Abstract
Gametogenesis is a complex biological process that is particularly sensitive to environmental insults such as chemicals. Many chemicals have a negative impact on the germline, either by directly affecting the germ cells, or indirectly through their action on the somatic nursing cells. Ultimately, these effects can inhibit fertility, and they may have negative consequences for the development of the offspring. Recently, nanomaterials such as nanotubes, nanowires, fullerene derivatives (buckyballs), and quantum dots have received enormous national attention in the creation of new types of analytical tools for biotechnology and the life sciences. Despite the wide application of nanomaterials, there is a serious lack of information concerning their impact on human health and the environment. Thus, there are limited studies available on toxicity of nanoparticles for risk assessment of nanomaterials. The purpose of this study was to assess the suitability of a mouse spermatogonial stem cell line as a model to assess nanotoxicity in the male germline in vitro. The effects of different types of nanoparticles on these cells were evaluated by light microscopy, and by cell proliferation and standard cytotoxicity assays. Our results demonstrate a concentration-dependent toxicity for all types of particles tested, whereas the corresponding soluble salts had no significant effect. Silver nanoparticles were the most toxic while molybdenum trioxide (MoO3) nanoparticles were the least toxic. Our results suggest that this cell line provides a valuable model with which to assess the cytotoxicity of nanoparticles in the germ line in vitro.
Keywords: nanoparticles, toxicity, cell line, spermatogonia, stem cells
Introduction
Nanotechnology involves the creation and manipulation of materials at the nanoscale level to create unique products that exploit novel properties. Recently, nanomaterials such as nanotubes, nanowires, fullerene derivatives (buckyballs), and quantum dots have received enormous national attention in the creation of new types of analytical tools for biotechnology and the life sciences (De Wild et al., 2003). Bionanomaterials, which are by definition in the 1–100 nm range, have been used to create materials that have novel physical/chemical properties and functions based on their advantageous, miniscule size. In particular, nanoparticles are now used to target synthetic peptides, proteins, oligonucleotides, and plasmids to specific cell type while protecting these macromolecules from enzymatic degradation (Chavany et al., 1994; Janes et al., 2001). In addition, nanoparticles have been proposed for the treatment of many diseases that need constant drug concentration in the blood or drug targeting to specific cells or organs (Moghimi et al., 2001; Panyam and Labhasetwar, 2003). In this respect, nanoencapsulated therapeutic agents such as antineoplastic drugs have been used with the aim of selectively targeting antitumor agents and obtaining higher drug concentration at the tumor site (Chawla and Amiji, 2002; Sahoo et al., 2004). This achievement appears to be important because many antineoplastic agents have several adverse side effects. Nanoparticles can be used to treat diseases that require a sustained presence of the drug at several anatomical sites (Panyam and Labhasetwar, 2003; Li et al., 2004). In addition, nanomaterials are of interest to defense and engineering programs because of their potential use in electronics, sensors, munitions, and energetic/reactive systems involved in the advancement of propulsion technology (Ringer and Ratinac, 2004). If formulated properly with other materials, nanomaterials may provide greater stability and efficiency for propellant systems.
Despite the wide application of nanomaterials, there is a serious lack of information concerning the impact of manufactured nanomaterials on human health and the environment. Typically, after systemic administration, the nanoparticles are small enough to penetrate even very small capillaries throughout the body, and therefore they offer the most effective approach to distribution in certain tissues. Because nanoparticles can pass through biological membranes, they can affect the physiology of any cell in an animal body (Brooking et al., 2001). This consideration is of importance for stem cells, where the effects of nanoparticles on their potential for self-renewal and differentiation is unknown. Data available from toxicity studies of nanoparticles, in particular in adult stem cells, are limited.
Gametogenesis is a complex biological process that is sensitive to environmental insult, for example, from chemicals (Adler, 2000; Iona et al., 2002). Chemical effects on germ cells and their maturation can inhibit fertility, cause cancer, and may have negative effects on the development of offspring. Mutagens, for example, produce heritable gene mutations, and heritable structural and numerical chromosome aberrations in germ cells (Allen et al., 1986; Tilly, 1998). The consequences of germ cell mutation for subsequent generations include the following: genetically determined phenotypic alterations without signs of illness; reduction in fertility; embryonic or perinatal death; congenital malformations with varying degrees of severity; and genetic diseases with varying degrees of health impairment (Brinkworth, 2000). Recent studies have shown that intravenous and/or intra-abdominal administration of nanoparticles to mice results in their accumulation in the cells of many tissues, including the brain and the testis, suggesting that they easily pass through the blood–brain and blood–testis barriers (Borm and Kreyling, 2004; Chen et al., 2003). The purpose of this study was to assess the suitability of a spermatogonial stem cell line as a model for the assessment of nanotoxicity in the male germ line in vitro. The effects of different types of nanoparticles on these cells was evaluated using light microscopy, cell proliferation and standard cytotoxicity assays. Our results suggest that this cell line provides a sensitive model with which to assess the cytotoxicity of nanoparticles in the germline. Furthermore, the relative toxicity of several types of widely used nanoparticles is discussed.
