Abstract
Inflammatory bowel disease (IBD), which includes Crohn's disease and ulcerative colitis, is an inflammatory autoimmune disease characterized by T-cell infiltration to the colon. Mesenchymal stem cells (MSCs) have the potential to rescue IBD owing to their immunosuppressive capabilities and clinical studies have shown positive influence on intestinal graft versus host disease. We demonstrate here a new method to coat MSCs with antibodies against addressins to enhance their delivery to the colon and thereby increase the therapeutic effectiveness. Bioluminescence imaging (BLI) demonstrated that vascular cell adhesion molecule antibody (Ab)-coated MSCs (AbVCAM-1- MSCs) had the highest delivery efficiency to inflamed mesenteric lymph node (MLN) and colon compared to untreated MSCs, Abisotype-MSCs, and AbMAdCAM-MSCs. Therapeutically, when mice with IBD were injected with addressin Ab-coated MSCs, they showed dramatically improved survival rates, higher IBD therapeutic scores, and significantly improved body weight gain compared to mice injected with MSCs only, isotype Ab, free Ab plus MSCs, or vehicle-only controls. These data demonstrate that anti-addressin Ab coating on MSC increased cell delivery to inflamed colon and increased the efficacy of MSC treatment of IBD. This is the first study showing an increased therapeutic efficacy when stem cells are first coated with antibodies specifically target them to inflamed sites.
Introduction
The efficiency of delivery of MSCs to injury sites is quite low,1 especially when delivered systemically. To overcome this limitation several laboratories have developed nongenetically engineering methodologies to enhance stem cell delivery, such as through the enzymatic modification of cell surface glycoproteins into E-selectin ligands2,3 or by biotinylating cell surface proteins to then coat the cells with streptavidin-linked ligands.4 This laboratory has developed a novel cell targeting method that has been shown to enhance cell binding when delivered locally,5 but has not yet been applied to systemic delivery. Recent in vitro studies in this laboratory showed that when addressin antibodies were incorporated onto MSC surface membranes, MSCs were specifically targeted to tumor necrosis factor–α-activated endothelial cells, and their binding is strong enough to resist the detachment of MSCs from endothelial cells while under physiological flow (shear).6 Based on these results, it was hypothesized that coating MSCs with anti-addressin antibodies would promote more efficient delivery of MSCs to sites of inflammation and would thereby improve therapeutic outcomes, and the results presented herein indicate that this is the case for MSCs targeted to treat IBD.
Various sources of mesenchymal stem cells (MSCs) have been tested as a treatment modality for inflammation-related diseases, such as inflammatory bowel disease (IBD),7,8,9,10 graft versus host disease,11,12,13 rheumatoid arthritis,14,15 type I diabetes,16,17 and multiple sclerosis.18,19 Newman et al.20 summarized that the mechanisms of action for the immunosuppressive effects observed in MSCs include both direct cellular contact, along with the secretion of soluble factors, such as transforming growth factor–β,21 prostaglandin E2,22 indoleamine-dioxygenase,23 nitric oxide,12 and tumor necrosis factor–a-stimulated gene-6.24 Recently, Ren et al.12 showed evidence that MSCs may arrest activated T cells in a graft versus host disease model via the secretion of proinflammatory factors and nitric oxide. Additional evidence indicates that MSC immune-suppressive activity is localized at the sites of inflammation and regulated by cells and factors present in local microenvironment,20 indicating that greater MSCs homing and infusion close to inflamed sites may enhance therapeutic results.
In the present study, this anti-addressin antibody (Ab) cell surface coating methodology was extended to target MSCs to IBD in vivo using experimental acute colitis model in mice. IBD, including Crohn's disease and ulcerative colitis, is an autoimmune disease characterized by dysfunction of mucosal T cells and altered cellular inflammation that ultimately leads to damage of the distal small intestine and the colonic mucosa.25 In this IBD model, activated T cells promote macrophage activation and neutrophil infiltration, resulting in a transmural inflamed intestinal mucosa, characterized by prolonged and uncontrolled production of proinflammatory cytokines and chemokines.25,26
Our results herein first validated the immune-modulatory capability of mouse MSCs, then demonstrated increased delivery of targeted firefly luciferase-expressing MSCs to colon, clinical efficacy based on survival, body weight, and histologic scoring and, finally, an increase in the proportion of Treg cells in the colon of targeted mice, indicating a possible mechanism of action of the delivered MSCs.
Results
Ab-coated MSCs suppress splenocyte proliferation
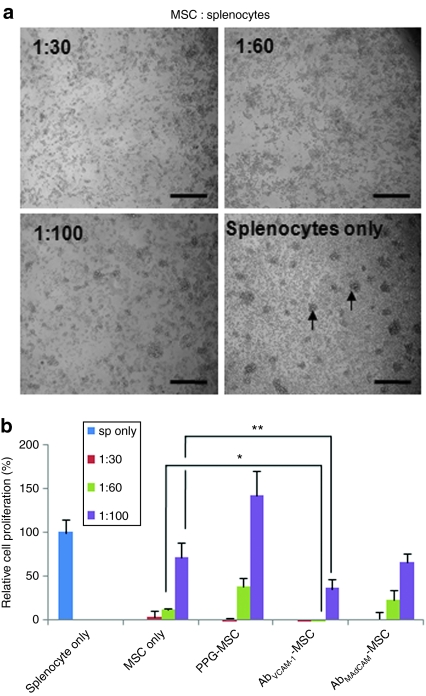
To examine the potential immunosuppressive capability by MSCs, MSCs were cocultured with freshly isolated splenocytes stimulated by CD3 Ab. In phase contrast microscopic images of 2-day cocultures (Figure 1a), the splenocytes-only group showed many colonies as indicated arrows, which is indicative of high-proliferative T-cell activity. A decrease in colonies correlated with the increase of MSC number, strongly indicating the inhibition of proliferation in the presence of MSCs.
