Abstract
We hypothesized that fusing granulocyte-macrophage colony-stimulation factor (GMCSF) and interleukin (IL)-21 as a single bifunctional cytokine (hereafter GIFT-21) would lead to synergistic anticancer immune effects because of their respective roles in mediating inflammation. Mechanistic analysis of GIFT-21 found that it leads to IL-21Rα-dependent STAT3 hyperactivation while also contemporaneously behaving as a dominant-negative inhibitor of GMCSF-driven STAT5 activation. GIFT-21's aberrant interactions with its cognate receptors on macrophages resulted in production of 30-fold greater amounts of IL-6, TNF-α, and MCP-1 when compared to controls. Furthermore, GIFT-21 treatment of primary B and T lymphocytes leads to STAT1-dependent apoptosis of IL-21Rα+ lymphocytes. B16 melanoma cells gene-enhanced to produce GIFT-21 were immune rejected by syngeneic C57Bl/6 mice comparable to the effect of IL-21 alone. However, a significant GIFT-21-driven survival advantage was seen when NOD-SCID mice were implanted with GIFT-21-secreting B16 cells, consistent with a meaningful role of macrophages in tumor rejection. Because GIFT-21 leads to apoptosis of IL-21Rα+ lymphocytes, we tested its cytolytic effect on IL-21Rα+ EL-4 lymphoma tumors implanted in C57Bl/6 mice and could demonstrate a significant increase in survival. These data indicate that GIFT-21 is a novel IL-21Rα agonist that co-opts IL-21Rα-dependent signaling in a manner permissive for targeted cancer immunotherapy.
Introduction
Cellular tumor vaccines can be generated by transfecting autologous or allogeneic tumor cells with cDNAs encoding for interleukins (ILs), cytokines, interferons, and molecules accessory to immune activation.1 The mechanism, by which the secretion of cytokines by tumor cells invokes an antitumor immune response, remains unclear. The reversal of “anergy,” the chemotactic, trophic, and activation effects on antigen-presenting cells, natural killer (NK) cells, macrophages, and lymphocytes at the site of an “immune” tumor vaccine are probably vital to the phenomena;2 cellular depletion studies have invoked a functional role for each of these cell types. Although the immunological underpinnings of the antitumor effect are not fully understood, phenomenological clinical studies in humans clearly demonstrate that previously immunologically silent tumors can be recognized following vaccination with tumor cells genetically engineered to express immunomodulatory proteins such as granulocyte-macrophage colony-stimulation factor (GMCSF).3 By comparing the antitumor effects of multiple cytokines against a mouse model of melanoma, it was found that GMCSF was the most effective cytokine in generating systemic immunity protecting against a distant tumor challenge and that IL-2 was the most effective cytokine at inducing locoregional tumor rejection.4 It is therefore sensible to test the combined use of GMCSF and IL-2 for cancer therapy. Because each of these cytokines has markedly distinct biological and pharmacokinetic properties, the likelihood of their contemporaneous physiological interaction with target immune effector cells is remote. The notion therefore arises that creating a fusion cytokine borne of the physical linkage of two unrelated cytokines—a “fusokine”—may possess pharmaceutical properties ascribable to each parental domain and may also acquire unheralded additive immune features. Indeed, we have previously demonstrated that a bifunctional chimeric protein borne from the fusion of GMCSF and IL-2 (hereafter GIFT-2) displayed novel and potent immunostimulatory properties that superseded those seen with either protein alone or expressed in combination.5 Although such a fusion protein is bereft of a true physiological role, the aim of cancer immunotherapy is to elicit as violent an immune reaction as possible against cancer, with minimal toxicity to normal tissue. The successful bioengineering of GIFT-2 demonstrates the feasibility of fusing GMCSF with ILs. Based on this premise, it is reasonable to expect that fusing GMCSF with other ILs, each with their own unique pleiotropic immune effects, may also be of interest.
IL-21 is the most recently identified member of the common y-chain family of cytokines, consisting also of IL-2, IL-4, IL-7, IL-9, IL-13, and IL-15 (ref. 6). IL-21's role is to promote the function of mature effector cells in the immune system. IL-21 differentiates CD4+ T cells down the Th17 pathway;7 it has been shown to activate NK cells8 and NK cell functions like antibody-dependent cell cytotoxicity8 and stimulate CD8+ T cells9 to mount an antitumor response;10 furthermore, IL-21 desensitizes responder cells to the inhibitory effects of regulatory T cells,11,12,13 and it acts as a switch for IgG production in B cells.14 We hypothesized that fusing GMCSF and IL-21 would lead to synergistic anticancer effects because of each cytokine's respective role in mediating inflammation. We provide evidence that the fusion protein synthetic transgene coupling GMCSF and IL-21, GIFT-21, is a potent and distinct immune stimulant from IL-21 and that GIFT-21 has novel pharmacological properties that are applicable to cancer immunotherapy and as a receptor-specific cytolytic compound.
Results
Design and characterization of murine GIFT-21
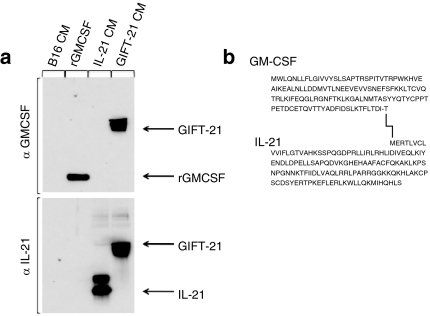
The fusokine was created by cloning the cDNA encoding for murine GMCSF in frame with the 5′ end of the cDNA encoding for murine IL-21. The last 30 base pairs at the 3′ end of the GMCSF cDNA were deleted to remove the stop codon, generating a cDNA encoding for a single 278 amino acid chain (Figure 1a). Denaturing immunoblotting was performed on the conditioned media of B16 melanoma cells retrovirally transduced to express GIFT-21 (B16 GIFT-21), demonstrating that both anti-mGMCSF and anti-mIL-21 antibodies recognized the same protein at a molecular weight of ~50 kd (Figure 1b). It has been previously reported that murine IL-21 could be differentially glycosylated.15 The IL-21 immunoblot pattern observed likely reflects this possibility.
