Abstract
Exercise capacity and skeletal muscle blood flow are diminished with ageing but little is known of underlying changes in microvascular haemodynamics. Further, it is not clear how the sympathetic nervous system affects the microcirculation of skeletal muscle with ageing or whether sex differences prevail in the regulation of arteriolar diameter in response to muscle contractions. In the gluteus maximus muscle of C57BL/6 mice, we tested the hypothesis that ageing would impair ‘rapid onset vasodilatation’ (ROV) in distributing arterioles (second-order, 2A) of old (20-month) males (OM) and females (OF) relative to young (3-month) males (YM) and females (YF). Neither resting (∼17 μm) nor maximum (∼30 μm) 2A diameters differed between groups. In response to single tetanic contractions at 100 Hz (duration, 100–1000 ms), ROV responses were blunted by half in OM relative to OF, YM or YF. With no effect in YM, blockade of α-adrenoreceptors with phentolamine (1 μm) restored ROV in OM. Topical noradrenaline (1 nm) blunted ROV in YM and YF to levels seen in OM and further suppressed ROV in OM (P < 0.05). To evaluate arteriolar blood flow, red blood cell velocity was measured in 2A of OM and YM; respective heart rates (353 ± 22 vs. 378 ± 15 beats min−1) and carotid arterial blood pressures (76 ± 3 vs. 76 ± 1 mmHg) were not different. Blood flows at rest (0.6 ± 0.1 vs. 1.6 ± 0.2 nl s−1) and during maximum dilatation (2.0 ± 0.8 vs. 5.4 ± 0.8 nl s−1) with sodium nitroprusside (10 μm) were attenuated >60% (P < 0.05) in OM. Blood flow at peak ROV was blunted by 75–80% in OM vs. YM (P < 0.05). In response to 30 s of rhythmic contractions at 2, 4 and 8 Hz, progressive dilatations did not differ with age or sex. Nevertheless, resting and peak blood flows in YM were 2- to 3-fold greater (P < 0.05) than OM. We suggest that ageing blunts ROV and restricts blood flow to skeletal muscle of OM through subtle activation of α-adrenoreceptors in microvascular resistance networks.
Introduction
A reduction in physical work capacity with ageing has long been attributed to the loss of muscle mass and function (Lexell, 1995). At the same time, physical activity requires adequate blood flow to support the contractile activity of muscle fibres. Human studies have demonstrated that the ability to increase blood flow to exercising skeletal muscle is impaired with ageing even when accounting for lean body mass (Proctor & Joyner, 1997; Proctor et al. 1998; Dinenno et al. 1999; Lawrenson et al. 2003). As this perfusion deficit is more pronounced in the legs than in the arms (Donato et al. 2006), the effect of ageing on blood flow appears to be greatest for muscles used for locomotion. In response to submaximal bicycling (Proctor & Joyner, 1997; Proctor et al. 1998) or single-leg rhythmic knee extension (Lawrenson et al. 2003; Parker et al. 2008), a restricted increase in vascular conductance to active muscles of ‘older’ (typically ∼60–70 years) vs.‘younger’ (typically ∼20–30 years) subjects reflects an increase in the resistance to blood flow, thereby implicating changes in the microcirculation. Studies of limb blood flow in humans are based primarily upon methods employing limb plethysmography, thermal dilution in venous effluent or Doppler ultrasound in proximal conduit arteries. While these data reflect regulatory events originating within the microcirculation, measurements performed for entire limbs and conduit vessels cannot ascertain where vasomotor responses occur or how they are regulated within microvascular resistance networks. In contrast, animal models have enabled more invasive approaches to investigate the effects of ageing on the microcirculation and the isolation of individual resistance vessels for in vitro studies has provided valuable insight into how key signalling events that underlie blood flow control are affected (Csiszar et al. 2002; Muller-Delp et al. 2002b; Woodman et al. 2002). However, surgical isolation for in vitro study eliminates key physiological interactions between microvessels, muscle fibres and the autonomic nervous system that are manifest in the intact organism (Thomas & Segal, 2004).
A characteristic response to the stress of physical exertion is an increase in muscle sympathetic nerve activity (SNA) (Seals, 1989), which is integral to the redistribution and augmentation of cardiac output to support the metabolic demands of active musculature (Rowell, 1974). An increase in SNA can impose restrictions on muscle blood flow (Thomas & Segal, 2004) and this effect appears to be augmented with ageing (Dinenno & Joyner, 2006; Proctor & Parker, 2006), even under resting conditions (Dinenno et al. 1999). Moreover, sex differences in how SNA changes with ageing are apparent. For example, although male subjects exhibited heightened SNA at an earlier age than did females, the increase in SNA with age increased more rapidly in females (Matsukawa et al. 1998; Narkiewicz et al. 2005). How the sympathetic nervous system affects the microcirculation of skeletal muscle with ageing, and whether sex differences prevail in the regulation of arteriolar diameter in response to muscle contractions, remain inadequately defined. Indeed, more subtle actions of sympathetic neuroeffector signalling may be manifest than previously recognized. Further insight into these relationships requires an experimental model that allows direct observation of arterioles in response to the contractile activity of skeletal muscle involved in locomotion.
Studies concerned with how the regulation of muscle blood flow is affected by ageing have focused primarily on cardiovascular responses to well-defined submaximal levels of rhythmic contractions of leg or forearm musculature. Thus our current understanding of how ageing impacts muscle perfusion reflects regulatory events occurring during steady-state levels of activity. In contrast, daily activities are more typically characterized by short bursts of activity with frequent transitions between different levels of energy expenditure. As reported in the human forearm (Corcondilas et al. 1964) and supported by ensuing studies (Shoemaker et al. 1998; Tschakovsky et al. 2004; Kirby et al. 2007) muscle blood flow can increase within the first cardiac cycle following the onset of muscle contraction. Direct observations of arterioles in the hamster cremaster (Mihok & Murrant, 2004) and cheek pouch retractor (VanTeeffelen & Segal, 2006) muscles confirm that dilatation can begin within the first 1–2 s of muscle contraction. Recent evidence from the human forearm indicates that such rapid vasodilatory responses to muscle contraction can be attenuated with ageing (Carlson et al. 2008). In turn, complementary observations of vasomotor kinetics in arterioles of the mouse gluteus maximus (GM) muscle have illustrated that both the initial increase in hyperaemia and the ability to sustain hyperaemia upon cessation of activity are impaired with ageing (Bearden et al. 2004; Bearden, 2007) with such effects predominating in males. Nevertheless, how ageing impacts both rapid and steady-state control of muscle blood flow within the microcirculation remains poorly understood, particularly in light of sympathetic regulation and the role of sex.