Figure 1.
Immune cell suppression by MSC. (a) Phase contrast images of MSCs and splenocytes stimulated by CD3 antibody and cocultured in a 96-well microplate for 3 days. Arrows indicate colony formation in the splenocyte-only culture, indicating T-cell proliferation. Bar = 200 µm. (b) Quantification of T-cell proliferation by 3H-thymidine uptake. The results show that T-cell suppression was MSC number dependent. Ab-coated MSCs showed greater T-cell suppression than MSCs only indicating a possible direct anti-VCAM-1 or anti-MAdCAM antibody effect. *P < 0.01, **P < 0.05 compared to MSC only. Data show mean ± SD and are representative of two independent experiments. Ab, antibody; MSC, mesenchymal stem cell; PPG, palmitated protein G.
To quantify the splenocyte proliferation in cocultures with MSCs, 3H-thymidine was added to cocultures and incorporation of 3H-thymidine was measured (Figure 1b). Relative cell proliferation is represented compared to that of the splenocytes-only group. The results demonstrate that MSCs inhibit splenocyte expansion in a dose-dependent fashion. Significant inhibition of splenocyte expansion was observed even at the ratio 1:100 of MSC to splenocytes. Interestingly, vascular cell adhesion molecule Ab-coated MSCs (AbVCAM-1-MSCs) showed statistically enhanced suppressive capability compared to MSC only at dilutions of 1:60 (*P < 0.01) and 1:100 (**P < 0.05; Student's t-test), possibly representing synergistic effects by anti-VCAM-1 antibodies released from MSCs. The same was not true for AbMAdCAM-MSCs, where the results were statistically indistinguishable from the MSCs only group.
More IV-delivered AbVCAM-1-MSCs are found in MLN and colon than uncoated MSC
To assess the biodistribution of intravenously injected MSCs, mice were imaged for bioluminescence [see Figure 2a for the overall bioluminescence imaging (BLI) study design]. C57BL6 mice treated with dextran sulfate sodium (DSS) for 5 days received luciferase-expressing (fluc)-MSCs (See Supplementary Materials and Methods and Supplementary Figure S1) dual-labeled with far-red dye, and were imaged at 2 hours after cell injection. After sacrifice, organs were collected, imaged, and quantified.
Figure 2.
In vivo bioluminescence imaging (BLI) of fluc-MSCs delivery. (a) Outline of experimental approach; mice were injected with 1.0 × 106 MSCs. (b) Representative BLI images. No signal was detected in mice without cell injection. AbVCAM-1-MSCs-injected mice showed the strongest signal among MSC only and AbMAdCAM-MSCs in MLN and colon. (c) Quantification of fluc-MSCs delivery to each organ. A statistically significant 1.8-fold increase by AbVCAM-1-MSCs relative to MSC only was demonstrated (n = 10–12, 3–4 independent experiments, *P < 0.02, Student's t-test). (d) Quantification of BLI for different organs comparing AbVCAM-1-MSCs to isotype Ab control. The signal from AbVCAM-1-MSCs in spleen, mesenteric lymph node (MLN), and colon was four-, three-, and twofold higher, respectively, than that of Abisotype-MSCs injected mice. *P < 0.05, Student's t-test. Similar delivery efficiency of Abisotype-MSCs and AbVCAM-1-MSCs was found in the lung. (e) Ex vivo imaging of far-red dye labeled MSCs in lung, spleen, mesenteric lymph node (MLN), and colon. Arrows indicate MSCs in organs of AbVCAM-1-MSCs injected mouse. Green color is autofluorescent background staining which is easily distinguished from far-red dye labeled MSCs. Ab, antibody; AbVCAM-1-MSCs, vascular cell adhesion molecule antibody-coated MSCs; MSC, mesenchymal stem cell.