Figure 1.
Characterization of GIFT-21. (a) Immunoblotting performed on the CM from B16 cells retrovirally transduced to express GIFT-21; rmGMCSF and CM from B16 expressing mIL-21 were used as controls. (b) Amino acid sequence of the GIFT-21 fusokine. CM, conditioned media; GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
GIFT-21 hyperactivates the IL-21Rα and competitively blocks the GMCSFR
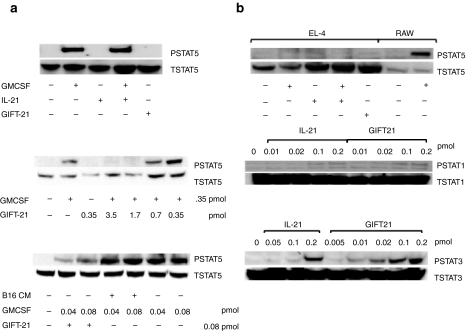
We utilized the responder murine cell lines EL-4 and RAW264.7 to analyze the cellular biochemistry of GIFT-21 on IL-21R and GMCSFR signaling independently of each one another in pure populations. The murine EL-4 lymphoma cell line expresses all the components of the IL-21R (IL-21Rα and the common γ-chain) but does not express the GMCSFR (data not shown). The murine myeloid RAW264.7 cell line expresses the GMCSFR, but not the IL-21Rα chain. When applying GIFT-21 conditioned media to RAW cells for 20 minutes, we observed a lack of STAT5 activation relative to the effect of GMCSF alone (Figure 2a, top). To investigate this further, we treated RAW cells with varying ratios of GIFT-21 to rmGMCSF. We observed that GIFT-21 acted as a dominant negative to rmGMCSF by preventing rmGMCSF from inducing STAT5 phosphorylation at GIFT-21 to GMCSF molar ratios of 5:1 and 10:1 (Figure 2a, middle and lower panels). The incubation of rmGMCSF with B16 conditioned media did not inhibit STAT5 phosphorylation in the absence of GIFT-21 (Figure 2a, lower panel). Upon treating EL-4 cells with GIFT-21 containing conditioned media for 20 minutes, we observed a hyperphosphorylation of IL-21Rα-dependent STAT3 relative to controls, whereas STAT1 and STAT5 activation was similar to that seen with IL-21 alone (Figure 2b). GMCSF-treated RAW cells were used as a positive control for STAT5 phosphorylation downstream of the γ-chain subunit of the IL-21R of EL-4 cells.
Figure 2.
Cellular biochemistry of GIFT-21. (a) STAT5 phosphorylation in RAW264.7 cells. Cells were stimulated for 20 minutes with rmGMCSF, rmIL-21, both cytokines, or with B16 GIFT-21 CM, and the cell lysates were probed for phosphorylated STAT5. Total STAT5 protein was used as a loading control. (b) STAT1/STAT3/STAT5 phosphorylation in EL-4 cells. Cells were stimulated for 20 minutes with a gradient of IL-21 and a gradient of B16 GIFT-21 CM, and the western blot of cell lysates was immunoblotted for phosphorylated STAT1 and STAT3. Total STAT1 or STAT3 protein was used as a loading control. EL-4 cells were stimulated for 20 minutes with B16 GIFT-21 CM and its controls, and the cell lysates were probed for phosphorylated STAT5. RAW264.7 cells were treated with rmGMCSF and used as a positive control for STAT5 phosphorylation in this experiment. CM, conditioned media; GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
GIFT-21 rapidly induces the production of pro-inflammatory cytokines by macrophages
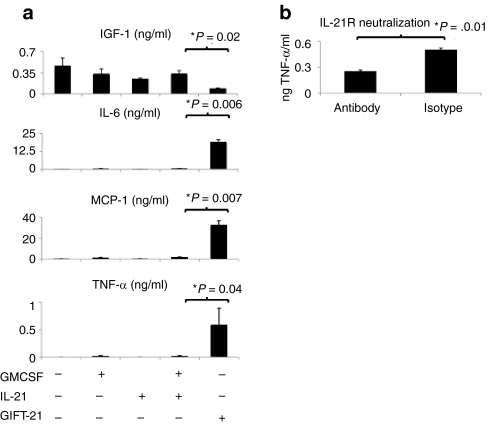
Peritoneal macrophages were harvested from the abdominal cavity of wild-type retired breeder C57Bl/6 mice as previously described.16 Enzyme-linked immunosorbent assays (ELISAs) were performed on the supernatant of peritoneal macrophages treated with cytokines for 24 and 48 hours. Following 24 hours of treatment with rmIL-21, rmGMCSF, both cytokines, or GIFT-21, IL-6, MCP-1, and TNF-α were produced in significantly larger quantities by macrophages treated with GIFT-21 than by those treated with the controls. Macrophages treated with GMCSF and IL-21 were found to produce 0.50 ± 0.15 ng/ml IL-6, 1.4 ± 1.1 ng/ml MCP-1, 0.014 ± 0.025 ng/ml TNF-α, whereas GIFT-21-treated macrophages produced 19 ± 3.2 ng/ml IL-6, 32 ± 7.3 ng/ml MCP-1, and 0.6 ± 0.5 ng/ml TNF-α. Macrophages treated with GIFT-21 produced significantly lower levels of IGF-1 than controls after 48 hours of incubation, 0.085 ± 0.012 ng/ml IGF-1 as compared to 0.33 ± 0.1 ng/ml for GMCSF and IL-21 (Figure 3a). IL-12, IFN-γ, and TGF-β were not detected at either time points (data not shown). Macrophages were treated with an anti-Fcy blocking antibody, then with 30 µg of anti-IL-21R antibody or isotype for 2 hours, then treated with GIFT-21, and TNF-α production was measured 24 hours later. Macrophages treated with anti-IL-21R antibody produced 250 pg/ml TNF-α, significantly less TNF-α than isotype-treated macrophages, 500 pg/ml TNF-α (Figure 3b).