Materials and Methods
Cell Line
The C18–4 cell line was previously established from type A spermatogonia isolated from 6-day-old mouse testes (Hofmann et al., 2005a). The cells were immortalized using the large T antigen gene and exhibit a phenotype characteristic of germline stem cells in vivo, such as the expression of the Ret and GFRα-1 surface receptors and of the nuclear factor Oct-4 (de Rooij and Russell, 2000; Pesce et al., 1998; Hofmann et al., 2005b). The cells are adherent and respond to the growth factor glial cell line–derived neurotrophic factor (GDNF) (Meng et al., 2000) by increasing their rate of proliferation (Hofmann et al., 2005a).
Cell Culture
The cells were maintained in D-MEM medium supplemented with 10% fetal calf serum (FCS), 1 mM sodium pyruvate, 2 mM glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin, and 100 mM non-essential amino acids. All tissue culture reagents were purchased from Atlanta Biologicals, (Norcross, GA) or Fisher Scientific (Pittsburgh, PA). For morphological studies, the cells were seeded in 6-well plates at a concentration of 3 × 105 cells/well in 2 ml of complete medium. For the MTS and LDH assays, the cells were seeded onto 96-well plates at a density of 1 × 104 cells per well. The cells were incubated in a humidified incubator at 33°C and 5% CO2 atmosphere. They were cultured for 2 days (60% confluency) before the assays. For the apoptosis/necrosis assay, the cells were seeded onto 6-well plates at a density of 1 × 105 cells/well and cultured for 2 days (80% confluency) before the assays.
Nanoparticles
Silver (Ag—15 nm), molybdenum (MoO3—30 nm), and aluminum (Al—30 nm) nanoparticles were obtained from the Air Force Research Laboratory, Brooks AFB, TX. The nanoparticles were made in a commercial pulsed-plasma reactor, which forms the particles in a gas phase process. As a positive control for toxicity, we used cadmium oxide, a large-sized material of ∼1000 nm known for its cytotoxic properties (Goering et al., 2000) (Fluka Chemicals/Sigma, St Louis, MO). Silver, molybdenum, and aluminum nanoparticles were first dispersed in phosphate buffered saline (PBS) and used at final concentrations of 5, 10, 25, 50, and 100 μg/ml culture medium. Cadmium oxide was also dispersed in PBS and used at final concentrations of 1, 2, 5, 10, and 25 μg/ml. For comparison, we also assessed the effect of soluble species such as cadmium chloride, silver carbonate, aluminum chloride, and sodium molybdate. Those chemicals were obtained from Sigma, St Louis, MO.
Cell Morphology
When cells reached 60% confluency, the different types of nanoparticles were added to the cells at different concentrations. After 48 h incubation, cell morphology was assessed with a Nikon Eclipse TS-100 phase-contrast microscope.
In Vitro Cell Viability/Cytotoxicity Studies
Mitochondrial function
Mitochondrial function was assessed using the CellTiter 96 AQueous One Solution Assay (Promega, Madison, WI). The solution reagent contains a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS(a)] and an electron coupling reagent (phenazine ethosulfate; PES). Fourty eight hours after seeding the cells, different concentrations of nanoparticles were added to the microtiter wells. The cultures were further incubated for 48 h, and then 20 μl of the AQueous One Solution reagent was directly added to the culture wells. After 3 h incubation, the absorbance at 490 nm was measured with a standard microplate reader (Victor3 multilabel counter, PerkinElmer, Boston, MA). The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture. Each experiment was done in triplicate. The relative cell viability (%) related to control wells containing cell culture medium without nanoparticles or PBS as a vehicle was calculated by [A]test/[A]control × 100. Where [A]test is the absorbance of the test sample and [A]control is the absorbance of control sample.
LDH leakage
For the lactase dehydrogenase (LDH) leakage assay, we used the CytoTox 96 Non-Radioactive Cytotoxicity Assay of Promega (Madison, WI). This is a colorimetric assay that quantitatively measures LDH, a stable cytosolic enzyme that is released upon cell lysis. Released LDH in culture supernatants is measured with a 30-min coupled enzymatic assay that results in the conversion of a tetrazolium salt (INT) into a red formazan product. The amount of color formed is proportional to the number of lysed cells. Fourty eight hours after seeding, the cells were treated with the different nanoparticles at different concentrations, and incubated for another 48 h. Then, 50 μl of cell culture medium was collected from each well, diluted 1:1 with fresh medium, and plated into a new microtiter plate. Next, 50 μl of substrate solution was added to the wells, and the plates were incubated for 30 min at room temperature. Absorbance at 490 nm was measured with a standard microplate reader (Victor3 multilabel counter, PerkinElmer, Boston, MA). Each experiment was done in triplicate. The spectrophotometer was calibrated to zero absorbance using culture medium without cells. The relative LDH leakage (%) related to control wells containing cell culture medium without nanoparticles or PBS as a vehicle was calculated by [A]test/[A]control × 100. Where [A]test is the absorbance of the test sample and [A]control is the absorbance of the control sample.
Apoptosis/necrosis assay
To determine cellular apoptosis or necrosis, we used the Vybrant Apoptosis Assay Kit #4 (Molecular Probes, Eugene, OR). Kit #4 detects changes in cell membrane permeability with YO-PRO-1 dye, a green-fluorescent nucleic acid stain that is permeant to apoptotic cells but not to live cells. Necrotic cells are labeled with red-fluorescent propidium iodide. Cells were cultured with sublethal concentrations of nanoparticles for 48 h. They were then washed with PBS and incubated with 2 μl of YO-PRO-1 dye and 2 μl of propidium iodide in 2 ml of PBS per well, and further incubated for 30 min. After three washes, the cultures were covered with 1 ml of PBS, and the numbers of apoptotic and necrotic cells were immediately evaluated with a phase-contrast fluorescence microscope. Results were expressed as percent control cells cultured without nanoparticles.