The goal of the present study was to provide new insight with respect to how ageing and sex interact to affect the ability of the microcirculation to increase muscle blood flow in response to contractile activity. Experiments were performed using intravital microscopy to study the mouse GM and thereby investigate arteriolar responses to contractile activity within a skeletal muscle that is actively recruited during locomotion, adapts to exercise conditioning (Bearden et al. 2004) and is common to both sexes. Moreover, activation of a single muscle minimizes requirements for adjustments in cardiac output, the demand for systemic cardiovascular regulation, or the possibility of activating baroreflexes and thereby emphasizes behaviour intrinsic to the peripheral circulation. We focused on second-order (2A) arterioles based upon their strategic location between proximal feed arteries and terminal arterioles as well as their essential role in controlling blood flow distribution within the muscle (Bearden et al. 2004). We tested the hypothesis that ageing would impair rapid onset vasodilatation (ROV) and the magnitude of arteriolar blood flow. Complementary experiments determined whether vasomotor responses were sex-specific, whether they were manifest for distinct forms of contractile activity, and whether an underlying role for sympathetic regulation could be resolved for any differences that emerged between groups according to age or sex.
Methods
Animal care and use
Experimental procedures were approved by the Animal Care and Use Committees of the John B. Pierce Laboratory (New Haven, CT, USA) and of the University of Missouri (Columbia, MO, USA) and were performed in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were performed using C57BL/6 mice with two age groups studied for each sex. The total number and body mass of mice studied in each group were as follows: old (20-month) males (OM: n= 18, 35 ± 3 g) and females (OF: n= 6, 35 ± 5 g) and young (3-month) males (YM: n= 23, 27 ± 2 g) and females (YF: n= 9, 23 ± 2 g). Mice were housed in animal care facilities of the John B. Pierce Laboratory or the University of Missouri, respectively, at ∼24°C on a 12 h–12 h light–dark cycle with food and water ad libitum. Mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) or Charles River Laboratories (Wilmington, MA, USA) and housed on site for at least 1 week before being studied. Body mass was measured prior to each experiment. Upon completion of experimental procedures each day, the anaesthetized mouse was killed by an overdose of pentobarbital sodium (intraperitoneal injection) and cervical dislocation.
Anaesthesia and muscle preparation
A mouse was anaesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg kg−1) which was supplemented as needed. During experiments, the depth of anaesthesia was maintained at a level that preserved sympathetic modulation of arterioles as confirmed by transient vasoconstriction in response to a firm toe pinch. The mouse was placed in the prone position on a heating plate to maintain oesophageal temperature at 37–38°C and viewed through a stereomicroscope to prepare the left GM for intravital microscopy. After carefully shaving the surgical area, the skin overlying the muscle was removed and exposed tissue was superfused continuously (3–5 ml min−1) with bicarbonate-buffered physiological salt solution (PSS; 35°C, pH 7.4) of the following composition (mm): NaCl 137, KCl 4.7, MgSO4 1.2, CaCl2 2, NaHCO3 18 and equilibrated with 5% CO2–95% N2. The origin of the GM was cut free from its insertion along the spine with great care taken to preserve the integrity of its neurovascular supply. Free edges of the muscle were gently reflected, spread evenly onto a transparent silicone rubber pedestal (Sylgard 184; Dow Corning, Midland, MI, USA) and pinned to approximate in situ dimensions. Insertion of the GM onto the femur remained intact.
Intravital microscopy
Upon completion of surgery, the mouse preparation was transferred to the stage of an intravital microscope (Zeiss ACM or Nikon E600FN). The superfused GM was equilibrated for at least 30 min before experimental observations. Using Köhler illumination, arterioles were observed through a Zeiss UD40 objective (numerical aperture = 0.41 or a Nikon 20× SLWD objective (numerical aperture = 0.35), respectively, coupled to a video camera (C2400; Hamamatsu, Japan). Final magnification on the video monitor was ∼1000×. Internal vessel diameter was determined as the distance between luminal edges using a video calliper with spatial resolution ≤1 μm calibrated with a stage micrometer (100 × 0.01 = 1 mm: Graticules Ltd, Tonbridge, Kent, UK).
Second-order arterioles (2A) were chosen for study for two reasons. First, these resistance microvessels are positioned anatomically to control the distribution of blood flow within the GM (Bearden et al. 2004). Second, the consistency of arteriolar network architecture across young and old animals enables data acquisition from approximately the same region of each muscle (Bearden et al. 2004). One arteriole was studied in each mouse. Following equilibration, the resting (baseline) diameter was recorded and arterioles were tested for oxygen sensitivity as follows: superfusate O2 was elevated from 0 to 21% (with 5% CO2, balance N2) for 10 min, arteriolar diameter was recorded, and equilibration with 5% CO2–95% N2 was restored for the duration of experimental procedures. Changes in arteriolar diameter (and blood flow in a subset of experiments; see Haemodynamic measurements, below) were evaluated in response to brief maximal tetanic contractions at 100 Hz as well as 30 s of rhythmic muscle contractions at 2, 4, or 8 Hz (see Skeletal muscle contractions, below). For these experiments, each muscle preparation underwent both contraction protocols with order randomized across experiments. At the end of each day's procedures, maximum arteriolar diameter was recorded by adding sodium nitroprusside (SNP, 10 μm) to the superfusate (Bearden et al. 2004; VanTeeffelen & Segal, 2006).
Skeletal muscle contractions
Contractions of the GM were evoked using electrical field stimulation (EFS). For this purpose, wire electrodes (90% Pt–10% Ir; diameter, 250 μm) were positioned in the superfusion solution on either side of the exposed muscle. Monophasic pulses (0.1 ms) were delivered at 10 V through a stimulus isolation unit (SIU5; Grass Technologies; Quincy, MA, USA) driven by a square wave stimulator (S48, Grass). Preliminary experiments confirmed that this voltage elicited reproducible contractions of the GM and of arteriolar responses for the duration of an experiment. Control experiments (n= 3) confirmed that muscle fibre contractions resulted from acetylcholine release from motor nerve terminals as the addition of a nicotinic receptor antagonist (d-tubocurarine, 10 μm) reversibly abolished muscle contractions in response to EFS (Jacobs & Segal, 2000). In the presence of d-tubocurarine, the absence of arteriolar constriction with EFS further serves to confirm that EFS did not activate perivascular sympathetic nerves.
