4 μm Bafilomycin A1 for 30 min at 37°C. Mucosal ATP release following the addition of the indicated PAR agonists (5 min at 37°C) was assessed using the luciferin–luciferase assay in the presence of blockers of ecto-nucleotidases. Experiments were performed in quadruplicate with three independent cultures. The results of a representative experiment are illustrated, and the data are expressed as the difference between PAR agonist and basal values (basal ATP values, 15 ± 5 nm) (mean ±s.e.m.; *P < 0.01 compared to thrombin stimulation; ##P < 0.01 compared to PAR1P and PAR2P stimulation).
Isolated mucin granules contain adenine nucleotides
These results, together with our previous observation that Ca2+-regulated mucin granule secretion is accompanied by enhanced ATP release (Kreda et al. 2007), suggest that mucin granules are a source of exocytotic ATP release. To more definitively assess this possibility, we utilized a strategy that takes advantage of the fluorescent dye quinacrine, which labels Calu-3 cell granules (Kreda et al. 2007), to isolate mucin granules from these cells. A representative image of quinacrine-labelled Calu-3 cells displaying strong granular fluorescence is shown in Fig. 6A. Stimulation of the cells with thrombin, PAR1P, or PAR2P resulted in loss of granules containing quinacrine fluorescence (Fig. 6B).
Figure 6. PAR agonists stimulate secretion of quinacrine-labelled granules.

Calu-3 cell mucin granules were loaded with quinacrine (10 μm, 20 min at 37°C). Cells were mounted in a confocal microscope and real-time images of the DIC/Nomarski illumination (grey) and fluorescence (green) channels acquired every 30 s (see Methods). Cells were challenged with vehicle (control), 50 nm thrombin, 100 μm PAR1P, or 100 μm PAR2P. A, overlay of the DIC and fluorescence confocal images of quinacrine-labelled Calu-3 cells in control conditions; bar = 10 μm. B, representation of the change in fluorescence intensity associated with 1 μm granules after 5 min incubation with vehicle or PAR agonists (n= 3; mean ±s.e.m.; *P < 0.01).
Next, we subjected quinacrine-labelled Calu-3 cells to subcellular fractionation and fluorescently labelled granules were isolated using two consecutive continuous Percoll gradients. Quinacrine-labelled granules (1–2 μm diameter) were concentrated within a fraction (Fig. 7A) that was also enriched in MUC5AC (Fig. 7B and C).
Figure 7. Isolation of mucin granules from Calu-3 cells.

Mucin granules were isolated from Calu-3 cells using two consecutive Percoll gradients as described in Methods; the results of a representative isolation experiment are illustrated. A, confocal microscopy image (DIC/fluorescence channel overlay) of an isolated mucin granule from Calu-3 cultures labelled with quinacrine. B, image of the immuno-slot blot for MUC5AC representing the first eight fractions of the second gradient. C, the profile of organelle distribution in the second gradient fractions was assessed by immuno-slot blot using specific antibodies that recognize the indicated cellular markers. The quantification of the densitometry data for each organelle marker is expressed as a percentage of the total lysate content. D, localization of VAMP-8 and MUC5AC was assessed by immunostaining under resting (control; left panels) or thrombin-stimulated (50 nm, 5 min at 37°C; right panels) conditions in Calu-3 cells; bar = 10 μm.
VAMP-8 has been proposed as the R-SNARE in goblet cell granule exocytosis (Davis & Dickey, 2008) based on its broad participation in exocrine secretion (Wang et al. 2007). Therefore, we investigated whether VAMP-8 is associated with airway epithelial mucin granule secretion and, hence, could serve as an additional marker for granule purification. RT-PCR analysis indicated that VAMP-8 transcripts were present in native airway epithelial tissues and in Calu-3 and WD-HBE cell cultures (not shown). Importantly, VAMP-8 immunoreactivity co-localized with MUC5AC in the mucin granule fraction (i.e. fraction 4, Fig. 7C), and in granules of intact Calu-3 cells (Fig. 7D, left panel). Addition of thrombin to Calu-3 cell cultures resulted in decreased VAMP-8 immunoreactivity, which re-distributed to a diffused intracellular pattern, concomitantly with the loss of MUC5AC granule staining (Fig. 7D, right panel). Collectively, these data suggest VAMP-8 should be a valid marker for mucin granule isolation.