Statistical Analysis
All experiments were done in triplicate, and the results were presented as mean ± standard deviation. The experimental data were analyzed by ANOVA using SPSS. Statistical significance was accepted at a level of p < 0.05. To calculate EC50 values, concentration–response curves were graphed using Excel. The EC50 of the curves was calculated by non-linear regression analysis and interpolation according to the method of Alexander et al. (1999).
Results
Cell Morphology
The general morphology of the C18–4 cells incubated with nanoparticles in phase-contrast microscopy is shown in Figure 1. The figure shows that the cells were well spread, and there was no distinct change in morphology after 48 h of incubation with any concentration of molybdenum nanoparticles relative to control cells (Fig. 1A and Fig. 1E). However, dramatic changes occur with cadmium oxide (Fig. 1B), which is considered as positive control for cytotoxicity in this report. Within 48 h of exposure at concentrations of 1 μg/ml CdO already, the cells shrink and become irregular. At concentrations higher than 5 μg/ml, they became necrotic and detached from the culture dishes. Figure 1C shows the dramatic changes induced by silver nanoparticles at concentrations of 10 μg/ml and above. In addition to necrotic areas, some cells retained an intact plasma membrane (arrows), indicating that apoptosis had occurred. Interestingly, aluminum nanoparticles did not induce shrinkage, necrosis, or apoptosis of the cells, at least at concentrations below 10 μg/ml. Instead, the particles accumulated in the cell cytoplasm, forming aggregates that were unable to enter the nucleus (Fig. 1D).
FIG. 1.
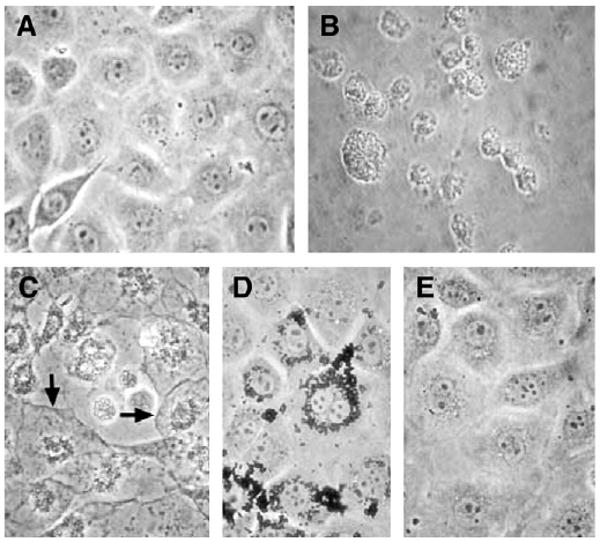
Morphology of the C18–4 spermatogonial stem cells after incubation with cadmium oxide and different types of nanoparticles for 48 h. A. Control. B. Cadmium oxide (10 μg/ml). C. Silver nanoparticles (Ag—15 nm, 10 μg/ml). D. Aluminum nanoparticles (AL—30 nm, 10 μg/ml). E. Molybdenum nanoparticles (MoO3—30 nm, 10 μg/ml).
Mitochondrial Function
The mitochondrial function—and by extension the viability of the germline stem cells—was measured by means of the MTS assay after culturing in presence of the nanoparticles for 48 h. As is evident from Figure 2, in general the cytotoxic effect of the nanoparticles on mitochondrial activity increases in relation to increasing concentration. Figure 2A shows that soluble cadmium chloride, a known toxicant, had no significant effect on mitochondrial activity at concentrations below 5 μg/ml. The EC50 was calculated at 21.3 μg/ml. In comparison, the cytotoxic effects of cadmium oxide were much stronger, with a significant inhibition of mitochondrial function starting at concentrations below 1 μg/ml. The EC50 was calculated at 0.5 μg/ml.
FIG. 2.
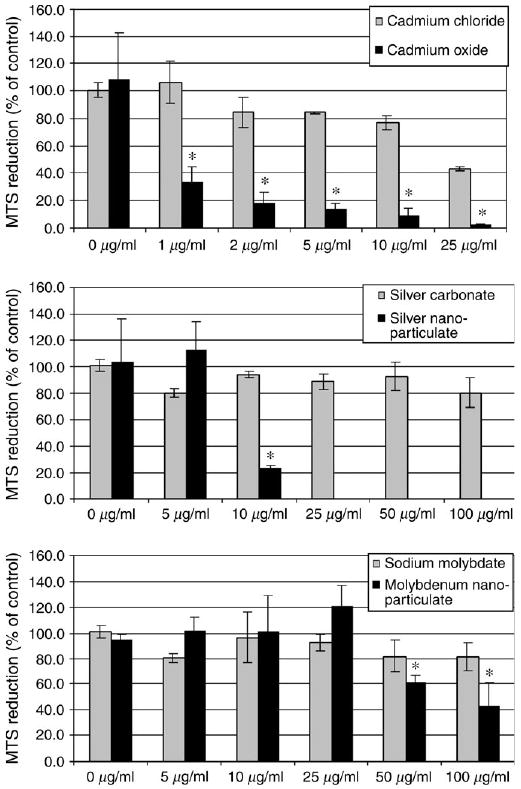
Influence of cadmium oxide and different types of nanoparticles on the metabolic activity of the C18–4 spermatogonial stem cells after 48 h incubation. At the end of the incubation period, mitochondrial function was determined by the MTS reduction assay as described in Materials and Methods. Each panel is a composite of two experiments, with n ≥ 3 for each point. *Statistically significant difference compared with controls (p < 0.05). A. MTS reduction in presence of different concentrations of cadmium chloride and cadmium oxide. B. MTS reduction in presence of different concentrations of silver carbonate and silver nanoparticles (Ag—15 nm). C. MTS reduction in presence of different concentration of sodium molybdate and molybdenum nanoparticles (MoO3—30 nm).