In BLI of individual organs (Figure 2b), mice without MSC injection showed no BLI signal. Among MSC injected mice, AbVCAM-1-MSCs showed the strongest signal in mesenteric lymph node (MLN) and colon. The relative BLI difference of AbVCAM-1-MSCs compared to MSCs only is shown in Figure 2c; data from four independent experiments (N = 3–4 mice in each experiment). The results showed a 1.3- and 1.8-fold (*P < 0.02, Student's t-test) increase in BLI signal of AbVCAM-1-MSCs mice compared to MSC only in MLN and colon, respectively (Figure 2c). To test the specificity of binding, AbVCAM-1-MSCs BLI signal was compared to that of isotype Ab control (Abisotype-MSCs) where AbVCAM-1-MSCs showed statistically significant three- to fourfold greater signal (*P < 0.05, Student's t-test) in spleen and MLN than Abisotype-MSCs (Figure 2d). However, although there was a twofold signal difference in colon, this was not statistically significant. The order of decreasing organ BLI signal intensity is: lung, spleen, MLN, and colon. Lung showed a similar signal between AbVCAM-1- MSCs and Abisotype-MSCs, indicating passive entrapment due to MSC size, which has been noted previously.27
To confirm the luminescent localization of MSCs in each organ, far-red dye labeled MSCs were imaged using near infrared light, which shows reduced background autofluorescence.28 Representative fluorescent microscopic images are shown in Figure 2e. Lung tissue was examined as a positive control and was shown to contain many MSCs (purple) in AbVCAM-1-MSCs-injected mice whereas the no MSCs injected mice were negative. Several far-red positive MSCs were detected in spleen, MLN, and colon, whereas no signal was detected in noninjected mice, confirming MSCs localization (Supplementary Figure S2) in those organs.
Addressin-MSCs show improved survival rates in IBD
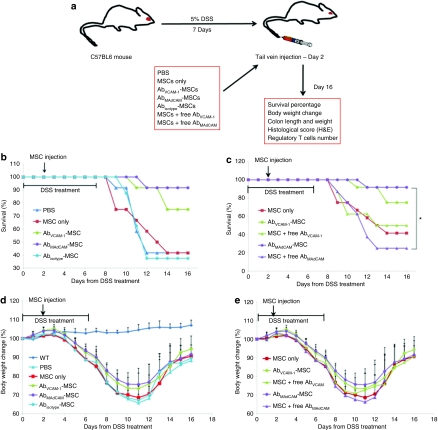
The potential therapeutic effect of MSCs was assessed in an acute experimental colitis model, wherein colitis is induced by 5% DSS in the drinking water over 7 days. At day 2 of oral administration of 5% DSS, mice were tail vein-injected with MSCs and survival rate, body weight change, colon length and weight, histological scores, and the number of regulatory T cells (Tregs) were determined (Figure 3a).
Figure 3.
Survival rate and body weight change in untreated controls, and 5% DSS-treated mice injected with vehicle only, MSCs only, or Ab-coated MSCs. (a) Experimental outline and groups. (b) Survival data showing statistically significant survival of AbMAdCAM-MSC compared to PBS (P < 0.01), MSC only (P < 0.01), Abisotype-MSC (P < 0.01), and a strong trend toward significance of AbVCAM-1-MSC compared to PBS (P = 0.063), MSC only (P = 0.078), Abisotype-MSC (P = 0.052). Survival percentage showed statistical significance in overall groups (P = 0.04) by Kaplan–Meier log-rank test. (c) Survival rate on MSCs coated with antibodies. Notably, surprising observation was made when Abs-coated MSCs injection was compared with the mixture of MSCs and free antibody. *indicates statistically significant difference between AbMAdCAM-MSC and the mixture of MSC and free MAdCAM antibody (P < 0.01). Similar results in body weight change (%) were obtained with survival rate, showing (d) antibody specific therapeutic effects and (e) importance of antibody incorporation on MSCs. Ab, antibody; AbVCAM-1-MSCs, vascular cell adhesion molecule antibody-coated MSCs; DSS, dextran sulfate sodium; MSC, mesenchymal stem cell; PBS, phosphate-buffered saline.
In the experimental set to investigate potential Ab-specific therapeutic effects (Figure 3b), AbMAdCAM-1-MSCs (91.7%, 11/12 mice) and AbVCAM-1-MSCs (75%, 9/12) injected mice showed a statistically significant increase on survival rate compared to phosphate-buffered saline (PBS) (41.7%, 5/12), MSCs only (41.7%, 5/12), and Abisotype-MSCs (37%, 3/8) injected mice (Kaplan–Meier log-rank, P = 0.036), indicating that anti-mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and VCAM-1 Ab coating specifically increased survival rate. To determine whether the increased survival rates were due to MSCs or due to the presence of anti-MAdCAM-1 or anti-VCAM-1 Ab, mixtures of MSCs and free Ab were injected and survival rate was compared with Ab-coated MSCs (Figure 3c). The Ab dose was calculated from the amount determined to be present in a typical MSC dose, which is 1.1 µg in 106 cells, or 4.4 × 106 Ab molecules per cell. This Ab dose was determined using flow cytometry to quantify the amount of fluorescein isothiocyanate-coated human immunoglobulin G that was bound to MSCs under conditions identical to that used for anti-MAdCAM-1 and anti-VCAM-1 Ab coating (Supplementary Figure S3). This high number of Ab molecules is likely due to some cellular internalization of antibodies.6 Interestingly, when free anti-MAdCAM-1 or anti-VCAM-1 Ab was injected along with uncoated MSCs, the survival rate for free anti-MAdCAM-1 plus MSCs was only 25% (2/8) compared to 91.7% for AbMAdCAM-1-MSCs (*P = 0.017) and, although not as impressive, for anti-VCAM-1 plus MSCs, only 50% (4/8). These results indicate that free anti-addressin antibodies alone (1.1 µg/mouse), even if MSCs are coinjected, are not able to increase survival rates in DSS-treated mice.