Haemodynamic measurements
Centreline red blood cell velocity (Vrbc) was monitored concomitant with diameter in 2A using an Optical Doppler Velocimeter as described (Jacobs & Segal, 2000; VanTeeffelen & Segal, 2006). To ensure fidelity of these recordings, we confirmed that the Vrbc signal was pulsatile with the cardiac cycle. Mean red blood cell velocity (Vm) was calculated as =Vrbc/1.6. Accurate velocimeter readings were not possible during tissue movement. Thus, for ROV experiments, peak Vrbc was recorded (for 1.5 s) during the peak of vasodilatation following a brief tetanic contraction. For rhythmic contractions, peak Vrbc was taken as that recorded for a similar interval immediately upon cessation of contractile activity (Welsh & Segal, 1997; Jacobs & Segal, 2000; VanTeeffelen & Segal, 2006). Blood flow was calculated as =π(D/2)2Vm, where D is diameter (Welsh & Segal, 1997; Jacobs & Segal, 2000; VanTeeffelen & Segal, 2006). Wall shear rate (WSR) during this period was calculated as = 8Vm/D.
Experimental protocols
Rapid onset vasodilatation (ROV)
Arteriolar responses to muscle contraction were first evaluated for ROV. Based upon preliminary experiments (data not shown) performed in light of earlier findings (Bearden et al. 2004; VanTeeffelen & Segal, 2006), a brief maximal tetanic contraction at 100 Hz was used to evoke ROV in each experimental group. To characterize arteriolar dilatations across a range of tetanic contraction durations, stimulus train durations were 100, 200, 400, 600, 800 and 1000 ms with the order randomized across experiments. The arteriole consistently returned to the initial resting baseline with 2–3 min of recovery between contractions. As tissue displacement occurred during contraction, diameter was recorded preceding each stimulus (resting baseline) and immediately following contraction with a delay of 1–2 s (Fig. 1) that reflected the time required to refocus and reposition the video calliper.
Figure 1. Rapid onset vasodilatation in response to brief tetanic contraction.
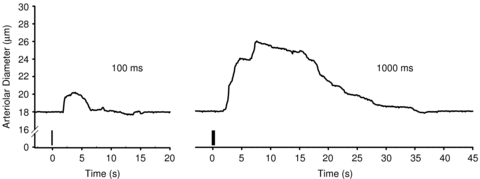
Representative traces of diameter responses to single tetanic contractions of 100 ms (left) and 1000 ms (right) duration from a young male mouse. Vertical bars indicate stimulation at 100 Hz. As ROV was nearly instantaneous, delays in diameter responses reflect time to refocus the microscope after contraction.
The ROV responses were first evaluated under control conditions. Steady-state vasodilatations to rhythmic contractions were then assessed as described in the next paragraph. In light of differences observed in ROV for OM compared to other groups (see Results for details), one of two pharmacological agents to inhibit (phentolamine; 1 μm) or activate (noradrenaline; NA, 1 nm) α-adrenoreceptors (αARs) was investigated for an effect on ROV. Following addition to the superfusion solution, each agent was first equilibrated for 15 min with no measureable effect on resting arteriolar diameter. It should be recognized that a 10-fold range of tetanic contractions plus a 4-fold range of rhythmic contractions was used to define 2A responses to exercise. Therefore only one pharmacological agent could be investigated reliably in a given preparation. To match these treatments by age, phentolamine was tested in OM and OF while NA was tested in YM and YF.
Steady-state vasodilatation to rhythmic contractions
As the nature of vasodilatation can vary with the pattern of muscle fibre activation (VanTeeffelen & Segal, 2000; Murrant, 2005), vasomotor responses to 30 s of rhythmic contractions at 2, 4 and 8 Hz (in randomized order) were also evaluated in each experimental group. Stimulation at these frequencies evokes unfused twitch contractions that correspond to ∼40% of peak tetanic tension (Bearden et al. 2004). Following each 30 s period of rhythmic twitch contractions, resting baseline was re-established consistently within 5 min. Arteriolar diameter was recorded preceding contractile activity, during rhythmic contractions, and following contractions throughout recovery.
Experimental emphasis on male mice
Throughout our initial experiments, the order in which age and sex were studied was varied across respective groups. However, based upon the clear distinction of ROV responses between YM and OM (see Fig. 2) and greater morbidity encountered for OF compared to OM, our experimental design was adjusted accordingly. To directly compare the effects of both activation and inhibition of αARs on ROV responses in the same GM preparations, a subset of experiments was performed on YM and OM (n= 5 per group). To preserve the integrity of preparations for these experiments, fewer tetanic contractions were performed (400, 600, 1000 ms; randomized) under each experimental condition. Thus, following equilibration, responses to each contraction were recorded under control conditions. The preparation was equilibrated with NA (1 nm) for 5 min and ROV responses were re-evaluated. After recovering from the final contraction, the preparation was equilibrated with phentolamine for 15 min and ROV responses were evaluated a final time.
Figure 2. Blunting of ROV in old male mice.
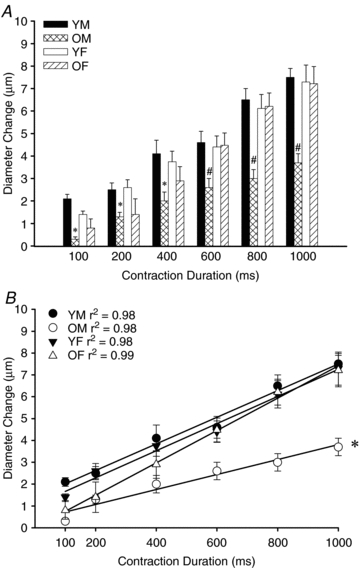
A, across the range of contraction durations, 2A diameter change in response to single tetanic contractions was attenuated by nearly half in OM as compared to YM, YF or OF, (n= 6–18 per group). *P < 0.05, OM vs. YM and YF; #P < 0.05, OM vs. other groups. B, in each experimental group, the duration of muscle contraction correlated well with the change in arteriolar diameter (n= 6–18 per group). However the slope of ROV responses to increasing stimulus duration was blunted in OM. *P < 0.05, OM vs. other groups. Abbreviations: YM, young male; YF: young female; OM: old male; OF: old female.
To provide further insight with respect to the impact of ageing on skeletal muscle perfusion, arteriolar blood flow was evaluated in subsets of OM and YM. In these animals, arterial blood pressure was measured by cannulating a carotid artery with polyethylene tubing (PE-10) connected to a pressure transducer (CDX III; COBE; Arvada, CO, USA) coupled to a Transbridge amplifier (World Precision Instruments, Sarasota, FL, USA). Blood pressures were averaged over 10 cardiac cycles to obtain a mean value for each mouse.