Figure 2B shows that, on the one hand, silver carbonate, generally considered to be non-hazardous, had no significant cytotoxic effect on mitochondrial function and cell viability up to concentrations of 100 μg/ml. The EC50 of silver carbonate was calculated at 408 μg/ml. On the other hand, silver added to the cells as a nanoparticulate (diameter of 15 nm) reduced drastically mitochondrial function and cell viability. The toxic effect of silver nanoparticles started between 5 and 10 μg/ml, with an EC50 calculated at 8.75 μg/ml. Higher concentrations of silver nanoparticles could not be tested because of particle clumping and precipitation above 10 μg/ml.
Figure 2C compares the influence of soluble and nanoparticulate molybdenum species on germline stem cell mitochondrial function and viability. In general, sodium molybdate is known to be moderately toxic for humans and other animal species. In the case of the C18–4 germline stem cells, the mitochondrial function and percentage viability of the germ cells decreased slightly at concentrations of 50 μg/ml and above, but the difference was not statistically different from the results obtained with lower concentrations. The EC50 of sodium molybdate was calculated at 322 μg/ml. In contrast, a significant decrease in mitochondrial function was seen when molybdenum was used as a nanoparticulate (30 nm in diameter). Our data indicate that in these cells, molybdenum nanoparticles exert toxic effects on cellular metabolic activity at concentrations of 50 μg/ml and above. The EC50 of molybdenum nanoparticles is 90 μg/ml.
The effects of aluminum nanoparticles on mitochondrial function could not be assessed because the particles accumulated in the cells and formed cytoplasmic aggregates at low concentrations (Fig. 1D), and the light-scattering effect of the particles interfered with the spectrophotometric readings.
Membrane Leakage
Figure 3A shows that soluble cadmium chloride has no effect on the plasma membrane at any of the concentrations tested. However, a dose-dependent increase in LDH leakage was observed with cadmium oxide. The EC50 of cadmium oxide was calculated at 2.5 μg/ml. In comparison, 48 h of treatment of the C18–4 germline stem cells with nanoparticles produced a dose-dependent increase of LDH leakage at lower concentrations only. In general, LDH leakage reached a plateau at higher concentrations. Figure 3B shows that soluble silver carbonate does not affect the plasma membrane, which was expected. A slight increase in LDH leakage was observed with silver nanoparticles, indicating that these particles interfere with cell metabolism rather than disrupting the plasma membrane. Thus, they might promote cell apoptosis rather than necrosis (Fig. 1). The EC50 of silver nanoparticles was evaluated at 2.5 μg/ml. Higher concentrations of silver nanoparticles were not assessed because of particle clumping and precipitation at levels above 10 μg/ml. Figure 3C indicates that aluminum chloride did not affect the integrity of the plasma membrane at any of the concentrations tested. In contrast, a statistically significant increase of LDH leakage was observed with aluminum nanoparticles. Leakage is dose-dependent at lower concentrations, and the values reach a plateau at around 25 μg/ml nanoparticles. The EC50 was measured at 4.7 μg/ml. Figure 3D shows the effects of sodium molybdate and molybdenum in nanoparticulate form on the C18–4 cells. Soluble sodium molybdate did not affect the integrity of the plasma membrane, whereas a significant increase of LDH leakage was observed with molybdenum nanoparticles. The leakage was dose-dependent at low concentrations until the values reached a plateau at 10 μg/ml nanoparticles. The EC50 was measured at 5 μg/ml.
FIG. 3.