Therapeutic measures of IBD
As a therapeutic index, body weight change was also determined. Similar to the survival rate, AbMAdCAM-1-MSCs and AbVCAM-1-MSCs showed higher body weight than PBS, MSCs only, and Abisotype-MSCs (Figure 3d). Also, compared with the mixture of MSC and free Ab, Ab-MSCs showed higher body weight recovery, as shown in Figure 3e. Both data sets in Figure 3d and e were statistically significant between 7 and 11 days [analysis of variance (ANOVA), P < 0.0001], after which nonsurviving mice skewed the data by being dropped from the body weight measurements. Furthermore, a repeated measures mixed model was conducted using SAS Proc mixed for pairwise tests comparing overall profiles of each group from 3 to 11 days. From this pairwise analysis, AbMAdCAM 1-MSCs showed a statistically significant difference compared to PBS (P < 0.02), MSC only (P < 0.01), Abisotype-MSCs (P < 0.05), and free MAdCAM Ab (P < 0.02). AbVCAM-1-MSCs showed significant statistical difference compared to PBS (P < 0.04), MSC only (P < 0.01), free VCAM-1 Ab (P < 0.04), and a strong trend toward significance compared to Abisotype-MSCs (P = 0.06).
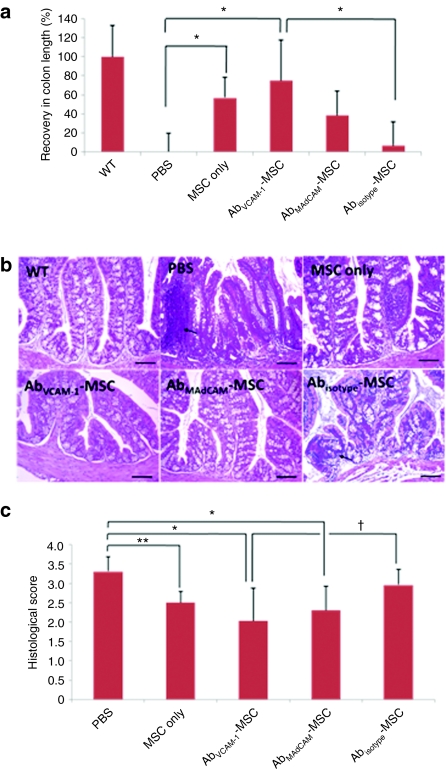
To assess colonic inflammation, colon length was assessed at day 16 in all surviving mice (Figure 4a). Recovery in colon length (%) was defined as relative difference compared to PBS-mice, where recovery in wild type and PBS shows 100 and 0%, respectively. Treatment DSS-mice with MSCs showed higher recovery than PBS (ANOVA, P = 0.001), indicating that MSCs alone are having at least some therapeutic effect. Notably, AbVCAM 1-MSCs showed a statistically significant difference compared to PBS and Abisotype-MSCs (ANOVA, *P < 0.05, Tukey's post hoc test). However, there are no significant differences between MSC only and Ab-MSCs-mice. It should be noted that although there was no significant difference here between MSCs only and addressin-MSCs, most of mice that were likely to show the lowest scores in the MSC-only group had not survived to day 16–only 4/12 MSCs only injected mice survived to day 16, whereas 11/12 AbMAdCAM-1-MSCs and 9/12 AbVCAM-1-MSCs survived.
Figure 4.
Colon length measurement and clinical scores of mice at day 16. (a) Colon length showed statistically improved recovery in MSCs only, AbVCAM-1-MSCs, AbMAdCAM-MSCs injected mice compared to PBS-injected mice; N = 8–12, three independent experiments, ANOVA (P = 0.001) with Tukey post hoc analysis. *indicates statistical significance at P < 0.05. (b) H&E staining of paraffin-embedded sections of colon. Damages of crypts, severe infiltration by immune cells (arrow), and high-vascular density were found in PBS-mice and Abisotype-MSCs; Bar = 100 µm. (c) Blinded histological scores of IBD levels based on H&E images; 0 indicates no damage, 4 indicates severe damage. AbVCAM-1-MSC-injected mice showed statistical significant improvement *P < 0.01 versus PBS (*P < 0.01), and versus Abisotype-MSCs (†P < 0.05). MSC only injected mice were improved compared to PBS-injected (**P < 0.05), as were AbMAdCAM-MSCs (*P < 0.01). All comparisons were via Mann–Whitney testing. Ab, antibody; AbVCAM-1-MSCs, vascular cell adhesion molecule antibody-coated MSCs; ANOVA, analysis of variance; DSS, dextran sulfate sodium; H&E, hematoxylin and eosin; MSC, mesenchymal stem cell; PBS, phosphate-buffered saline.
Examination of the colon from DSS-untreated mice showed a histologically normal structure. However, PBS-injected DSS-mice showed transmural necrosis, diffuse loss of crypts, extensive fibrosis, and immune cell infiltration indicated as an arrow (Figure 4b). Injection of MSCs into DSS-mice significantly decreased the extent and severity of damage in colon although some mild immune cells infiltration is observed in MSC-treated mice. However, AbVCAM-1-MSCs, AbMAdCAM-MSCs injected mice retained normal colon morphology. In blinded histological scores (Figure 4c), AbVCAM-1-MSCs showed statistical significance with *P < 0.01 versus PBS and P < 0.05 versus Abisotype-MSCs. MSC-only and AbMAdCAM-1-MSCs also significantly presented lower histological scores than PBS, P < 0.05 and P < 0.01 versus PBS, respectively. However, there was no difference between PBS and Abisotype-MSCs.