The MUC5AC/VAMP-8-containing fraction exhibited negligible amounts of MUC1 (plasma membrane marker), GM 130 and p230 (Golgi markers), protein disulfide isomerase (endoplasmic reticulum marker), LAMP-1 (lysosomal marker), or cytochrome oxidase subunit III/IV and ATP-synthase α-subunit (mitochondrial markers) (Fig. 7C). Thus, we conclude by these independent markers, MUC5AC and VAMP-8, that we had purified a mucin granule population.
We next measured the adenyl nucleotide content of the various fractions. ATP was concentrated in the same fraction as MUC5AC and VAMP-8 (i.e. fraction 4, Fig. 8A). Based on (i) the number of Calu-3 cells per culture cm2 (150,000–300,000), (ii) the yield (60 ± 20%) of MUC5AC in the isolated mucin granule fraction relative to the total cell culture homogenate, (iii) the percentage of goblet cells within a Calu-3 cell culture (30–40%), and (iv) the amount of ATP recovered in the mucin granule fraction (∼2 pmol cm−2), the mucin granule ATP content was calculated as 0.008–0.025 fmol per goblet cell or 500–900 pmol (mg protein)−1, and represented <2% of the ATP content in the whole Calu-3 cell culture lysate.
Figure 8. Isolated mucin granules contain ATP and other nucleotides.
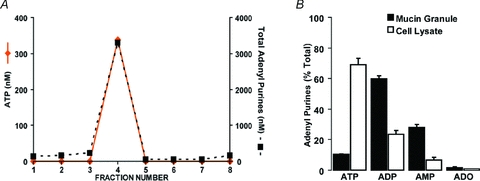
A, quantification of the amounts of ATP and total adenyl purine in each of the fractions collected in the second gradient was performed by etheno-derivatization and HPLC analysis. Data are expressed as the concentration of ATP (left axis) and total adenine-containing species (right axis); note that left and right axes represent different concentration ranges. B, quantification of the content of adenyl purine species (ATP, ADP, AMP and adenosine) in the total cell lysate and isolated mucin granule fraction (i.e. fraction 4) was performed by etheno-derivatization and HPLC analysis. Data are the average of four independent granule isolations and represent the percentage distribution of each species with respect to the total adenyl purine content in the fraction (mean ±s.e.m.). Note, the total adenyl purine mass in the mucin granule fraction represented <5% of the cell lysate content.
In addition, ATP metabolites were abundant in the MUC5AC/VAMP-8-enriched fraction (Fig. 8A). Indeed, in isolated mucin granules, ADP and AMP were the prevalent species (approximately 60% and 30%, respectively), while ATP and adenosine comprised ∼10% and ∼2% of total purines, respectively (Fig. 8B). This pattern of nucleotide distribution within mucin granules clearly contrasted with that observed in the whole cell lysate, where ATP represented the dominant (∼70%) adenyl species (Fig. 8B). These data suggest that an ATP hydrolysing activity was present in the lumen of the mucin granule. Using [3H]ATP as radiotracer, an ATPase activity was revealed in mucin granules permeabilized with Triton X-100 (150 ± 16 nmol ATP min−1 (mg protein)−1, n= 2).
ADP and AMP accompanied ATP release from PAR-stimulated cells
The relatively high levels of ADP and AMP, relative to ATP, in mucin granules (Fig. 8B) suggest that these nucleotide species are co-released with ATP and mucins. To examine this possibility directly, the adenyl purine content of Calu-3 cell surface liquid was assessed by etheno-derivatization. A marked increase in ADP, AMP and ATP accumulation was observed in mucosal samples from cells stimulated with thrombin (50 nm, 5 min), relative to control cells (Fig. 9). The net increase in ATP concentration (28 ± 3 nm) measured with this assay was ∼50% lower than that observed using the luciferase assay (57 ± 5 nm, Fig. 5). This difference is consistent with the fact that β,γ-met-ATP and ebselen, which efficiently block extracellular ATP hydrolysis on airway epithelia (Okada et al. 2006; Seminario-Vidal et al. 2009), were included in the luciferase assay but not in the derivatization protocol, due to interference of the blockers in the detection of etheno-species. Importantly, the net increase in mass of ATP, ADP and AMP combined following thrombin addition (95 ± 11 nm, Fig. 9) substantially exceeded the mass of ATP release detected in the presence of ATPase inhibitors (57 ± 5 nm, Fig. 5). The most likely interpretation of these data is that mucin granule release of ADP/AMP contributed, at least in part, to the accumulation of these species in Calu-3 cell surface liquid.