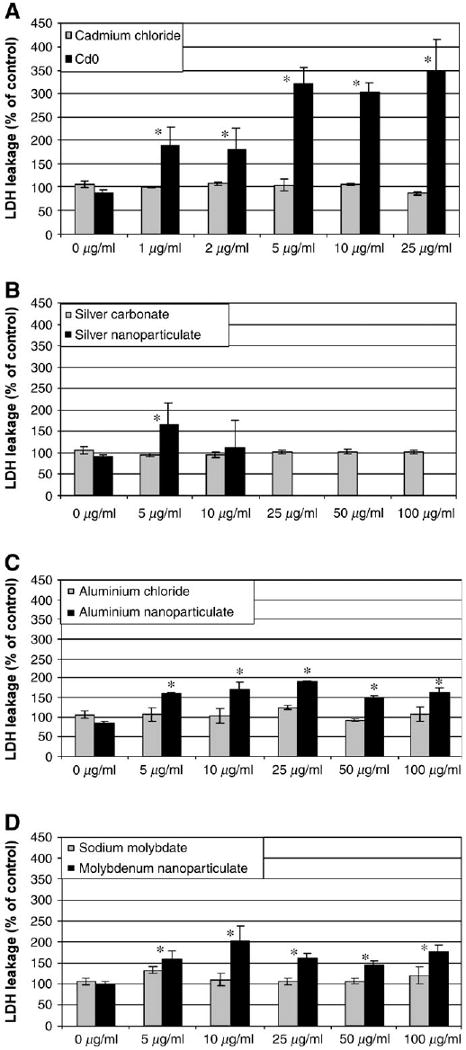
Influence of cadmium oxide and different types of nanoparticles on the membrane integrity of the C18–4 spermatogonial stem cells after 48 h incubation. At the end of the incubation period, the amount of LDH released into the medium was measured by spectrophotometric analysis as described in Materials and Methods. Each panel is a composite of two experiments, with n ≥ 3 for each point.*Statistically significant difference compared with controls (p < 0.05). A. LDH membrane leakage in presence of different concentrations of cadmium chloride and cadmium oxide. B. LDH membrane leakage in presence of different concentrations of silver carbonate and silver nanoparticles (Ag—15 nm). C. LDH membrane leakage in presence of different concentrations of aluminum chloride and aluminum nanoparticles (Al—30 nm). D. LDH membrane leakage in presence of different concentrations of sodium molybdate and molybdenum nanoparticles (MoO3—30 nm).
Apoptosis Assays
Because the cellular metabolic activity seemed affected by the nanoparticles but the plasma membrane remained relatively intact, we assessed the possibility of apoptosis caused by the nanoparticles, especially at lower concentrations. Using the Vybrant apopotosis assay that measures the number of cells in apoptosis versus necrosis, we confrmed that apopotosis took place at low nanoparticle concentrations. Figure 4A shows an increased number of apoptotic cells, and that increase is dose-dependent at lower concentrations (1–5 μg/ml for cadmium oxide, and 10–50 μg/ml for the nanoparticulates tested). More cells became necrotic as the concentrations increased. In the case of molybdenum nanoparticles, a small number of apoptotic cells began to be observed starting at a concentration above 25 μg/ml, and few necrotic cells were observed at concentrations below 50 μg/ml. Figure 4B and 4C illustrates apoptosis in the C18–4 cells treated with molybdenum nanoparticles at a concentration of 50 μg/ml. Most cells looked intact in phase-contrast microscopy (Fig. 4B). However, these same cells exhibited positive nuclei in fluorescence microscopy when the YO-PRO-1 dye was used (Fig. 4C), but they were negative for propidium iodide that can penetrate only necrotic cells.
FIG. 4.
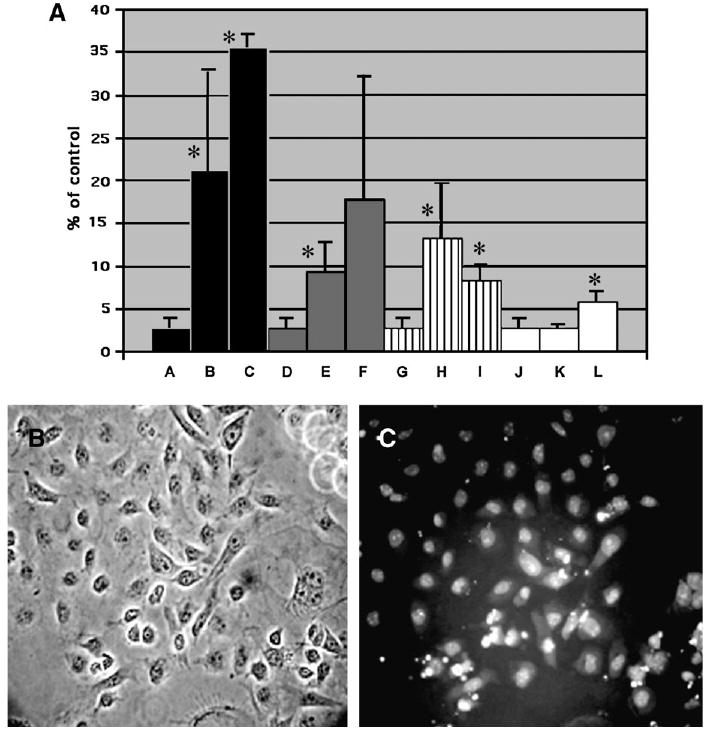
Induction of apoptosis by cadmium oxide and different types of nanoparticles in the C18–4 spermatogonial stem cells after 48 h incubation. A. At the end of the incubation period, the percentage of cells in apoptosis was measured with the Vybrant assay that uses the YO-PRO-1 dye and propidium iodide as described in Materials and Methods. The graph is a composite of two experiments, with n ≥ 3 for each point.*Statistically significant difference compared with controls (p < 0.05). Lanes: A. control (medium +PBS); B. cadmium oxide (1 μg/ml); C. cadmium oxide (5 μg/ml); D. control (medium + PBS); E. silver nanoparticles (5 μg/ml); F. silver nanoparticles (10 μg/ml); G. control (medium + PBS); H. aluminum nanoparticles (5 μg/ml); I. aluminum nanoparticles (10 μg/ml); J. control (medium + PBS); K. molybdenum nanoparticles (25 μg/ml). L. molybdenum nanoparticles (50 μg/ml). B. Morphology of the C18–4 spermatogonial stem cells in phase contrast microscopy after incubation with 10 μg/ml molybdenum nanoparticles for 48 h. (400×). C. Same field in fluoresence microscopy showing cells positive for the YO-PRO-1 dye (apoptotic cells). These cells are negative for propidium iodide.