Chemicals and solutions
All chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA) and dissolved in purified deionized water (dH2O, 18.2 MΩ), with the exception of phentolamine, which was first dissolved in 100% ethanol. Stock solutions were prepared weekly, stored at 4°C and diluted at least 100-fold in PSS to final working concentrations on the day of an experiment. Based upon preliminary experiments, NA (1 nm) was added to the superfusion solution at to subtly activate αARs without producing arteriolar constriction. In accord with our earlier studies of αAR inhibition in microvessels (Haug & Segal, 2005), the concentration of phentolamine in the superfusion solution was 1 μm (with 0.1% ethanol). Vehicle controls confirmed that 0.1% ethanol in PSS was without affect on baseline diameter or vasomotor responses.
Data acquisition, statistical analyses and presentation
Data were collected at 100 Hz using a PowerLab system (AD Instruments; Colorado Springs, CO, USA) coupled to a personal computer. Data were analysed using SigmaStat software (v. 3.11; Systat Software Inc., Point Richmond, CA, USA) and differences were accepted as statistically significant with P < 0.05. For ROV data, one- and two-way (age, sex) repeated measures analysis of variance (RMANOVA) was used to test for the main effect of tetanic contraction duration across the four experimental groups. Linear regression was performed to evaluate the correlation between the duration of contraction and the magnitude of ROV for each animal. Slopes of these responses were compared across groups using ANOVA. The main effect of stimulus frequency during rhythmic contractions was analysed using RMANOVA. Where treatment conditions differed between Young (NA exposure) and Old (phentolamine exposure), pair-wise comparisons were performed using multiple t tests with a Bonferroni correction to maintain total P < 0.05. To compare OM and YM with respect to the effect of NA and phentolamine on ROV in the same preparations, a 3-way (age, treatment, contraction duration) RMANOVA was used. When significant F-ratios were obtained with ANOVA or RMANOVA, Tukey's tests were performed for post hoc comparisons. Summary data are presented as mean values ± standard error (s.e.).
Results
Arteriolar responses to O2 and haemodynamic characteristics in Young and Old mice
Neither baseline nor maximum arteriolar diameters differed significantly with age or sex (Table 1). However, arteriolar constriction in response to elevating superfusate O2 from 0 to 21% was significantly greater (P < 0.05) in YM compared to other groups (Table 1), consistent with earlier observations (Bearden et al. 2004). There were no differences in respective heart rates (378 ± 15 vs. 353 ± 22 beats min−1) or mean arterial blood pressures (76 ± 1 vs. 76 ± 3 mmHg) between YM and OM.
Table 1.
Diameters and oxygen response of second-order arterioles in mouse gluteus maximus muscle
| Baseline diameter (μm) |
Maximum diameter (μm) |
O2 response (μm) |
||||
|---|---|---|---|---|---|---|
| Age-group | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ |
| Young | 17 ± 1 | 17 ± 1 | 31 ± 1 | 29 ± 1 | −10 ± 1* | −5 ± 1 |
| Old | 17 ± 1 | 17 ± 1 | 31 ± 1 | 30 ± 1 | −6 ± 1 | −4 ± 1 |
Baseline values were recorded under control conditions (superfusate equilibrated with 5% CO2–95% N2). Maximum values were recorded during superfusion with SNP (10 μm). O2 response indicates the change in diameter in response to elevating superfusate O2 from 0 to 21%.
Arterioles of YM constricted more than other groups, P < 0.05 (n= 6–22 per cell).
Rapid onset vasodilatation and blood flow
Vasomotor responses
The diameter change with ROV increased consistently with the duration of tetanic contraction (P < 0.01; Figs. 2–4). Across the entire range of single tetanic contractions, there were no significant differences in ROV responses between YM, YF or OF (Fig. 2A), though a subtle effect was apparent for OF at shorter durations. In striking contrast, ROV responses of OM were attenuated by nearly half (P < 0.05) across the entire range of contraction durations (Fig. 2A). The slope of the regression line determined for mean responses across the range of contractions was taken as an index of the sensitivity of ROV responses for each experimental group. As illustrated in Fig. 2B, there was a strong positive correlation between increasing contraction duration and the change in arteriolar diameter. Nevertheless, the sensitivity of ROV to single tetanic contractions was reduced significantly (P < 0.05) in OM as compared to YM, YF and OF, which were not different from each other.
Figure 4. Effects of NA and phentolamine on ROV in Young and Old male mice.
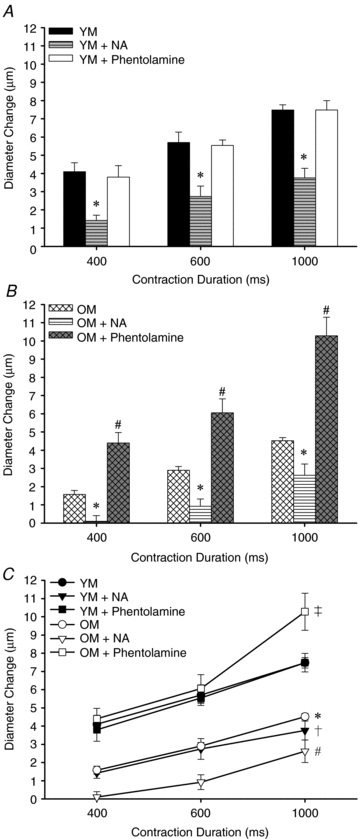
A, young males: superfusion of NA (1 nm) blunted ROV (*P < 0.01) while superfusion of phentolamine (1 μm) had no significant effect on ROV (n= 5). B, old males: superfusion of NA (1 nm) blunted ROV (*P < 0.01) while superfusion of phentolamine (1 μm) enhanced ROV vs. OM under control conditions (#P < 0.01; n= 5). C, comparisons of YM vs. OM (data in A and B re-plotted): under control conditions, ROV was blunted in OM vs. YM (*P < 0.01). For YM + NA, ROV was blunted significantly below control (†P < 0.01) and these levels were not significantly different from OM. For OM + NA, ROV was further depressed for each contraction (#P < 0.01). Phentolamine had no effect in YM yet increased ROV significantly in OM (‡P < 0.01). Note that 1 OM had a particularly large response to 1000 ms contraction with phentolamine that elevated the mean ±s.e. for this data point.
Given that SNA can inhibit vasodilatation (Thomas & Segal, 2004), we tested the hypothesis that inhibition of αARs would restore the magnitude of ROV responses in OM. Remarkably, with no change in resting diameter, superfusion with phentolamine (1 μm) increased ROV of OM such that these responses were no longer different from those of YM (Fig. 3A). For age-matched controls, phentolamine had no effect on ROV in OF (Fig. 3B). In complementary experiments, superfusion with NA (1 nm) consistently depressed ROV in YM to levels observed in OM (Fig. 3A) and did so without changing resting diameter. Though less pronounced, the effect of NA in YF was similar to that observed in YM (Fig. 3B).
Figure 3. Roles of α-adrenoreceptor inhibition and activation in ROV.