Figure 3.
GIFT-21 induces the secretion of pro-inflammatory cytokines by macrophages. (a) 5 × 104 macrophages were treated for 24 or 48 hours with equimolar concentrations of GIFT-21 versus controls. Production of secreted IGF-1, IL-6, MCP-1, and TNF-α were measured by enzyme-linked immunosorbent assay (mean ± SEM, n = 3). Graphs represent the average of three independent experiments. (b) 5 × 104 macrophages were treated with GIFT-21 with and without IL-21R blockade. GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
GIFT-21 induces a phenotypic change in the morphology of macrophages
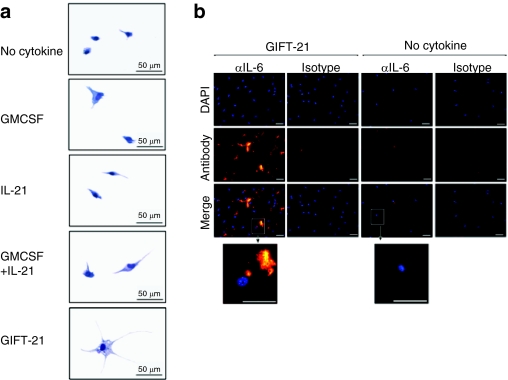
Macrophages treated with GIFT-21 were observed to adopt an altered appearance characterized by an enlarged volume, increased surface area, and the formation of long dendritic processes, vesicles, and granules (Figure 4a). Immunofluorescent intracellular staining of GIFT-21-treated macrophages was also performed for IL-6 (Figure 4b).
Figure 4.
GIFT-21 induces macroscopic changes in macrophage morphology. (a) Giemsa staining of macrophages treated with GIFT-21 versus controls. Bar = 50 µm. (b) Intracellular immunofluorescent staining of IL-6 (versus isotype control) in peritoneal macrophages treated with B16 GIFT-21 conditioned media (left panels) versus no treatment (right panels). Bar = 10 µm. GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
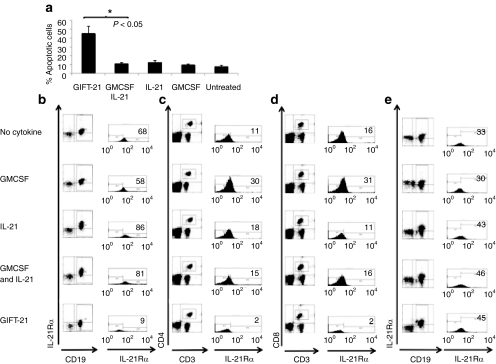
GIFT-21 induces apoptosis of IL-21Rα+ lymphocytes in a STAT1-dependent manner
Unfractionated spleen cells were harvested from wild-type C57Bl/6 mice, and 2 × 105 splenocytes were treated in vitro with GIFT-21 and controls at equimolar concentrations (1 nmol/l) for 24 hours. We observed that GIFT-21-treated splenocytes contained significantly more annexin V positive and annexin V propidium iodide (PI) double positive cells than the controls (Figure 5a). IL-21Rα-expressing subpopulations were gated upon in CD19+ B cells (Figure 5b), CD3+CD4+ (Figure 5c), and CD3+CD8+ (Figure 5d) T cells. We observed a significant increase in annexin V and PI+ splenocytes in the GIFT-21-treated group only. Compared to untreated controls, we observed an IL-21Rα-specific depletion in each lymphoid subgroup analyzed. We isolated splenocytes from STAT1−/− mice and found that GIFT-21 treatment had no significant effect on the number of CD19+/IL-21Rα+ B cells relative to controls (Figure 5e).
Figure 5.