Discussion
Despite the many benefits of nanotechnology, some studies indicate that certain nanoparticles may cause adverse effects because of their small size and unique properties (Service, 2003; Hoet et al., 2004). Indeed, their size makes them highly mobile in both the human body and the environment. Nanomaterials can enter human tissues through several ports via the lungs after inhalation (Oberdorster, 2001), through the digestive system (Jani et al., 1990), and possibly through the skin (Kreilgaard, 2002; Lademann et al., 1999). Systemic distribution of nanoparticles has been demonstrated after inhalation and oral uptake (Jani et al., 1990; Oberdorster et al., 2002), and nanoparticles have been found to cross the blood–brain barrier, reaching the olfactory bulb and the cerebellum (Borm and Kreyling, 2004; Oberdorster et al., 2004). Chen and colleagues also reported that nanoparticles can penetrate the blood-testis barrier (Chen et al., 2003). Although organ- or cell-specific drug delivery through nanoparticles is a promising area of medicine, and nanoparticles might be used some day as sensors for intracellular mechanisms, few toxicology studies are available. Many of the artificially manufactured nanoparticles are made of nonbiodegradable pollutants, such as carbon black and metals, and the long-term behavior of such substances is not known.
Toxicants that impair normal reproductive functions are an important public health issue. A decrease in semen quality of approximately 2% per year over the preceding 50 years has been reported for industrialized countries (Carlsen et al., 1992). It has been hypothesized that exposure to toxic chemicals is an important cause of the decline, although great regional differences exist for the same level of environmental contamination. Nevertheless, studies have shown that high exposure of men to various chemicals in certain occupational settings resulted in lower semen quality. For example, dibromochloropropane (DBCP), a nemotocide, resulted in lower sperm counts because of the destruction of undifferentiated spermatogonia (Potashnik et al., 1978). Therefore, it has now become critical to understand the molecular mechanisms leading to reproductive toxicity. Several in vivo animal models have been used to assess the testicular toxicity of many compounds. However, these models necessitate the sacrifice of animals at the end of experimentation, and they do not allow the manipulation or dissection of intracellular pathways to elucidate mechanisms of toxicity at the molecular level. In vitro model alternatives have been established, and some of them have tried to reproduce in the petri dish the complex cell–cell interactions that take place between the different germ cells and Sertoli cells (Hadley et al., 1985; Yu et al., 2005). However, these models are limited by the poor viability of the freshly isolated germ cells.
In this study, we used a cell line with spermatogonial stem cell characteristics to evaluate the toxicity of different types of nanoparticles on the germline. We used three parameters widely used in toxicological studies, such as the ability of mitochondria to reduce MTS, the integrity of the plasma membrane, and the activation of apoptotic pathways.
Our results indicate that the C18–4 cells provide a suitable test system for cytotoxicity in the germline. The MTS and LDH assays can be used for rank ordering of chemical and nanoparticle toxicity on mitochondrial function and plasma membrane integrity. Cadmium is a recognized toxicant that has been classified as a probable human carcinogen. It is a heavy metal that has the potential to cause lysosomal damage and DNA breakage in mammalian hepatocytes (Fotakis et al., 2005) and many other cells and tissues (Satoh et al., 2002). Cadmium also disrupts mitochondrial function both in vivo (Belyaeva et al., 2002) and in vitro (Pourahmad and O'Brien, 2000), and promotes apoptosis (Pulido and Parrish, 2003). In the testis, cadmium induces lysosomal damage in testicular Sertoli cells (Boscolo et al., 1985), but its main toxic effects appear in germ cells. In male rats subcutaneously injected with 0.6 mg cadmium chloride/kg body weight, histological examination of the testes revealed an accumulation of cadmium only in spermatogonia and spermatocytes, but not in somatic cells (Aoyagi et al., 2002). Subsequently, a decrease in the number of spermatogonia in relation to the time of exposure was observed, followed by a decrease in the number of spermatocytes and, ultimately, sperm cells. In humans, male infertility is strongly linked to cadmium exposure, but is rather due to a failure of the acrosomal reaction in sperm cells (Benoff et al., 2000). In our spermatogonial stem cell line, exposure of the cells to cadmium chloride induced a significant decrease in their metabolic activity. However, like hepatocytes (Fotakis et al., 2005), cadmium chloride had no effect on the integrity of the plasma membrane, as monitored by the LDH release assay. Importantly, the deleterious effects of cadmium were enhanced for cells exposed to the particulate, insoluble form of cadmium, such as cadmium oxide. These effects mimic well-documented data obtained on macrophages and other cell lines (Goering et al., 2000). In this case, not only mitochondrial function decreased drastically, but LDH was released in the cell environment as a function of cadmium oxide concentration. Because oxidative stress and lipid peroxidation have been reported after exposure to both cadmium chloride and cadmium oxide, the higher toxicity of cadmium oxide might be related to the size of the particles entering into contact with the plasma membrane. Furthermore, our study shows that the sensitivity of the C18–4 cells to cadmium oxide is comparable to the sensitivity of the BRL 3A liver cells regarding their metabolic activity (Hussain et al. in press) (Table 1). The membrane integrity of the C18–4 cells is less affected. This is corroborated by the fact that at lower concentrations cadmium oxide promotes apoptosis rather than necrosis in the C18–4 cells, thus leaving the plasma membrane intact.