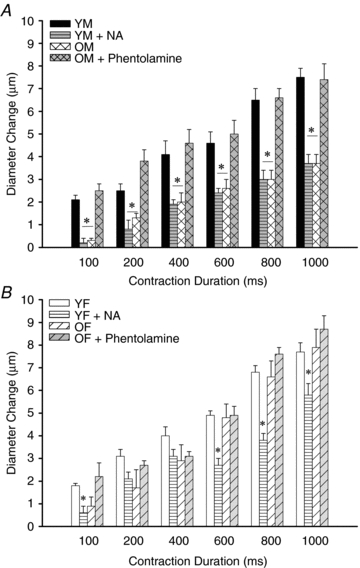
A, superfusion of phentolamine (1 μm) increased ROV in OM to levels not different from those recorded in YM. Conversely, superfusion of NA (1 nm) blunted ROV in YM to levels not different from those recorded in OM. Resting diameters (Table 1) were not different between conditions. *P < 0.05 for OM and YM + NA vs. YM and OM + phentolamine (n= 6–8 per group). B, superfusion of NA (1 nm) blunted ROV in YF (consistent with its effect in YM, panel A), particularly at longer durations of contraction. However, superfusion of phentolamine (1 μm) had no effect on ROV responses in OF, which were not different from those in YF. Resting diameters (Table 1) were not different between conditions. *P < 0.05, YF + NA vs. other groups (n= 4–6 per group). Abbreviations are defined in Fig. 2 legend.
Adrenoreceptor treatments compared between OM and YM
Our initial experiments investigated the effect of αAR inhibition or activation by testing phentolamine in Old mice and NA in Young mice. These data are limited as they did not determine whether inhibition of αARs could affect ROV in Young mice or whether stimulation of αARs could affect ROV in Old mice. Therefore a subset of experiments was performed to compare the effect of both αAR inhibition and activation on ROV in the same preparations of OM and YM (Fig. 4). Consistent with findings in Fig. 2, control ROV responses of OM were blunted consistently relative to those of YM. Despite having no effect in YM, phentolamine again increased ROV in OM (P < 0.05) to levels not different from responses recorded in YM. In turn, NA blunted ROV in YM to levels not different from OM under control conditions and attenuated ROV even further in OM (P < 0.01) with responses to 400 and 600 ms contractions nearly abolished.
Haemodynamic responses
At rest, 2A blood flow was attenuated by >50% in OM vs. YM (Fig. 5A), as was maximum blood flow during topical exposure to SNP (2.0 ± 0.8 vs. 5.4 ± 0.8 nls−1 respectively, P < 0.05; n= 6 per group). As shown in Fig. 5A, baseline blood flow recovered consistently between each tetanic contraction. Blood flow increased slightly but significantly with the duration of contraction in both OM and YM. However, the hyperaemic response to tetanic contraction was also attenuated greatly in OM vs. YM (P < 0.05; Fig. 5A).
Figure 5. Arteriolar haemodynamic responses during ROV were blunted in OM vs. YM.
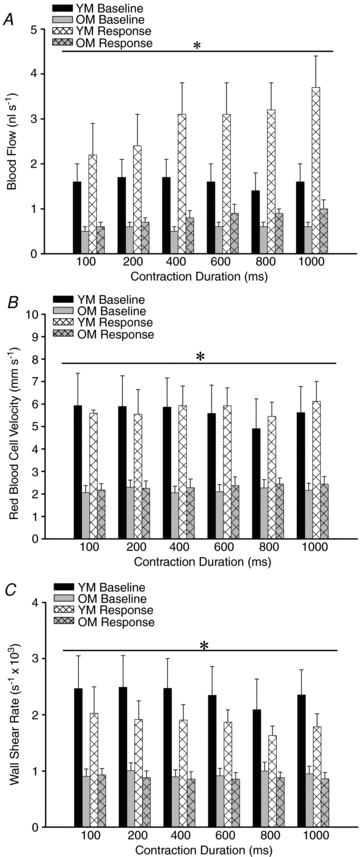
A, at rest and during peak dilatation in response to single tetanic contractions, blood flow was greatly attenuated in OM vs. YM. *P < 0.05, OM vs. YM. B, mean red blood cell velocity during peak dilatation did not change with increasing stimulus duration in either YM or OM. However, baseline and response values were blunted consistently in OM vs. YM. *P < 0.05, OM vs. YM. C, wall shear rate during peak dilatation did not change with increasing stimulus duration in either YM or OM. However, WSR was consistently lower in OM vs. YM. *P < 0.05, OM vs. YM. For all panels, n= 6 per group. Abbreviations are defined in Fig. 2 legend.
With no difference in resting diameter (Table 1), the reduction in 2A blood flow in OM vs. YM is explained by lower velocity of red blood cells. Baseline Vm remained stable during experiments but was reduced by more than half (P < 0.05) in OM vs. YM (Fig. 5B). Recorded during the peak of ROV diameter responses, there was no significant effect of contractile activity on Vm in either YM or OM. Thus, Vm was consistently reduced in OM and this is consistent with flow restriction by vessels located upstream from the exposed muscle (e.g. proximal feed arteries; VanTeeffelen & Segal, 2003). Nearly identical relationships were apparent for WSR (Fig. 5C), with OM having consistently lower values relative to YM (P < 0.05) at rest and in response to tetanic contraction.
Steady-state vasodilatation and blood flow with rhythmic contractions
From similar baseline diameters (Table 1), arteriolar dilatations consistently reached a stable plateau during 30 s of rhythmic contractions at 2, 4 and 8 Hz. The magnitude of dilatation increased with stimulation frequency for all experimental groups (P < 0.05). In marked contrast to ROV responses (Figs 2 and 3), neither age nor sex had a significant effect on the magnitude of diameter changes to rhythmic contractions (Fig. 6A) as arterioles of YM, YF, OM and OF all exhibited similar dilatations under these conditions.
Figure 6. With rhythmic contractions, arteriolar dilatations are independent of age or sex yet blood flow is blunted in OM.
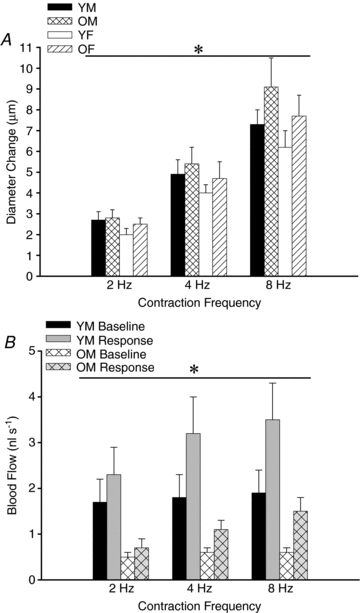
A, with no difference in resting or maximal diameters (Table 1), the magnitude of arteriolar dilatation increased with contraction frequency in a similar manner for each experimental group. *Main effect of stimulus frequency, P < 0.05 (n= 6–18 per group). B, blood flows at rest (baseline) and in response to 30 s of rhythmic contractions were restricted by more than half in OM compared to YM. *P < 0.05, OM vs. YM (n= 6 per group). Abbreviations are defined in Fig. 2 legend.