Effect of GIFT-21 on IL-21Rα expressing lymphoid subsets. Unfractionated splenocytes from normal C57Bl/6 mice were treated with GIFT-21 versus controls for 24 hours; (a) apoptosis was measured by flow cytometry using annexin V and propidium iodide staining; and analysis of IL-21Rα expressing lymphocyte subsets analyzed by flow cytometry (mean ± SEM, n = 3); (b) CD19+, (c) CD3+CD4+, and (d) CD3+CD8+ cells were gated for IL-21Rα expression 24 hours following treatment with GIFT-21. Percentage of the IL-21Rα expressing fraction is indicated in top right of each flow histogram. (e) Splenocytes isolated from STAT1−/− mice were treated with GIFT-21 versus controls, and the expression of the IL-21Rα was measured 24 hours later. The figures are representative of two independent experiments. GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
GIFT-21 effect on B16 melanoma tumorigenicity
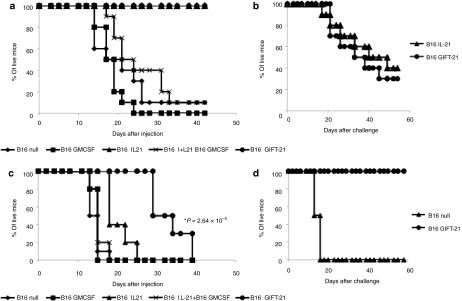
In order to evaluate GIFT-21's effectiveness as an antitumor agent in vivo, we retrovirally transduced B16 melanoma cells to express GIFT-21(ref. 5) and monitored survival following subcutaneous implantation in wild-type immunocompetent syngeneic C57BI6 recipients. Five groups of mice (n = 10 each) were implanted with 106 gene-enhanced B16 cells: (i) untransduced B16 control; (ii) B16 expressing 5 ng/106 cells/24-hour GMCSF (B16 GMCSF); (iii) B16 expressing 4 ng/106 cells/24-hour IL-21 (B16 IL-21); (iv) a 1:1 mixture of B16 GMCSF and B16 IL-21; and (v) B16 expressing 2 ng/106 cells/24-hour GIFT-21 (B16 GIFT-21). Both B16 GIFT-21 and B16 IL-21 groups remained tumor free for >40 days in significant contrast to all other test groups and controls (Figure 6a). Thirty days following B16 implantation, in order to ascertain whether an adaptive response against B16 had developed, survivors of the B16 IL-21 and B16 GIFT-21 groups were then challenged with 106 unmodified B16 cells subcutaneously in the contralateral flank relative to the first implantation. There was no significant difference in tumor growth between these two groups (Figure 6b). To test the effect of GIFT-21 in mice with impaired lymphoid system, yet normal myeloid function, we implanted B16 cells subcutaneously in NOD-SCID mice and monitored survival over time. Five groups of mice (n = 10 each) were implanted with 106 gene-enhanced B16 cells: (i) untransduced B16 control; (ii) B16 expressing 5 ng/106 cells/24-hour GMCSF (B16 GMCSF); (iii) B16 expressing 4 ng/106 cells/24-hour IL-21 (B16 IL-21); (iv) a 1:1 mixture of B16 GMCSF and B16 IL-21; and (v) B16 expressing 2 ng/106 cells/24-hour GIFT-21 (B16 GIFT-21). There was no statistically significant difference in survival between the mice injected with B16 IL-21 and the rest of the controls. However, there was a significant increase in NOD-SCID mouse survival implanted with B16 GIFT-21 (logrank P = 2.64 × 10−5) (Figure 6c). To test whether STAT1 function is essential for rejection of B16 GIFT-21 in immunocompetent mice, we implanted 106 unmodified B16 and 106 B16 GIFT-21 subcutaneously STAT1−/− mice (n = 5) and monitored survival over time. B16 GIFT-21 cells were rejected in 100% of STAT1−/− mice (logrank P = 0.002) (Figure 6d).
Figure 6.
GIFT-21 elicits a robust immune response against cancer. Implantation of cytokine secreting B16 tumors in vivo. (a) B16 cells producing GIFT-21 versus all controls were implanted in WT C57Bl/6 mice. B16 IL-21 and B16 GIFT-21 were robustly rejected. Data are representative of three separate experiments. (b) B16 IL-21 and B16 GIFT-21 survivors were challenged with unmodified B16, and the challenge was rejected at equal rates in both groups. (c) Only NOD-SCID mice injected with B16 GIFT-21 survived significantly longer than the controls. Data are the pooling of two separate experiments. (d) B16 GIFT-21 was rejected from STAT1−/− mice. GMCSF, granulocyte-macrophage colony-stimulation factor; IL-21, interleukin-21.
GIFT-21 effect on IL-21Rα+ EL-4 lymphoma in vitro and in vivo
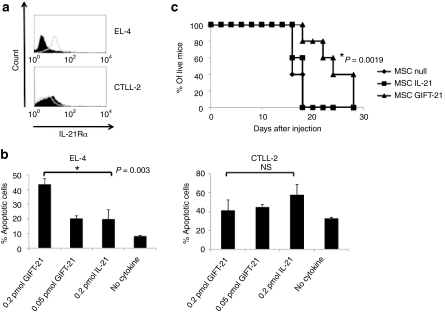
The murine EL-4 lymphoma cell line is syngeneic to the C57BI6 strain. We performed cytometric analysis and demonstrate that EL-4 express the IL-21Rα, and that the control CTLL-2 cells are IL-21Rα− (Figure 7a). Following in vitro treatment with GIFT-21 and rmIL-21 for 24 hours, we measured the fraction of apoptotic cells by analysis of PI/annexin V positivity by flow cytometry. We demonstrate that GIFT-21 induces significant dose-dependent apoptosis of IL-21Rα+ EL-4 cells relative to controls and that this effect is absent in IL-21Rα− CTLL-2 cells (Figure 7b). We have previously demonstrated that mesenchymal stromal cells (MSCs) could serve as an effective vehicle for the continuous delivery of cytokines such as IL-2 and IL-12 in vivo.5,17 Therefore, we used GIFT-21 engineered MSCs as a method to deliver the GIFT-21 fusokine systemically in mice and to determine its effect, as a plasma borne protein, on EL-4 tumor cell growth in wild-type immunocompetent C57BI6 mice. Three test groups of C57BI6 mice (n = 5 each) were implanted intraperitoneally with gene-enhanced MSCs: (i) 12 × 106 C57BI6 MSCs; (ii) 12 × 106 MSCs producing 850 pg GIFT-21/106 cells/24 hours, and: (iii) 12 × 106 MSCs producing 850 pg IL-21/106 cells/24 hours. One day after MSC implantation, all mice were inoculated subcutaneously with 106 EL-4 cells. Tumor volume was measured over time. We observed a statistically significant increase in survival time in mice treated with MSCs expressing GIFT-21 as compared with MSC IL-21 (P = 0.0019) (Figure 7c). There was no statistically significant difference between the unmodified MSC and MSC IL-21.
Figure 7.