TABLE 1. Calculated EC50 Values of CdO and Ag Nanoparticles for C18–4 Spermatogonial Stem Cells Compared to BRL 3A Liver Cells.
| Chemical | MTT EC50 (μg/ml) | LDH EC50 (μg/ml) | ||
|---|---|---|---|---|
| C18–4 cells | BRL 3A cells | C18–4 cells | BRL 3A cells | |
| CdO | 0.50 | 0.83 | 2.50 | 0.75 |
| Ag-15 nm | 8.75 | 24.00 | 2.50 | 50.00 |
Because the C18–4 cells showed an increased sensitivity to the particulate form of cadmium rather than the soluble form, we next studied the effects of metal nanoparticles on these germline stem cells. As predicted, silver carbonate, which was used as a control, was not toxic as shown by the MTS and LDH leakage assays. In contrast, silver nanoparticles (15 nm) reduced mitochondrial function drastically and increased membrane leakage. Our data show that the C18–4 cells are more sensitive than the BRL 3A cells in that respect (Table 1). There are studies showing that silver nanoparticles could be used in bone cement or other implantable devices as antimicrobial agents (Alt et al., 2004), but our study shows that silver in nanoparticulate form could be toxic for the bone-lining cells and other tissues.
Although molybdenum in soluble form is considered to be a mildly toxic substance, it did not significantly affect the metabolic activity or the membrane integrity of the C18–4 cells. Molybdenum as a nanoparticulate did not affect metabolic activity either, at least up to a concentration of 40 μg/ml (EC50 = 90 μg/ml). At higher concentrations (over 50 μg/ml), the molybdenum nanoparticles become significantly toxic. Interestingly, whereas the effect on mitochondrial function is mild, molybdenum nanoparticles seem to promote some plasma membrane leakage at very low concentrations (5 μg/ml and 10 μg/ml). The same pattern of toxicity is shown for aluminum nanoparticles; however, the morphology of the cells did not change, indicating that at these concentrations apoptosis still occurs. This finding was corroborated by the Vybrant assay. It is known that extreme and visible membrane damage occurs only in the late stages of apoptosis.
In conclusion, we have demonstrated that the C18–4 germline stem cells are a valuable tool with which to study in vitro toxicity in the germline. The sensitivity of these cells to Ag nanoparticles is greater than that of BRL 3A liver cells, which are widely used in toxicity studies. However, in the case of cadmium and the other nanoparticles tested, the sensitivities of the two cell lines are comparable. The molecular mechanisms of nanoparticles toxicity are still poorly understood, and the availability of a cell line with which to gain an understanding of these processes in the germline is of paramount importance.
Acknowledgments
We thank Jeffrey Calhoun for valuable technical assistance. The work was funded in part by the Lance Armstrong Foundation. Laura Braydich-Stolle is a Consortium Research Fellow funded by the AFRL Human Effectiveness Directorate. Conflict of interest: none declared.
Footnotes
For Permissions, please email: journals.permissions@oupjournals.org
References
- Adler ID. Spermatogenesis and mutagenicity of environmental hazards: Extrapolation of genetic risk from mouse to man. Andrologia. 2000;32:233–237. doi: 10.1046/j.1439-0272.2000.00390.x. [DOI] [PubMed] [Google Scholar]
- Alexander B, Browse DJ, Reading SJ, Benjamin IS. A simple and accurate mathematical method for calculation of the EC50. J Pharmacol Toxicol Methods. 1999;41:55–58. doi: 10.1016/s1056-8719(98)00038-0. [DOI] [PubMed] [Google Scholar]
- Allen JW, Liang JC, Carrano AV, Preston RJ. Review of literature on chemical-induced aneuploidy in mammalian male germ cells. Mutat Res. 1986;167:123–137. doi: 10.1016/0165-1110(86)90013-8. [DOI] [PubMed] [Google Scholar]
- Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Ishikawa H, Miyaji K, Hayakawa K, Hata M. Cadmium-induced testicular damage in a rat model of subchronic intoxication. Reprod Med Biol. 2002;1:59–63. doi: 10.1046/j.1445-5781.2002.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva EA, Glazunov VV, Korotkov SM. Cyclosporin A–sensitive permeability transition pore is involved in Cd(2+)-induced dysfunction of isolated rat liver mitochondria: Doubts no more. Arch Biochem Biophys. 2002;405:252–264. doi: 10.1016/s0003-9861(02)00400-9. [DOI] [PubMed] [Google Scholar]
- Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update. 2000;6:107–121. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Kreyling W. Toxicological hazards of inhaled nanoparticles—potential implications for drug delivery. J Nanosci Nanotechnol. 2004;4:521–531. doi: 10.1166/jnn.2004.081. [DOI] [PubMed] [Google Scholar]
- Boscolo P, Sacchettoni-Logroscino G, Ranelletti FO, Gioia A, Carmignani M. Effects of long-term cadmium exposure on the testis of rabbits: Ultrastructural study. Toxicol Lett. 1985;24:145–149. doi: 10.1016/0378-4274(85)90050-5. [DOI] [PubMed] [Google Scholar]