Figure 9. Nucleotide composition of Calu-3 cell secretions.
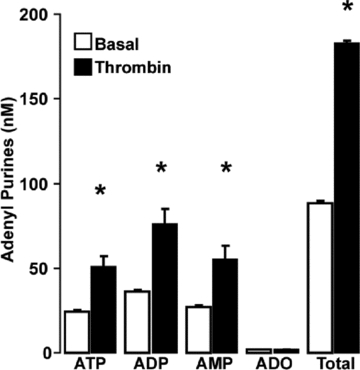
Calu-3 cells were stimulated with thrombin (50 nm, 5 min at 37°C) and the apical bath was collected and analysed for adenyl purines as above. The results of a representative experiment are illustrated, and data are expressed as the mean ±s.e.m.; *P < 0.01 compared to non-stimulated (basal) levels. Similar results were obtained in two independent experiments performed in quadruplicate.
Recent studies from our laboratory and others suggested that connexin/pannexin hemichannels represent a pathway for cytosolic ATP release from human bronchial epithelial cells (Seminario-Vidal et al. 2009; Ransford et al. 2009). To assess the potential involvement of hemichannels in nucleotide release from thrombin-stimulated Calu-3 cells, the effect of the connexin/pannexin inhibitor carbenoxolone (CBX) was investigated. Pre-treatment of cells with 10 μm CBX robustly reduced ATP release (82% inhibition) and partially decreased ADP (53%) and AMP (41%) accumulations (Supplemental Fig. 1). It is worth nothing that ATP/ADP/AMP measurements were carried out in the absence of ecto-nucleotidase inhibitors (as explained above in Fig. 9) and, therefore, ATP release and hydrolysis is likely to have contributed to ADP/AMP levels in these ASLs. However, a robust ADP/AMP mass increase in response to thrombin persisted in CBX-treated cells, suggesting that a hemichannel-independent pathway, e.g. mucin granules, represents an important source of released nucleotides.
Discussion
Gel-forming mucins, the principal polymeric species of the airway mucus, are condensed inside specialized granules and released from cells via Ca2+-regulated exocytotic mechanisms (Verdugo, 1991; Davis et al. 1992). Mucin release requires synchronized secretion of ions/water for mucin dispersion into the ASL, but these transport activities are not expressed on goblet cells (Verdugo, 1991). Thus, the mechanisms by which electrolyte transport and mucin secretion activities are synchronized are poorly understood. Given that mucin secretion is accompanied by enhanced nucleotide release (Kreda et al. 2007) and that ASL nucleotides and nucleosides regulate airway epithelial electrolyte transport activities (Kreda et al. 2007; Lazarowski & Boucher, 2009), we hypothesized that mucin granules themselves are the source of coordinately released nucleotides. We tested this hypothesis by (i) characterizing the contribution of receptor-mediated mucin secretion to nucleotide release, and (ii) quantifying the nucleotide content within isolated mucin granules.
Our results demonstrate that primary cultures of WD-HBE cells and immortalized Calu-3 cells express functional PAR1 and PAR2, which upon activation, promote Ca2+-dependent mucin secretion and ATP release onto the apical surface. The observation that thrombin-promoted ATP release was partially inhibited by manoeuvres that deplete ATP from vesicular compartments (e.g. bafilomycin A1) suggests that thrombin-elicited ATP release was mediated, at least in part, by an exocytotic mechanism (Fig. 5). Moreover, ATP release was reduced under conditions that inhibited mucin exocytosis (i.e. intracellular calcium chelating with BAPTA AM, actin cytoskeleton disruption with cytochalasin D, and inhibition of Rho and myosin light chain kinases with H1152 and ML7, respectively; Fig. 5), also consistent with a mucin granule secretion contribution to ATP release.