GIFT-21 can act as an IL-21R-specific chemotherapeutic drug. (a) EL-4 express the IL-21Rα and CTLL-2 do not. (b) GIFT-21 induces the apoptosis of EL-4, but not CTLL-2 (mean ± SEM, n = 3). (c) Only mice treated with MSC GIFT-21 survived significantly longer than the unmodified MSC control when challenged with a 106 injection of EL-4 subcutaneously. IL-21, interleukin-21; MSC, mesenchymal stromal cell; NS, not significant.
Discussion
Our hypothesis was that a fusokine borne of the marriage of GMCSF and IL-21 would serve as an innovative biopharmaceutical for cancer immunotherapy by its theoretical ability to recruit the antisuppressive and pro-inflammatory properties of IL-21 and enhance them through partnering with GMCSF, a powerful activator of macrophages and dendritic cells. We demonstrated that GIFT-21 was more potent than IL-21 in the way that it enhances STAT3 signaling downstream of the IL-21Rα chain and, unexpectedly, found that this molecule acts as a dominant negative for STAT5 phosphorylation downstream of the GMCSFR. This observation suggests that GIFT-21 has unheralded immunomodulatory properties because the IL-21 and GMCSF moieties both influence the other's ability to properly bind to their respective receptor complexes.
As a proof-of-concept experiment evaluating GIFT-21's ability to induce an antitumor immune response, B16 melanoma was implanted into syngeneic C57Bl/6 mice. IL-21 had previously been shown to be remarkably effective in eliciting an immune reaction against B16 and thus preventing the development of cancer in vivo.18,19 GIFT-21 was demonstrated to also potently induce the rejection of B16. One of the most interesting features of this experiment is the fact that combining B16 GMCSF with B16 IL-21 unexpectedly inhibited IL-21's pro-inflammatory properties. In retrospect, this is not surprising because IL-21 has been shown to mediate part of its effects through NK cells and GMCSF is a negative regulator of NK cell activation.20 It is also possible that the combination of IL-21 and GMCSF influences local macrophages to produce cytokines that promote tumor growth instead of promoting an inflammatory response, as seen with the production of IGF-1 by peritoneal macrophages treated with GMCSF and IL-21. GMCSF has also been shown that tumors recruit myeloid-derived suppressor cells as a means of altering inflammatory reactions,21 and it also plays a key role in the generation of myeloid-derived suppressor cells.22 The observation that the combination of B16 GMCSF and B16 IL-21 does not induce tumor rejection stands in stark contrast to how GIFT-21 was capable of inducing robust antitumor immunity. When we challenged the surviving WT C57Bl/6 mice that had been implanted with B16 GIFT-21 and B16 IL-21, we saw comparable long-term antitumor memory. It has previously been reported that IL-21 recruits CD8+ T cells and NK cells to mediate its effects against tumors,18 but GIFT-21 induces apoptosis of the IL-21Ra+ lymphoid cells it should activate. It was therefore surprising that B16 GIFT-21 would elicit tumor memory equivalent to that of B16 IL-21. Although GIFT-21's stimulatory effects were insufficient to induce the complete rejection of the tumors in NOD-SCID mice, they were sufficient to significantly increase the survival of the mice. The characterization of the macrophage response to GIFT-21 likely explains why B16 GIFT-21 elicited a partial response in NOD-SCID mice. We therefore hypothesize that the adaptive immune system is recruited secondarily to the activation of macrophages by GIFT-21. We believe that if the macrophages were directly killing tumor cells, the impact would have been more profound in the NOD-SCID mice because other cells would not be required to achieve the full effect seen in immunocompetent mice.
We believe that GIFT-21 mediates its effects downstream of the IL-21R and not the GMCSFR because of how GIFT-21 not only acts as a dominant negative for GMCSFR signaling, but also because IL-21R neutralization was capable of reducing TNF-α production by primary macrophages treated with GIFT-21. We hypothesized that STAT1 also played a key role in GIFT-21-mediated inflammation as it did in apoptosis, but B16 GIFT-21 was rejected from STAT1−/− mice, indicating that GIFT-21 most likely functions by hyperphosphorylating STAT3.
We investigated the production of MCP-1, IL-6, TNF-α, IGF-1, TGF-β, IFN-γ, and IL-12 by macrophages because macrophages play a critical role in modulating the immune response. The production of these cytokines is an important part of defining classically activated macrophages involved in inflammation and alternately activated macrophages involved in wound healing.23 We also investigated the production of IFN-y and IL-12 by macrophages because macrophages respond to LPS by producing IFN-y and to IFN-y by producing IL-12 in order to drive Th1 activation of CD4 T cells.24,25 TNF-α and IL-6 are known to activate macrophages and lymphocytes26,27 through their respective receptors and to recruit lymphocytes to areas of inflammation by inducing nearby endothelial cells to upregulate VCAM-1, permitting VLA-4-mediated migration of lymphocytes.28,29 The production of TNF-α and IL-6 is primarily macrophage driven and the cytokines are key players in models of rheumatoid arthritis and colitis.30,31,32 It has also been shown that MCP-1 was an important chemokine involved in recruiting inflammatory macrophages and lymphocytes in rodent models of rheumatoid arthritis.33,34 Finally, it has previously been reported that IL-21 stimulated the alternative pathway of macrophage activation, known to include the production of IGF-1, shown to be a negative regulator of TNF-α and IL-6 production by macrophages in a murine model of atherosclerosis.35,36,37 We believe GIFT-21-activated macrophages possess many features of classically activated macrophages, whereas the controls promote alternative macrophage activation. GIFT-21 provides a strong base for activating lymphoid and myeloid cells and recruiting activated lymphocytes against cancer through the production of TNF-α, IL-6, and MCP-1, and the downregulation of anti-inflammatory molecules such as IGF-1 by macrophages. Finally, we have also documented that IL-12, IFN-γ, and TGF-β were not produced under any condition.