- Brinkworth MH. Paternal transmission of genetic damage: Findings in animals and humans. Int J Androl. 2000;23:123–135. doi: 10.1046/j.1365-2605.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- Brooking J, Davis SS, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. B M J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavany C, Saison-Behmoaras T, Le DT, Puisieux F, Couvreur P, Helene C. Adsorption of oligonucleotides onto polyisohexylcyanoacrylate nanoparticles protects them against nucleases and increases their cellular uptake. Pharm Res. 1994;11:1370–1378. doi: 10.1023/a:1018923301967. [DOI] [PubMed] [Google Scholar]
- Chawla JS, Amiji MM. Biodegradable poly(epsilon-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xue Z, Zheng D, Xia K, Zhao Y, Liu T, Long Z, Xia J. Sodium chloride modified silica nanoparticles as a non-viral vector with a high efficiency of DNA transfer into cells. Curr Gene Ther. 2003;3:273–279. doi: 10.2174/1566523034578339. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- De Wild M, Berner S, Suzuki H, Ramoino L, Baratoff A, Jung TA. Molecular assembly and self-assembly: Molecular nanoscience for future technologies. Ann N Y Acad Sci. 2003;1006:291–305. doi: 10.1196/annals.1292.020. [DOI] [PubMed] [Google Scholar]
- Fotakis G, Cemeli E, Anderson D, Timbrell JA. Cadmium chloride–induced DNA and lysosomal damage in a hepatoma cell line. Toxicol In Vitro. 2005;19:481–489. doi: 10.1016/j.tiv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Goering PL, Kuester RK, Neale AR, Chapekar MS, Zaremba TG, Gordon EA, Hitchins VM. Effects of particulate and soluble cadmium species on biochemical and functional parameters in cultured murine macrophages. In Vitro Mol Toxicol. 2000;13:125–136. doi: 10.1089/109793300440712. [DOI] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suarez-Quian CA, Kleinman HK, Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles—known and unknown health risks. J Nanobiotechnol. 2004;2:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005a;23:200–210. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M. Isolation of mouse germ line stem cells; influence of GDNF. Dev Biol. 2005b;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Hess K, Gearhart J, Geiss K, Schlager J. In vitro toxicity of nanoparticles in BRL3A rat liver cells. Toxicol In vitro. doi: 10.1016/j.tiv.2005.06.034. in press. [DOI] [PubMed] [Google Scholar]
- Iona S, Klinger FG, Sisti R, Ciccalese R, Nunziata A, De FM. A comparative study of cytotoxic effects of N-ethyl-N-nitrosourea, Adriamycin, and mono-(2-ethylhexyl)phthalate on mouse primordial germ cells. Cell Biol Toxicol. 2002;18:131–145. doi: 10.1023/a:1015336318623. [DOI] [PubMed] [Google Scholar]
- Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev. 2001;47:83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54(Suppl 1):S77–S98. doi: 10.1016/s0169-409x(02)00116-3. [DOI] [PubMed] [Google Scholar]
- Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol. 1999;12:247–256. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Wen LX, Shao L, Chen JF. Fabrication of porous hollow silica nanoparticles and their applications in drug release control. J Control Release. 2004;98:245–254. doi: 10.1016/j.jconrel.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Potashnik G, Ben-Aderet N, Israeli R, Yanai-Inbar I, Sober I. Suppressive effect of 1,2-dibromo-3-chloropropane on human spermatogenesis. Fertil Steril. 1978;30:444–447. doi: 10.1016/s0015-0282(16)43580-6. [DOI] [PubMed] [Google Scholar]
- Pourahmad J, O'Brien PJ. A comparison of hepatocyte cytotoxic mechanisms for Cu2+ and Cd2+ Toxicology. 2000;143:263–273. doi: 10.1016/s0300-483x(99)00178-x. [DOI] [PubMed] [Google Scholar]
- Pulido MD, Parrish AR. Metal-induced apoptosis: Mechanisms. Mutat Res. 2003;533:227–241. doi: 10.1016/j.mrfmmm.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Ringer SP, Ratinac KR. On the role of characterization in the design of interfaces in nanoscale materials technology. Microsc Microanal. 2004;10:324–335. doi: 10.1017/S1431927604040504. [DOI] [PubMed] [Google Scholar]
- Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112:335–340. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- Satoh M, Koyama H, Kaji T, Kito H, Tohyama C. Perspectives on cadmium toxicity research. Tohoku J Exp Med. 2002;196:23–32. doi: 10.1620/tjem.196.23. [DOI] [PubMed] [Google Scholar]
- Service RF. Nanomaterials show signs of toxicity. Science. 2003;300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Molecular and genetic basis of normal and toxicant-induced apoptosis in female germ cells. Toxicol Lett. 1998;102-103:497–501. doi: 10.1016/s0378-4274(98)00240-9. [DOI] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: An improved in vitro model for assessment of male reproductive toxicity. Toxicol Sci. 2005;84:378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]