Arteriolar blood flow increased with stimulation frequency (P < 0.05) for both YM and OM. Peak responses in YM (2.3 ± 0.6 to 3.5 ± 0.8 nl s−1) were 2- to 3-fold greater (P < 0.05) than those recorded in OM (Fig. 6B) despite similar increases in arteriolar diameter, providing additional evidence for an upstream restriction of blood flow in OM compared to YM.
Discussion
The present study was undertaken using the C57BL/6 mouse as a model for investigating how ageing influences the arteriolar control of blood flow within a mammalian hindlimb locomotor muscle and to determine whether such effects differ between sexes. In second-order arterioles (2A) of the gluteus maximus muscle, we demonstrate that ROV in response to single tetanic contractions was blunted consistently and selectively in 20-month-old males relative to age-matched females and to 3-month-old mice of both sexes. Remarkably, the inhibition of αARs with phentolamine restored ROV in OM to levels seen in the other three groups. In turn, the blunted ROV observed in OM was reproduced in YM and YF by exposure to a concentration of NA (1 nm) that had no effect on resting diameter. Thus the blunting of ROV of 2A of OM can be explained by a subtle elevation in the resting level of arteriolar smooth muscle cell activation via αARs. In distinct contrast, there were no differences between respective groups for 2A dilatations during rhythmic submaximal contractions. Nevertheless, arteriolar blood flows were attenuated significantly under all conditions for OM as compared to YM, which can be explained by a blunted ability to evoke dilatation in proximal segments of the resistance network.
Rapid onset vasodilatation and its impairment with ageing
The present data are the first to demonstrate ROV in the mouse microcirculation and do so in a hindlimb muscle that is active during locomotion and adapts to physical training (Bearden et al. 2004). Elucidating ROV in response to a brief muscular contraction in humans (Corcondilas et al. 1964) has stimulated ongoing interest in understanding such rapid responsiveness of the peripheral resistance vasculature. Direct observations of the microcirculation in hamster cremaster (Mihok & Murrant, 2004; Armstrong et al. 2007b) and retractor (VanTeeffelen & Segal, 2006) muscles have resolved rapid dilatation of individual arterioles in response to single contractions, providing a direct link between events described for intact limbs in humans and the ability to resolve underlying mechanisms of blood flow control in the microcirculation.
That ROV is impaired with ageing in human subjects (Carlson et al. 2008) underscores the relevance of the present findings in the mouse GM as a model that enables direct insight into signalling events that modulate such phenotypic changes in vasomotor control. In accord with studies of the human forearm, where blood flow increased linearly with the intensity of single contractions (Tschakovsky et al. 2004), we observed ROV in the mouse GM to increase in direct proportion to the duration of single tetanic contractions (Fig. 2). Moreover, these dilatory responses were blunted selectively in OM across a 10-fold range of contraction duration (Fig. 2A). Attenuation of this relationship in OM relative to OF, YM or YF (Fig. 2B) indicates a reduction in sensitivity of ROV in OM. In turn, the difference between sexes that emerged here with ageing suggests that the determinants of ROV are similar in male and female mice until key events associated with ageing occur in OM. As active tension produced by the GM in male mice is maintained with ageing throughout the frequency-force relationship (Bearden et al. 2004), the blunting of ROV is not likely to be explained by a loss of contractile function. Instead, our findings suggest a subtle effect of αAR activation in OM that was not present in other experimental groups. Additional support for sex differences in the kinetics of vasomotor responses with ageing comes from demonstrating the slowing of vasodilatation onset (and acceleration of recovery) in arterioles of OM relative to other groups (Bearden, 2007). Such phenotypic changes may contribute to the lag in oxygen uptake at the onset of moderate cycling in older vs. younger subjects (DeLorey et al. 2004).
Role for α-adrenoreceptor activation in blunting ROV and arteriolar blood flow
A unique finding of this study is restoration of ROV in OM when αARs were inhibited with phentolamine (Figs 3 and 4). This gain of function led us to test whether the sympathetic neurotransmitter NA could mediate such an effect and was tested in young animals of both sexes. As illustrated in Fig. 3, ROV responses were attenuated by NA in YM and YF without altering resting arteriolar diameter. While internal diameter was not changed measurably by NA, arteriolar smooth muscle cells adopted a ‘crinkly’ appearance similar to that observed for arterioles in OM. The present findings thereby indicate a subtle yet constitutively higher level of arteriolar smooth muscle cell activation via αARs in OM as compared to the other groups studied here. In turn, exposing OM to NA resulted in even greater attenuation of ROV (Fig. 4) despite no effect on resting diameter.
The use of phentolamine as a non-selective αAR antagonist precludes the ability to resolve specific roles for α1- vs.α2-adrenoreceptors in modulating ROV. Nevertheless, as shown in feed arteries of hamster skeletal muscle, the two adrenoreceptor subtypes can interact additively to suppress rapid dilatations. For example, acetylcholine initiates hyperpolarization and rapid relaxation of microvascular smooth muscle cells (Emerson & Segal, 2000) through activating calcium-sensitive K+ channels (Domeier & Segal, 2007). Indeed, early studies in the dog hindlimb (Mohrman et al. 1973; Mohrman & Sparks, 1974) implicated a key role for K+ in rapid hyperaemic responses to single brief tetanic contractions. This conclusion is supported by recent findings in the hamster cremaster muscle illustrating the role of K+ in mediating rapid arteriolar dilatation in response to single contractions of skeletal muscle fibres (Armstrong et al. 2007a).