Combining these different observations, we conclude that GIFT-21 acts in a paracrine fashion through the IL-21R on the surface of myeloid cells to activate lymphocytes to mount an antitumor response with an equivalent antitumor memory as the one generated by IL-21. It is likely that lymphocytes are responding to macrophages activated by GIFT-21 and not GIFT-21 itself because of how GIFT-21 rapidly induces apoptosis of IL-21Rα+ lymphocytes. These unexpected results open up different applications for GIFT-21, either through pharmacological administration of the fusokine or through cell therapy using ex vivo–activated macrophages to treat cancer or infectious diseases that sustain themselves by suppressing the host innate immune response, such as tuberculosis.38
Although GIFT-21 was expected to directly amplify the functions of cytotoxic T lymphocytes and NK cells as well as enhance IL-21's modulation of antibody-dependent cell cytotoxicity, these are unlikely scenarios because GIFT-21 rapidly induces IL-21Rα lymphocytes to undergo apoptosis. It has previously been shown that IL-21 could induce apoptosis of IL-21R+ B-cell chronic lymphocytic leukemia, but this was only shown in vitro after the cells had been stimulated with a cocktail including CD40L or in conjunction with other drugs.39 Furthermore, it has never been shown that IL-21 could have such effects in vivo, and our experimental data indicate that this is unlikely as only MSC GIFT-21 could prolong the survival of mice implanted with EL-4 lymphoma. This observation is important because GIFT-21 carries the potential to be a receptor-specific immunotherapeutic drug. This is significant because there are several lymphoid malignancies, such as Hodgkin's lymphoma,40 multiple myeloma,41 and B-cell chronic lymphocytic leukemia,39 that often express the IL-21R. Furthermore, IL-21R signaling has been implicated in a worse prognosis in multiple myeloma,41 making the IL-21R an excellent target for therapies utilizing GIFT-21.
Two fusokines marrying GMCSF to a common γ-chain cytokine have been previously described. The first such fusokine: GIFT-2 was characterized by its pro-inflammatory anticancer effect, mediated mainly through responsive NK cells5 and altered signaling through the IL-2 receptor.42 A second-generation fusokine, GIFT-15, was designed by linking GMCSF with IL-15; this paradoxically led to immune suppression due to its markedly aberrant signaling through the IL-15 receptor on lymphomyeloid cells.16 GIFT-21 distinguishes itself from GIFT-2 and GIFT-15 by the remarkable fact that the GMCSF domain has acquired a loss-of-function phenotype, behaving as a dominant-negative inhibitor of GMCSFR signaling while also inducing hyper IL-21R signaling. The sum of these unheralded effects is a profoundly pro-inflammatory effect on myeloid cells manifested by the exuberant production of IL-6, MCP-1, and TNF-α. Despite GIFT-21's effect as an inducer of apoptosis in IL-21-Rα+ lymphocytes, the sum of its activating properties on myeloid cells leads to an unambiguous pro-inflammatory anticancer immune effect in vivo. The latter feature, the induction of apoptosis of IL-21Rα+ lymphocytes, suggested that GIFT-21 could be utilized for the selective targeting of IL-21Rα+ lymphoid malignancy in vivo. In conclusion, GIFT-21 represents a novel agent that co-opts IL-21R signaling in a manner useful for the enhancement of myeloid effector function as well as for targeted cancer immunotherapy.
Materials and Methods
Animals, cell lines, recombinant proteins, antibodies, ELISA kits, and cDNA. Female 6- to 8-week-old C57Bl/6 and C57Bl/6 retired breeder mice were purchased from Harlan Laboratories (Indianapolis, IN), and NOD.CB17-Prkdcscid/J (NOD-SCID) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). STAT1−/− mice were obtained with permission from Joan Durbin. The B16F0 (B16) cell line was cultured in Dulbecco's modified Eagle's medium (DMEM) (Wisent Technologies, Rocklin, CA) supplemented with 10% fetal bovine serum (FBS) (Wisent Technologies) and 100 U/ml of penicillin/streptomycin (Wisent Technologies). The CTLL-2 cell line was purchased from ATCC, Manassas, VA and was cultured in RPMI supplemented with 10% FBS, 1 mmol/l sodium pyruvate, 10% T-Stim purchased from BD Biosciences (San Diego, CA) and 100 U/ml of penicillin/streptomycin. The EL-4 cell line was purchased from ATCC and was cultured in RPMI (Wisent Technologies) supplemented with 10% FBS, and 100 U/ml of penicillin/streptomycin. The RAW264.7 cell line was purchased from ATCC, and it was cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 100 U/ml penicillin/streptomycin. Recombinant proteins (rGMCSF/rIL-21), antibodies, and ELISA kits for mIL-21, mIFN-γ, mTNF-α, mTGF-β, mIL-6, mIL-12, mJE/MCP-1, mIGF-1, mIL-21, mIL-21R, and STAT1 were purchased from R&D Systems (Minneapolis, MN). Anti-mouse Fcγ III/II, CD3, CD4, CD8, CD19, IL-21Rα, and isotype control antibodies for flow cytometry were purchased from BD Biosciences. Antibodies against STAT3/5 and phosphorylated STAT3/5 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against phosphorylated STAT1 were purchased from Abcam (Cambridge, MA). The mGMCSF ELISA was ordered from eBioscience (San Diego, CA). Apoptosis detection kits were purchased from Invitrogen (Burlington, Ontario, Canada). The murine IL-21 cDNA was purchased from Invivogen (San Diego, CA).