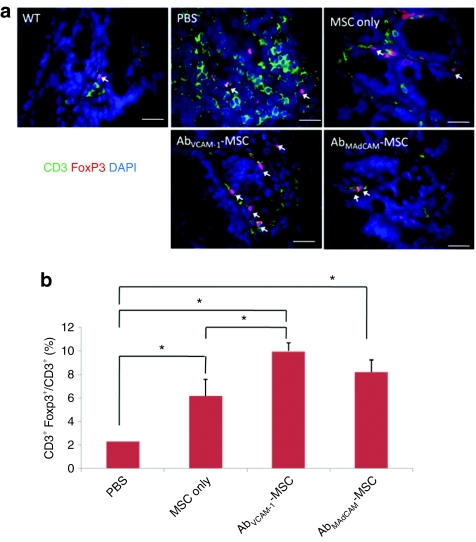
Percentage of Treg cells is increased in mice targeted with MSCs
To examine the number of Treg in treated mice, paraffin-sectioned colons were double-immunostained with CD3 Ab (T-cell marker), Foxp3 (Treg, intracellular marker) and 4′-6-diamidino-2-phenylindole, and fluorescent microscopic images were captured, and the differently stained cells quantified. A representative CD3+Foxp3+ cell image is shown in Figure 5a, where Tregs are colocalized with Foxp3 Ab staining, 4′-6-diamidino-2-phenylindole nuclear staining, and surface expression of CD3. PBS-injected mice showed a high number of CD3+ T cell in the colon whereas MSC-mice showed a low number as shown in Figure 5a. Total percentage of Treg is shown in Figure 5b, demonstrating that treatment of DSS-mice with MSCs increased the percentage of Tregs with statistical significance (ANOVA, P < 0.0001). Interestingly, AbVCAM-1-MSCs showed statistical difference compared to PBS and MSC only (ANOVA, *P < 0.05, Tukey's post hoc test).
Figure 5.
Ratio of regulatory T cells (Treg) and total T cells in Ab-MSC- mice. Paraffin-embedded colon sections were immunostained against CD3 (green, T cells), Foxp3 (red, Treg), and DAPI (nucleus). (a) Representative images of Treg are shown in each panel (arrows). Treg cells are double-stained with red fluorescence (Foxp3+, intracellular marker) and blue staining (nucleus) surrounded by green fluorescence (CD3+). (b) Ratio of Tregs to total T cells. The percentage of Treg cells compared to total T cells (CD3+Foxp3+ T cells/CD3+ T cells) is shown on the y-axis. AbVCAM 1-MSC-injected mice showed nearly five times greater percentage of Tregs compared to PBS-only (ANOVA, P < 0.0001), whereas MSC only injected showed nearly a tripling of the Treg percentage compared to PBS-injected mice (P < 0.05); ANOVA, analysis with Tukey post hoc analysis. Interestingly, AbVCAM-1-MSCs showed a statistical difference compared to PBS and MSC only (ANOVA, *P < 0.05, Tukey's post hoc test). Ab, antibody; AbVCAM-1-MSCs, vascular cell adhesion molecule antibody-coated MSCs; ANOVA, analysis of variance; DAPI, 4′-6-diamidino-2-phenylindole; MSC, mesenchymal stem cell; PBS, phosphate-buffered saline.
Discussion
An essential component of any stem cell therapy is the efficient delivery to a location within the host where those cells have the greatest chance of producing a positive effect. Few studies2,4 have looked at ways to increase stem cell delivery and engraftment, and none, prior to this study, have shown significant difference in clinical outcome measures. The goal of this study is to test a methodology to target stem cells to specific sites of inflammation. MSCs were targeted to sites of inflammation in an IBD model with the goal of having those engrafted MSCs produce a “quieting” effect on the inflammatory process owing to their immunosuppressive potential.7,8,9,10,11,12,13,14,15,16,17,18,19 It was hypothesized that a set of cell paints, antibodies to addressins, would direct MSCs to sites of inflammation and thereby increase efficacy to IBD treatment.
From our results, MSCs targeted by Ab coating showed increased efficacy of treatment, as indicated from survival rate, body weight recovery, colon length, weight/length measurement, and histological scores. Interestingly, targeted MSCs were also shown to modulate the balance between Tregs and T cells within the target tissue, as demonstrated by immunofluorescent quantification. Taken together, this targeting methodology has been shown to increase delivery of stem cells to inflamed organs and may provide a means of increasing the efficiency of cell delivery in other disease models using stem cells as a therapy.