Vasodilatation can ascend the resistance network into proximal feed arteries in response to muscle contraction (Hilton, 1959) and thereby increase muscle blood flow by reducing vascular resistance located upstream from intramuscular arterioles (Folkow et al. 1971; Segal & Jacobs, 2001). In older humans, blood flow to exercising skeletal muscle is attenuated (Dinenno & Joyner, 2006; Proctor & Parker, 2006), though the site(s) of flow restriction were not defined. As feed arteries supplying the GM were not exposed in our surgical preparations, the elevated activation of αARs that was apparent in arterioles was also likely to be manifested in proximal branches of the vascular supply. We therefore propose that the restriction of arteriolar perfusion in OM reflects the impairment of ascending vasodilatation by αAR activation, such that feed artery tone remained high and thereby limited blood flow into arterioles downstream. The attenuated Vm (Fig. 5B) and WSR (Fig. 5C) recorded at rest and during arteriolar responses in OM are consistent with such an effect and supported by earlier findings. First, sympathetic nerve stimulation reduced blood flow into contracting skeletal muscle even during robust arteriolar dilatation and was associated with inhibition of feed artery dilatation (VanTeeffelen & Segal, 2003). Second, activation of αARs inhibited the ability of vasodilatation to conduct along feed arteries (Haug & Segal, 2005). Third, conducted vasodilatation is impaired in OM relative to YM (Bearden et al. 2004, 2007). Though the mechanism underlying the inhibition of vasodilatation in proximal segments of the resistance vasculature remains to be defined, we suggest that it too is associated with higher levels of αAR activation in vascular smooth muscle cells.
Rhythmic twitch contractions dilated arterioles to similar steady-state levels irrespective of age or sex (Fig. 6A). This similarity across experimental groups contrasts with the selective blunting of ROV for OM in response to single tetanic contractions. Thus the effect of ageing on arteriolar dilatation depends upon the nature of contractile activity. This conclusion is consistent with the interpretation that signalling events mediating sustained vasodilatation differ from those mediating rapid vasodilatation (Haddy & Scott, 1975; Morganroth et al. 1975). Even with similar arteriolar dilatations across experimental groups, and consistent with attenuated blood flow during ROV, peak blood flow responses were consistently 2- to 3-fold greater in YM versus OM (Fig. 5B). In accord with flow limitations apparent during ROV, such behaviour during rhythmic contractions (Fig. 6B) and during maximum dilatation with topical SNP (see Results, Haemodynamic responses) further implies that total blood flow into the arteriolar network was restricted by proximal segments of the resistance network (Jacobs & Segal, 2000; VanTeeffelen & Segal, 2003). As these conclusions are based entirely upon haemodynamic measurements performed in male animals due to the greater morbidity we encountered with OF vs. OM mice, future experiments are required to ascertain the nature of microvascular blood flow in female animals and how it may be affected by ageing.
Experiments performed on the cremaster muscle of rats have shown that differences in the ability to evoke dilatation in proximal vs. distal resistance microvessels may also reflect corresponding differences in the regional distribution of αAR subtypes (Anderson & Faber, 1991). Whether regional distribution of αAR subtypes is manifest in the mouse GM remains to be investigated. Nevertheless, the present findings help to explain how augmented αAR activation with ageing effectively blunts resting blood flow as well as exercise hyperaemia in humans (Dinenno & Joyner, 2006; Proctor & Parker, 2006). Indeed, a similar mechanism may well contribute to delaying the attainment of functional vasodilatation as well as the impaired ability to sustain vasodilatation into the recovery period (Bearden et al. 2004; Bearden, 2007).
Sympathetic neuroeffector signalling, muscle blood flow and the role of sex
While there is general agreement that enhanced actions of the sympathetic nervous system contribute to attenuated blood flow responses with ageing (Dinenno & Joyner, 2006; Proctor & Parker, 2006), it remains controversial as to whether vasomotor responsiveness to neuroeffector signalling is altered. For example, when acute sympathetic vasoconstriction (via cold pressor test) was evaluated during bicycle ergometry, older men demonstrated greater reduction in leg vascular conductance as compared to their younger counterparts (Koch et al. 2003). In contrast, when postjunctional αARs in the leg of male subjects at rest were stimulated with tyramine to release NA, ageing was associated with reduced responsiveness of both α1- and α2-AR subtypes (Smith et al. 2007). These recent findings support earlier observations showing attenuated forearm blood flow reductions of older vs. young subjects (both male and female) at rest during infusions of NA into the brachial artery (Hogikyan & Supiano, 1994). In accord with the present data, lower basal vascular conductance in the legs of older vs. younger males (Dinenno et al. 1999; Smith et al. 2007) reflected greater tonic vasoconstriction mediated by enhanced SNA recorded from the peroneal nerve (Dinenno et al. 1999). However, when the responsiveness of isolated skeletal muscle arterioles to adrenergic receptor agonists has been evaluated directly in vitro, vasoconstriction to NA was similar between vessels isolated from young (4-month) and aged (24-month) rats (Muller-Delp et al. 2002a). Similarly, in the mouse GM, constriction to phenylephrine (an α1-selective adrenoreceptor agonist) was not different between YM and OM (Bearden et al. 2004). Thus age-related differences in vasomotor responsiveness that have been identified in awake human subjects at rest or during exercise may involve cardiovascular reflexes that are avoided in isolated vessels or individual muscles of anaesthetized animals as studied here.
Consistent with the present findings, recent observations in older humans indicate that ROV in the forearm was blunted relative to younger subjects (Carlson et al. 2008). However, unlike the present study, this effect of ageing was apparent for both sexes. Leg blood flow during moderate cycling has been reported to be reduced in both older male subjects (Proctor et al. 1998) and female subjects (Proctor et al. 2003a) even when accounting for active muscle mass. Nevertheless, impaired blood flow in older men relative to their younger counterparts has not been consistently observed (Proctor et al. 2003b) and more recent findings emphasize that attenuated vasodilatation during rhythmic single-leg knee extensions (which minimize central limitations of cardiac output) is greater in older women than in older men (Parker et al. 2008). Thus apparent differences between sexes may also depend upon the nature of exercise used for evaluating muscle blood flow and whether or not systemic cardiovascular reflexes are invoked. As oestrogen therapy was found to reverse augmented sympathetic vasoconstriction in postmenopausal women (Fadel et al. 2004), the role of oestrogen in maintaining arteriolar perfusion (and ROV) with ageing also remains to be ascertained.
The present data showing selective blunting of arteriolar blood flow in the GM of OM (Figs 5A and 6B) are supported by earlier studies in the hindlimb of Fischer 344 rats, where the ability to increase blood flow during muscle contractions as well as during vasodilator infusion was depressed in 24-month vs. 12-month males (Irion et al. 1987) but not in females (Irion et al. 1988). In these studies, greater muscle fatigue was associated with the reduction in muscle blood flow. As neuropeptide Y (NPY) is a co-transmitter released with NA from sympathetic nerve terminals, evidence from adult Sprague–Dawley rats suggests that such sex differences in muscle blood flow may be related to a greater role for NPY in mediating sympathetic actions on the vasculature for males as compared to females (Jackson et al. 2005a,b;). However, the effects of ageing on the contributions of NPY to noradrenergic vasoreactivity also remain to be elucidated.