Previously, the presence of ATP in the mucin granules had been hypothesized based on the premise that mucin molecule packaging and granule integrity are energy-dependent processes (Perez-Vilar, 2007). Direct testing of this hypothesis has been challenging because the scant numbers of goblet cells within normal airway epithelia and granule fragility has hampered the isolation of intact mucin granules. Taking advantage of Calu-3 cell cultures that comprise up to 40% goblet-like mucin granule expressing cells (Kreda et al. 2007), we were able to obtain a subcellular fraction highly enriched with mucin granules. Employing a cell cavitation method and applying two successive continuous Percoll gradients, mucin granules were isolated devoid of measurable amounts of other cellular components. Importantly, a population of isolated mucin granules were intact, since ATP was enriched in the mucin granule-containing fraction (Fig. 8A). Moreover, AMP and to a greater extent ADP were more abundant than ATP in this fraction (Fig. 8B), strongly suggesting that a spectrum of adenyl nucleotides (rather than ATP alone) are released from mucin granules during mucin exocytosis.
It could be argued that the relative high content of ADP/AMP observed in the mucin granule may be due to an artefact consequent to granule isolation, e.g. a phosphatase activity associated with the cytosol-facing side of the granule membrane could have rapidly hydrolysed ATP upon disruption of the mucin granule. This possibility seems unlikely since, for nucleotide measurements, mucin granules were disrupted in the presence of trichloroacetic acid, which rapidly inactivates enzyme activities. Thus, the relative high content of ADP/AMP relative to ATP in the lumen of granules suggests the presence of metabolic activities, e.g. energy-dependent and/or phosphorylation reactions, inside the granule. Supporting this notion, an activity capable of hydrolysing exogenous [3H]ATP with a rate of 150 ± 16 nmol ATP min−1 (mg protein)−1 was detected in isolated granules following granule permeabilization with Triton X-100. Thus, ATP, ADP and AMP are likely to co-exist within intact mucin granules. While mucin granule isolation was performed at 4°C, a condition that minimizes ATPase activities, the relative abundance of adenyl species within isolated mucin granules in living cells remains to be determined.
Nevertheless, the net increase in mass of ADP/AMP in secretions from PAR-stimulated cells (Fig. 9) surpassed that predicted from the hydrolysis of ATP released alone (Fig. 5). Thus, the data strongly suggest that an intracellular pool contributed to ADP and AMP release. This conclusion is consistent with the predictions of a recently described mathematical model of nucleotide regulation in ASL. According to this model, AMP and ADP are predicted to be released from airway epithelia via an exocytotic mechanism (Zuo et al. 2008). Indeed, based on the nucleotide contents in the mucin granule fraction (Figs 7 and 8), we would predict that mucin granule exocytosis contributes 1.4 pmol ATP, 8.4 pmol ADP and 4.2 pmol AMP per cm2 culture. However, this estimate does not take into account that during cell fractionation a subpopulation of mucin granules could have been partially disrupted, resulting in loss of small soluble molecules (e.g. ATP/ADP/AMP) while retaining their MUC5AC content. Thus, the above prediction is likely to underestimate the actual contribution of mucin granules to ASL.
Our results suggest that mucin granules release ADP/AMP in preference to ATP. Such a pattern of nucleotide release from goblet cells offers a potential physiological advantage to the airway of selectively activating ion/water transport activities on neighbouring ciliated cells, while minimizing autocrine feedback on mucin secretion from goblet cells (see a proposed model of ASL nucleotide regulation in Fig. 10). Specifically, released ADP and AMP can be rapidly hydrolysed to produce adenosine, which selectively promotes liquid secretion via an A2b receptor/CFTR-mediated mechanism on ciliated cells (Lazarowski et al. 2004; Tarran et al. 2005). In contrast, adenosine receptors are not expressed in goblet cells, and this feature, plus the relatively low levels of ATP release, prevents autocrine stimulation of further mucin release from goblet cells. Such a mechanism maximizes the capacity to hydrate mucins in normal airways, but allows fine control of mucin secretion.
Figure 10. Model of adenyl nucleotide regulation in ASL.