A possible mechanism of enhanced therapeutic effects mediated by Ab-coated MSCs treatment is proposed (Supplementary Figure S4). First, it is suggested that improved therapeutic effects are induced from the increased MSC delivery to sites of inflammation by addressin Ab coating, as indicated by the addressin Ab coating on MSCs exhibiting highly increased MSCs adhesion on upregulated addressins on activated endothelial cells, as observed previously in vitro6 and, now, in vivo. An in vitro cell binding assay showed that increased cell binding of AbVCAM-1-MSCs on activated mouse endothelial cells by tumor necrosis factor–α treatment (Supplementary Figure S5) was seen compared to MSC only in a VCAM-1 expression dependent manner (Supplementary Figure S6). Moreover, in BLI for MSC trafficking in vivo, AbVCAM-1- MSCs showed highest cell delivery among MSC only, AbMAdCAM-MSCs, and Abisotype-MSCs. Once delivered to inflamed sites, MSCs are thought to ameliorate inflammatory responses through contact-dependent and soluble factors.20 A recent publication7 directly demonstrated a role for MSCs in reducing inflammation in IBD, wherein data was presented showing reduced inflammation by MSC activity is associated with downregulation of the production of a wide panel of inflammatory/cytotoxic mediators by mucosal immune cells, and by increased levels of the anti-inflammatory cytokine, interleukin-10.
In addition to direct reduction of colonic inflammation by infused MSCs, MSCs have been reported to induce T cell into Treg in vitro29,30 and in vivo7 to ameliorate inflammation. Treg cells, characterized by CD4+CD25+Foxp3+, play a major role in the maintenance of immune tolerance to self and in regulating T-cell homeostasis.31,32 Therefore, it was hypothesized that the presence of MSCs would affect the number of Treg and AbVCAM-1-MSCs, which showed the strongest BLI signal in vivo, would generate higher numbers of Treg cells in colon than any other controls. In the Treg quantification studies (Figure 5), it was confirmed that the highest Treg numbers and ratio to T cells were found in the colon from AbVCAM-1-MSCs injected mice. This is the first report to show evidence that MSC infusion increased Treg numbers and ratio to T cells in acute colitis colon.
The incorporation of VCAM-1 Ab onto MSCs is another factor to improve therapeutic results, independent of enhanced cell targeting. This interpretation is supported by the in vitro immunosuppression assay (Figure 1), where it was demonstrated that AbVCAM-1-MSCs showed higher T-cell suppression than MSC only, strongly indicating that the released antibodies themselves may contribute to this synergistic suppressive effect. Regarding Ab release from MSC, flow cytometric analysis of MSC coated with fluorescein isothiocyanate-labeled human immunoglobulin G demonstrated that palmitated protein G (PPG)-anchored antibodies have 3.4 hours half-life in 37 °C culture (Supplementary Figure S7). In the free Ab in vivo experiment (Figure 3a), it was shown that the significantly improved therapeutic effect (Figure 3) is mediated by Ab anchored MSCs, not by the mixture of MSCs and free Ab. One interpretation is that Ab-coated MSCs can travel to inflamed sites and then antibodies released from the coated MSCs are locally delivered to the inflamed sites, whereas free antibodies diffuse rapidly throughout the body in the case of the mixture of MSCs and free Ab. There are two possible actions in reducing inflammation by released antibodies. One is that released antibodies in the bloodstream can effectively block T-cell adhesion on activated endothelium. The other is that Ab release from MSCs, which transmigrated to sites of inflammation, is localized within inflammation areas. These results indicate the importance of Ab anchoring in exhibiting therapeutic effects compared to free Ab.
For AbMAdCAM-MSCs injected mice, it was shown that a single-systemic injection of AbMAdCAM-MSCs ameliorated the clinical and histological signs of IBD, and increased survival rate from 40 to 92%, which was an unexpected finding in light of the fact that AbVCAM-MSCs showed a greater localization to the colon based on BLI results. Therefore, it cannot be concluded that increased survival rate is simply a result of more efficient MSC delivery to colon, because AbMAdCAM-MSCs delivery was observed to be as poor as that of the MSCs only group (Figure 2). This raises the possibility that locally released MAdCAM Ab have a independent effect on the local immune-modulatory milieu. However, while AbVCAM-MSCs showed significantly greater suppression of T-cell proliferation, AbMAdCAM-MSCs did not (Figure 1), which would support the hypothesis that MAdCAM Ab, used as an anti-inflammatory drug, to treat experimental colitis in IBD, works via a mechanism of endothelium blocking.33,34,35
In summary, it is suggested that MSCs can be used as an immunosuppressive cell and also as a drug delivery vehicle—in this case by delivering antibodies that were included to increase MSC delivery. Based on these conclusions, several possible strategies are available to improve IBD treatment using MSC targeting: (i) a combination of dual targeting molecules using VCAM-1 and MAdCAM Ab on the same cells, which has proven to be a more effective delivery method in cartilage studies;5 and (ii) a combination of targeting Ab and therapeutic Ab, such as anti-tumor necrosis factor–α (Infliximab) or anti-α4 Ab (Natalizumab), which have currently been using as anti-inflammatory drugs.36 The possibility of transducing MSCs with modulatory cytokines, like interleukin-10, is another option.
In conclusion, these data indicate that targeting MSCs to IBD by Ab coating against addressins has opened a new therapeutic technique for suppressing inflammation using cell-based therapies that can be applied to other inflammation-related diseases. This cell delivery methodology is universal in that it can be applied to any potentially therapeutic cells and, in addition, provides a mechanism to deliver potential therapeutic molecules along with the delivered cells.