Fusokine design and expression. Mouse GMCSF cDNA was amplified by PCR, aligned in frame with the cDNA encoding IL-21, also amplified by PCR from its original vector. The GIFT-21 cDNA was cloned in a bicistronic retrovector allowing the expression of the fusokine and green fluorescent protein.5 Infectious retroparticles were generated through transfection of 293-GP2 packaging cells (Clontech, Mountain View, CA) using PolyFect (Qiagen, Mississauga, Ontario, Canada). Concentrated retroparticles were used to transduce B16 and MSCs. B16 were expanded, and the supernatant was collected and concentrated using Amicon centrifugation columns (Millipore, Cambridge, Ontario, Canada). GIFT-21 expression levels were quantified using a mIL-21 ELISA kit (R&D Systems).
GIFT-21-mediated biochemical responses in splenocytes, EL-4 and RAW264.7 cells. Splenocytes were collected from C57Bl/6 mice. EL-4 cells were maintained in RPMI 10% FBS and RAW264.7 cells in Dulbecco's modified Eagle's medium 10% FBS. Media supplemented with cytokines was used to stimulate 2 × 106 cells for 20 min with the different test conditions. Cell lysates were separated by 4–20% gradient SDS-PAGE (Thermo Scientific, Pittsburgh, PA), and western blot analysis was performed with antiphosphorylated STAT1, STAT3, and STAT5 according to manufacturer's instructions. For apoptosis assays, 2 × 105 EL-4 cells were cultured for 24 hours and stained with PI and annexin V. Cells positive for annexin V and double positive for PI and annexin V by flow cytometric analysis were considered apoptotic. To ascertain IL-21R depletion of lymphocytes, 2 × 105 splenocytes were cultured in a 96-well plate for 24 hours supplemented with equimolar concentrations (1 nmol/l) of GIFT-21, rmIL-21, rmGMCSF, or a combination of rmIL-21 and rmGMCSF. After incubation for 1 hour with a blocking anti-mouse Fcγ III/II antibody at 4 °C, splenocytes were labeled with appropriate antibodies and analyzed by flow cytometry using a Becton Dickinson FACScan and the CellQuest software (BD Biosciences, San Jose, CA).
Macrophage collection and phenotyping. Peritoneal macrophages were collected by peritoneal lavage of C57Bl/6 retired breeder mice. 4 × 106 cells were then plated into 6-well flat bottom plates and left to adhere overnight in RPMI 10% FBS. The nonadherent cells were washed from the plates using sterile PBS, and the remaining adherent cells were treated with RPMI 10% FBS and equimolar concentrations of GIFT-21, recombinant mouse (rm)IL-21, rmGMCSF, or a combination of rmGMCSF and rmIL-21 for 24 and 48 hours. The cell culture medium was collected and analyzed by ELISA. In order to neutralize the IL-21R, 30 µg/ml of anti-IL-21R antibody or isotype (R&D Systems) was applied to the macrophages 2 hours prior to 24-hour stimulation with GIFT-21, and the cell culture medium was collected and analyzed by ELISA.
Peritoneal macrophages were incubated with GIFT-21 and controls in Lab-Tek II chamber slides (Nalge Nunc International, Naperville, IL) for 24 hours. Giemsa staining was performed by the Department of Hematology of the Jewish General Hospital. For immunofluorescence, the cells were fixed in 4% paraformaldehyde in PBS and permeabilized using 0.1% Triton X-100 in PBS. The cells were stained using an anti-mIL-6 antibody or isotype, then coupled with an Alexa 555 conjugated secondary antibody (Invitrogen Molecular Probes, Eugene, OR). The slides were stained with DAPI (Invitrogen) and mounted using Immu-Mount (Thermo Scientific) and visualized at room temperature using a Leica DM LB2 microscope (Leica Microsystems, Deerfield, IL) mounted with a DFC480 camera, using the Leica application software v3.4.0.
Murine B16 melanoma modeling. B16 cells were transduced to express GMCSF, IL-21, and GIFT-21 as previously described.5 106 B16 cells were injected subcutaneously in syngeneic C57Bl/6 mice or immunodeficient (NOD-SCID) mice, and survival was monitored over time. Mice were killed when the tumor volume reached 500 mm3 or if the tumor ulcerated. Tumor volume was measured as [(length × width2)/2]. Surviving mice were challenged with an injection of 106 unmodified B16 cells subcutaneously on the contralateral flank.
Gene-enhanced MSCs for in vivo delivery of GIFT-21. Unmodified IL-21 and GIFT-21 engineered MSCs were generated as previously described17,43 and were injected intraperitoneally (12 × 106) into naive syngeneic C57Bl/6 mice. The mice were subsequently injected with 106 IL-21Rα+ EL-4 tumor cells subcutaneously 24 hours later, and tumor growth and survival was evaluated over time.
Statistical analysis. P values were calculated by paired Student's t-test and logrank tests where applicable.
Acknowledgments
We thank Joan Durbin for the STAT1−/− mice; Francois Mercier, Dimitri Coutsinos, Lilian Amrein, Christian Young, Marie-Noëlle Boivin, Jing Zhao, and Claudia Penafuerte-Diaz for their advice; and Harald Biessman for his support. We also thank the Department of Hematology of the Jewish General Hospital for their assistance with the histochemistry. This work was funded by CIHR grant MOP-15017. Patrick Williams holds an MD/PhD CIHR studentship. The authors declared no conflict of interest.