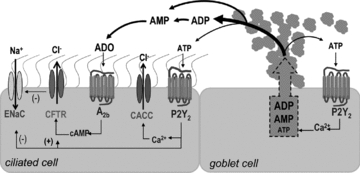
The schematic diagram represents a ciliated and a goblet cell of the airway surface epithelium. Mucin exocytosis from goblet cells is accompanied by release of adenyl nucleotides present in mucin granules as co-cargo molecules. ADP is the prevalent species followed by AMP and ATP. In ASL, ADP and AMP (and ATP) are rapidly metabolized by ecto-nucleotidases into adenosine. Adenyl purines have autocrine and paracrine regulatory activities on epithelial cells. For example, adenosine stimulates the A2b receptor on ciliated cells. CFTR, which is expressed in ciliated cells, is activated by A2b receptor-promoted cAMP formation (and PKA activation, not shown). Thus, chloride secretion is increased and sodium absorption is reduced (by CFTR-mediated inhibition of ENaC), which generates the driving gradient for water secretion necessary to disperse newly secreted mucins into the ASL. ATP released from mucin granules stimulates P2Y2 receptors on goblet cells for further mucin secretion, and on ciliated cells resulting in activation of TMEM16A or CACC (calcium activated chloride channel), activation of CFTR (via PKC), and inhibition of ENaC.
It is worth noting that non-exocytotic mechanisms are likely to also contribute to ATP release in airway epithelia. Recently we reported that thrombin promotes robust release of ATP from WD-HBE cells, which are largely dominated by non-mucous cells, as well as from lung epithelial A549 cells, which are devoid of mucin granules (Seminario-Vidal et al. 2009). ATP release from these cells was partially inhibited by inhibitors of connexin/pannexin hemichannels, suggesting the involvement of conductive mechanisms (Seminario-Vidal et al. 2009). The results in Supplemental Fig. 1 illustrating that the CBX partially reduced thrombin-elicited nucleotide release from Calu-3 cells are in agreement with this hypothesis. A prediction in this scenario is that conductive nucleotide release, e.g. from non-mucous cells, would reflect cytosolic nucleotide concentrations, i.e. ATP would be the predominant released species. Our observation that significant amounts of AMP and ADP are stored within and released from mucin granules in thrombin-stimulated Calu-3 cells is predicted to reduce the contribution of the cytosolic pool to nucleotide release in goblet cell metaplastic airway epithelia.
An additional important contribution of our study was the identification of VAMP-8 as the vesicle SNARE protein associated with MUC5AC granules in airway epithelial goblet cells (Fig. 7). Although investigation of the contribution of VAMP-8 to mucin secretion is beyond the scope of the current study, the fact that VAMP-8 immunostaining redistributed upon agonist-stimulated MUC5AC secretion (Fig. 7D) provides the first experimental evidence of a functional role for VAMP-8 in goblet cell granule exocytosis, as previously speculated (Davis & Dickey, 2008).
Thrombin was utilised as a tool to initiate agonist-mediated ATP release in our studies. However, thrombin has been reported to be present in the airways of patients with bronchial asthma and allergic rhinitis (Reed & Kita, 2004; Shimizu et al. 2008) and to stimulate mucin secretion through PAR1 activation in airway epithelial cells (Shimizu et al. 2008). PAR2 is not activated by thrombin but is activated by trypsin, tryptase, cathepsin G and proteinase 3, and its expression is up-regulated in respiratory epithelium subsequent to inflammation in asthma and COPD (Reed & Kita, 2004). Activation of PAR2 has been linked to mucin secretion in gastrointestinal epithelial cells (Kawabata et al. 2001; Kim et al. 2008), but, according to one study, PAR2 promotes only modest mucin secretory responses in airway epithelial cells (Lin et al. 2008). Our data, however, suggest that, like PAR1, PAR2 promotes robust mucin secretion (and ATP release) in two airway epithelial cell models. Because chronic airway inflammation is accompanied by goblet cell metaplasia (Davis & Dickey, 2008), a thrombin and/or trypsin-like PAR-dependent mucin and ATP secretagogue activity may be a significant feature of chronic lung diseases.
In summary, our results demonstrate that mucin granules are an important source of releasable ATP, ADP and AMP, providing paracrine signalling to ciliated cells for mucin hydration. By releasing predominantly ADP and AMP, mucin granules have the capacity to minimize autocrine stimulation of mucin release, while favouring adenosine formation, selectively activating ion/water secretion from ciliated cells. Lastly, the observation that both PAR1 and PAR2 agonists elicited robust mucin secretion from polarized monolayers of goblet-like Calu-3 cells suggests that similar processes may be a feature of chronic muco-obstructive lung diseases.