Materials and Methods
Cell culture. The murine MSCs used here are from a conditionally immortalized mouse MSC line, termed bone marrow cell-9 cells, that have been shown to retain multipotentiality37 and were originally derived from C57BL-10 × CBA/CA background.38 Bone marrow cell-9 cells were cultured at 33 °C, 5% CO2 in low-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1 µg/ml γ-interferon, penicillin, and streptomycin. For these studies, murine MSCs were transduced with a triple-fusion reporter, fluc–mrfp–ttk (encoding firefly luciferase, monomeric red fluorescent protein, and truncated herpes simplex virus type 1 sr39 thymidine kinase) by use of a lentiviral vector, as per Love et al.39 Transduced MSCs were sorted by fluorescent-activated cell sorting (excitation wavelength: 575 nm, emission wavelength: 618 ± 20 nm) at the Flow Cytometry Facility at the University of Pittsburg School of Medicine (St. Pittsburgh, PA). Luciferase enzyme activity was assayed by incubating fluc-MSCs in a 96-well microplate with 50 µl of -luciferin (Promega, Fitchburg, WI) and imaging with a Xenogen IVIS 200 System (Xenogen, Alamada, CA), followed by analysis using the Xenogen Living Image software.
Ab coating on cells. The cell coating method followed was described previously.6 In brief, MSCs were harvested with 0.25% trypsin, 0.25 mmol/l EDTA for 5 minutes at 37 °C, washed, incubated in 50 µg/ml of PPG, and washed with Dulbecco's modified Eagle's medium to remove unreacted PPG. The PPG-coated cells (PPG-MSCs) were then incubated in goat anti-mouse VCAM-1 Ab or MAdCAM (R&D Systems, Minneapolis, MN) at 100 µg/ml in Dulbecco's modified Eagle's medium on 4 °C for 1 hour. Finally, Ab-coated cells (Ab-MSCs) were washed with Dulbecco's modified Eagle's medium to remove unreacted Ab to PPG-MSCs.
Immune suppression assay. For the immune cell suppression assay by MSCs,40 3 × 103, 5 × 103, and 1 × 104 MSCs were plated in a flat-bottom 96-well plate (Falcon; BD Bioscience, San Jose, CA) and cultured for 2 hours, and then 3 × 105 splenocytes per well (freshly isolated from C57BL6 female mice) were added to preadhered MSCs and cocultured for 3 days. To stimulate T cells, anti-CD3ε (2C11) (BD Pharmingen, San Jose, CA) was added to each well with a 1 µg/ml of concentration. Proliferation was assessed by pulsing cells for 12–16 hours with 1 µCi/well of tritiated thymidine (Perkin Elmer, Waltham, MA). Cells were harvested using a cell harvester (Packard Instrument) and incorporated tritiated thymidine was measured by scintillation spectrometry (Packard Instrument, Waltham, MA). As a control, MSCs only were cultured and the proliferation was assessed with the same way as cocultures with splenocytes above.
In vivo BLI and fluorescent imaging. For BLI and fluorescent imaging, luciferase-expressing MSCs were labeled with far-red dye (CellTrace DDAO-SE; Invitrogen, Carlsbad, CA) as protocol; far-red dye shows minimal autofluorescence in frozen tissue sections.28 Abs were coated onto MSCs, as described above, and were administered to C57BL6 mice (female, 7 weeks) via tail vein injection (1.0 × 106 cells/mouse) treated with 5% DSS for 5 days. At 2 hours postinjection, each animal was given an intraperitoneal injection of 0.2 mg of -luciferin (Promega) in 0.2 ml of sterile PBS and then and then was imaged with an IVIS 200 System (Xenogen), mice were sacrificed, and isolated lung, spleen, MLN, and colon were imaged and then frozen for cryosectioning. For fluorescent MSC localization, cryosectioned tissue was counter-stained with 4′-6-diamidino-2-phenylindole and observed with a fluorescent microscope (Leica, DM6000, Leica Microsystems, Bannockburn, IL) equipped with a near infrared 622-nm wavelength filter.
IBD model. All IBD mice were housed in the Animal Resource Facility at Case Western Reserve University (Cleveland, OH), and all procedures were performed under an approved IACUC protocol. C57BL/6 mice were chosen as hosts due to their similarity in genetic background to bone marrow cell-9 cells which, along with the documented immune-suppressive qualities of MSCs,41 minimized the likelihood of immune rejection. Colitis is induced in mice by the addition of 5% DSS in the drinking water for 7 days. Body weight and survival were monitored over 16 days, and at day 16, mice were sacrificed, colon length and weight were measured, and tissues were fixed in formalin and prepared for histology. Recovery in colon length (%) is defined as an equation: 100 × (Lsample − LPBS)/LWT – LPBS). Lsample, LWT, and LPBS are the colon length of sample, wild type, and PBS-injected mice, respectively. From this equation, recovery in colon length in wild type and PBS-injected mice is 100 and 0%, respectively. The degree of IBD severity was determined from histological scores based on hematoxylin and eosin-stained images of colon.42 In brief, inflammation was graded from 0 to 4 as follows in a blinded fashion: 0, no signs of inflammation; 1, low leukocyte infiltration; 2, moderate leukocyte infiltration; 3, high leukocyte infiltration, moderate fibrosis, high vascular density; 4, transmural infiltration, extensive fibrosis. Immunostaining against CD3 and Foxp3 was used to identify Tregs. For immunostaining, paraffin sections were deparaffinized and underwent heat induced epitope retrieval by boiling sections in 0.01 mol/l citrate buffer. Sections were then treated with peroxidase quenching solution (Sigma, St Louis, MO) and then with primary Ab of rabbit anti-human CD3 (1:600; DAKO, Carpinteria, CA) and rat anti-mouse Foxp3 antibody (1:50; eBioscience, San Diego, CA) at the same time, washed with PBS and treated with Alexa 488 goat anti-rabbit (1:200) and Alexa568 goat anti-rat (1:200) secondary antibodies together. The double-immunostained section was observed with a fluorescent microscope (Leica, DM6000) and images were processed with Adobe photoshop CS3 software.