REFERENCES
- Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong HT., and, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J. Vaccines in cancer: GVAX, a GM-CSF gene vaccine. Expert Rev Vaccines. 2005;4:259–274. doi: 10.1586/14760584.4.3.259. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Wu JH, Bouganim N., and, Galipeau J. Granulocyte-macrophage colony-stimulating factor and interleukin-2 fusion cDNA for cancer gene immunotherapy. Cancer Res. 2004;64:8795–8799. doi: 10.1158/0008-5472.CAN-04-1776. [DOI] [PubMed] [Google Scholar]
- Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S., and, Carson WE., 3rd Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- Casey KA., and, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- Kishida T, Asada H, Itokawa Y, Cui FD, Shin-Ya M, Gojo S, et al. Interleukin (IL)-21 and IL-15 genetic transfer synergistically augments therapeutic antitumor immunity and promotes regression of metastatic lymphoma. Mol Ther. 2003;8:552–558. doi: 10.1016/s1525-0016(03)00222-3. [DOI] [PubMed] [Google Scholar]
- Kim-Schulze S, Kim HS, Fan Q, Kim DW., and, Kaufman HL. Local IL-21 promotes the therapeutic activity of effector T cells by decreasing regulatory T cells within the tumor microenvironment. Mol Ther. 2009;17:380–388. doi: 10.1038/mt.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT.et al. (2007IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes J Immunol 178732–739. [DOI] [PubMed] [Google Scholar]
- Li Y., and, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–235. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konforte D., and, Paige CJ. Identification of cellular intermediates and molecular pathways induced by IL-21 in human B cells. J Immunol. 2006;177:8381–8392. doi: 10.4049/jimmunol.177.12.8381. [DOI] [PubMed] [Google Scholar]
- Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, et al. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- Rafei M, Wu JH, Annabi B, Lejeune L, François M., and, Galipeau J. A GMCSF and IL-15 fusokine leads to paradoxical immunosuppression in vivo via asymmetrical JAK/STAT signaling through the IL-15 receptor complex. Blood. 2007;109:2234–2242. doi: 10.1182/blood-2006-07-037473. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Francois M, Boivin MN, Martineau D., and, Galipeau J. Neo-organoid of marrow mesenchymal stromal cells secreting interleukin-12 for breast cancer therapy. Cancer Res. 2008;68:4810–4818. doi: 10.1158/0008-5472.CAN-08-0160. [DOI] [PubMed] [Google Scholar]
- Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- Søndergaard H, Frederiksen KS, Thygesen P, Galsgaard ED, Skak K, Kristjansen PE, et al. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunol Immunother. 2007;56:1417–1428. doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal M, Cumberland W, Champlin R., and, Fahey JL. Effect of recombinant human granulocyte-macrophage colony-stimulating factor administration on the lymphocyte subsets of patients with refractory aplastic anemia. Blood. 1990;76:1580–1585. [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM., and, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C., and, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS., and, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz MJ, Barber SA, Dieffenbach CW., and, Vogel SN. Induction of IFN-gamma in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A., and, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Andrade RM, Wessendarp M, Portillo JA, Yang JQ, Gomez FJ, Durbin JE, et al. TNF receptor-associated factor 6-dependent CD40 signaling primes macrophages to acquire antimicrobial activity in response to TNF-alpha. J Immunol. 2005;175:6014–6021. doi: 10.4049/jimmunol.175.9.6014. [DOI] [PubMed] [Google Scholar]
- Renauld JC, Vink A., and, Van Snick J. Accessory signals in murine cytolytic T cell responses. Dual requirement for IL-1 and IL-6. J Immunol. 1989;143:1894–1898. [PubMed] [Google Scholar]
- Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Iademarco MF, McQuillan JJ, Rosen GD., and, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- Yamamoto M, Yoshizaki K, Kishimoto T., and, Ito H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J Immunol. 2000;164:4878–4882. doi: 10.4049/jimmunol.164.9.4878. [DOI] [PubMed] [Google Scholar]
- Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Alvaro-Gracia JM, Maki R., and, Alvaro-Garcia JM. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- Ogata H, Takeya M, Yoshimura T, Takagi K., and, Takahashi K. The role of monocyte chemoattractant protein-1 (MCP-1) in the pathogenesis of collagen-induced arthritis in rats. J Pathol. 1997;182:106–114. doi: 10.1002/(SICI)1096-9896(199705)182:1<106::AID-PATH816>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA., and, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–120. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynes MW., and, Riches DW. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J Immunol. 2003;171:3550–3559. doi: 10.4049/jimmunol.171.7.3550. [DOI] [PubMed] [Google Scholar]
- Beltan E, Horgen L., and, Rastogi N. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb Pathog. 2000;28:313–318. doi: 10.1006/mpat.1999.0345. [DOI] [PubMed] [Google Scholar]
- Gowda A, Roda J, Hussain SR, Ramanunni A, Joshi T, Schmidt S.et al. (2008IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro Blood 1114723–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht B, Kreher S, Anagnostopoulos I, Jöhrens K, Monteleone G, Jundt F, et al. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3alpha. Blood. 2008;112:3339–3347. doi: 10.1182/blood-2008-01-134783. [DOI] [PubMed] [Google Scholar]
- Brenne AT, Fagerli UM, Shaughnessy JD, Jr, Våtsveen TK, Rø TB, Hella H, et al. High expression of BCL3 in human myeloma cells is associated with increased proliferation and inferior prognosis. Eur J Haematol. 2009;82:354–363. doi: 10.1111/j.1600-0609.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penafuerte C, Bautista-Lopez N, Mohamed-Rachid B, Routy JP., and, Galipeau J. The human ortholog of granulocyte macrophage colony-stimulating factor and interleukin-2 fusion protein induces potent ex vivo natural killer cell activation and maturation. Cancer Res. 2009;69:9020–9028. doi: 10.1158/0008-5472.CAN-09-2322. [DOI] [PubMed] [Google Scholar]
- Stagg J, Lejeune L, Paquin A., and, Galipeau J. Marrow stromal cells for interleukin-2 delivery in cancer immunotherapy. Hum Gene Ther. 2004;15:597–608. doi: 10.1089/104303404323142042. [DOI] [PubMed] [Google Scholar]