Acknowledgments
We thank Dr. Pedro Vedugo for useful discussions and suggestions, Dr. Robert Tarran for the use of the Leica SP5 confocal microscope system, and Dr. Scott Randell and Leslie Fulcher for providing primary bronchial cells. We are indebted to Lisa Brown for editorial assistance of the manuscript. This work was supported by National Institute of Health grant P01-HL034322 and the Cystic Fibrosis Foundation grant CFF-SEMINA08FO.
Glossary
Abbreviations
- ASL
airway surface liquid
- CFTR
cystic fibrosis transmembrane conductance regulator
- MCC
mucociliary clearance
- PAR
protease-activated receptor
- PARP
PAR activating peptide
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP8
vesicle associated membrane protein 8
Author contributions
Conception and design of the experiments: S.M.K. and E.R.L. Collection, analysis and interpretation of data: S.M.K., L.S.-V., C.A.vanH., W.O., L.J. and E.R.L. Drafting the article or revising it critically for important intellectual content: S.M.K., R.C.B. and E.R.L. All the authors approved the final version of this manuscript.
Supplemental material
Supplementary Figure 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- Davis CW, Dowell ML, Lethem MI, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: Response to exogenous ATP, ADP, and adenosine. Am J Physiol Cell Physiol. 1992;262:C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kinoshita M, Nishikawa H, Kuroda R, Nishida M, Araki H, Arizono N, Oda Y, Kakehi K. The protease-activated receptor-2 agonist induces gastric mucus secretion and mucosal cytoprotection. J Clin Invest. 2001;107:1443–1450. doi: 10.1172/JCI10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Choi BH, Jung SR, Sernka TJ, Kim S, Kim KT, Hille B, Nguyen TD, Koh DS. Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. J Biol Chem. 2008;283:18711–18720. doi: 10.1074/jbc.M801655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild-type and ΔF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153:1528–1537. doi: 10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. Purinergic receptors in airway epithelia. Curr Opin Pharmacol. 2009;9:262–267. doi: 10.1016/j.coph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KW, Park J, Crews AL, Li Y, Adler KB. Protease-activated receptor-2 (PAR-2) is a weak enhancer of mucin secretion by human bronchial epithelial cells in vitro. Int J Biochem Cell Biol. 2008;40:1379–1388. doi: 10.1016/j.biocel.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer ML, Lee SY, Maniak PJ, Carlson D, Fahrenkrug SC, O’Grady SM. Protease-activated receptor regulation of Cl− secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am J Physiol Cell Physiol. 2006;290:C1189–C1198. doi: 10.1152/ajpcell.00464.2005. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J. Mucin granule intraluminal organization. Am J Respir Cell Mol Biol. 2007;36:183–190. doi: 10.1165/rcmb.2006-0291TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- Russo A, Soh UJ, Trejo J. Proteases display biased agonism at protease-activated receptors: location matters! Mol Interv. 2009;9:87–96. doi: 10.1124/mi.9.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signalling pathways. J Biol Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER. Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signalling molecules. J Biol Chem. 2009;284:12572–12583. doi: 10.1074/jbc.M806759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shimizu T, Morser J, Kobayashi T, Yamaguchi A, Qin L, Toda M, Essandro-Gabazza C, Maruyama T, Takagi T, Yano Y, Sumida Y, Hayashi T, Takei Y, Taguchi O, Suzuki K, Gabazza EC. Role of the coagulation system in allergic inflammation in the upper airways. Clin Immunol. 2008;129:365–371. doi: 10.1016/j.clim.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis: The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P. Mucin exocytosis. Am Rev Respir Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Wang CC, Shi H, Guo K, Ng CP, Li J, Qi Gan B, Chien Liew H, Leinonen J, Rajaniemi H, Hong Zhou Z, Zeng Q, Hong W. VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol Biol Cell. 2007;18:1056–1063. doi: 10.1091/mbc.E06-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia. J Biol Chem. 2008;283:26805–26819. doi: 10.1074/jbc.M801516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