Statistical analyses. Student's t-test analysis was applied to cell binding assay and BLI data. Survival rate was analyzed by Kaplan–Meier log-rank test. Body weight change was tested for significance on different days by ANOVA and additional statistical analysis was performed with the SAS Proc Mixed test in order to include the data from multiple time points for a more statistically robust analysis. ANOVA analysis and Tukey post hoc testing was applied to colon length, colon weight/length, and percentage of Treg number in colon. Histological scores were analyzed by the nonparametric Mann–Whitney test.
SUPPLEMENTARY MATERIAL Figure S1. Preparation of Fluc-MSC. Figure S2. MSC localization in lung and colon 2 hours postinjection. Figure S3. Quantification of antibody incorporation on MSCs. Figure S4. Schematic diagram of effective targeting Ab-MSCs to inflammatory sites. Figure S5. MSC binding to bEnd.3. Figure S6. Addressin expression on bEnd.3 cells. Figure S7. Antibody release test from cell membrane. Materials and Methods.
Acknowledgments
We thank Dr Lee for lentivirus for MSC transduction, Drs Schluchter and Panneerselvam for statistical analysis, Dr Hyoung-Gon Lee for assistance with Treg immunostaining (Department of Pathology, CWRU), and Dr Donnenberg (Pittsburg School of Medicine) for help with mrfp FACS analysis. This publication was made possible by grant number R01R49785-01A1 from NIAMS/NIH.
Supplementary Material
Preparation of Fluc-MSC.
MSC localization in lung and colon 2 hours postinjection.
Quantification of antibody incorporation on MSCs.
Schematic diagram of effective targeting Ab-MSCs to inflammatory sites.
MSC binding to bEnd.3.
Addressin expression on bEnd.3 cells.
Antibody release test from cell membrane.
REFERENCES
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- Xia L, McDaniel JM, Yago T, Doeden A., and, McEver RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Vemula PK, Teo GS, Spelke D, Karnik R, Wee le Y, et al. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug Chem. 2008;19:2105–2109. doi: 10.1021/bc800345q. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Cohen N, Goldberg VM., and, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res. 2004;22:735–741. doi: 10.1016/j.orthres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ko IK, Kean TJ., and, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702–3710. doi: 10.1016/j.biomaterials.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D., and, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, et al. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol. 2009;218:350–359. doi: 10.1002/path.2535. [DOI] [PubMed] [Google Scholar]
- García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451–454. doi: 10.1007/s00384-003-0490-3. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Aksu AE, Horibe E, Sacks J, Ikeguchi R, Breitinger J, Scozio M, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol. 2008;127:348–358. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- Mueller MB., and, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- Abdi R, Fiorina P, Adra CN, Atkinson M., and, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA., and, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Aggarwal S., and, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC., and, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ., and, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Karp JM., and, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Troy T, Jekic-McMullen D, Sambucetti L., and, Rice B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 2004;3:9–23. doi: 10.1162/15353500200403196. [DOI] [PubMed] [Google Scholar]
- Prevosto C, Zancolli M, Canevali P, Zocchi MR., and, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M., and, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP., and, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E., and, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- Farkas S, Hornung M, Sattler C, Edtinger K, Steinbauer M, Anthuber M, et al. Blocking MAdCAM-1 in vivo reduces leukocyte extravasation and reverses chronic inflammation in experimental colitis. Int J Colorectal Dis. 2006;21:71–78. doi: 10.1007/s00384-004-0709-y. [DOI] [PubMed] [Google Scholar]
- Kato S, Hokari R, Matsuzaki K, Iwai A, Kawaguchi A, Nagao S, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–189. [PubMed] [Google Scholar]
- Siegmund B., and, Zeitz M. Clinical aspects of inflammatory bowel disease. Eur J Immunol. 2009;39:2026–2030. doi: 10.1002/eji.200939601. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love Z, Wang F, Dennis J, Awadallah A, Salem N, Lin Y, et al. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J Nucl Med. 2007;48:2011–2020. doi: 10.2967/jnumed.107.043166. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., and, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A., and, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preparation of Fluc-MSC.
MSC localization in lung and colon 2 hours postinjection.
Quantification of antibody incorporation on MSCs.
Schematic diagram of effective targeting Ab-MSCs to inflammatory sites.
MSC binding to bEnd.3.
Addressin expression on bEnd.3 cells.
Antibody release test from cell membrane.