Summary and perspective
The present study provides evidence that ageing is associated with microvascular dysfunction that is selective for male C57BL/6 mice. Deleterious effects of ageing on dynamic blood flow control in the GM of OM were highlighted by blunted ROV in response to single tetanic contractions when compared to the GM of OF, YM or YF. As blockade of αARs with phentolamine restored ROV in OM and topical NA attenuated ROV in YM and YF, we suggest that ageing is associated with constitutively elevated levels of αAR activation in resistance microvessels of OM. Furthermore, arteriolar blood flow was attenuated in OM relative to YM at rest, during ROV, during steady state dilatations to rhythmic twitch contractions and during maximal dilatation with topical SNP. As these effects were manifest despite no significant differences in systemic arterial blood pressure, resting or maximal arteriolar diameters between experimental groups, we propose that even without overt vasoconstriction or vascular remodelling, subtle levels of αAR activation can effectively compromise rapid adjustments in tissue blood flow and could thereby impair daily activities. Moreover, blunted red blood cell velocity with no difference in arteriolar diameters implicates proximal segments of the resistance network as key sites for restricting muscle blood flow with ageing.
Acknowledgments
This work was supported by Grant RO1-HL086483 from the Heart, Lung, and Blood Institute of the National Institutes of Health, United States Public Health Service. D.N.J. was supported by a postdoctoral fellowship from the Heart and Stroke Foundation of Canada. A.W.M. was supported by a Life Sciences Fellowship from the University of Missouri. The helpful comments of Dr Paul Fadel are greatly appreciated.
Glossary
Abbreviations
- αAR
α-adrenoreceptor
- EFS
electrical field stimulation
- GM
gluteus maximus
- NA
noradrenaline
- ROV
rapid onset vasodilatation
- SNA
sympathetic nerve activity
Author contributions
D.N.J., A.W.M. and S.S.S. contributed to the conception, experimental design and execution of the study, and the analysis and interpretation of data. Experiments were performed at the John B. Pierce Laboratory (D.N.J. and S.S.S.) and at the University of Missouri (A.W.M. and S.S.S.). D.N.J. and S.S.S. prepared the original submission. A.W.M. contributed additional text and all co-authors have approved the final version submitted.
Author's present address
D. N. Jackson: Department of Medical Biophysics, Schulich School of Medicine & Dentistry, The University of Western Ontario, London, Ontario, Canada N6A 5C1.
References
- Anderson KM, Faber JE. Differential sensitivity of arteriolar α1- and α2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007a;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong ML, Dua AK, Murrant CL. Time course of vasodilation at the onset of repetitive skeletal muscle contractions. Am J Physiol Regul Integr Comp Physiol. 2007b;292:R505–515. doi: 10.1152/ajpregu.00381.2006. [DOI] [PubMed] [Google Scholar]
- Bearden SE. Advancing age produces sex differences in vasomotor kinetics during and after skeletal muscle contraction. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1274–1279. doi: 10.1152/ajpregu.00213.2007. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Linn E, Ashley BS, Looft-Wilson RC. Age-related changes in conducted vasodilation: effects of exercise training and role in functional hyperemia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1717–1721. doi: 10.1152/ajpregu.00827.2006. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol. 2008;294:H1963–1970. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol. 1964;19:142–146. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol. 2004;97:165–172. doi: 10.1152/japplphysiol.01179.2003. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. α-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13:329–341. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol. 2004;561:893–901. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenic and metabolic effects on precapillary vessels of skeletal muscle. Acta Physiol Scand. 1971;81:459–471. doi: 10.1111/j.1748-1716.1971.tb04924.x. [DOI] [PubMed] [Google Scholar]
- Haddy FJ, Scott JB. Metabolic factors in peripheral circulatory regulation. Fed Proc. 1975;34:2006–2011. [PubMed] [Google Scholar]
- Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of α1- and α2-adrenoreceptor activation. J Physiol. 2005;563:541–555. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton SM. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. J Physiol. 1959;149:93–111. doi: 10.1113/jphysiol.1959.sp006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogikyan RV, Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab. 1994;266:E717–724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol. 1987;42:660–665. doi: 10.1093/geronj/42.6.660. [DOI] [PubMed] [Google Scholar]
- Irion GL, Vasthare US, Tuma RF. Preservation of skeletal muscle hyperemic response to contraction with aging in female rats. Exp Gerontol. 1988;23:183–188. doi: 10.1016/0531-5565(88)90005-8. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Milne KJ, Noble EG, Shoemaker JK. Gender-modulated endogenous baseline neuropeptide Y Y1-receptor activation in the hindlimb of Sprague-Dawley rats. J Physiol. 2005a;562:285–294. doi: 10.1113/jphysiol.2004.076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DN, Milne KJ, Noble EG, Shoemaker JK. Neuropeptide Y bioavailability is suppressed in the hindlimb of female Sprague-Dawley rats. J Physiol. 2005b;568:573–581. doi: 10.1113/jphysiol.2005.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Segal SS. Attenuation of vasodilatation with skeletal muscle fatigue in hamster retractor. J Physiol. 2000;524:929–941. doi: 10.1111/j.1469-7793.2000.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fibre type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1600–1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol. 2004;82:282–287. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Cant JR, Sparks HV. Time course of vascular resistance and venous oxygen changes following brief tetanus of dog skeletal muscle. Circ Res. 1973;33:323–336. doi: 10.1161/01.res.33.3.323. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res. 1974;35:384–390. doi: 10.1161/01.res.35.3.384. [DOI] [PubMed] [Google Scholar]
- Morganroth ML, Mohrman DE, Sparks HV. Prolonged vasodilation following fatiguing exercise of dog skeletal muscle. Am J Physiol. 1975;229:38–43. doi: 10.1152/ajplegacy.1975.229.1.38. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002a;282:H1843–1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002b;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Murrant CL. Stimulation characteristics that determine arteriolar dilation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R505–R513. doi: 10.1152/ajpregu.00571.2004. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104:655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol. 1997;82:1411–1415. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003a;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003b;94:1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Seals DR. Influence of muscle mass on sympathetic neural activation during isometric exercise. J Appl Physiol. 1989;67:1801–1806. doi: 10.1152/jappl.1989.67.5.1801. [DOI] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol. 1998;76:418–427. doi: 10.1139/cjpp-76-4-418. [DOI] [PubMed] [Google Scholar]
- Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional α-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol. 2007;582:63–71. doi: 10.1113/jphysiol.2007.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Effect of motor unit recruitment on functional vasodilatation in hamster retractor muscle. J Physiol. 2000;524:267–278. doi: 10.1111/j.1469-7793.2000.t01-1-00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibres with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol. 1997;273:H156–163. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]


